When Two Worlds Collide: The Contribution and Association Between Genetics (APOEε4) and Neuroinflammation (IL-1β) in Alzheimer’s Neuropathogenesis
Abstract
1. Introduction
2. Methods
2.1. Patients and Specimens
2.2. Ethics, Consent, and Permissions
2.3. Tissue Culture and Maintenance
2.4. Human Hippocampal Immunohistochemistry
2.5. Image Analysis
2.6. RT-PCR Amplification
2.7. Western Blot
2.8. Statistical Analysis
3. Results
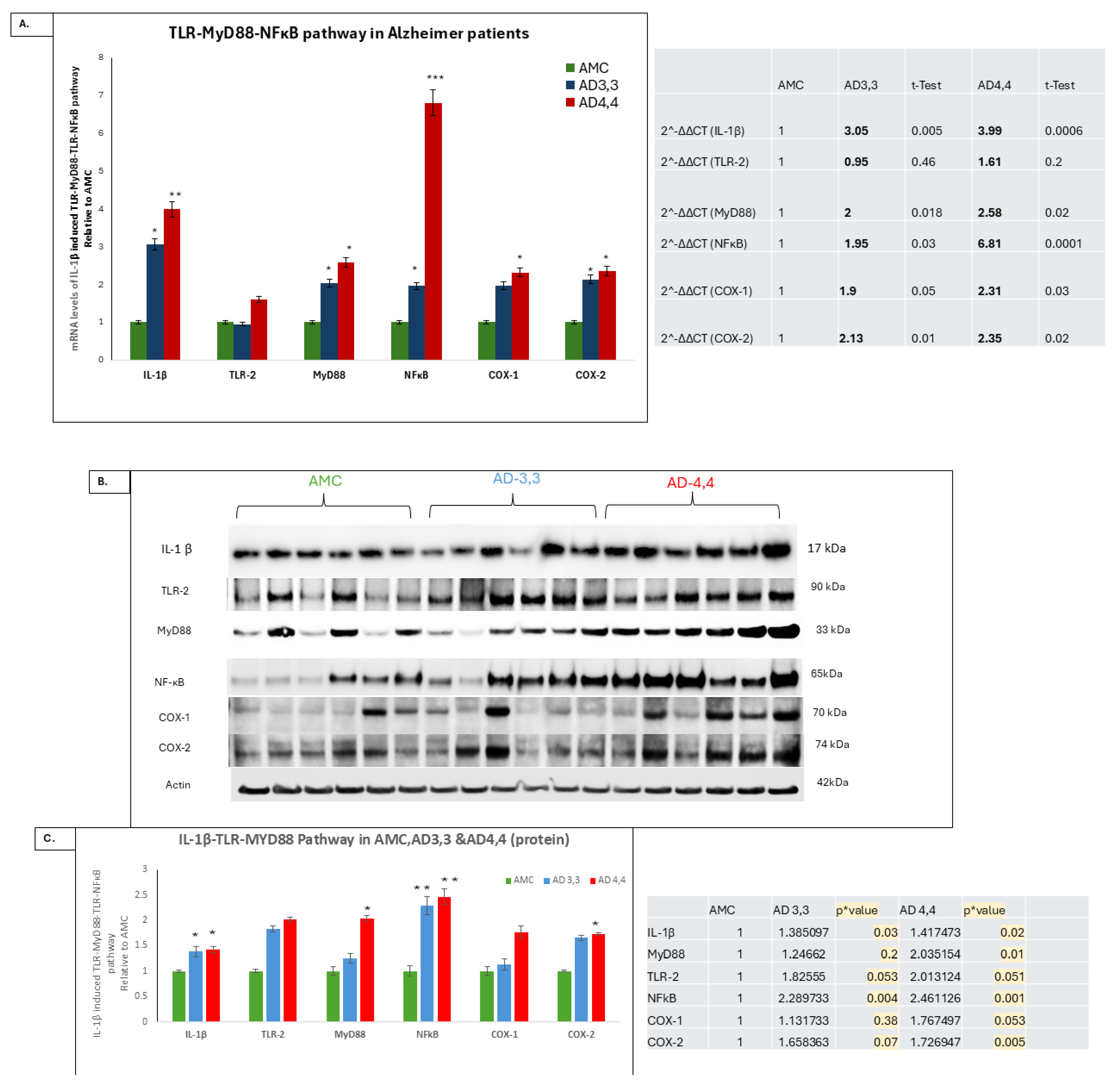
3.1. The Levels of an IL-1β-Driven Pathway, TLR, MyD88, and NFκB Were Higher in AD 4,4 Carriers than in Either AD 3,3 or AMC
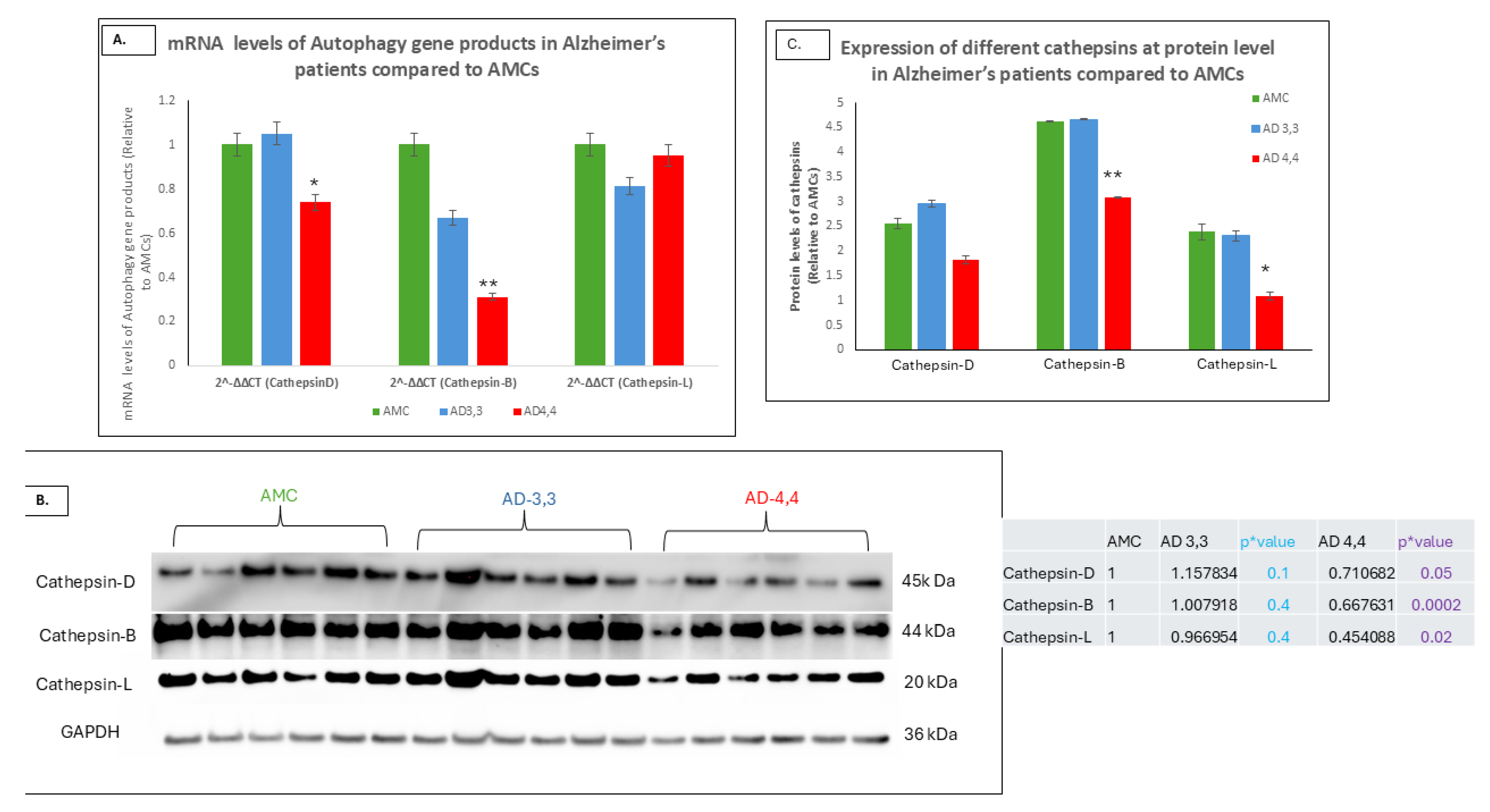
3.2. Autophagy Was Dysregulated in APOεE4 Carriers
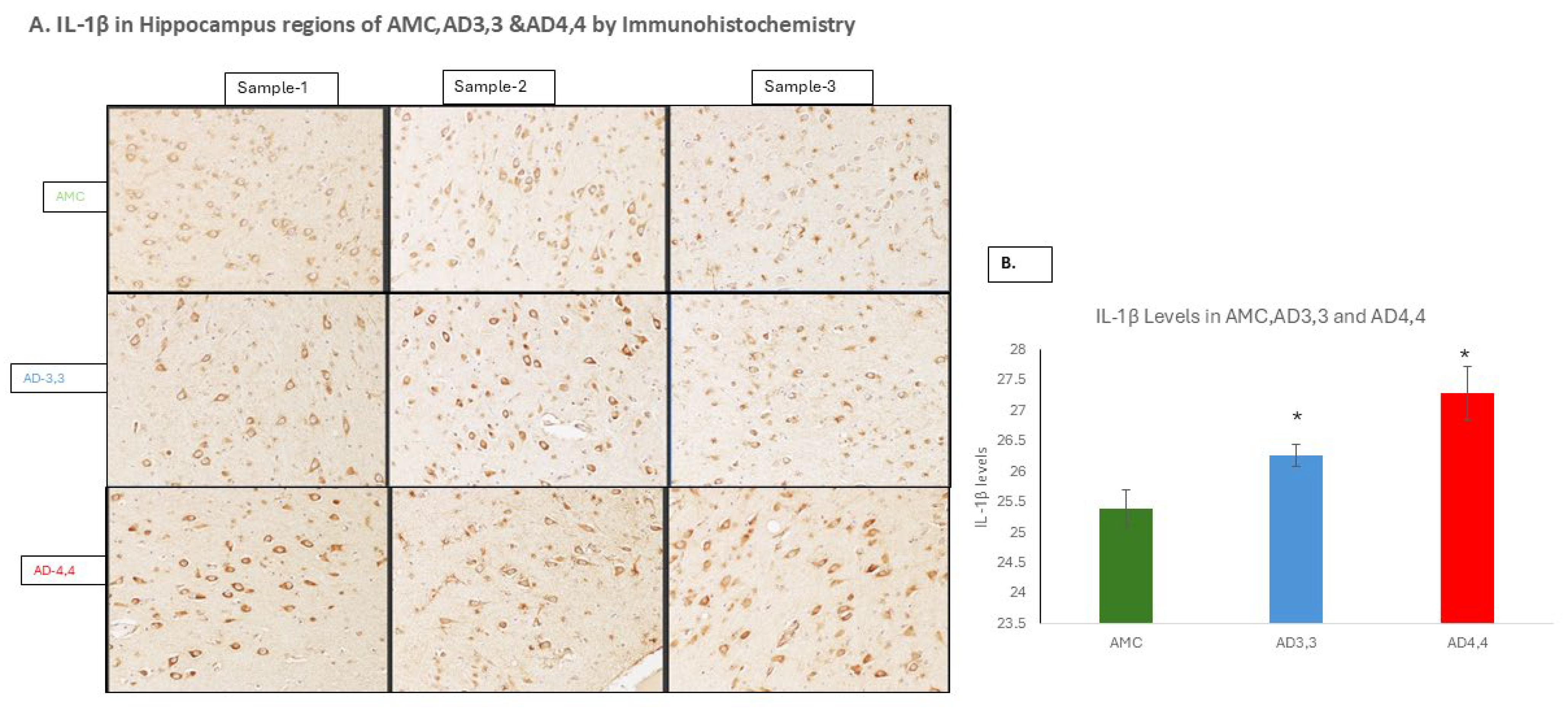
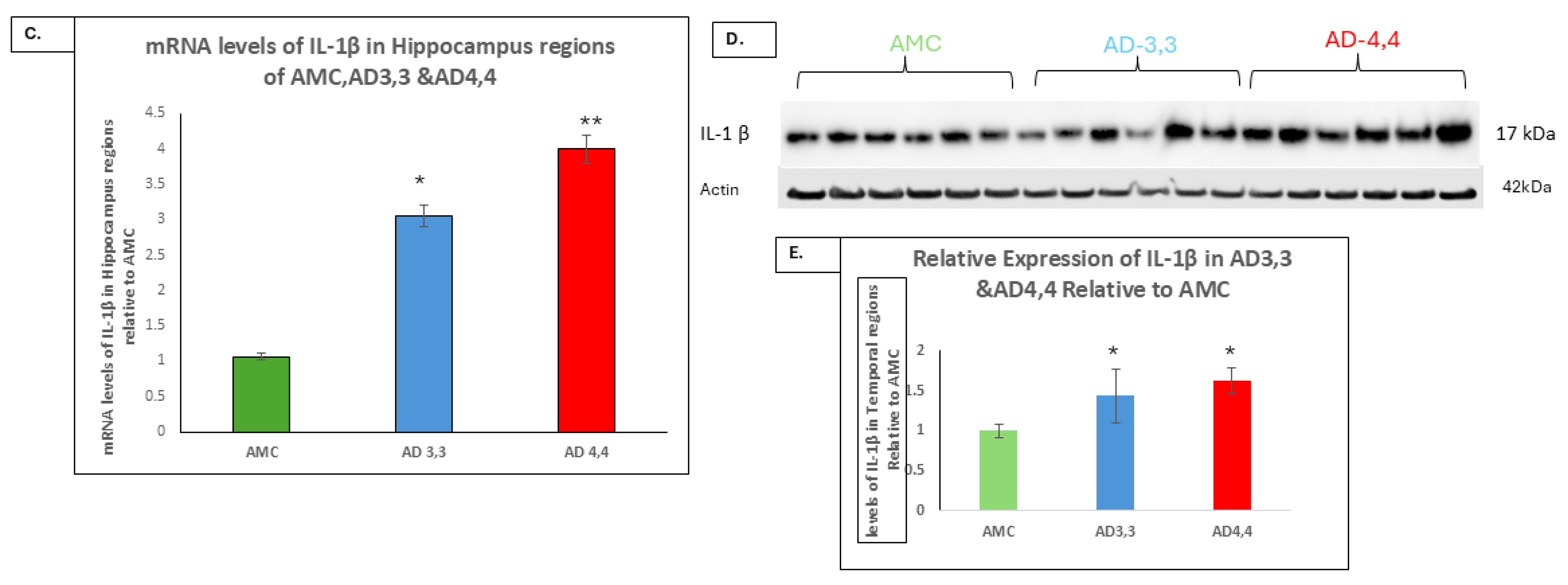
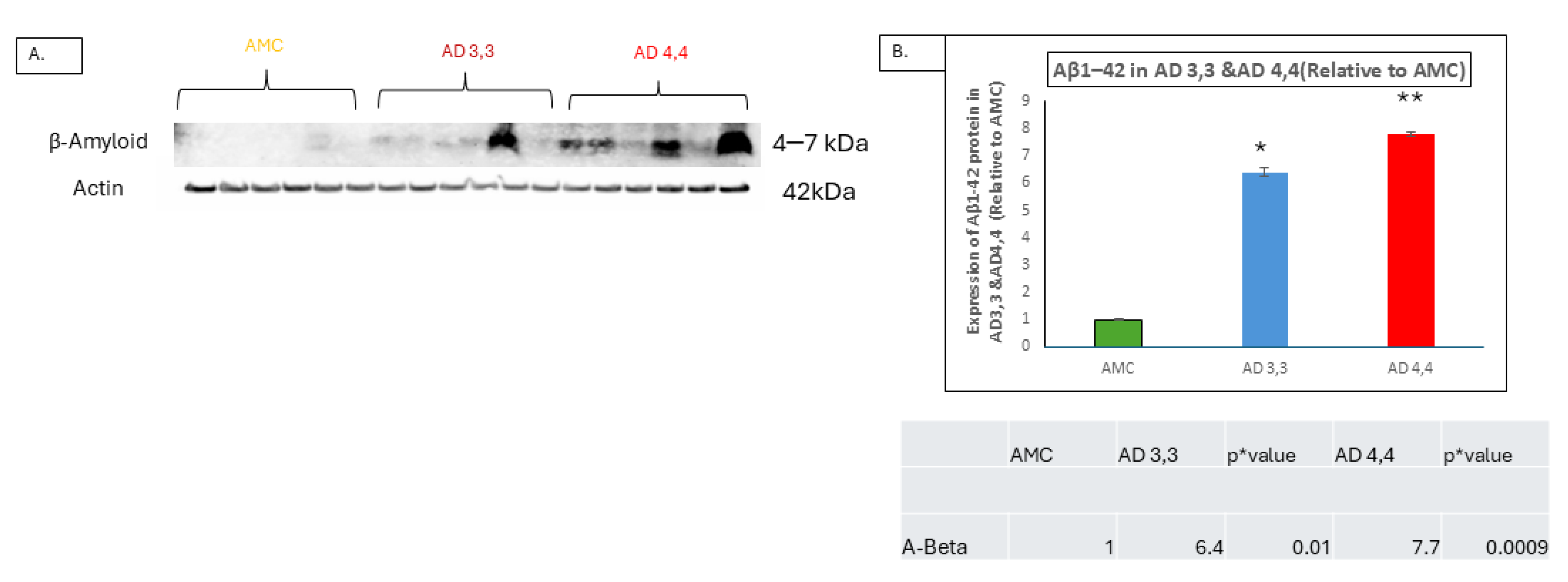
3.3. Interleukin-1β and Aβ Accumulation
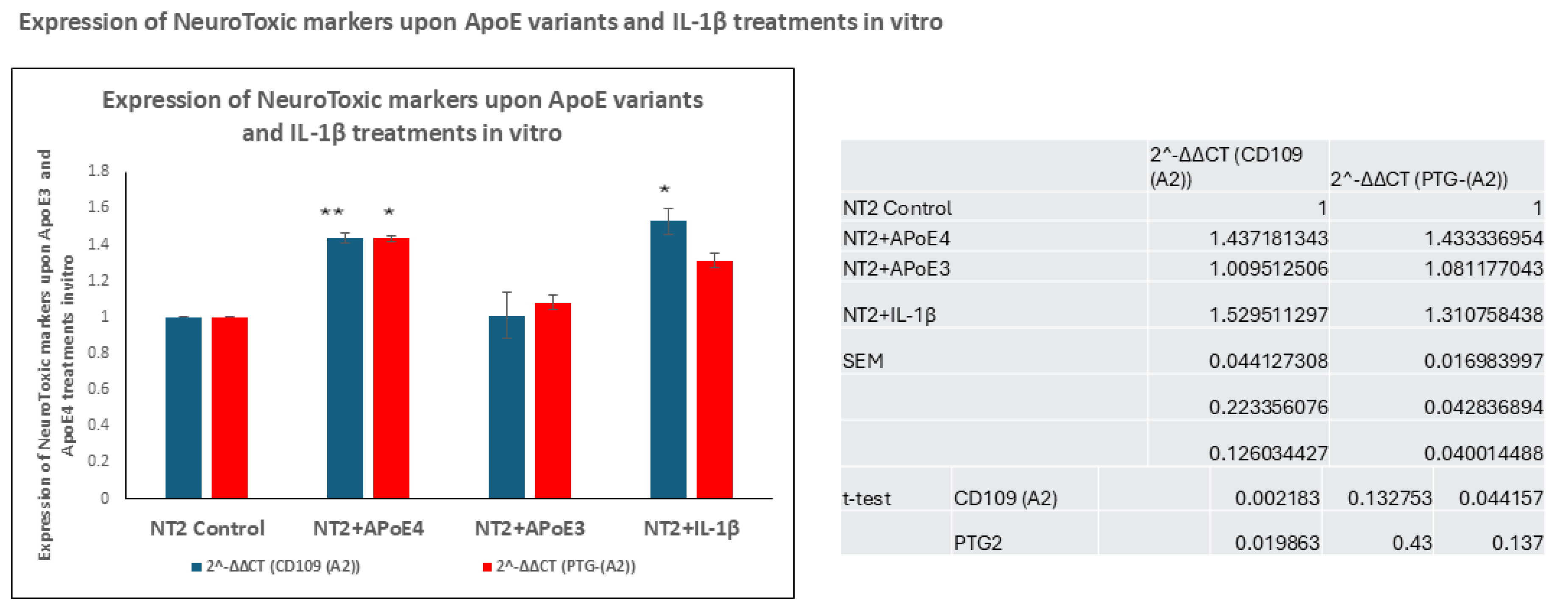
3.4. In Vitro Treatment of NT2 Cells with ApoE4 Increased the Transcription and Production of Neurotoxic Markers, Including CD109 and PTG-(A2)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoogmartens, J.; Cacace, R.; Van Broeckhoven, C. Insight into the genetic etiology of Alzheimer′s disease: A comprehensive review of the role of rare variants. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12155. [Google Scholar]
