Microbiome in Neuroblastoma: A Virgin Island in the World of Onco-Microbiome
Abstract
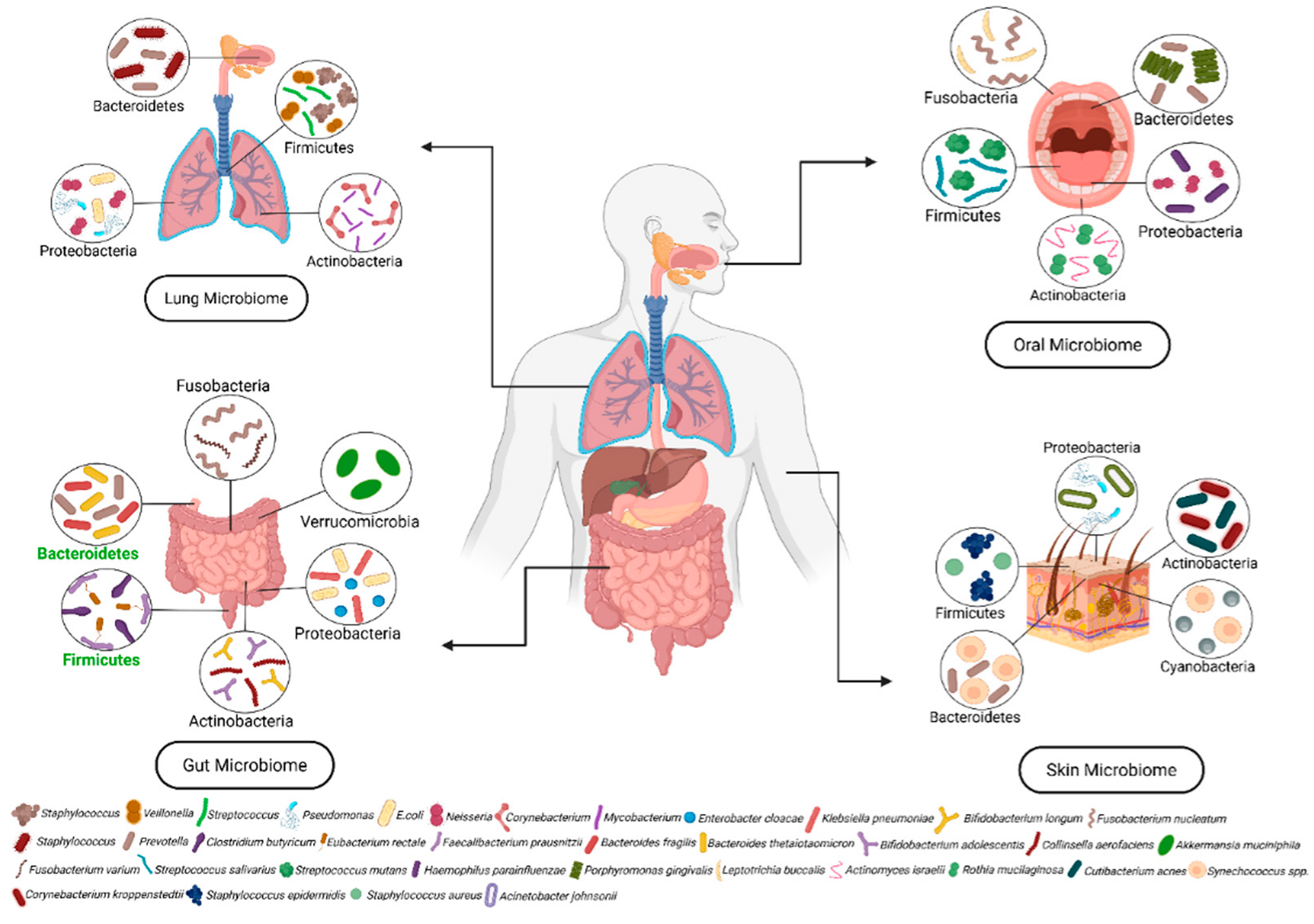
1. Microbiome
1.1. Composition of Microbiota
1.2. Traits of Microbial Diversity
1.3. Microbiota in Human Health
| Disease | Microbiome Involved | Factors Contributing to Dysbiosis | Effect of Dysbiosis | Reference |
|---|---|---|---|---|
| Parkinson’s disease | ↑ Akkermansia, Lactobacillus ↓ Prevotella, Roseburia | Genetic traits, pesticide exposure, antibiotic use, diet | Exacerbates neuroinflammation via gut–brain axis | [48] |
| Alzheimer’s disease | ↑ Escherichia/Shigella, Bacteroides ↓ Faecalibacterium prausnitzii | Aging, poor nutrition, inflammation, chronic disease | Promotes neuroinflammation and amyloid-beta deposition | [49] |
| Multiple Sclerosis | ↑ Methanobrevibacter smithii, Akkermansia ↓ Clostridia, | Vitamin D deficiency, antibiotics, environmental factors | Alters immune regulation and gut permeability | [50] |
| Huntington’s Disease | ↑ Proteobacteria, ↓ Lactobacillus | Genetic mutation, oxidative stress, altered nutrition | Disrupts metabolic and neuroimmune homeostasis | [51] |
| Autism Spectrum Disorder | ↑ Clostridia, Desulfovibrio ↓ Bifidobacterium | Cesarean birth, early antibiotic exposure, formula feeding | Impairs neurodevelopment via microbial metabolite imbalance | [52] |
| Chronic Fatigue Syndrome | ↑ Enterobacteriaceae ↓ Faecalibacterium, SCFA-producing bacteria | Viral infections, chronic stress, gut permeability | Drives systemic inflammation and immune dysfunction | [53] |
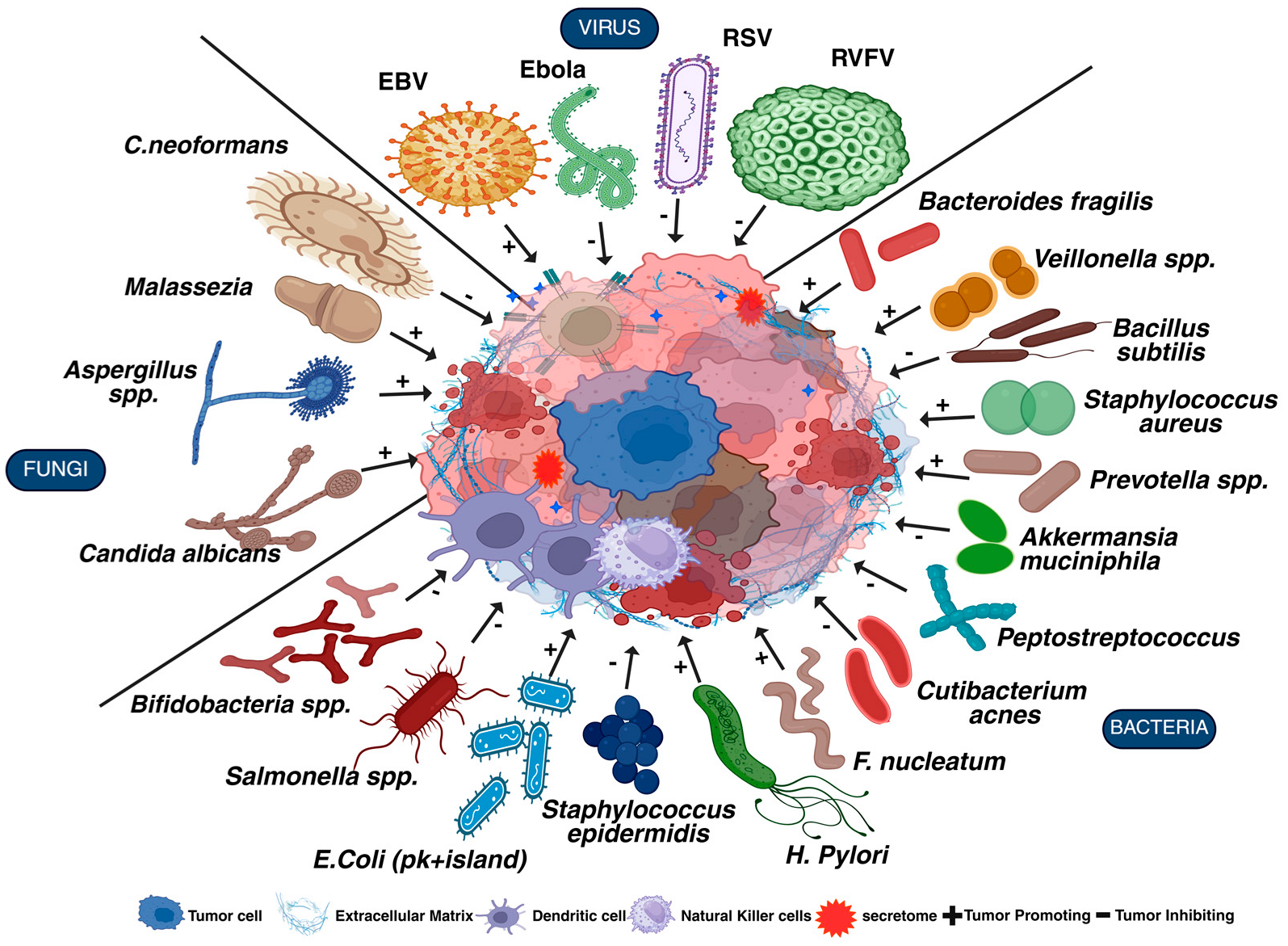
2. Microbiome in Cancer
| Cancer | Microbes | Pathway | Mechanism | Effects | Reference |
|---|---|---|---|---|---|
| Colorectal Cancer | Fusobacterium nucleatum | Wnt/β-catenin | FadA from F. nucleatum activates β-catenin via E-cadherin binding | Promote proliferation and tumor initiation | [63] |
| Enterotoxigenic Bacteroides fragilis | NFκB/STAT3 | BFT toxin and LPS stimulate chronic inflammation, IL-6 upregulates STAT3 | Drives inflammation and immune evasion | [64] | |
| E. coli (pks+) island | PI3K/AKT/mTOR | Colibactin and ROS activate PI3K/Akt Promotes tumorigenesis Alters tumor suppressor genes TP53 and proto-oncogenes KRAS | Enhance survival, angiogenesis | [65] | |
| F. nucleatum, E. coli | TLR/MyD88/MAPK | Microbial ligands activate TLRs → MyD88 → MAPK cascade | Inflammatory signaling, cytokine production | [66] | |
| Gastric Cancer | H. pylori (CagA) | SHP-2/Ras/ERK | CagA protein activates NFκB (direct injection into epithelial cells); LPS induces inflammation loss of polarity and hyperproliferation | Promotes cell proliferation and transformation | [67] |
| H. pylori, Peptostreptococcus | NFκB/STAT3 | IL-6, TNFα driven by H. pylori and others → survival signaling | Chronic inflammation and immune modulation | [68] | |
| H. pylori (VacA) | PI3K/AKT | SCFAs and VacA promote survival and immune suppression | Cell survival and metabolic shift | [69] | |
| F. nucleatum Peptostreptococcus, | EMT-related (Snail, Twist) | Inflammation triggers EMT programs via NFκB and others | Invasion and metastasis | [70] | |
| Esophageal Cancer | P. gingivalis, F. nucleatum | NFκB/IL-6/STAT3 | Chronic exposure to P. gingivalis LPS and F. nucleatum leads to NFκB activation → IL-6 secretion → STAT3 phosphorylation via TLR4 signaling Drives cell survival, angiogenesis, and immune evasion | Inflammation-driven survival, growth, and angiogenesis | [71] |
| Candida albicans | EGFR/STAT3 | Candida albicans and oral dysbiosis increase EGF, HER2 receptor kinase, TGF-α (EGFR ligand expression) → EGFR activation → STAT3-driven survival and proliferation | Proliferation, inhibition of apoptosis | [72] | |
| Candida albicans, Prevotella, Veillonella | TLR/MyD88/MAPK | TLR2/4 recognize PAMPs from fungi and Gram-negative bacteria → activate MyD88 → downstream MAPKs (ERK, JNK, p38) → cytokine storm and inflammation | Immune modulation and inflammation | [73] | |
| Pancreatic Cancer | Malassezia | KRAS/MAPK | Mutant KRAS is central to PDAC. Microbiota-driven inflammation (e.g., IL-1β, TNFα) enhances KRAS downstream signaling (ERK, MEK) Increased proliferation and survival | Drives oncogenesis | [74,75] |
| E. coli, Malassezia | Hedgehog (Shh/Gli) | Microbial imbalance can dysregulate Shh/Gli signaling in the tumor microenvironment, leading to excessive stroma formation, reducing drug delivery and enhancing immune exclusion | Stromal reprogramming and desmoplasia | [76] | |
| F. nucleatum, Malassezia, E. coli | TLR/MyD88 | Bacterial and fungal components (LPS, β-glucans) bind TLRs → MyD88-dependent signaling → NFκB/MAPK activation → inflammation Macrophage reprogramming to pro-tumor M2 phenotype | Inflammation and immune evasion | [77] | |
| Breast Cancer | Firmicutes, Proteobacteria | Estrogen Metabolism | Altered microbiome composition increases β-glucuronidase activity → deconjugates estrogen glucuronide → promotes reabsorption of active estrogen | Increased risk of ER+ breast cancer | [78] |
| Bacteroides spp. | Estrobolome | Produces β-glucuronidase enzyme that deconjugates estrogens in the gut → estrogen re-enters circulation (enterohepatic recycling) | Elevated estrogen levels promote ER+ breast cancer | [78,79] | |
| E. coli, S. aureus | NFκB, STAT3 | Induce ROS and inflammatory cytokines Promote DNA double-strand breaks Activate survival and proliferation pathways | Promotes tumorigenesis via genomic instability and inflammation | [80] | |
| Lung Cancer | Akkermansia muciniphila | TLR2–IL-10 axis | Stimulates TLR2 → promotes anti-inflammatory cytokine IL-10 Enhances gut barrier integrity and Treg induction | Protectiv—reduces inflammation and promotes immune surveillance | [81] |
| Prevotella spp, Veillonella, | TLR/MyD88/NFκB | Bacterial PAMPs (e.g., LPS) bind TLRs (TLR2, TLR4), activates MyD88 → NFκB pathway, cytokine release (IL-6, TNFα) → chronic inflammation | Promotes inflammation, DNA damage, immune suppression → tumor initiation and progression | [82] | |
| Bifidobacterium spp. | TLR9/IFN-γ signaling | Stimulates TLR9 on dendritic cells → Increases IFN-γ, CD8+ T cell activation Enhances antigen presentation | Enhances anti-tumor immunity, reduces immunosuppressive microenvironment | [83] | |
| Haemophilus, Streptococcus, | MAPK/ERK | TLR signaling and microbial cytokines activate MAPK cascade Activates ERK, p38 → gene expression for proliferation | Increases proliferation, survival, and tissue remodeling favorable for tumor development | [84] | |
| Skin Cancer | Staphylococcus aureus | TLR2/TLR4 | Lipoteichoic acid and peptidoglycan activate TLR2/TLR4 → MyD88-dependent NFκB activation → IL-6, IL-1β secretion Produces toxins that induce reactive oxygen species (ROS) → oxidative stress | Enhances tumor progression and immune evasion and DNA damage, genomic instability | [85] |
| Staphylococcus epidermidis | 6-HAP-mediated Inhibition | Produces 6-HAP (6-N-hydroxyaminopurine) → inhibits DNA polymerase activity → reduces DNA synthesis in tumor cells | Suppresses tumor growth and exhibits protective effects | [86] | |
| Cutibacterium acnes | TLR2/NFκB | Activates TLR2 on keratinocytes → NFκB activation → pro-inflammatory cytokine release Induces chronic inflammation and oxidative stress | Promotes DNA damage and tumor initiation | [87] | |
| Brain Cancer | Bacteroides fragilis | Kynurenine/AHR Pathway | Alters tryptophan metabolism → increases kynurenine → activates aryl hydrocarbon receptor (AHR) in brain tissues | Favors glioblastoma progression | [88,89] |
| Clostridium spp | Epigenetic Modulation via SCFAs | Produces butyrate → inhibits histone deacetylases (HDACs) → promotes apoptosis and DNA repair in glial cells | Opposes tumor proliferation in gliomas | [90,91] | |
