Therapeutic Opportunities in Overcoming Premature Termination Codons in Epidermolysis Bullosa via Translational Readthrough
Abstract
1. Epidermolysis Bullosa
2. Introduction to Readthrough Therapies
3. Aminoglycoside-Mediated Readthrough for EB
3.1. Introduction to Aminoglycosides
3.2. Studies on RDEB
3.3. Studies on JEB
3.4. Studies on EB Simplex
3.5. Limitations and Future Directions with Aminoglycoside Therapy
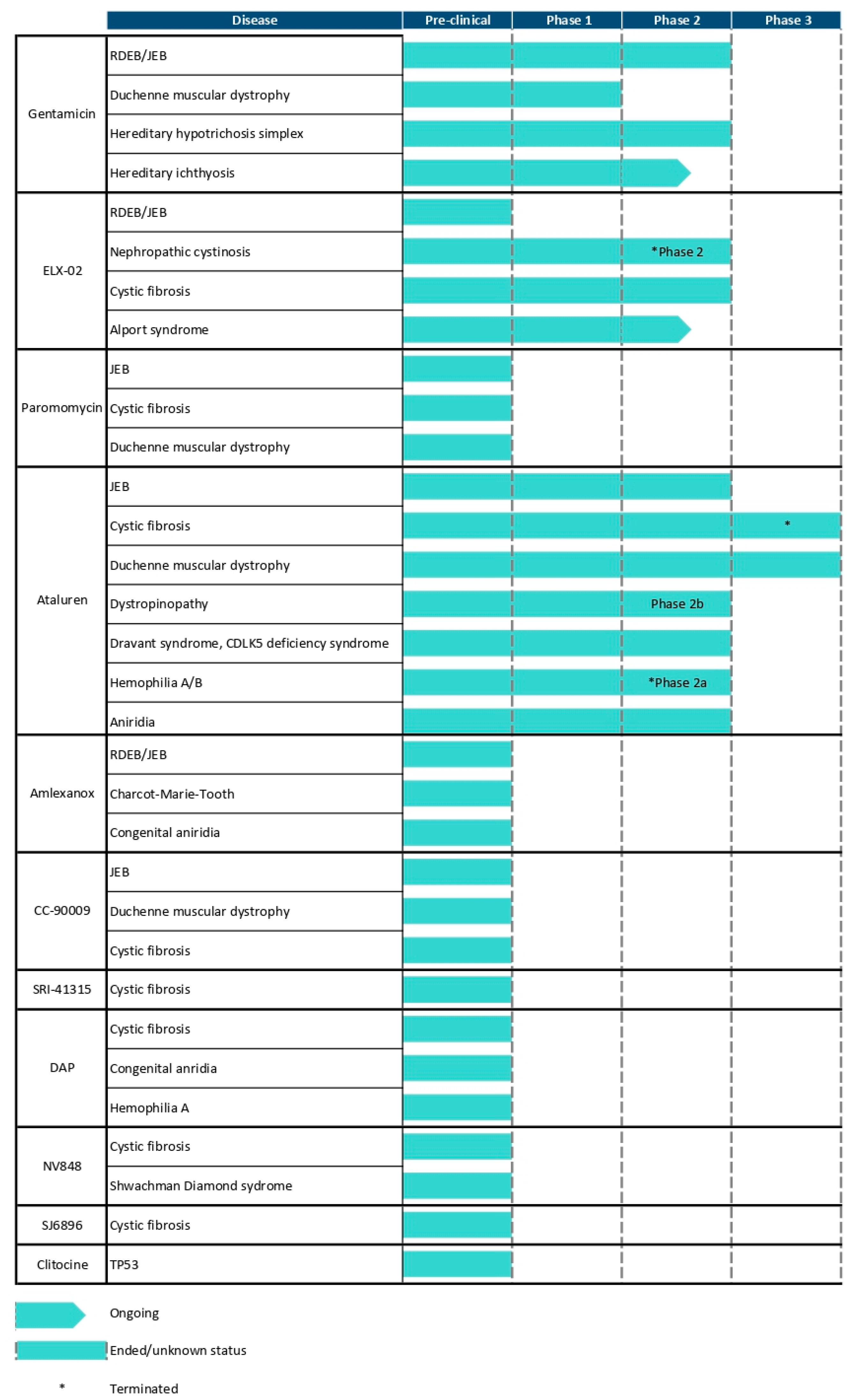
4. Evaluating Novel Readthrough Agents for EB in Preclinical Studies
4.1. Aminoglycoside Derivatives
4.2. Ataluran/PTC124
4.3. Amlexanox
4.4. Translation Termination Factor Inhibitors
5. Other Promising Readthrough Therapies
6. Limitations in Amino Acid Selection at PTC Sites During Readthrough Induced by Small Molecules
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAP | 2,6-diaminopurine |
| AFs | Anchoring fibrils |
| ATM | Ataxia Telangiectasia Mutated |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| DEJ | Dermal–epidermal junction |
| DMD | Duchenne Muscular Dystrophy |
| DEB | Dystrophic Epidermolysis Bullosa |
| EB | Epidermolysis Bullosa |
| EBDASI | Epidermolysis Bullosa Disease Activity and Scarring Index |
| FTSJ1 | FtsJ RNA 2′-O-methyltransferase |
| Gln | Glutamine |
| H-JEB | Herlitz Junctional Epidermolysis Bullosa |
| IM | Intramuscular |
| IV | Intravenous |
| JEB | Junctional Epidermolysis Bullosa |
| Lys | Lysine |
| MFI | Mean Fluorescence Intensity |
| NMD | Nonsense-Mediated Decay |
| PTC | Premature Termination Codons |
| RDEB | Recessive Dystrophic Epidermolysis Bullosa |
| Trp | Tryptophan |
| C7 | Type VII collagen |
| C17 | Type XVII collagen |
| Tyr | Tyrosine |
References
- Mariath, L.M.; Santin, J.T.; Schuler-Faccini, L.; Kiszewski, A.E. Inherited Epidermolysis Bullosa: Update on the Clinical and Genetic Aspects. An. Bras. Dermatol. 2020, 95, 551–569. [Google Scholar] [CrossRef]
- Has, C.; Bauer, J.W.; Bodemer, C.; Bolling, M.C.; Bruckner-Tuderman, L.; Diem, A.; Fine, J.-D.; Heagerty, A.; Hovnanian, A.; Marinkovich, M.P.; et al. Consensus Reclassification of Inherited Epidermolysis Bullosa and Other Disorders with Skin Fragility. Br. J. Dermatol. 2020, 183, 614–627. [Google Scholar] [CrossRef]
- Fine, J.-D.; Mellerio, J.E. Extracutaneous Manifestations and Complications of Inherited Epidermolysis Bullosa. J. Am. Acad. Dermatol. 2009, 61, 367–384. [Google Scholar] [CrossRef]
- Bardhan, A.; Bruckner-Tuderman, L.; Chapple, I.L.C.; Fine, J.-D.; Harper, N.; Has, C.; Magin, T.M.; Marinkovich, M.P.; Marshall, J.F.; McGrath, J.A.; et al. Epidermolysis Bullosa. Nat. Rev. Dis. Primers 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, E.; Ruppert, R.; Fässler, R. The Kindlin Family: Functions, Signaling Properties and Implications for Human Disease. J. Cell Sci. 2016, 129, 17–27. [Google Scholar] [CrossRef]
- Fine, J.-D.; Bruckner-Tuderman, L.; Eady, R.A.J.; Bauer, E.A.; Bauer, J.W.; Has, C.; Heagerty, A.; Hintner, H.; Hovnanian, A.; Jonkman, M.F.; et al. Inherited Epidermolysis Bullosa: Updated Recommendations on Diagnosis and Classification. J. Am. Acad. Dermatol. 2014, 70, 1103–1126. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Weisman, A.; King, A.; Maksomski, S.; Shotwell, C.; Bailie, C.; Weaver, H.; Bodan, R.; Guerrero, E.; Zmazek, M.; et al. Occupational Therapy for Epidermolysis Bullosa: Clinical Practice Guidelines. Orphanet J. Rare Dis. 2019, 14, 129. [Google Scholar] [CrossRef]
- Goldschneider, K.R.; Good, J.; Harrop, E.; Liossi, C.; Lynch-Jordan, A.; Martinez, A.E.; Maxwell, L.G.; Stanko-Lopp, D. Pain Care for Patients with Epidermolysis Bullosa: Best Care Practice Guidelines. BMC Med. 2014, 12, 178. [Google Scholar] [CrossRef]