- Cacace, R.; Sleegers, K.; Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer’s Dement. 2016, 12, 733–748. [Google Scholar]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar]
- Wisniewski, K.E.; Dalton, A.J.; McLachlan, D.C.; Wen, G.Y.; Wisniewski, H.M. Alzheimer’s disease in Down’s syndrome: Clinicopathologic studies. Neurology 1985, 35, 957. [Google Scholar]
- Huang, Y.; Liu, X.Q.; Wyss-Coray, T.; Brecht, W.J.; Sanan, D.A.; Mahley, R.W. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 8838–8843. [Google Scholar]
- Shin, J.H. Dementia epidemiology fact sheet 2022. Ann. Rehabil. Med. 2022, 46, 53–59. [Google Scholar]
- Parcon, P.A.; Balasubramaniam, M.; Ayyadevara, S.; Jones, R.A.; Liu, L.; Reis, R.J.; Barger, S.W.; Mrak, R.E.; Griffin, W.S. Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs. Alzheimer’s Dement. 2018, 14, 230–242. [Google Scholar]
- Ganne, A.; Mainali, N.; Balasubramaniam, M.; Atluri, R.; Pahal, S.; Asante, J.; Nagel, C.; Vallurupalli, S.; Reis, R.J.; Ayyadevara, S. Ezetimibe lowers risk of Alzheimer’s and related dementias over sevenfold, reducing aggregation in model systems by inhibiting 14–3-3G:: Hexokinase interaction. Aging Biol. 2024, 2, 20240028. [Google Scholar]
- Hou, M.; Zhang, Z.; Fan, Z.; Huang, L.; Wang, L. The mechanisms of Ca2+ regulating autophagy and its research progress in neurodegenerative diseases: A review. Medicine 2024, 103, e39405. [Google Scholar]