| Prevotella spp. | Th17/IL-17 | Promotes IL-17-producing Th17 cells via mucosal stimulation → enhances systemic inflammation and disrupts blood–brain barrier (BBB) integrity | Facilitates immune cell infiltration and may promote glioma invasiveness | [92] | |
| Neuroblastoma | Bacteroides fragilis | STAT3/NFκB | Accumulations of myeloid-derived suppressor cells and inhibition of dendritic cell differentiation | Supports tumor progression | [93] |
2.1. Gut Microbiome in Cancer Development
2.1.1. Gastrointestinal Cancer (GI)
2.1.2. Non-Gastrointestinal (Non-GI) Cancer
2.2. Direct and Indirect Effects of Microbiome
| Cancer | Microbial Metabolites | Microbes Involved | Role of Microbial Metabolites in Cancer | Reference |
|---|---|---|---|---|
| Colorectal cancer | SCFA-butyrate | Clostridium butyricum | Butyrate enhances immune responses and inhibits tumor progression by altering T cell differentiation and stemness. | [127] |
| Ferroptosis Inhibitors-Lactate | E. coli, Klebsiella | Inhibit ferroptosis to prevent iron-dependent oxidative damage and promote CRC cell survival and growth. | [128,129] | |
| Indole-3-propionic acid | Lactobacillus, Bifidobacterium | Indole-3-acetate modulates immunological responses via the gut homeostasis-maintaining aryl hydrocarbon receptor (AhR) and enhances mucosal barrier integrity. | [130] | |
| Tryptophan Metabolites- kynurenine | E. faecalis | Inhibit immune responses and contribute to tumor immune evasion. | [131] | |
| Phenylacetic Acid (PAA) | Bacteroides, Lactobacillus | Affects tumor microenvironment and metabolic pathways by modulating immune responses for cell proliferation. | [123] | |
| Gastric cancer | N-nitroso Compounds (NOCs) | E. coli, Enterococcus faecalis | Nitrosamines are mutagenic, generating DNA adducts that induce mutations and genetic instability. | [132] |
| tryptophan, arginine | Lactobacillus, streptococcus | Upregulated in neoplastic tissues; facilitate tumor proliferation and immune evasion through metabolic reprogramming. | [133] | |
| Esophageal cancer | Perfluorooctanoate | Clostridium leptum | Increased PFOA levels influenced by C. leptum are linked to heightened EC risk. Endocrine disruptor | [134] |
| SCFAs | Phascolarctobacterium, Fusobacterium nucleatum | SCFAs can modulate inflammation and support tumor growth by promoting lipid synthesis and maintaining epithelial proliferation | [107] | |
| Lipopolysaccharides | Fusobacterium nucleatum | Promotes chronic inflammation, leading to epithelial damage and carcinogenesis and activates TLR4/NFκB signaling pathway, increasing IL-6, TNFα | [135] | |
| Pancreatic cancer | Trimethylamine N-oxide | Clostridium sporogenes, Anaerococcus hydrogenalis | Enhances anti-tumor immunity; administration in PDAC-bearing mice reduced tumor growth and activated effector T cell responses | [136] |
| Indole-3-acetate | Enterococcus faecalis, Lactobacillus spp. | Suppress the anti-tumor activity by inducing immunosuppressive tumor-associated macrophages | [137] | |
| Breast cancer | SCFA- butyrate, propionate, acetate | Eubacterium rectale, Clostridium perfringen, Faecalibacterium prausnitzii | SCFA act as HDAC inhibitors, inducing apoptosis, cell cycle arrest, and epigenetic changes and modulate IL-10 and TGF-β. | [138] |
| Trimethylamine N-oxide | Clostridiales, Faecalibacterium, Ruminococcaceae | Induces ferroptosis or pyroptosis in tumor cells and promotes anti-tumor immunity | [138] | |
| Lung cancer | kynurenine, indoles | Clostridium sporogenes, Lactobacillus | Regulate pulmonary immune microenvironment via aryl hydrocarbon receptor signaling | [139] |
| Secondary bile acids | Clostridium, Eubacterium | Influence lung immunity via gut–lung circulation and improve immunotherapy outcomes | [139] | |
| Skin cancer | Lipoteichoic acid | Staphylococcus epidermidis | Inhibits UV-induced skin tumor formation via TLR2 signaling. | [140] |
| Phenol-soluble modulins | Staphylococcus aureus, Cutibacterium acnes | Promote inflammation and immune evasion, contributing to squamous cell carcinoma development | [140] | |
| Brain cancer | Polyunsaturated fatty acids | Alistipes, Bacteroides | Dysregulated PUFA metabolism leads to neuroinflammation, which is a shared mechanism in glioma and brain tumors | [141] |
| Arachidonic acid, Phenylacetic acid | Bacteroides, Clostridium scindens | Promotes neuroinflammation and amyloid-beta aggregation | [142] |
2.2.1. Microbial Metabolites (Direct Effect)
2.2.2. Reactive Oxygen and Nitrogen Species (Direct Effect)
2.2.3. Immune Modulation (Indirect Effect)
2.2.4. Cancer Therapy Modulation
3. The Role of Gut Microbiota in Pediatric Cancer: Implications for Immune Modulation, Dysbiosis, and Therapeutic Interventions
4. Neuroblastoma
4.1. Microbiome and Neuroblastoma
4.1.1. Gut Microbiome Predicts the Risk for NB
4.1.2. Postnatal (And Not Maternal Transmission) Programming of Microbiome in NB Patients
4.1.3. Function of Gut Microbe on NB Pathogenesis
4.1.4. Presence of NB Alters Gut Microbiome Composition
4.1.5. Microbial Composition Predicts Treatment Outcome in NB (Murine Model)
4.1.6. Prebiotic Treatment Mitigates NB-Steered Microbial Mayhem
4.1.7. Microbial Composition in TME Predicts NB Outcome
5. Clinical Research Landscape: Challenges and Ongoing Trials in Neuroblastoma Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| SCFAs | Short-chain fatty acids |
| HMOs | Human milk oligosaccharides |
| VOCs | Volatile organic compounds |
| IBD | Inflammatory bowel disease |
| TME | Tumor microenvironment |
| EBV | Epstein–Barr virus |
| HTLV | Human T cell lymphotropic virus |
| CRC | Colorectal cancer |
| TLRs | Toll-like receptors |
| BFT | Bacteroides fragilis toxin |
| NFκB | Nuclear factor kappa light chain enhancer of activated B cell |
| HDAC | Histone deacetylases |
| GC | Gastric cancer |
| CagAA | Cytotoxin-associated gene |
| VacA | Vacuolating cytotoxin A |
| CNS | Central nervous system |
| ICB | Immunological checkpoint blockade |
| NOCs | N-nitroso Compounds |
| DCA | Deoxycholic acid |
| HCC | Hepatocellular carcinoma |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| TNFα | Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| STAT3 | Signal transducer and activator of transcription 3 |
| Tregs | Regulatory T cells |
| PD-1 | Programmed Cell Death Protein 1 |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| ALL | Acute lymphoblastic leukemia |
| GALT | Gut-associated lymphoid tissue |
| BDNF | Brain-derived neurotrophic factor |
| LPS | Lipopolysaccharides |
| BBB | Blood-brain barrier |
| IL-10 | Interleukin-10 |
| HL | Hodgkin’s lymphoma |
| NHL | Non-Hodgkin’s lymphoma |
| NCC | Neural crest cells |
| NB | Neuroblastoma |
| IgA | immunoglobulin A |
| HAIs | Hospital-associated infections |
| GOS | Galactooligosaccharides |
| FOS | Fructooligosaccharides |
| IMCT | Intensive multi-modal clinical therapy |
| INRGSS | International Neuroblastoma Risk Group Staging System |
| INSS | International Neuroblastoma Staging System |
| DC | Dendritic cell |
| MHC-I | Major histocompatibility complex class I |
| TGF-β | Transforming growth factor-beta |
| PGE2 | Prostaglandin E2 |
| IFN-γ | Interferon-gamma |
| NKG2D | Natural killer group 2 member D |
| GWAS | Genome-wide association study |
| SNPs | Single nucleotide polymorphisms |
| MR | Mendelian randomization |
| LCA | Lithocholic acid |
| DCA | Deoxycholic acid |
| UDC | Ursodeoxycholic acid |
| CTX | Cyclophosphamide |
| TAC | Tumor-associated cachexia |
| COG | Children’s Oncology Group |
| CREB | cAMP response element-binding protein |