- Piñón Hofbauer, J.; Wally, V.; Guttmann-Gruber, C.; Gratz, I.; Koller, U. Therapy Development for Epidermolysis Bullosa. In Rare Diseases—Diagnostic and Therapeutic Odyssey; Valarmathi, M.T., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83962-930-3. [Google Scholar]
- Pope, E.; Lara-Corrales, I.; Mellerio, J.; Martinez, A.; Schultz, G.; Burrell, R.; Goodman, L.; Coutts, P.; Wagner, J.; Allen, U.; et al. A Consensus Approach to Wound Care in Epidermolysis Bullosa. J. Am. Acad. Dermatol. 2012, 67, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Laimer, M.; Prodinger, C.; Bauer, J.W. Hereditary Epidermolysis Bullosa. J. Dtsch. Derma. Gesell. 2015, 13, 1125–1133. [Google Scholar] [CrossRef]
- Welponer, T.; Prodinger, C.; Pinon-Hofbauer, J.; Hintersteininger, A.; Breitenbach-Koller, H.; Bauer, J.W.; Laimer, M. Clinical Perspectives of Gene-Targeted Therapies for Epidermolysis Bullosa. Dermatol. Ther. 2021, 11, 1175–1197. [Google Scholar] [CrossRef] [PubMed]
- Guide, S.V.; Gonzalez, M.E.; Bağcı, I.S.; Agostini, B.; Chen, H.; Feeney, G.; Steimer, M.; Kapadia, B.; Sridhar, K.; Quesada Sanchez, L.; et al. Trial of Beremagene Geperpavec (B-VEC) for Dystrophic Epidermolysis Bullosa. N. Engl. J. Med. 2022, 387, 2211–2219. [Google Scholar] [CrossRef]
- Murrell, D.F.; Bodemer, C.; Bruckner, A.L.; Cunningham, T.; Davis, C.; Fernández, M.F.; Kiritsi, D.; Maher, L.; Sprecher, E.; Torres-Pradilla, M.; et al. Long-Term Safety and Efficacy of Oleogel-S10 (Birch Bark Extract) in Epidermolysis Bullosa: 24-Month Results from the Phase III EASE Study. Br. J. Dermatol. 2025, 192, 1007–1017. [Google Scholar] [CrossRef]
- Tang, J.Y.; Marinkovich, M.P.; Wiss, K.; McCarthy, D.; Truesdale, A.; Chiou, A.S.; Eid, E.; McIntyre, J.K.; Bailey, I.; Furukawa, L.K.; et al. Prademagene Zamikeracel for Recessive Dystrophic Epidermolysis Bullosa Wounds (VIITAL): A Two-Centre, Randomised, Open-Label, Intrapatient-Controlled Phase 3 Trial. Lancet 2025, 406, 163–173. [Google Scholar] [CrossRef]
- Hammersen, J.; Has, C.; Naumann-Bartsch, N.; Stachel, D.; Kiritsi, D.; Söder, S.; Tardieu, M.; Metzler, M.; Bruckner-Tuderman, L.; Schneider, H. Genotype, Clinical Course, and Therapeutic Decision Making in 76 Infants with Severe Generalized Junctional Epidermolysis Bullosa. J. Investig. Dermatol. 2016, 136, 2150–2157. [Google Scholar] [CrossRef]
- Varki, R.; Sadowski, S.; Pfendner, E.; Uitto, J. Epidermolysis Bullosa. I. Molecular Genetics of the Junctional and Hemidesmosomal Variants. J. Med. Genet. 2006, 43, 641–652. [Google Scholar] [CrossRef]
- Celik, A.; Kervestin, S.; Jacobson, A. NMD: At the Crossroads between Translation Termination and Ribosome Recycling. Biochimie 2015, 114, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N.; Pearce, D.A. Nonsense-Mediated Decay in Genetic Disease: Friend or Foe? Mutat. Res./Rev. Mutat. Res. 2014, 762, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Lejeune, F. Deciphering the Molecular Mechanism of Stop Codon Readthrough. Biol. Rev. 2021, 96, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Frizzell, R.A.; Bedwell, D.M. Aminoglycoside Antibiotics Restore CFTR Function by Overcoming Premature Stop Mutations. Nat. Med. 1996, 2, 467–469. [Google Scholar] [CrossRef]
- Lee, H.-L.R.; Dougherty, J.P. Pharmaceutical Therapies to Recode Nonsense Mutations in Inherited Diseases. Pharmacol. Ther. 2012, 136, 227–266. [Google Scholar] [CrossRef] [PubMed]