- Epstein, F.H.; Dinarello, C.A.; Wolff, S.M. The role of interleukin-1 in disease. N. Engl. J. Med. 1993, 328, 106–113. [Google Scholar]
- Head, E.T.; Lott, I.M.; Wilcock, D.A.; Lemere, C. Aging in Down syndrome and the development of Alzheimer’s disease neuropathology. Curr. Alzheimer Res. 2016, 13, 18–29. [Google Scholar]
- Griffin, W.S.; Stanley, L.C.; Ling, C.H.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., 3rd.; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar]
- Aboud, O.; Mrak, R.E.; Boop, F.A.; Griffin, W.S. Epilepsy: Neuroinflammation, neurodegeneration, and APOE genotype. Acta Neuropathol. Commun. 2013, 1, 41. [Google Scholar]
- Liu, L.; Aboud, O.; Jones, R.A.; Mrak, R.E.; Griffin, W.S.; Barger, S.W. Apolipoprotein E expression is elevated by interleukin 1 and other interleukin 1-induced factors. J. Neuroinflamm. 2011, 8, 175. [Google Scholar]
- Griffin, W.S.; Sheng, J.G.; Royston, M.C.; Gentleman, S.M.; McKenzie, J.E.; Graham, D.I.; Roberts, G.W.; Mrak, R.E. Glial-neuronal interactions in Alzheimer’s disease: The potential role of a ‘cytokine cycle’in disease progression. Brain Pathol. 1998, 8, 65–72. [Google Scholar]
- Mirra, S.S.; Heyman, A.; McKeel, D.; Sumi, S.M.; Crain, B.J.; Brownlee, L.M.; Vogel, F.S.; Hughes, J.P.; Belle, G.V.; Berg, L. Participating CERAD Neuropathologists. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer′s disease. Neurology 1991, 41, 479. [Google Scholar]