References
- Kennedy, M.S.; Chang, E.B. The microbiome: Composition and locations. Prog. Mol. Biol. Transl. Sci. 2020, 176, 1–42. [Google Scholar]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Laterza, L.; Rizzatti, G.; Gaetani, E.; Chiusolo, P.; Gasbarrini, A. The Gut Microbiota and Immune System Relationship in Human Graft-versus-Host Disease. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016025. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral. Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef] [PubMed]
- Natalini, J.G.; Singh, S.; Segal, L.N. The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 2023, 21, 222–235. [Google Scholar] [CrossRef]
- Dickson, R.P.; Martinez, F.J.; Huffnagle, G.B. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014, 384, 691–702. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Dasaraju, P.V.; Liu, C. Infections of the respiratory system. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Bassis, C.; Erb-Downward, J.; Dickson, R. Analysis of the Upper Respiratory Tract Microbiotas as the Source of the Lung and Gastric Microbiotas in Healthy Individuals. MBio 2015, 6, e37. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Sun, Q.; Wei, J.; Li, B.; Qiu, Y.; Liu, K.; Shao, D.; Ma, Z. Targeting the pulmonary microbiota to fight against respiratory diseases. Cells 2022, 11, 916. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S.S. Diet, microbiota and gut-lung connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Auchtung, T.A.; Fofanova, T.Y.; Stewart, C.J.; Nash, A.K.; Wong, M.C.; Gesell, J.R.; Auchtung, J.M.; Ajami, N.J.; Petrosino, J.F. Investigating Colonization of the Healthy Adult Gastrointestinal Tract by Fungi. mSphere 2018, 3, 00092-18. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jin, B.; Xu, X.; Zuo, X.; Li, Y.; Li, Z. The Effects of Delivery Mode on the Gut Microbiota and Health: State of Art. Front. Microbiol. 2021, 12, 724449. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.B.; Jordan, S.; Baker, B.J.; Carlson, N.S. The Maternal Infant Microbiome: Considerations for Labor and Birth. MCN Am. J. Matern. Child. Nurs. 2017, 42, 318–325. [Google Scholar] [CrossRef]
- Wernroth, M.L.; Peura, S.; Hedman, A.M.; Hetty, S.; Vicenzi, S.; Kennedy, B.; Fall, K.; Svennblad, B.; Andolf, E.; Pershagen, G.; et al. Development of gut microbiota during the first 2 years of life. Sci. Rep. 2022, 12, 9080. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.-C.; Kiilerich, P.; Akrami, R.; Krämer, M.; Uhlén, M. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 2021, 29, 765–776.e3. [Google Scholar] [CrossRef]
- Ronan, V.; Yeasin, R.; Claud, E.C. Childhood Development and the Microbiome-The Intestinal Microbiota in Maintenance of Health and Development of Disease During Childhood Development. Gastroenterology 2021, 160, 495–506. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Gupta, A. Influence of Early Life, Diet, and the Environment on the Microbiome. Clin. Gastroenterol. Hepatol. 2019, 17, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Mörkl, S.; Lackner, S.; Meinitzer, A.; Mangge, H.; Lehofer, M.; Halwachs, B.; Gorkiewicz, G.; Kashofer, K.; Painold, A.; Holl, A. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur. J. Nutr. 2018, 57, 2985–2997. [Google Scholar] [CrossRef]
- Hartmann, E.M.; Hickey, R.; Hsu, T.; Betancourt Román, C.M.; Chen, J.; Schwager, R.; Kline, J.; Brown, G.; Halden, R.U.; Huttenhower, C. Antimicrobial chemicals are associated with elevated antibiotic resistance genes in the indoor dust microbiome. Environ. Sci. Technol. 2016, 50, 9807–9815. [Google Scholar] [CrossRef]
- Gupt, A.; Naudiyal, S.; Rani, A.; Kumar, S. Mental health and the microbiome: A review of psychological impacts of gut microflora. Curr. Pharmacol. Rep. 2024, 10, 223–236. [Google Scholar] [CrossRef]
- Oldereid, T.S.; Jiang, X.; Nordhus, K.S.; Ponzetta, A.; Bjornholt, J.V.; Bjorkstrom, N.K.; Melum, E.; Rasmussen, H. Role of bacteria and microbial metabolites in immune modulation during early life. Scand. J. Immunol. 2024, 99, e13336. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fulling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Dadgar, N.; Edlukudige Keshava, V.; Raj, M.S.; Wagner, P.L. The Influence of the Microbiome on Immunotherapy for Gastroesophageal Cancer. Cancers 2023, 15, 4426. [Google Scholar] [CrossRef] [PubMed]
- Todor, S.B.; Ichim, C. Microbiome Modulation in Pediatric Leukemia: Impact on Graft-Versus-Host Disease and Treatment Outcomes: A Narrative Review. Children 2025, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Franzin, M.; Stefančič, K.; Lucafò, M.; Decorti, G.; Stocco, G. Microbiota and drug response in inflammatory bowel disease. Pathogens 2021, 10, 211. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837. [Google Scholar] [CrossRef]
- Li, W.-Z.; Stirling, K.; Yang, J.-J.; Zhang, L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes 2020, 11, 293. [Google Scholar] [CrossRef]
- Akagawa, S.; Kaneko, K. Gut microbiota and allergic diseases in children. Allergol. Int. 2022, 71, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.Y.; Yeo, X.Y.; Bae, H.G.; Lee, D.P.S.; Ho, R.C.; Kim, J.E.; Jo, D.G.; Jung, S. Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases? Life 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The gut–brain axis and its relation to Parkinson’s disease: A review. Front. Aging Neurosci. 2022, 13, 782082. [Google Scholar] [CrossRef]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.-H. Crosstalk between gut and brain in Alzheimer’s disease: The role of gut microbiota modulation strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef]
- Campagnoli, L.I.M.; Marchesi, N.; Varesi, A.; Morozzi, M.; Mascione, L.; Ricevuti, G.; Esposito, C.; Galeotti, N.; Pascale, A. New therapeutic avenues in multiple sclerosis: Is there a place for gut microbiota-based treatments? Pharmacol. Res. 2024, 209, 107456. [Google Scholar] [CrossRef]
- Ekwudo, M.N.; Gubert, C.; Hannan, A.J. The microbiota–gut–brain axis in Huntington’s disease: Pathogenic mechanisms and therapeutic targets. FEBS J. 2025, 292, 1282–1315. [Google Scholar] [CrossRef]
- De Sales-Millán, A.; Aguirre-Garrido, J.F.; González-Cervantes, R.M.; Velázquez-Aragón, J.A. Microbiome–Gut–Mucosal–Immune–Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology. Behav. Sci. 2023, 13, 548. [Google Scholar] [CrossRef]
- Varesi, A.; Deumer, U.-S.; Ananth, S.; Ricevuti, G. The emerging role of gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Current evidence and potential therapeutic applications. J. Clin. Med. 2021, 10, 5077. [Google Scholar] [CrossRef]
- Bultman, S.J. The microbiome and its potential as a cancer preventive intervention. Semin. Oncol. 2016, 43, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Cullin, N.; Azevedo Antunes, C.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef] [PubMed]
- Boccellato, F.; Meyer, T.F. Bacteria Moving into Focus of Human Cancer. Cell Host Microbe 2015, 17, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, X.; Guo, Y.; Yan, J.; Abuduwaili, A.; Aximujiang, K.; Yan, J.; Wu, M. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1, 3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monogr. Eval. Carcinog. Risks Hum. 2008, 97, 3. [Google Scholar]
- Bagga, S.; Bouchard, M.J. Cell cycle regulation during viral infection. In Cell Cycle Control: Mechanisms and Protocols; Springer: Berlin/Heidelberg, Germany, 2014; pp. 165–227. [Google Scholar]