- Keeling, K.M.; Xue, X.; Gunn, G.; Bedwell, D.M. Therapeutics Based on Stop Codon Readthrough. Annu. Rev. Genom. Hum. Genet. 2014, 15, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T. Aminoglycoside Antibiotics: Old Drugs and New Therapeutic Approaches. Cell. Mol. Life Sci. 2007, 64, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.T.; Anderson, C.B.; Fass, U.; Khatri, S.; Gesteland, R.F.; Atkins, J.F.; Flanigan, K.M. Readthrough of Dystrophin Stop Codon Mutations Induced by Aminoglycosides. Ann. Neurol. 2004, 55, 422–426. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.R.; Cotton, C.U.; Hodges, C.A. Synergy between Readthrough and Nonsense Mediated Decay Inhibition in a Murine Model of Cystic Fibrosis Nonsense Mutations. IJMS 2020, 22, 344. [Google Scholar] [CrossRef] [PubMed]
- Cogan, J.; Weinstein, J.; Wang, X.; Hou, Y.; Martin, S.; South, A.P.; Woodley, D.T.; Chen, M. Aminoglycosides Restore Full-Length Type VII Collagen by Overcoming Premature Termination Codons: Therapeutic Implications for Dystrophic Epidermolysis Bullosa. Mol. Ther. 2014, 22, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Sayar, S.B.; Zheng, S.; Chacón-Solano, E.; Condrat, I.; Yadav, A.; Roberge, M.; Larcher Laguzzi, F. Read-Through for Nonsense Mutations in Type XVII Collagen—Deficient Junctional Epidermolysis Bullosa. J. Investig. Dermatol. 2022, 142, 1227–1230.e4. [Google Scholar] [CrossRef]
- Sayar, S.B.; Has, C. Strategy for the Optimization of Read-Through Therapy for Junctional Epidermolysis Bullosa with COL17A1 Nonsense Mutation. J. Investig. Dermatol. 2024, 144, 2221–2229.e1. [Google Scholar] [CrossRef]
- Lai, C.-H.; Chun, H.H.; Nahas, S.A.; Mitui, M.; Gamo, K.M.; Du, L.; Gatti, R.A. Correction of ATM Gene Function by Aminoglycoside-Induced Read-through of Premature Termination Codons. Proc. Natl. Acad. Sci. USA 2004, 101, 15676–15681. [Google Scholar] [CrossRef]
- Barton-Davis, E.R.; Cordier, L.; Shoturma, D.I.; Leland, S.E.; Sweeney, H.L. Aminoglycoside Antibiotics Restore Dystrophin Function to Skeletal Muscles of Mdx Mice. J. Clin. Investig. 1999, 104, 375–381. [Google Scholar] [CrossRef]
- Wilschanski, M.; Famini, C.; Blau, H.; Rivlin, J.; Augarten, A.; Avital, A.; Kerem, B.; Kerem, E. A Pilot Study of the Effect of Gentamicin on Nasal Potential Difference Measurements in Cystic Fibrosis Patients Carrying Stop Mutations. Am. J. Respir. Crit. Care Med. 2000, 161, 860–865. [Google Scholar] [CrossRef]
- Clancy, J.P.; Bebök, Z.; Ruiz, F.; King, C.; Jones, J.; Walker, L.; Greer, H.; Hong, J.; Wing, L.; Macaluso, M.; et al. Evidence That Systemic Gentamicin Suppresses Premature Stop Mutations in Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2001, 163, 1683–1692. [Google Scholar] [CrossRef]
- Wilschanski, M.; Yahav, Y.; Yaacov, Y.; Blau, H.; Bentur, L.; Rivlin, J.; Aviram, M.; Bdolah-Abram, T.; Bebok, Z.; Shushi, L.; et al. Gentamicin-Induced Correction of CFTR Function in Patients with Cystic Fibrosis and CFTR Stop Mutations. N. Engl. J. Med. 2003, 349, 1433–1441. [Google Scholar] [CrossRef]
- Malik, V.; Rodino-Klapac, L.R.; Viollet, L.; Wall, C.; King, W.; Al-Dahhak, R.; Lewis, S.; Shilling, C.J.; Kota, J.; Serrano-Munuera, C.; et al. Gentamicin-induced Readthrough of Stop Codons in Duchenne Muscular Dystrophy. Ann. Neurol. 2010, 67, 771–780. [Google Scholar] [CrossRef]
- Wagner, K.R.; Hamed, S.; Hadley, D.W.; Gropman, A.L.; Burstein, A.H.; Escolar, D.M.; Hoffman, E.P.; Fischbeck, K.H. Gentamicin Treatment of Duchenne and Becker Muscular Dystrophy Due to Nonsense Mutations. Ann. Neurol. 2001, 49, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Woodley, D.T.; Cogan, J.; Hou, Y.; Lyu, C.; Marinkovich, M.P.; Keene, D.; Chen, M. Gentamicin Induces Functional Type VII Collagen in Recessive Dystrophic Epidermolysis Bullosa Patients. J. Clin. Investig. 2017, 127, 3028–3038. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Manjunath, S.; Madakshira, M.G.; De, D.; Handa, S.; Chatterjee, D.; Radotra, B.D. Topical Gentamicin 0.1% Promotes Collagen 7 Expression in Recessive Dystrophic Epidermolysis Bullosa. Indian Dermatol. Online J. 2022, 13, 480–483. [Google Scholar] [CrossRef]