- Braak, H.; Braak, E. Staging of Alzheimer′s disease-related neurofibrillary changes. Neurobiol. Aging 1995, 271, 10. [Google Scholar]
- Wang, W.; Moerman-Herzog, A.M.; Slaton, A.; Barger, S.W. Presenilin 1 mutations influence processing and trafficking of the ApoE receptor apoER2. Neurobiol. Aging 2017, 49, 145–153. [Google Scholar]
- Dutta, D.; Jana, M.; Majumder, M.; Mondal, S.; Roy, A.; Pahan, K. Selective targeting of the TLR2/MyD88/NF-κB pathway reduces α-synuclein spreading in vitro and in vivo. Nat. Commun. 2021, 12, 5382. [Google Scholar] [CrossRef]
- Theendakara, V.; Peters-Libeu, C.A.; Spilman, P.; Poksay, K.S.; Bredesen, D.E.; Rao, R.V. Direct Transcriptional Effects of Apolipoprotein E. J. Neurosci. 2016, 36, 685–700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drobny, A.; Huarcaya, S.P.; Dobert, J.; Kluge, A.; Bunk, J.; Schlothauer, T.; Zunke, F. The role of lysosomal cathepsins in neurodegeneration: Mechanistic insights, diagnostic potential and therapeutic approaches. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2022, 1869, 119243. [Google Scholar]
- Wang, C.; Sun, B.; Zhou, Y.; Grubb, A.; Gan, L. Cathepsin B degrades amyloid-β in mice expressing wild-type human amyloid precursor protein. J. Biol. Chem. 2012, 287, 39834–39841. [Google Scholar]