- Zhang, Y.; Guo, W.; Zhan, Z.; Bai, O. Carcinogenic mechanisms of virus-associated lymphoma. Front. Immunol. 2024, 15, 1361009. [Google Scholar] [CrossRef]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Gu, X.; Zhang, J.; Nomoto, D.; Okadome, K.; Baba, H.; Qiu, P. Gut microbiome in gastrointestinal cancer: A friend or foe? Int. J. Biol. Sci. 2022, 18, 4101. [Google Scholar] [CrossRef]
- Pevsner-Fischer, M.; Tuganbaev, T.; Meijer, M.; Zhang, S.H.; Zeng, Z.R.; Chen, M.H.; Elinav, E. Role of the microbiome in non-gastrointestinal cancers. World J. Clin. Oncol. 2016, 7, 200–213. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Hu, T.; Huang, H.; Chen, G.; Jin, B.; Zeng, G.; Liu, J. Entero-toxigenic Bacteroides fragilis contributes to intestinal barrier injury and colorectal cancer progression by mediating the BFT/STAT3/ZEB2 pathway. Cell Cycle 2024, 23, 70–82. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Muhlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor−κB, and up-regulating expression of microRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, Y.; Dou, Y.; Xu, D. Helicobacter pylori and gastric cancer: Mechanisms and new perspectives. J. Hematol. Oncol. 2025, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Lamb, A.; Chen, L.F. Role of the Helicobacter pylori-Induced inflammatory response in the development of gastric cancer. J. Cell. Biochem. 2013, 114, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, N.J.; Caston, R.R.; McClain, M.S.; Ohi, M.D.; Cover, T.L. An overview of Helicobacter pylori VacA toxin biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Yáñez, L.; Soto, C.; Tapia, H.; Pacheco, M.; Tapia, J.; Osses, G.; Salinas, D.; Rojas-Celis, V.; Hoare, A.; Quest, A.F. Co-culture of P. gingivalis and F. nucleatum synergistically elevates IL-6 expression via TLR4 signaling in oral keratinocytes. Int. J. Mol. Sci. 2024, 25, 3611. [Google Scholar] [CrossRef]
- Zhu, W.; Phan, Q.T.; Boontheung, P.; Solis, N.V.; Loo, J.A.; Filler, S.G. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc. Natl. Acad. Sci. USA 2012, 109, 14194–14199. [Google Scholar] [CrossRef]
- Liu, J.; Geng, F.; Sun, H.; Wang, X.; Zhang, H.; Yang, Q.; Zhang, J. Candida albicans induces TLR2/MyD88/NF-κB signaling and inflammation in oral lichen planus-derived keratinocytes. J. Infect. Dev. Ctries 2018, 12, 780–786. [Google Scholar] [CrossRef]
- Elaskandrany, M.; Patel, R.; Patel, M.; Miller, G.; Saxena, D.; Saxena, A. Fungi, host immune response, and tumorigenesis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 321, G213–G222. [Google Scholar] [CrossRef] [PubMed]
- Bansod, S.; Dodhiawala, P.B.; Lim, K.-H. Oncogenic KRAS-induced feedback inflammatory signaling in pancreatic cancer: An overview and new therapeutic opportunities. Cancers 2021, 13, 5481. [Google Scholar] [CrossRef] [PubMed]
- Iriana, S.; Asha, K.; Repak, M.; Sharma-Walia, N. Hedgehog signaling: Implications in cancers and viral infections. Int. J. Mol. Sci. 2021, 22, 1042. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.; Kong, X.; Wu, R.; Peng, Q.; Zhang, Y.; Zhou, L.; Duan, L. Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-κ B/S100A9 Cascade. Front. Immunol. 2021, 12, 658681. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, J.; Chen, J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Jiang, Z.; Tong, H.; Ma, X.; Liu, Y. Tumor microbiome: Roles in tumor initiation, progression, and therapy. Mol. Biomed. 2025, 6, 1–43. [Google Scholar] [CrossRef]
- Souza, V.G.P.; Forder, A.; Pewarchuk, M.E.; Telkar, N.; de Araujo, R.P.; Stewart, G.L.; Vieira, J.; Reis, P.P.; Lam, W.L. The Complex Role of the Microbiome in Non-Small Cell Lung Cancer Development and Progression. Cells 2023, 12, 2801. [Google Scholar] [CrossRef]
- Paudel, K.R.; Dharwal, V.; Patel, V.K.; Galvao, I.; Wadhwa, R.; Malyla, V.; Shen, S.S.; Budden, K.F.; Hansbro, N.G.; Vaughan, A. Role of lung microbiome in innate immune response associated with chronic lung diseases. Front. Med. 2020, 7, 554. [Google Scholar] [CrossRef]
- Xuan, S.; Ma, Y.; Zhou, H.; Gu, S.; Yao, X.; Zeng, X. The implication of dendritic cells in lung diseases: Immunological role of toll-like receptor 4. Genes Dis. 2023, 11, 101007. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S. Targeting MAPK signaling: A promising approach for treating inflammatory lung disease. Pathol.-Res. Pract. 2024, 254, 155122. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z. Bacterial and Host Factors in Staphylococcus aureus Septic Arthritis. Master’s Thesis, University of Gothenburg, Gothenburg, Sweden, 2024. [Google Scholar]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef]
- Lee, E.; Min, K.; Ahn, H.; Jeon, B.-N.; Park, S.; Yun, C.; Jeon, H.; Yeon, J.-S.; Kim, H.; Park, H. Potential therapeutic skin microbiomes suppressing Staphylococcus aureus-derived immune responses and upregulating skin barrier function-related genes via the AhR signaling pathway. Int. J. Mol. Sci. 2022, 23, 9551. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Lauro, C.; Quaglio, D.; Ghirga, F.; Botta, B.; Trettel, F.; Limatola, C. Neuro-Signals from Gut Microbiota: Perspectives for Brain Glioma. Cancers 2021, 13, 2810. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Heng, B.; Guillemin, G.J. The gut microbiota, kynurenine pathway, and immune system interaction in the development of brain cancer. Front. Cell Dev. Biol. 2020, 8, 562812. [Google Scholar] [CrossRef]
- Peng, F.; Li, S.; Wang, L.; Han, M.; Fan, H.; Tang, H.; Peng, C.; Du, J.; Zhou, Z. Combination of Hdac Inhibitor Sodium Butyrate and Immunotherapy in Glioma: Regulation of Immunologically Hot and Cold Tumors Via Gut Microbiota and Metabolites. Front Immunol. 2025, 16, 1532528. [Google Scholar] [CrossRef]
- Stein, R.A.; Riber, L. Epigenetic effects of short-chain fatty acids from the large intestine on host cells. Microlife 2023, 4, uqad032. [Google Scholar] [CrossRef]
- Bellone, M.; Brevi, A.; Huber, S. Microbiota-propelled T helper 17 cells in inflammatory diseases and cancer. Microbiol. Mol. Biol. Rev. 2020, 84, e00064-19. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.S. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Yu, M.R.; Kim, H.J.; Park, H.R. Fusobacterium nucleatum Accelerates the Progression of Colitis-Associated Colorectal Cancer by Promoting EMT. Cancers 2020, 12, 2728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X. Advances of Wnt signalling pathway in colorectal cancer. Cells 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A. Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome in Colorectal Cancer Progression. Front. Immunol. 2021, 12, 807648. [Google Scholar] [CrossRef] [PubMed]
- Hartl, K.; Sigal, M. Microbe-Driven Genotoxicity in Gastrointestinal Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 7439. [Google Scholar] [CrossRef]
- Jeon, J.I.; Ko, S.H.; Kim, J.M. Intestinal epithelial cells exposed to Bacteroides fragilis enterotoxin regulate NF-κB activation and inflammatory responses through β-catenin expression. Infect. Immun. 2019, 87, e00312-19. [Google Scholar] [CrossRef]
- Nakkarach, A.; Foo, H.L.; Song, A.A.-L.; Mutalib, N.E.A.; Nitisinprasert, S.; Withayagiat, U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb. Cell Factories 2021, 20, 36. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Akbari, S.K.A.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef]
- Javanmard, A.; Ashtari, S.; Sabet, B.; Davoodi, S.H.; Rostami-Nejad, M.; Esmaeil Akbari, M.; Niaz, A.; Mortazavian, A.M. Probiotics and their role in gastrointestinal cancers prevention and treatment; an overview. Gastroenterol. Hepatol. Bed Bench 2018, 11, 284–295. [Google Scholar]
- Ansari, S.; Yamaoka, Y. Role of vacuolating cytotoxin A in Helicobacter pylori infection and its impact on gastric pathogenesis. Expert. Rev. Anti-Infect. Ther. 2020, 18, 987–996. [Google Scholar] [CrossRef]