- Sandanger, Ø.; Torp, M.K.; Glesaaen, A.; Nilsen, H.R.; Sitek, J.C. Nonintensive Topical Gentamicin Treatment of Patients with Severe Epidermolysis Bullosa Caused by Nonsense Mutations. JEADV Clin. Pract. 2024, 3, 536–546. [Google Scholar] [CrossRef]
- Woodley, D.T.; Hao, M.; Kwong, A.; Levian, B.; Cogan, J.; Hou, Y.; Mosallaei, D.; Kleinman, E.; Zheng, K.; Chung, C.; et al. Intravenous Gentamicin Therapy Induces Functional Type VII Collagen in Patients with Recessive Dystrophic Epidermolysis Bullosa: An Open-Label Clinical Trial. Br. J. Dermatol. 2024, 191, 267–274. [Google Scholar] [CrossRef]
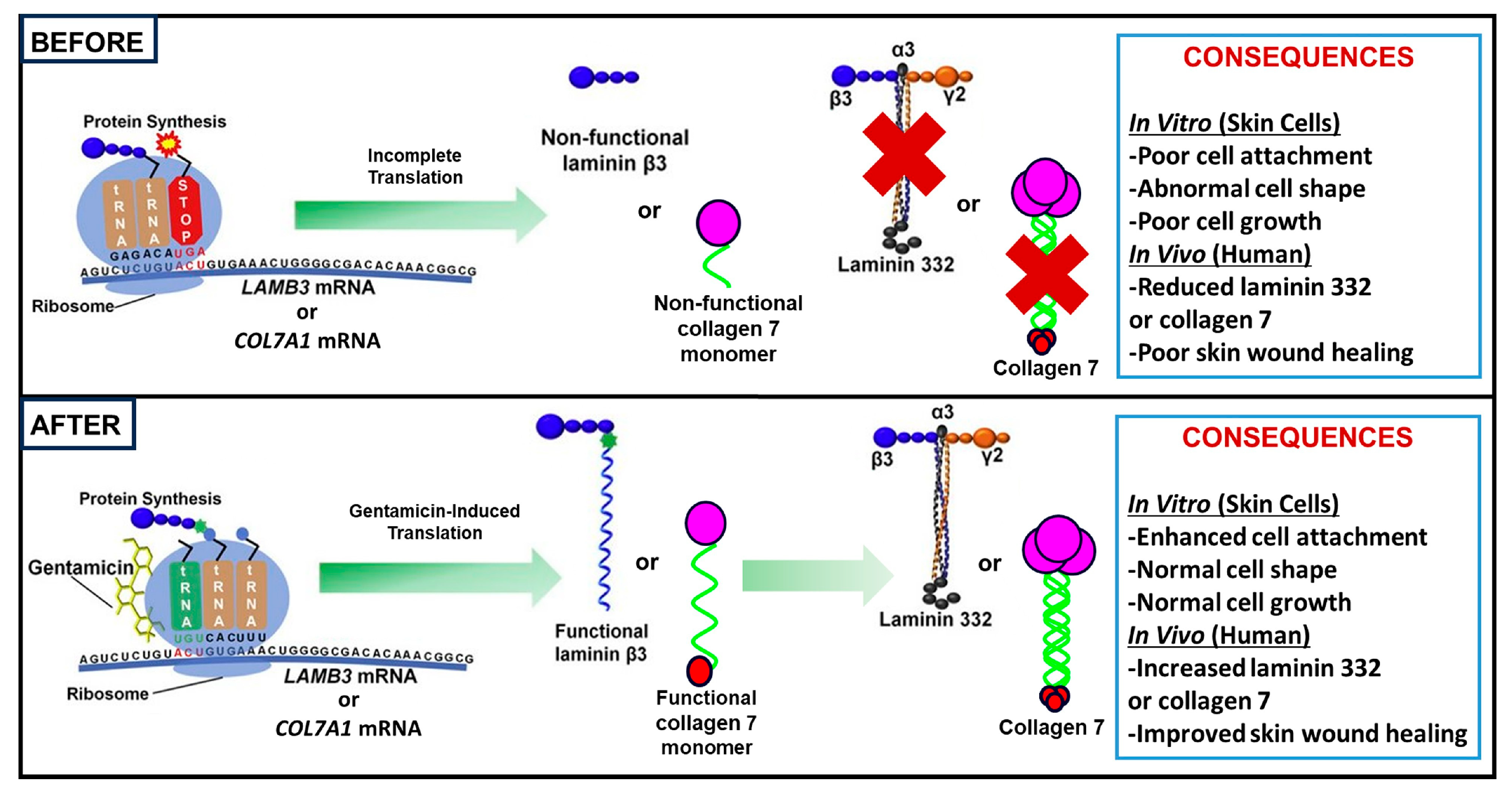
- Lincoln, V.; Cogan, J.; Hou, Y.; Hirsch, M.; Hao, M.; Alexeev, V.; De Luca, M.; De Rosa, L.; Bauer, J.W.; Woodley, D.T.; et al. Gentamicin Induces LAMB3 Nonsense Mutation Readthrough and Restores Functional Laminin 332 in Junctional Epidermolysis Bullosa. Proc. Natl. Acad. Sci. USA 2018, 115, E6536–E6545. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.; Cogan, J.; Hou, Y.; Antaya, R.; Hao, M.; Kim, G.; Lincoln, V.; Chen, Q.; Woodley, D.T.; Chen, M. Gentamicin Induces Laminin 332 and Improves Wound Healing in Junctional Epidermolysis Bullosa Patients with Nonsense Mutations. Mol. Ther. 2020, 28, 1327–1338. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.; Liang, J.; Zheng, L.; Chen, F.; Yao, Z.; Li, M. Gentamicin Induces COL17A1 Nonsense Mutation Readthrough in Junctional Epidermolysis Bullosa. J. Dermatol. 2020, 47, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Hou, P.; Huang, F.; Hsu, C. Topical Gentamicin Ointment Induces LAMB3 Nonsense Mutation Readthrough and Improves Corneal Erosions in a Patient with Junctional Epidermolysis Bullosa. Clin. Exp. Ophthalmol. 2021, 49, 309–312. [Google Scholar] [CrossRef]
- Hammersen, J.; Neuner, A.; Wild, F.; Schneider, H. Attenuation of Severe Generalized Junctional Epidermolysis Bullosa by Systemic Treatment with Gentamicin. Dermatology 2019, 235, 315–322. [Google Scholar] [CrossRef]
- Mosallaei, D.; Hao, M.; Antaya, R.J.; Levian, B.; Kwong, A.; Cogan, J.; Hamilton, C.; Schwieger-Briel, A.; Tan, C.; Tang, X.; et al. Molecular and Clinical Outcomes After Intravenous Gentamicin Treatment for Patients With Junctional Epidermolysis Bullosa Caused by Nonsense Variants. JAMA Dermatol. 2022, 158, 366. [Google Scholar] [CrossRef]
- Martínez-Santamaría, L.; Maseda, R.; De Arriba, M.D.C.; Membrilla, J.A.; Sigüenza, A.I.; Mascías, J.; García, M.; Quintana, L.; Esteban-Rodríguez, I.; Hernández-Fernández, C.P.; et al. Evaluation of Systemic Gentamicin as Translational Readthrough Therapy for a Patient With Epidermolysis Bullosa Simplex With Muscular Dystrophy Owing to PLEC1 Pathogenic Nonsense Variants. JAMA Dermatol. 2022, 158, 439. [Google Scholar] [CrossRef]
- Guthrie, O.W. Aminoglycoside Induced Ototoxicity. Toxicology 2008, 249, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-Induced Nephrotoxicity. J. Pharm. Pract. 2014, 27, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kerem, E. ELX-02: An Investigational Read-through Agent for the Treatment of Nonsense Mutation-Related Genetic Disease. Expert Opin. Investig. Drugs 2020, 29, 1347–1354. [Google Scholar] [CrossRef]
- Kandasamy, J.; Atia-Glikin, D.; Shulman, E.; Shapira, K.; Shavit, M.; Belakhov, V.; Baasov, T. Increased Selectivity toward Cytoplasmic versus Mitochondrial Ribosome Confers Improved Efficiency of Synthetic Aminoglycosides in Fixing Damaged Genes: A Strategy for Treatment of Genetic Diseases Caused by Nonsense Mutations. J. Med. Chem. 2012, 55, 10630–10643. [Google Scholar] [CrossRef] [PubMed]