- Suire, C.N.; Abdul-Hay, S.O.; Sahara, T.; Kang, D.; Brizuela, M.K.; Saftig, P.; Dickson, D.W.; Rosenberry, T.L.; Leissring, M.A. Cathepsin D regulates cerebral Aβ42/40 ratios via differential degradation of Aβ42 and Aβ40. Alzheimer’s Res. Ther. 2020, 12, 80. [Google Scholar]
- Wang, H.; Sang, N.; Zhang, C.; Raghupathi, R.; Tanzi, R.E.; Saunders, A. Cathepsin L mediates the degradation of novel APP C-terminal fragments. Biochemistry 2015, 54, 2806–2816. [Google Scholar]
- Fessel, J. Caveolae, CD109, and endothelial cells as targets for treating Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12066. [Google Scholar]
- Tesseur, I.; Zou, K.; Esposito, L.; Bard, F.; Berber, E.; Van Can, J.; Lin, A.H.; Crews, L.; Tremblay, P.; Mathews, P.; et al. Deficiency in neuronal TGF-β signaling promotes neurodegeneration and Alzheimer’s pathology. J. Clin. Investig. 2006, 116, 3060–3069. [Google Scholar]
- El-Sahar, A.E.; Shiha, N.A.; El Sayed, N.S.; Ahmed, L.A. Alogliptin attenuates lipopolysaccharide-induced neuroinflammation in mice through modulation of TLR4/MYD88/NF-κB and miRNA-155/SOCS-1 signaling pathways. Int. J. Neuropsychopharmacol. 2021, 24, 158–169. [Google Scholar]
- Vom Hofe, I.; Stricker, B.H.; Ikram, M.K.; Wolters, F.; Ikram, M.A. Long-Term Exposure to Non-Steroidal Anti-Inflammatory Medication in Relation to Dementia Risk. J. Am. Geriatr. Soc. 2025, 73, 1484–1490. [Google Scholar]