- Fakharian, F.; Asgari, B.; Nabavi-Rad, A.; Sadeghi, A.; Soleimani, N.; Yadegar, A.; Zali, M.R. The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 953718. [Google Scholar] [CrossRef]
- Muszynski, D.; Kudra, A.; Sobocki, B.K.; Folwarski, M.; Vitale, E.; Filetti, V.; Dudzic, W.; Kazmierczak-Siedlecka, K.; Polom, K. Esophageal cancer and bacterial part of gut microbiota—A multidisciplinary point of view. Front. Cell Infect. Microbiol. 2022, 12, 1057668. [Google Scholar] [CrossRef]
- Lv, J.; Guo, L.; Liu, J.-J.; Zhao, H.-P.; Zhang, J.; Wang, J.-H. Alteration of the esophageal microbiota in Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 2019, 25, 2149. [Google Scholar] [CrossRef]
- Moe, K.T.; Tan, K.S.-W. Mechanistic Insights on Microbiota-Mediated Development and Progression of Esophageal Cancer. Cancers 2024, 16, 3305. [Google Scholar] [CrossRef]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Bacterial translocation in gastrointestinal cancers and cancer treatment. Biomedicines 2022, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, D.L.; Zenoniani, A.; Umme, S.; Piattelli, A.; Curia, M.C. Intratumoral Fusobacterium nucleatum in Pancreatic Cancer: Current and Future Perspectives. Pathogens 2024, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Orlacchio, A.; Mazzone, P. The role of toll-like receptors (TLRs) mediated inflammation in pancreatic cancer pathophysiology. Int. J. Mol. Sci. 2021, 22, 12743. [Google Scholar] [CrossRef] [PubMed]
- Mladosievičová, B.; Čierniková, S.; Mego, M. Molecular Mechanisms of Cancer Pathogenesis; Comenius University Bratislava: Bratislava, Slovakia, 2024. [Google Scholar]
- Fernandez, M.F.; Reina-Perez, I.; Astorga, J.M.; Rodriguez-Carrillo, A.; Plaza-Diaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public. Health 2018, 15, 1747. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. The microbiome–estrogen connection and breast cancer risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic. Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef]
- Summer, M.; Ali, S.; Fiaz, U.; Tahir, H.M.; Ijaz, M.; Mumtaz, S.; Mushtaq, R.; Khan, R.; Shahzad, H.; Fiaz, H. Therapeutic and immunomodulatory role of probiotics in breast cancer: A mechanistic review. Arch. Microbiol. 2023, 205, 296. [Google Scholar] [CrossRef]
- Fan, S.; Jiang, Z.; Zhang, Z.; Xing, J.; Wang, D.; Tang, D. Akkermansia muciniphila: A potential booster to improve the effectiveness of cancer immunotherapy. J. Cancer Res. Clin. Oncol. 2023, 149, 13477–13494. [Google Scholar] [CrossRef]
- Pei, B.; Peng, S.; Huang, C.; Zhou, F. Bifidobacterium modulation of tumor immunotherapy and its mechanism. Cancer Immunol. Immunother. 2024, 73, 94. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef] [PubMed]
- Tomela, K.; Pietrzak, B.; Schmidt, M.; Mackiewicz, A. The tumor and host immune signature, and the gut microbiota as predictive biomarkers for immune checkpoint inhibitor response in melanoma patients. Life 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Coggshall, K.; Brooks, L.; Nagarajan, P.; Arron, S.T. The microbiome and its contribution to skin cancer. Microbiome Cancer 2019, 87–106. [Google Scholar] [CrossRef]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Galus, Ł.; Mackiewicz, J.; Kaczmarek, M.; Mackiewicz, A.; Schmidt, M. A clinical outcome of the anti-PD-1 therapy of melanoma in polish patients is mediated by population-specific gut microbiome composition. Cancers 2022, 14, 5369. [Google Scholar] [CrossRef]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef]
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 2020, 6, 192–204. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Nunez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Jia, D.; Wang, Q.; Qi, Y.; Jiang, Y.; He, J.; Lin, Y.; Sun, Y.; Xu, J.; Chen, W.; Fan, L. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 2024, 187, 1651–1665.e21. [Google Scholar] [CrossRef]
- Cui, W.; Guo, M.; Liu, D.; Xiao, P.; Yang, C.; Huang, H.; Liang, C.; Yang, Y.; Fu, X.; Zhang, Y. Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition. Nat. Cell Biol. 2024, 26, 124–137. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Yan, X.-D.; Wang, Y.; Wang, Z.-Z.; Mao, X.-L.; Ye, L.-P.; Li, S.-W. Role of ferroptosis in colorectal cancer. World J. Gastrointest. Oncol. 2023, 15, 225. [Google Scholar] [CrossRef]
- Niekamp, P.; Kim, C.H. Microbial metabolite dysbiosis and colorectal cancer. Gut Liver 2023, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- Ala, M. Tryptophan metabolites modulate inflammatory bowel disease and colorectal cancer by affecting immune system. Int. Rev. Immunol. 2022, 41, 326–345. [Google Scholar] [CrossRef]
- Phakathi, M. The Production of N-Nitrosamines by Gut Bacteria. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2020. [Google Scholar]
- Dai, D.; Yang, Y.; Yu, J.; Dang, T.; Qin, W.; Teng, L.; Ye, J.; Jiang, H. Interactions between gastric microbiota and metabolites in gastric cancer. Cell Death Dis. 2021, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, B.; Yang, H.; Zhu, Z. Gut Microbiota, Human Blood Metabolites, and Esophageal Cancer: A Mendelian Randomization Study. Genes 2024, 15, 729. [Google Scholar] [CrossRef]
- Chiang, H.C.; Hughes, M.; Chang, W.L. The role of microbiota in esophageal squamous cell carcinoma: A review of the literature. Thorac. Cancer 2023, 14, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Mirji, G.; Worth, A.; Bhat, S.A.; El Sayed, M.; Kannan, T.; Goldman, A.R.; Tang, H.-Y.; Liu, Q.; Auslander, N.; Dang, C.V. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 2022, 7, eabn0704. [Google Scholar] [CrossRef]
- Hezaveh, K.; Shinde, R.S.; Klötgen, A.; Halaby, M.J.; Lamorte, S.; Quevedo, R.; Neufeld, L.; Liu, Z.Q.; Jin, R.; Grünwald, B.T. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 2022, 55, 324–340.e8. [Google Scholar] [CrossRef]
- Filippou, C.; Themistocleous, S.C.; Marangos, G.; Panayiotou, Y.; Fyrilla, M.; Kousparou, C.A.; Pana, Z.-D.; Tsioutis, C.; Johnson, E.O.; Yiallouris, A. Microbial therapy and breast cancer management: Exploring mechanisms, clinical efficacy, and integration within the one health approach. Int. J. Mol. Sci. 2024, 25, 1110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shang, S.; Wu, M.; Song, Q.; Chen, D. Gut microbial metabolites in lung cancer development and immunotherapy: Novel insights into gut-lung axis. Cancer Lett. 2024, 598, 217096. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Hou, Y.; Gohel, D.; Zhou, Y.; Xu, J.; Bykova, M.; Yang, Y.; Leverenz, J.B.; Pieper, A.A.; Nussinov, R. Systematic characterization of multi-omics landscape between gut microbial metabolites and GPCRome in Alzheimer’s disease. Cell Rep. 2024, 43, 114128. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Yang, R.; Qian, L. Research on gut microbiota-derived secondary bile acids in cancer progression. Integr. Cancer Ther. 2022, 21, 15347354221114100. [Google Scholar] [CrossRef]
- Song, Y.; Lau, H.C.; Zhang, X.; Yu, J. Bile acids, gut microbiota, and therapeutic insights in hepatocellular carcinoma. Cancer Biol. Med. 2024, 21, 144–162. [Google Scholar] [CrossRef]
- Mahdi, A.K. Molecular pathology: The Roles of P53 in the Oxidative Stress and DNA Damage Responses in Chronic Liver Disease and Hepatocellular Carcinoma. Master’s Thesis, Newcastle University, Newcastle upon Tyne, UK, 2018. [Google Scholar]
- DCA Dichloroacetate Breakthrough Anticancer Agent. Available online: https://truemedmd.com/2019/11/dca-dichloroacetate-breakthrough-anticancer-agent/ (accessed on 25 March 2025).