- Haverty, T.; Wyatt, D.J.; Porter, K.M.; Leubitz, A.; Banks, K.; Goodyer, P.; Hu, M. Phase 1 Renal Impairment Trial Results Supports Targeted Individualized Dosing of ELX-02 in Patients With Nephropathic Cystinosis. J. Clin. Pharmacol. 2021, 61, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Leubitz, A.; Vanhoutte, F.; Hu, M.; Porter, K.; Gordon, E.; Tencer, K.; Campbell, K.; Banks, K.; Haverty, T. A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Escalation Study to Evaluate the Safety and Pharmacokinetics of ELX-02 in Healthy Subjects. Clin. Pharm. Drug Dev. 2021, 10, 859–869. [Google Scholar] [CrossRef]
- Leubitz, A.; Frydman-Marom, A.; Sharpe, N.; Van Duzer, J.; Campbell, K.C.M.; Vanhoutte, F. Safety, Tolerability, and Pharmacokinetics of Single Ascending Doses of ELX-02, a Potential Treatment for Genetic Disorders Caused by Nonsense Mutations, in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2019, 8, 984–994. [Google Scholar] [CrossRef]
- Levian, B.; Hou, Y.; Tang, X.; Bainvoll, L.; Zheng, K.; Badarinarayana, V.; Aghamohammadzadeh, S.; Chen, M. Novel Readthrough Agent. Suppresses Nonsense Mutations and Restores Functional Type VII Collagen and Laminin 332 in Epidermolysis Bullosa. Mol. Ther. Nucleic Acids 2024, 35, 102334. [Google Scholar] [CrossRef]
- Brasell, E.J.; Chu, L.L.; Akpa, M.M.; Eshkar-Oren, I.; Alroy, I.; Corsini, R.; Gilfix, B.M.; Yamanaka, Y.; Huertas, P.; Goodyer, P. The Novel Aminoglycoside, ELX-02, Permits CTNSW138X Translational Read-through and Restores Lysosomal Cystine Efflux in Cystinosis. PLoS ONE 2019, 14, e0223954. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Juan, L.; Shi, W.; Nie, Z.; Zhang, S.; Ma, F.; Hu, J.; Chen, J.; Li, P.; Xie, X. Pharmaceuticals Promoting Premature Termination Codon Readthrough: Progress in Development. Biomolecules 2023, 13, 988. [Google Scholar] [CrossRef]
- Welch, E.M.; Barton, E.R.; Zhuo, J.; Tomizawa, Y.; Friesen, W.J.; Trifillis, P.; Paushkin, S.; Patel, M.; Trotta, C.R.; Hwang, S.; et al. PTC124 Targets Genetic Disorders Caused by Nonsense Mutations. Nature 2007, 447, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Friesen, W.J.; Tomizawa, Y.; Leszyk, J.D.; Zhuo, J.; Johnson, B.; Dakka, J.; Trotta, C.R.; Xue, X.; Mutyam, V.; et al. Ataluren Stimulates Ribosomal Selection of Near-Cognate tRNAs to Promote Nonsense Suppression. Proc. Natl. Acad. Sci. USA 2016, 113, 12508–12513. [Google Scholar] [CrossRef]
- Huang, S.; Bhattacharya, A.; Ghelfi, M.D.; Li, H.; Fritsch, C.; Chenoweth, D.M.; Goldman, Y.E.; Cooperman, B.S. Ataluren Binds to Multiple Protein Synthesis Apparatus Sites and Competitively Inhibits Release Factor-Dependent Termination. Nat. Commun. 2022, 13, 2413. [Google Scholar] [CrossRef]
- Berger, J.; Li, M.; Berger, S.; Meilak, M.; Rientjes, J.; Currie, P.D. Effect of Ataluren on Dystrophin Mutations. J. Cell. Mol. Med. 2020, 24, 6680–6689. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, X.; Welch, E.M.; Hirawat, S.; Peltz, S.W.; Bedwell, D.M. PTC124 Is an Orally Bioavailable Compound That Promotes Suppression of the Human CFTR -G542X Nonsense Allele in a CF Mouse Model. Proc. Natl. Acad. Sci. USA 2008, 105, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Beryozkin, A.; Samanta, A.; Gopalakrishnan, P.; Khateb, S.; Banin, E.; Sharon, D.; Nagel-Wolfrum, K. Translational Read-Through Drugs (TRIDs) Are Able to Restore Protein Expression and Ciliogenesis in Fibroblasts of Patients with Retinitis Pigmentosa Caused by a Premature Termination Codon in FAM161A. IJMS 2022, 23, 3541. [Google Scholar] [CrossRef]