- Laivut, S.; Jarusintanakorn, S.; Sripha, K. Exploring novel indomethacin-derived compounds via investigation of NSAIDs through molecular docking and in vitro testing for anti-amyloid beta aggregation. Pharm. Sci. Asia 2024, 51, 59. [Google Scholar]
- Narasimhappagari, J.; Liu, L.; Balasubramaniam, M.; Ayyadevara, S.; Aboud, O.; Griffin, W.S.T. The Seminal Role of the Proinflammatory Cytokine IL-1β and Its Signaling Cascade in Glioblastoma Pathogenesis and the Therapeutic Effect of Interleukin-1β Receptor Antagonist (IL-1RA) and Tolcapone. Int. J. Mol. Sci. 2025, 26, 6893. [Google Scholar] [CrossRef]
- Shvetcov, A.; Johnson, E.C.; Winchester, L.M.; Walker, K.A.; Wilkins, H.M.; Thompson, T.G.; Rothstein, J.D.; Krish, V.; Imam, F.B.; Burns, J.M. APOE ε4 carriers share immune-related proteomic changes across neurodegenerative diseases. Nat. Med. 2025, 1–12. [Google Scholar]
- Balasubramaniam, M.; Narasimhappagari, J.; Liu, L.; Ganne, A.; Ayyadevara, S.; Atluri, R.; Ayyadevara, H.; Caldwell, G.; Reis, R.J.; Barger, S.W.; et al. Rescue of ApoE4-related lysosomal autophagic failure in Alzheimer’s disease by targeted small molecules. Commun. Biol. 2024, 7, 60. [Google Scholar]
| Gene (Direction) | Sequence | Species |
|---|---|---|
| MyD88 | (F)5′-CCA GCA TTG AGG AGG ATT GC-3′ (R)5′-GCT CTG CTG TCC GTG GGA-3′ | Human |