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Ye, J.; Wu, W.; Li, Y.; Li, L. Influences of the gut microbiota on DNA methylation and histone modification. Dig. Dis. Sci. 2017, 62, 1155–1164. [Google Scholar] [CrossRef]
- Chen, X.; Kong, Q.; Zhao, X.; Zhao, C.; Hao, P.; Irshad, I.; Lei, H.; Kulyar, M.F.; Bhutta, Z.A.; Ashfaq, H.; et al. Sodium acetate/sodium butyrate alleviates lipopolysaccharide-induced diarrhea in mice via regulating the gut microbiota, inflammatory cytokines, antioxidant levels, and NLRP3/Caspase-1 signaling. Front. Microbiol. 2022, 13, 1036042. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Y Aboul-Enein, H. Reactive oxygen and nitrogen species in carcinogenesis: Implications of oxidative stress on the progression and development of several cancer types. Mini Rev. Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Huycke, M.M.; Abrams, V.; Moore, D.R. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 2002, 23, 529–536. [Google Scholar] [CrossRef]
- Gagniere, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Veeraraghavan, J.; Natarajan, M.; Herman, T.S.; Aravindan, N. Low-dose γ-radiation-induced oxidative stress response in mouse brain and gut: Regulation by NFκB–MnSOD cross-signaling. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2011, 718, 44–55. [Google Scholar] [CrossRef]
- Aravindan, N.; Aravindan, S.; Pandian, V.; Khan, F.H.; Ramraj, S.K.; Natt, P.; Natarajan, M. Acquired tumor cell radiation resistance at the treatment site is mediated through radiation-orchestrated intercellular communication. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 677–685. [Google Scholar] [CrossRef]
- Bahrami, A.; Khalaji, A.; Bahri Najafi, M.; Sadati, S.; Raisi, A.; Abolhassani, A.; Eshraghi, R.; Khaksary Mahabady, M.; Rahimian, N.; Mirzaei, H. NF-kappaB pathway and angiogenesis: Insights into colorectal cancer development and therapeutic targets. Eur. J. Med. Res. 2024, 29, 610. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef]
- Charles, A.; Thomas, R.M. The Influence of the microbiome on the innate immune microenvironment of solid tumors. Neoplasia 2023, 37, 100878. [Google Scholar] [CrossRef]
- Shen, M.; Du, Y.; Ye, Y. Tumor-associated macrophages, dendritic cells, and neutrophils: Biological roles, crosstalk, and therapeutic relevance. Med. Rev. 2021, 1, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Geis, A.L.; Fan, H.; Wu, X.; Wu, S.; Huso, D.L.; Wolfe, J.L.; Sears, C.L.; Pardoll, D.M.; Housseau, F. Regulatory T-cell Response to Enterotoxigenic Bacteroides fragilis Colonization Triggers IL17-Dependent Colon Carcinogenesis. Cancer Discov. 2015, 5, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Suraya, R.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Microbiome as a Target for Cancer Therapy. Integr. Cancer Ther. 2020, 19, 1534735420920721. [Google Scholar] [CrossRef]
- Bouferraa, Y.; Fares, C.; Bou Zerdan, M.; Boyce Kennedy, L. Microbial Influences on Immune Checkpoint Inhibitor Response in Melanoma: The Interplay between Skin and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 9702. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Wang, W.; Fan, J.; Zhang, C.; Huang, Y.; Chen, Y.; Fu, S.; Wu, J. Targeted modulation of gut and intra-tumor microbiota to improve the quality of immune checkpoint inhibitor responses. Microbiol. Res. 2024, 282, 127668. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Moreno, C.M.; Boeree, E.; Freitas, C.M.T.; Weber, K.S. Immunomodulatory role of oral microbiota in inflammatory diseases and allergic conditions. Front. Allergy 2023, 4, 1067483. [Google Scholar] [CrossRef]
- Lan, Z.; Liu, W.J.; Cui, H.; Zou, K.L.; Chen, H.; Zhao, Y.Y.; Yu, G.T. The role of oral microbiota in cancer. Front. Microbiol. 2023, 14, 1253025. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Tai, E.W.; Ward, K.C.; Bonaventure, A.; Siegel, D.A.; Coleman, M.P. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017, 123 (Suppl. S24), 5178–5189. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef] [PubMed]
- Bemark, M.; Pitcher, M.J.; Dionisi, C.; Spencer, J. Gut-associated lymphoid tissue: A microbiota-driven hub of B cell immunity. Trends Immunol. 2024, 45, 211–223. [Google Scholar] [CrossRef]
- Ignacio, A.; Czyz, S.; McCoy, K.D. Early life microbiome influences on development of the mucosal innate immune system. Semin. Immunol. 2024, 73, 101885. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Goswami, M.; Bose, P.D. Gut microbial dysbiosis in the pathogenesis of leukemia: An immune-based perspective. Exp. Hematol. 2024, 133, 104211. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Thorbinson, C.; Kilday, J.P. Childhood Malignant Brain Tumors: Balancing the Bench and Bedside. Cancers 2021, 13, 6099. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Ye, Z.; Ye, Z.; Wang, M.; Cao, Z.; Gao, R.; Zhang, Y. Gut microbiota in brain tumors: An emerging crucial player. CNS Neurosci. Ther. 2023, 29 (Suppl. S1), 84–97. [Google Scholar] [CrossRef] [PubMed]
- Molska, M.; Mruczyk, K.; Cisek-Woźniak, A.; Prokopowicz, W.; Szydełko, P.; Jakuszewska, Z.; Marzec, K.; Trocholepsza, M. The influence of intestinal microbiota on BDNF levels. Nutrients 2024, 16, 2891. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- du Chatinier, A.; Velilla, I.Q.; Meel, M.H.; Hoving, E.W.; Hulleman, E.; Metselaar, D.S. Microglia in pediatric brain tumors: The missing link to successful immunotherapy. Cell Rep. Med. 2023, 4, 127668. [Google Scholar] [CrossRef]
- Li, D.; Lan, X.; Xu, L.; Zhou, S.; Luo, H.; Zhang, X.; Yu, W.; Yang, Y.; Fang, X. Influence of gut microbial metabolites on tumor immunotherapy: Mechanisms and potential natural products. Front. Immunol. 2025, 16, 1552010. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Camberos-Barraza, J.; Guadrón-Llanos, A.M.; De la Herrán-Arita, A.K. The gut microbiome-neuroglia axis: Implications for brain health, inflammation, and disease. Neuroglia 2024, 5, 254–273. [Google Scholar] [CrossRef]
- Keane, L.; Cryan, J.F.; Gleeson, J.P. Exploiting the gut microbiome for brain tumour treatment. Trends Mol. Med. 2025, 31, 213–223. [Google Scholar] [CrossRef]
- Marcotte, E.L.; Ritz, B.; Cockburn, M.; Clarke, C.A.; Heck, J.E. Birth characteristics and risk of lymphoma in young children. Cancer Epidemiol. 2014, 38, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.W.; Hill, D.G.; Jones, S.A. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front. Immunol. 2016, 7, 401. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Han, Y.; Fu, Z.; Chai, Y.; Guo, X.; Du, S.; Li, C.; Wang, D. The causal relationship between gut microbiota and lymphoma: A two-sample Mendelian randomization study. Front. Immunol. 2024, 15, 1397485. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Evans, J. Childhood cancers and immunodeficiency syndromes. Clin. Paediatr. Diet. 2020, 371–392. [Google Scholar] [CrossRef]