- Bezzerri, V.; Lentini, L.; Api, M.; Busilacchi, E.M.; Cavalieri, V.; Pomilio, A.; Diomede, F.; Pegoraro, A.; Cesaro, S.; Poloni, A.; et al. Novel Translational Read-through–Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome. Biomedicines 2022, 10, 886. [Google Scholar] [CrossRef]
- Miller, J.N.; Kovács, A.D.; Pearce, D.A. The Novel Cln1R151X Mouse Model of Infantile Neuronal Ceroid Lipofuscinosis (INCL) for Testing Nonsense Suppression Therapy. Hum. Mol. Genet. 2015, 24, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Stingl, K.; Kohl, S.; Ries, J.; Linnert, J.; Nagel-Wolfrum, K. Ataluren for the Treatment of Usher Syndrome 2A Caused by Nonsense Mutations. IJMS 2019, 20, 6274. [Google Scholar] [CrossRef]
- Brumm, H.; Mühlhaus, J.; Bolze, F.; Scherag, S.; Hinney, A.; Hebebrand, J.; Wiegand, S.; Klingenspor, M.; Grüters, A.; Krude, H.; et al. Rescue of Melanocortin 4 Receptor (MC4R) Nonsense Mutations by Aminoglycoside-Mediated Read-Through. Obesity 2012, 20, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Dranchak, P.K.; Di Pietro, E.; Snowden, A.; Oesch, N.; Braverman, N.E.; Steinberg, S.J.; Hacia, J.G. Nonsense Suppressor Therapies Rescue Peroxisome Lipid Metabolism and Assembly in Cells from Patients with Specific PEX Gene Mutations. J. Cell. Biochem. 2011, 112, 1250–1258. [Google Scholar] [CrossRef]
- Harmer, S.C.; Mohal, J.S.; Kemp, D.; Tinker, A. Readthrough of Long-QT Syndrome Type 1 Nonsense Mutations Rescues Function but Alters the Biophysical Properties of the Channel. Biochem. J. 2012, 443, 635–642. [Google Scholar] [CrossRef]
- Auld, D.S.; Thorne, N.; Maguire, W.F.; Inglese, J. Mechanism of PTC124 Activity in Cell-Based Luciferase Assays of Nonsense Codon Suppression. Proc. Natl. Acad. Sci. USA 2009, 106, 3585–3590. [Google Scholar] [CrossRef]
- McElroy, S.P.; Nomura, T.; Torrie, L.S.; Warbrick, E.; Gartner, U.; Wood, G.; McLean, W.H.I. A Lack of Premature Termination Codon Read-Through Efficacy of PTC124 (Ataluren) in a Diverse Array of Reporter Assays. PLoS Biol. 2013, 11, e1001593. [Google Scholar] [CrossRef]
- Ryan, N.J. Ataluren: First Global Approval. Drugs 2014, 74, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Kerem, E.; Konstan, M.W.; De Boeck, K.; Accurso, F.J.; Sermet-Gaudelus, I.; Wilschanski, M.; Elborn, J.S.; Melotti, P.; Bronsveld, I.; Fajac, I.; et al. Ataluren for the Treatment of Nonsense-Mutation Cystic Fibrosis: A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet Respir. Med. 2014, 2, 539–547. [Google Scholar] [CrossRef]
- Konstan, M.W.; VanDevanter, D.R.; Rowe, S.M.; Wilschanski, M.; Kerem, E.; Sermet-Gaudelus, I.; DiMango, E.; Melotti, P.; McIntosh, J.; De Boeck, K. Efficacy and Safety of Ataluren in Patients with Nonsense-Mutation Cystic Fibrosis Not Receiving Chronic Inhaled Aminoglycosides: The International, Randomized, Double-Blind, Placebo-Controlled Ataluren Confirmatory Trial in Cystic Fibrosis (ACT CF). J. Cyst. Fibros. 2020, 19, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Peabody Lever, J.E.; Mutyam, V.; Hathorne, H.Y.; Peng, N.; Sharma, J.; Edwards, L.J.; Rowe, S.M. Ataluren/Ivacaftor Combination Therapy: Two N-of-1 Trials in Cystic Fibrosis Patients with Nonsense Mutations. Pediatr. Pulmonol. 2020, 55, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, G.M.; Amano, S.U.; Flanagan, K.E.; Rieger, K.E.; Marinkovich, M.P.; Wiss, K. Treatment With Ataluren for Wound Healing and Health Complications in a Patient With Junctional Epidermolysis Bullosa. JAMA Dermatol. 2023, 159, 1145. [Google Scholar] [CrossRef]
- Maquat, L.E. Nonsense-Mediated mRNA Decay; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-1-4987-1339-9. [Google Scholar]
- Bell, J. Amlexanox for the Treatment of Recurrent Aphthous Ulcers. Clin. Drug Investig. 2005, 25, 555–566. [Google Scholar] [CrossRef]