| TLR-2 | (F)5′-AAG GGC AGC TCA GGA TCT TT-3′ (R)5′-AGA CTG CCC AGG GAA GAA AA-3′ | Human |
| NF-κB p65 | (F)5′-AGA TAC CAC CAA GAC CCA CC-3′ (R)5′-CTG TCC CTG GTC CTG TGT AG-3′ | Human |
| COX-1 | (F)5′-GCT GAG TGG CTA TTT CCT GC-3′ (R)5′-CTC GTA GCT GTA CTC CTG GG-3′ | Human |
| COX-2 | (F)5′-CAA TCT GGC TGA GGG AAC ACA ACA-3′ (R)5′-ATC TGC CTG CTC TGG TCA ATG GA-3′ | Human |
| Cathepsin B | (F)5′-TCT CTG ACC GGA TCT GCA TC 3′ (R)5′-TCA CAG GGA ATG GAG TA-3′ | Human |
| Cathepsin D | (F)5′-CAG AAG CTG GTG GAC CAG AAC-3′ (R)5′-TGC GGG TGA CAT TCA GGT AC-3′ | Human |
| Cathepsin L | (F)5′-TAG AGG CAC AGT GGA CCA AG-3′ (R)5′-TGC GGG TGA CAT TCA GGT AG-3′ | Human |
| 18S | (F)5′-TTC GGA CGT CTG CCC TAT CAA-3′ (R)5′-ATG GTA GGC ACG GCG ACT A-3′ | Human |
| Antibody | Species | Cat# | Company |
|---|---|---|---|
| IL-1β | Mouse mAb | 12242S | Cell Signaling Technology IL USA |
| TLR-2 | Rabbit mAb | #13744 | Cell Signaling Technology |
| MyD88 | Rabbit mAb | Ab133739 | Abcam |
| NF-κB p65 | Rabbit mAb | Ab32536 | Abcam |
| COX-1 | Rabbit mAb | Ab109025 | Abcam |
| COX-2 | Rabbit mAb | Ab179800 | Abcam |
| Cathepsin B | Rabbit mAb | 3383S | Cell Signaling Technology |
| Cathepsin D | Rabbit mAb | 740892 | Cell Signaling Technology |
| Cathepsin L | Rabbit mAb | 55914 | Cell Signaling Technology |
| GAPDH | Mouse mAb | sc47724 | Santa Cruz |