- Cheung, N.K.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Subramanian, P.; Mohanvelu, S.; Somasundaram, D.B.; Aravindan, S.; Aravindan, N. Acquired RD3 loss regulates immune surveillance in high-risk and therapy defying progressive neuroblastoma. Cancer Commun. 2024, 44, 1427. [Google Scholar] [CrossRef]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Jarvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J. Allergy Clin. Immunol. 2022, 150, 523–534. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Tissing, W.J.; Dun, C.A.; Meessen, N.E.; Kamps, W.A.; de Bont, E.S.; Harmsen, H.J. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef]
- Wilde, J.; Slack, E.; Foster, K.R. Host control of the microbiome: Mechanisms, evolution, and disease. Science 2024, 385, eadi3338. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Nurgaziyev, M.; Sergazy, S.; Chulenbayeva, L.; Nurgozhina, A.; Gulyayev, A.; Kozhakhmetov, S.; Kartbayeva, G.; Kushugulova, A. The effects of antibiotics on the gut microbiome and the immune system (Review). Georgian Med. News 2020, 167–173. [Google Scholar] [PubMed]
- Pedretti, L.; Massa, S.; Leardini, D.; Muratore, E.; Rahman, S.; Pession, A.; Esposito, S.; Masetti, R. Role of Nutrition in Pediatric Patients with Cancer. Nutrients 2023, 15, 710. [Google Scholar] [CrossRef]
- Joffe, L.; Ladas, E.J. Nutrition during childhood cancer treatment: Current understanding and a path for future research. Lancet Child. Adolesc. Health 2020, 4, 465–475. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Iyer, N.; Vaishnava, S. Vitamin A at the interface of host-commensal-pathogen interactions. PLoS Pathog. 2019, 15, e1007750. [Google Scholar] [CrossRef]
- Zhai, Z.; Dong, W.; Sun, Y.; Gu, Y.; Ma, J.; Wang, B.; Cao, H. Vitamin-Microbiota Crosstalk in Intestinal Inflammation and Carcinogenesis. Nutrients 2022, 14, 3383. [Google Scholar] [CrossRef]
- Dutta, A.; Flores, R. Infection prevention in pediatric oncology and hematopoietic stem cell transplant recipients. In Healthcare-Associated Infections in Children: A Guide to Prevention and Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 281–299. [Google Scholar]
- Lax, S.; Gilbert, J.A. Hospital-associated microbiota and implications for nosocomial infections. Trends Mol. Med. 2015, 21, 427–432. [Google Scholar] [CrossRef]
- Thompson, K.N.; Oulhote, Y.; Weihe, P.; Wilkinson, J.E.; Ma, S.; Zhong, H.; Li, J.; Kristiansen, K.; Huttenhower, C.; Grandjean, P. Effects of Lifetime Exposures to Environmental Contaminants on the Adult Gut Microbiome. Environ. Sci. Technol. 2022, 56, 16985–16995. [Google Scholar] [CrossRef]
- Simiakova, M.; Bielik, V. The pros and cons of probiotic use in pediatric oncology patients following treatment for acute lymphoblastic leukemia. Front. Pediatr. 2024, 12, 1427185. [Google Scholar] [CrossRef]
- Zolkiewicz, J.; Marzec, A.; Ruszczynski, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferrau, F.; Libra, M. Lactobacillus rhamnosus GG: An Overview to Explore the Rationale of Its Use in Cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Galacto-oligosaccharides and Colorectal Cancer: Feeding our Intestinal Probiome. J. Funct. Foods 2015, 12, 92–108. [Google Scholar] [CrossRef]
- Ohno, H. The impact of metabolites derived from the gut microbiota on immune regulation and diseases. Int. Immunol. 2020, 32, 629–636. [Google Scholar] [CrossRef]
- Slizewska, K.; Markowiak-Kopec, P.; Slizewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health benefits of prebiotics, probiotics, synbiotics, and postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, P.; Duan, H.; Wang, J.; Qiu, Y.; Cui, Z.; Yin, Y.; Wan, D.; Xie, L. Gut microbiota in muscular atrophy development, progression, and treatment: New therapeutic targets and opportunities. Innovation 2023, 4, 100479. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, N.; Terence, H.; and Aravindan, S. Emerging therapeutic targets for neuroblastoma. Expert. Opin. Ther. Targets 2020, 24, 899–914. [Google Scholar] [CrossRef]
- Trainor, P. Neural Crest Cells: Evolution, Development and Disease; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Rubenstein, J.; Puelles, L. Development of the nervous system. In Epstein’s Inborn Errors of Development: The Molecular Basis of Clinical Disorders of Morphogenesis; Oxford University Press: Oxford, UK, 2016; p. 63. [Google Scholar]
- Morgenstern, D.A.; Baruchel, S.; Irwin, M.S. Current and future strategies for relapsed neuroblastoma: Challenges on the road to precision therapy. J. Pediatr. Hematol./Oncol. 2013, 35, 337–347. [Google Scholar] [CrossRef]
- Simon, T.; Berthold, F.; Borkhardt, A.; Kremens, B.; De Carolis, B.; Hero, B. Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: Results of German trials. Pediatr. Blood Cancer 2011, 56, 578–583. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Garaventa, A.; Parodi, S.; De Bernardi, B.; Dau, D.; Manzitti, C.; Conte, M.; Casale, F.; Viscardi, E.; Bianchi, M.; D’Angelo, P. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur. J. Cancer 2009, 45, 2835–2842. [Google Scholar] [CrossRef]
- London, W.B.; Castel, V.; Monclair, T.; Ambros, P.F.; Pearson, A.D.; Cohn, S.L.; Berthold, F.; Nakagawara, A.; Ladenstein, R.L.; Iehara, T. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J. Clin. Oncol. 2011, 29, 3286–3292. [Google Scholar] [CrossRef]
- Caldas, C. Cancer sequencing unravels clonal evolution. Nat. Biotechnol. 2012, 30, 408–410. [Google Scholar] [CrossRef]
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef]
- Suita, S.; Tajiri, T.; Kaneko, M.; Hirai, M.; Mugishima, H.; Sugimoto, T.; Tsuchida, Y. Implications of MYCN amplification in patients with stage 4 neuroblastoma who undergo intensive chemotherapy. J. Pediatr. Surg. 2007, 42, 489–493. [Google Scholar] [CrossRef]
- Pandian, V.; Ramraj, S.; Khan, F.H.; Azim, T.; Aravindan, N. Metastatic neuroblastoma cancer stem cells exhibit flexible plasticity and adaptive stemness signaling. Stem Cell Res. Ther. 2015, 6, 400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- UT Southwestern Medical Center. Neuroblastoma Diagnosis and Stages. Available online: https://utswmed.org/conditions-treatments/neuroblastoma/neuroblastoma-diagnosis-and-stages/ (accessed on 25 March 2025).