- Gonzalez-Hilarion, S.; Beghyn, T.; Jia, J.; Debreuck, N.; Berte, G.; Mamchaoui, K.; Mouly, V.; Gruenert, D.C.; Déprez, B.; Lejeune, F. Rescue of Nonsense Mutations by Amlexanox in Human Cells. Orphanet J. Rare Dis. 2012, 7, 58. [Google Scholar] [CrossRef]
- Benslimane, N.; Miressi, F.; Loret, C.; Richard, L.; Nizou, A.; Pyromali, I.; Faye, P.-A.; Favreau, F.; Lejeune, F.; Lia, A.-S. Amlexanox: Readthrough Induction and Nonsense-Mediated mRNA Decay Inhibition in a Charcot–Marie–Tooth Model of hiPSCs-Derived Neuronal Cells Harboring a Nonsense Mutation in GDAP1 Gene. Pharmaceuticals 2023, 16, 1034. [Google Scholar] [CrossRef]
- Atanasova, V.S.; Jiang, Q.; Prisco, M.; Gruber, C.; Piñón Hofbauer, J.; Chen, M.; Has, C.; Bruckner-Tuderman, L.; McGrath, J.A.; Uitto, J.; et al. Amlexanox Enhances Premature Termination Codon Read-Through in COL7A1 and Expression of Full Length Type VII Collagen: Potential Therapy for Recessive Dystrophic Epidermolysis Bullosa. J. Investig. Dermatol. 2017, 137, 1842–1849. [Google Scholar] [CrossRef]
- Chauvin, C.; Jean-Jean, O. Proteasomal Degradation of Human Release Factor eRF3a Regulates Translation Termination Complex Formation. RNA 2008, 14, 240–245. [Google Scholar] [CrossRef]
- Lee, R.E.; Lewis, C.A.; He, L.; Bulik-Sullivan, E.C.; Gallant, S.C.; Mascenik, T.M.; Dang, H.; Cholon, D.M.; Gentzsch, M.; Morton, L.C.; et al. Small-Molecule eRF3a Degraders Rescue CFTR Nonsense Mutations by Promoting Premature Termination Codon Readthrough. J. Clin. Investig. 2022, 132, e154571. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.P.L.; Yip, M.C.J.; Oltion, K.; Taunton, J.; Shao, S. The eRF1 Degrader SRI-41315 Acts as a Molecular Glue at the Ribosomal Decoding Center. Nat. Chem. Biol. 2024, 20, 877–884. [Google Scholar] [CrossRef]
- Baradaran-Heravi, A.; Balgi, A.D.; Hosseini-Farahabadi, S.; Choi, K.; Has, C.; Roberge, M. Effect of Small Molecule eRF3 Degraders on Premature Termination Codon Readthrough. Nucleic Acids Res. 2021, 49, 3692–3708. [Google Scholar] [CrossRef] [PubMed]
- Palomar-Siles, M.; Yurevych, V.; Bykov, V.J.N.; Wiman, K.G. Pharmacological Induction of Translational Readthrough of Nonsense Mutations in the Retinoblastoma (RB1) Gene. PLoS ONE 2023, 18, e0292468. [Google Scholar] [CrossRef]
- Heldin, A.; Cancer, M.; Palomar-Siles, M.; Öhlin, S.; Zhang, M.; Sun-Zhang, A.; Mariani, A.; Liu, J.; Bykov, V.J.N.; Wiman, K.G. Novel Compounds That Synergize with Aminoglycoside G418 or eRF3 Degraders for Translational Readthrough of Nonsense Mutant TP53 and PTEN. RNA Biol. 2023, 20, 368–383. [Google Scholar] [CrossRef]
- Gurzeler, L.-A.; Link, M.; Ibig, Y.; Schmidt, I.; Galuba, O.; Schoenbett, J.; Gasser-Didierlaurant, C.; Parker, C.N.; Mao, X.; Bitsch, F.; et al. Drug-Induced eRF1 Degradation Promotes Readthrough and Reveals a New Branch of Ribosome Quality Control. Cell Rep. 2023, 42, 113056. [Google Scholar] [CrossRef]
- Sharma, J.; Du, M.; Wong, E.; Mutyam, V.; Li, Y.; Chen, J.; Wangen, J.; Thrasher, K.; Fu, L.; Peng, N.; et al. A Small Molecule That Induces Translational Readthrough of CFTR Nonsense Mutations by eRF1 Depletion. Nat. Commun. 2021, 12, 4358. [Google Scholar] [CrossRef]
- Hou, Y.; Levian, B.; Chung, C.; Woodley, D.; Chen, M. 756 Enhancing Readthrough Therapy for Epidermolysis Bullosa: The Synergistic Potential of SRI-41315 and Gentamicin. J. Investig. Dermatol. 2024, 144, S132. [Google Scholar] [CrossRef]
- Levian, B.; Hou, Y.; Chung, C.; Filzen, R.; Zheng, K.; Chen, M. 405 CC-90009 Potentiates Gentamicin-Induced Premature Termination Codon Readthrough Activity in Epidermolysis Bullosa. J. Investig. Dermatol. 2024, 144, S70. [Google Scholar] [CrossRef]