| β-Actin | Rabbit mAb | #4970 | Cell Signaling Technology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narasimhappagari, J.; Liu, L.; Balasubramaniam, M.; Ayyadevara, S.; Griffin, W.S.T. When Two Worlds Collide: The Contribution and Association Between Genetics (APOEε4) and Neuroinflammation (IL-1β) in Alzheimer’s Neuropathogenesis. Cells 2025, 14, 1216. https://doi.org/10.3390/cells14151216
Narasimhappagari J, Liu L, Balasubramaniam M, Ayyadevara S, Griffin WST. When Two Worlds Collide: The Contribution and Association Between Genetics (APOEε4) and Neuroinflammation (IL-1β) in Alzheimer’s Neuropathogenesis. Cells. 2025; 14(15):1216. https://doi.org/10.3390/cells14151216
Chicago/Turabian StyleNarasimhappagari, Jagadeesh, Ling Liu, Meenakshisundaram Balasubramaniam, Srinivas Ayyadevara, and W. Sue T. Griffin. 2025. "When Two Worlds Collide: The Contribution and Association Between Genetics (APOEε4) and Neuroinflammation (IL-1β) in Alzheimer’s Neuropathogenesis" Cells 14, no. 15: 1216. https://doi.org/10.3390/cells14151216
APA StyleNarasimhappagari, J., Liu, L., Balasubramaniam, M., Ayyadevara, S., & Griffin, W. S. T. (2025). When Two Worlds Collide: The Contribution and Association Between Genetics (APOEε4) and Neuroinflammation (IL-1β) in Alzheimer’s Neuropathogenesis. Cells, 14(15), 1216. https://doi.org/10.3390/cells14151216