- Domingo-Fernandez, R.; Watters, K.; Piskareva, O.; Stallings, R.L.; Bray, I. The role of genetic and epigenetic alterations in neuroblastoma disease pathogenesis. Pediatr. Surg. Int. 2013, 29, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, N.; Subramanian, K.; Somasundaram, D.B.; Herman, T.S.; Aravindan, S. MicroRNAs in neuroblastoma tumorigenesis, therapy resistance, and disease evolution. Cancer Drug Resist. 2019, 2, 1086. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: Roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef]
- Ma, K.; Wang, L.; Li, W.; Tang, T.; Ma, B.; Zhang, L.; Zhang, L. Turning cold into hot: Emerging strategies to fire up the tumor microenvironment. Trends Cancer 2024, 11, 117–134. [Google Scholar] [CrossRef]
- Vanichapol, T.; Chutipongtanate, S.; Anurathapan, U.; Hongeng, S. Immune escape mechanisms and future prospects for immunotherapy in neuroblastoma. BioMed Res. Int. 2018, 2018, 1812535. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Bonavida, B. Activation of natural killer cells by probiotics. Onco Ther. 2016, 7, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Keyel, M.E.; Reynolds, C.P. Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: Development and place in therapy. Biologics 2018, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.-M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Pathania, A.S.; Prathipati, P.; Murakonda, S.P.; Murakonda, A.B.; Srivastava, A.; Byrareddy, S.N.; Coulter, D.W.; Gupta, S.C.; Challagundla, K.B. Immune checkpoint molecules in neuroblastoma: A clinical perspective. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–258. [Google Scholar]
- Bao, R.; Spranger, S.; Hernandez, K.; Zha, Y.; Pytel, P.; Luke, J.J.; Gajewski, T.F.; Volchenboum, S.L.; Cohn, S.L.; Desai, A.V. Immunogenomic determinants of tumor microenvironment correlate with superior survival in high-risk neuroblastoma. J. Immunother. Cancer 2021, 9, e002417. [Google Scholar] [CrossRef]
- Chu, J. Potential cause-and-effect relationship between gut microbiota and childhood neuroblastoma: A mendelian randomization analysis. Indian J. Pediatr. 2024, 92, 717–724. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Manghi, P.; Cumbo, F.; Masetti, G.; Armanini, F.; Asnicar, F.; Blanco-Miguez, A.; Pinto, F.; Punčochář, M.; Garaventa, A. Neuroblastoma is associated with alterations in gut microbiome composition subsequent to maternal microbial seeding. EBioMedicine 2024, 99, 104917. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Xie, F.; Zhang, H. The causal relationship between gut microbiota and neuroblastoma: A bidirectional Mendelian randomization analysis and meta-analysis. Microbiol. Spectr. 2024, 12, e03656-23. [Google Scholar] [CrossRef]
- Castellani, C.; Singer, G.; Kaiser, M.; Kaiser, T.; Huang, J.; Sperl, D.; Kashofer, K.; Fauler, G.; Guertl-Lackner, B.; Höfler, G. Neuroblastoma causes alterations of the intestinal microbiome, gut hormones, inflammatory cytokines, and bile acid composition. Pediatr. Blood Cancer 2017, 64, e26425. [Google Scholar] [CrossRef]
- Castellani, C.; Singer, G.; Eibisberger, M.; Obermüller, B.; Warncke, G.; Miekisch, W.; Kolb-Lenz, D.; Summer, G.; Pauer, T.M.; ElHaddad, A. The effects of neuroblastoma and chemotherapy on metabolism, fecal microbiome, volatile organic compounds, and gut barrier function in a murine model. Pediatr. Res. 2019, 85, 546–555. [Google Scholar] [CrossRef]
- Obermüller, B.; Singer, G.; Kienesberger, B.; Klymiuk, I.; Sperl, D.; Stadlbauer, V.; Horvath, A.; Miekisch, W.; Gierschner, P.; Grabherr, R. The effects of prebiotic supplementation with OMNi-LOGiC® FIBRE on fecal microbiome, fecal volatile organic compounds, and gut permeability in murine neuroblastoma-induced tumor-associated cachexia. Nutrients 2020, 12, 2029. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Huang, R.; Stucky, A.; Chen, X.; Sun, L.; Wen, Q.; Zeng, Y.; Fletcher, H.; Wang, C. The machine-learning-mediated interface of microbiome and genetic risk stratification in neuroblastoma reveals molecular pathways related to patient survival. Cancers 2022, 14, 2874. [Google Scholar] [CrossRef]

| Stage | Microbiome Development | Influencing Factors |
|---|---|---|
| Birth | Colonization commences with germs originating from the maternal body (vagina, feces, skin). | Method of delivery (vaginal versus caesarean) |
| Newborn infant (1–4 weeks) | Initially dominated by Staphylococcus and Enterobacteriaceae, followed by subsequent succession by Bifidobacterium. | Feeding technique (breast milk versus formula), gestational age, antibiotic administration |
| Infancy (2 years) | Enrichment of Bifidobacterium and incorporation of lactic acid bacteria | Duration of breastfeeding, adoption of solid meals |
| Childhood (2–4 years) | Change to mature microbes, rise of Bacteroides | Environmental exposures |
| Adulthood (above 18 years) | Firmicutes and Bacteroidetes | Lifestyle, nutrition, environmental conditions |
| INRGSS | Features | Risk Groups | Event-Free Survival |
|---|---|---|---|
| L1 | Locoregional tumor without any identified risk factors based on imaging | Very low—low risk | 5-year—>75–85% |
| L2 | Tumor cells have metastasized to adjacent tissues | Low risk | 5-year—75–85% |
| M | NB cells spread to distant organs | Low risk—high risk | 5-year—50–75% |
| MS | Metastatic disease localized to the skin, liver, or bone marrow. | High risk | 5-year—<50% |
| INSS Stage | Description | Risk Group | 5-Year Survival Rate (%) |
|---|---|---|---|
| Stage 1 | Localized tumor, completely resected by surgery | Low Risk | 90–95% |
| Stage 2A | Tumor localized but cannot be completely removed by surgery | Low/Intermediate Risk | 80–90% |
| Stage 2B | Tumors on one side may not always be fully resectable | Intermediate Risk | 75–85% |
| Stage 3 | Unresectable tumor that may involve lymph nodes but has not spread distantly | High Risk | 50–70% |
| Stage 4 | Cancer has spread to distant sites (e.g., bone, liver, bone marrow) | High Risk | 20–40% |
| Stage 4S | In children <1 year, cancer has spread to liver, skin, and/or bone marrow (≤10% involvement) | Low/Intermediate Risk | 80–95% |
| TME Subtype | Immune Characteristics | Genomic Features | Pathway Enrichment | Clinical Implication |
|---|---|---|---|---|
| T cell-inflamed | High CD8+ T cell infiltration, IFN- γ signature, immune checkpoint molecules | High neoantigen load, diverse TCR repertoire | Immune-related pathways (e.g., IFN signaling) | Best overall and event-free survival |
| Intermediate | Moderate immune cell markers | Variable neoantigen burden | Moderate immune and oncogenic signaling | Intermediate prognosis |
| Non-T cell-inflamed | Low immune gene expression, T cell exclusion signatures | Activation of MYCN, ASCL1, SOX11, KMT2A, even without MYCN amplification | Neurodevelopmental and cell cycle pathways | Poor survival; resistant to immunotherapy |
| Trial Title | Description | Eligibility Criteria | Objective | Lead Organization | Phase |
|---|---|---|---|---|---|
| Dinutuximab with Chemo-therapy, Surgery and Stem Cell Transplantation for the Treatment of Children with Newly Diagnosed High Risk NB | Tests the addition of dinutuximab to induction chemotherapy and standard care in high-risk NB | ≤30 years, newly diagnosed high-risk NB, specific renal/liver/cardiac function criteria | To determine if early chemoimmunotherapy improves event-free survival | Children’s Oncology Group | Phase III |
| Eflornithine (DFMO) and Etoposide for Relapsed/Refractory NB | DFMO + etoposide in relapsed/refractory NB | ≤30.99 years, relapsed/refractory NB, prior multi-drug chemotherapy | Evaluate safety and efficacy of DFMO + etoposide | Giselle Sholler | Phase I/II |
| A Study of Therapeutic Iobenguane (131-I) and Vorinostat for Recurrent or Progressive High-Risk NB Subjects | 131I-MIBG + Vorinostat for recurrent/progressive NB | Iobenguane-avid high-risk NB, prior induction therapy, stem cell availability | Evaluate efficacy and safety of combination therapy | DRAXIMAGE | Phase II |
| A Study of a Vaccine in Combination with Beta-glucan in People with NB | OPT-821 vaccine + beta-glucan for high-risk NB | HR-NB in CR, ≥21 and ≤180 days post systemic therapy, adequate organ function | Assess anti-GD2 antibody titers | Memorial Sloan Kettering Cancer Center | Phase II |
| Naxitamab Added to Induction for Newly Diagnosed High-Risk NB | Naxitamab added to 5 cycles of induction chemotherapy | ≤21 years, newly diagnosed high-risk NB, specific INSS stages | Evaluate efficacy and safety of naxitamab in induction | Giselle Sholler | Phase II |
| Autologous hALK. Chimeric Antigen Receptor T Cells (hALK.CAR T) for the Treatment of Relapsed or Refractory High-Risk NB | hALK.CAR T cell therapy for relapsed/refractory NB | ≥12 months and <30 years, relapsed/refractory high-risk NB | Identify MTD and assess safety and efficacy | Dana-Farber Harvard Cancer Center | Phase I/II |
| 67Cu-SARTATE™ Peptide Receptor Radionuclide Therapy Administered to Pediatric Patients With High-Risk, Relapsed, Refractory NB | Adaptive trial of 67Cu-SARTATE in pediatric high-risk NB | High-risk NB, adequate organ function, stem cell product available | Evaluate safety and efficacy of 67Cu-SARTATE | Clarity Pharmaceuticals | Phase I/II |
| Donor Immune Cells (Allogenic Ex Vivo Expanded Gamma Delta T Cells), Dinutuximab, Temozolomide, Irinotecan and Zoledronate for the Treatment of Refractory, Relapsed, or Progressive NB or Osteosarcoma in Children | Gamma delta T cells + dinutuximab + chemo for refractory/relapsed NB | ≥12 months, high-risk NB or osteosarcoma, measurable disease | Determine MTD and define toxicities | Emory University Hospital | Phase I |
| Reduced Chemotherapy (N10) for the Treatment of High-Risk NB in Children | N10 chemo regimen for high-risk NB | <19 years, HR-NB, ≤1 prior HR-NB chemo cycle | Assess early CR rate and survival outcomes | Memorial Sloan Kettering Cancer Cente | Phase II |
| High Risk NB, a Study 1.8 of SIOP-Europe (SIOPEN) | Multimodal treatment protocol with randomized immunotherapy arms | High-risk NB (stages 2–4s, MYCN+ or >12 months) | Improve EFS with BuMel MAT and immunotherapy, including immunotherapy (e.g., IL-2) which may interact with gut microbiome. | St. Anna Kinderkrebsforschung | Phase I/II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, A.K.; Subramanian, P.; Periyasamy, L.; Aravindan, S.; Aravindan, N. Microbiome in Neuroblastoma: A Virgin Island in the World of Onco-Microbiome. Cells 2025, 14, 1218. https://doi.org/10.3390/cells14151218
Giri AK, Subramanian P, Periyasamy L, Aravindan S, Aravindan N. Microbiome in Neuroblastoma: A Virgin Island in the World of Onco-Microbiome. Cells. 2025; 14(15):1218. https://doi.org/10.3390/cells14151218
Chicago/Turabian StyleGiri, Ashwath Keshav, Poorvi Subramanian, Loganayaki Periyasamy, Sivaroopan Aravindan, and Natarajan Aravindan. 2025. "Microbiome in Neuroblastoma: A Virgin Island in the World of Onco-Microbiome" Cells 14, no. 15: 1218. https://doi.org/10.3390/cells14151218
APA StyleGiri, A. K., Subramanian, P., Periyasamy, L., Aravindan, S., & Aravindan, N. (2025). Microbiome in Neuroblastoma: A Virgin Island in the World of Onco-Microbiome. Cells, 14(15), 1218. https://doi.org/10.3390/cells14151218