- Trzaska, C.; Amand, S.; Bailly, C.; Leroy, C.; Marchand, V.; Duvernois-Berthet, E.; Saliou, J.-M.; Benhabiles, H.; Werkmeister, E.; Chassat, T.; et al. 2,6-Diaminopurine as a Highly Potent Corrector of UGA Nonsense Mutations. Nat. Commun. 2020, 11, 1509. [Google Scholar] [CrossRef]
- Leroy, C.; Spelier, S.; Essonghe, N.C.; Poix, V.; Kong, R.; Gizzi, P.; Bourban, C.; Amand, S.; Bailly, C.; Guilbert, R.; et al. Use of 2,6-Diaminopurine as a Potent Suppressor of UGA Premature Stop Codons in Cystic Fibrosis. Mol. Ther. 2023, 31, 970–985. [Google Scholar] [CrossRef]
- Miao, K.L. Use of 2,6-Diaminopurine as a Highly Potent Corrector of UGA Nonsense Mutations in Recessive Dystrophic Epidermolysis Bullosa. Presented at the Society of Investigate Dermatology Annual Meeting, San Diego, CA, USA, 7–10 May 2025. [Google Scholar]
- Carollo, P.S.; Tutone, M.; Culletta, G.; Fiduccia, I.; Corrao, F.; Pibiri, I.; Di Leonardo, A.; Zizzo, M.G.; Melfi, R.; Pace, A.; et al. Investigating the Inhibition of FTSJ1, a Tryptophan tRNA-Specific 2′-O-Methyltransferase by NV TRIDs, as a Mechanism of Readthrough in Nonsense Mutated CFTR. IJMS 2023, 24, 9609. [Google Scholar] [CrossRef] [PubMed]
- Corrao, F.; Zizzo, M.G.; Tutone, M.; Melfi, R.; Fiduccia, I.; Carollo, P.S.; Leonardo, A.D.; Caldara, G.; Perriera, R.; Pace, A.; et al. Nonsense Codons Suppression. An Acute Toxicity Study of Three Optimized TRIDs in Murine Model, Safety and Tolerability Evaluation. Biomed. Pharmacother. 2022, 156, 113886. [Google Scholar] [CrossRef] [PubMed]
- Fiduccia, I.; Corrao, F.; Zizzo, M.G.; Perriera, R.; Genovese, F.; Vitale, E.; Ricci, D.; Melfi, R.; Tutone, M.; Pace, A.; et al. Promoting Readthrough of Nonsense Mutations in CF Mouse Model: Biodistribution and Efficacy of NV848 in Rescuing CFTR Protein Expression. Mol. Ther. 2024, 32, 4514–4523. [Google Scholar] [CrossRef] [PubMed]
- Friesen, W.J.; Trotta, C.R.; Tomizawa, Y.; Zhuo, J.; Johnson, B.; Sierra, J.; Roy, B.; Weetall, M.; Hedrick, J.; Sheedy, J.; et al. The Nucleoside Analog Clitocine Is a Potent and Efficacious Readthrough Agent. RNA 2017, 23, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Zandanell, J.; Wießner, M.; Bauer, J.W.; Wagner, R.N. Stop Codon Readthrough as a Treatment Option for Epidermolysis Bullosa—Where We Are and Where We Are Going. Exp. Dermatol. 2024, 33, e15042. [Google Scholar] [CrossRef]
- Toledano, I.; Supek, F.; Lehner, B. Genome-Scale Quantification and Prediction of Pathogenic Stop Codon Readthrough by Small Molecules. Nat. Genet. 2024, 56, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Varki, R.; Sadowski, S.; Uitto, J.; Pfendner, E. Epidermolysis Bullosa. II. Type VII Collagen Mutations and Phenotype-Genotype Correlations in the Dystrophic Subtypes. J. Med. Genet. 2006, 44, 181–192. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, K.L.; Huynh, R.; Woodley, D.; Chen, M. Therapeutic Opportunities in Overcoming Premature Termination Codons in Epidermolysis Bullosa via Translational Readthrough. Cells 2025, 14, 1215. https://doi.org/10.3390/cells14151215
Miao KL, Huynh R, Woodley D, Chen M. Therapeutic Opportunities in Overcoming Premature Termination Codons in Epidermolysis Bullosa via Translational Readthrough. Cells. 2025; 14(15):1215. https://doi.org/10.3390/cells14151215
Chicago/Turabian StyleMiao, Kathleen L., Ryan Huynh, David Woodley, and Mei Chen. 2025. "Therapeutic Opportunities in Overcoming Premature Termination Codons in Epidermolysis Bullosa via Translational Readthrough" Cells 14, no. 15: 1215. https://doi.org/10.3390/cells14151215
APA StyleMiao, K. L., Huynh, R., Woodley, D., & Chen, M. (2025). Therapeutic Opportunities in Overcoming Premature Termination Codons in Epidermolysis Bullosa via Translational Readthrough. Cells, 14(15), 1215. https://doi.org/10.3390/cells14151215



