Epimedium-Derived Exosome-Loaded GelMA Hydrogel Enhances MC3T3-E1 Osteogenesis via PI3K/Akt Pathway
Abstract
1. Background
2. Methods
2.1. Materials
2.2. Epimedium Plant Material Processing and Exosome Isolation
2.3. Preparation of Gelatin Hydrogels
2.4. Preparation of GelMA@Exo (Exosome-Loaded GelMA Hydrogels)
2.5. MC3T3-E1 Cell Culture and Osteogenic Induction
2.6. Transwell Co-Culture with GelMA@Exo
2.7. Nanoparticle Tracking Analysis (NTA)
2.8. Transmission Electron Microscopy (TEM)
2.9. Nucleic Acid Electrophoresis
2.10. SDS-PAGE Protein Analysis
2.11. Fluorescent Labeling of Exosomes and Cellular Uptake
2.12. Scanning Electron Microscopy (SEM) of Hydrogels
2.13. Swelling Behavior of GelMA Hydrogels
2.14. Degradation Behavior of GelMA Hydrogels
2.15. Distribution of Labeled Exosomes in GelMA Hydrogels
2.16. Exosome Release Kinetics from GelMA Hydrogels
2.17. Optimization of Exosome and GelMA Concentrations (CCK-8 Assays)
2.18. Synergistic Effect of Gelatin Hydrogels and Exosomes on Cell Proliferation
2.19. Alkaline Phosphatase (ALP) Staining
2.20. Alizarin Red S Staining for Mineralization
2.21. Endothelial Tube Formation Assay
2.22. Wound Healing (Cell Migration) Assay
2.23. SA-β-Gal Staining for Cellular Senescence
2.24. Quantitative Real-Time PCR (qRT-PCR)
2.25. Western Blot Analysis
2.26. Transcriptomic Sequencing and Pathway Analysis
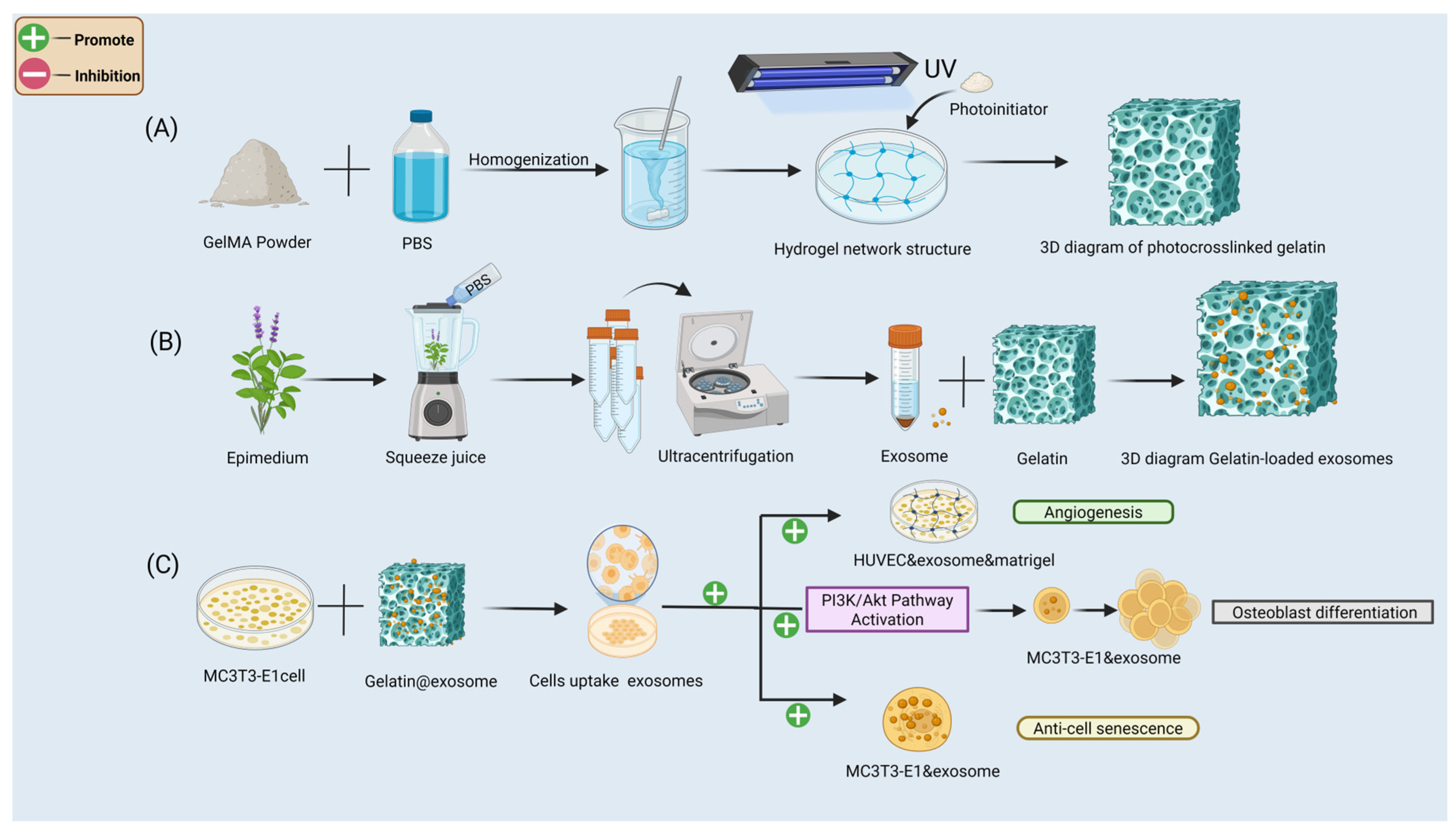
3. Construction and Biological Mechanism of the GelMA@Exosome System
- (A)
- Preparation of photocrosslinked GelMA hydrogels: GelMA prepolymer (homogenization of methacryloyl gelatin in PBS with LAP photoinitiator) is UV-crosslinked to form a stable 3D porous network.
- (B)
- Exosome isolation and loading: exosomes are extracted from Epimedium leaf tissues (by homogenization and ultracentrifugation) and then mixed into the GelMA prepolymer solution before UV-crosslinking to create the GelMA@Exo composite hydrogel.
- (C)
- In vitro functional validation: the GelMA@Exo system promotes angiogenesis in HUVECs, activates the PI3K/Akt signaling pathway in MC3T3-E1 preosteoblasts, enhances osteogenic differentiation, and reduces cellular senescence.
4. Results
4.1. Comprehensive Characterization of Epimedium-Derived Exosomes and GelMA Hydrogel Structure
4.2. In Vitro Evaluation of Exosome Release and Cellular Uptake from GelMA Hydrogels
4.3. Synergistic Enhancement of Cell Proliferation, Angiogenesis, and Migration by GelMA@Exo
4.4. GelMA@Exo Enhances Late-Stage Osteogenic Mineralization
4.5. GelMA@Exo Enhances Early Osteogenic Differentiation (ALP Activity)
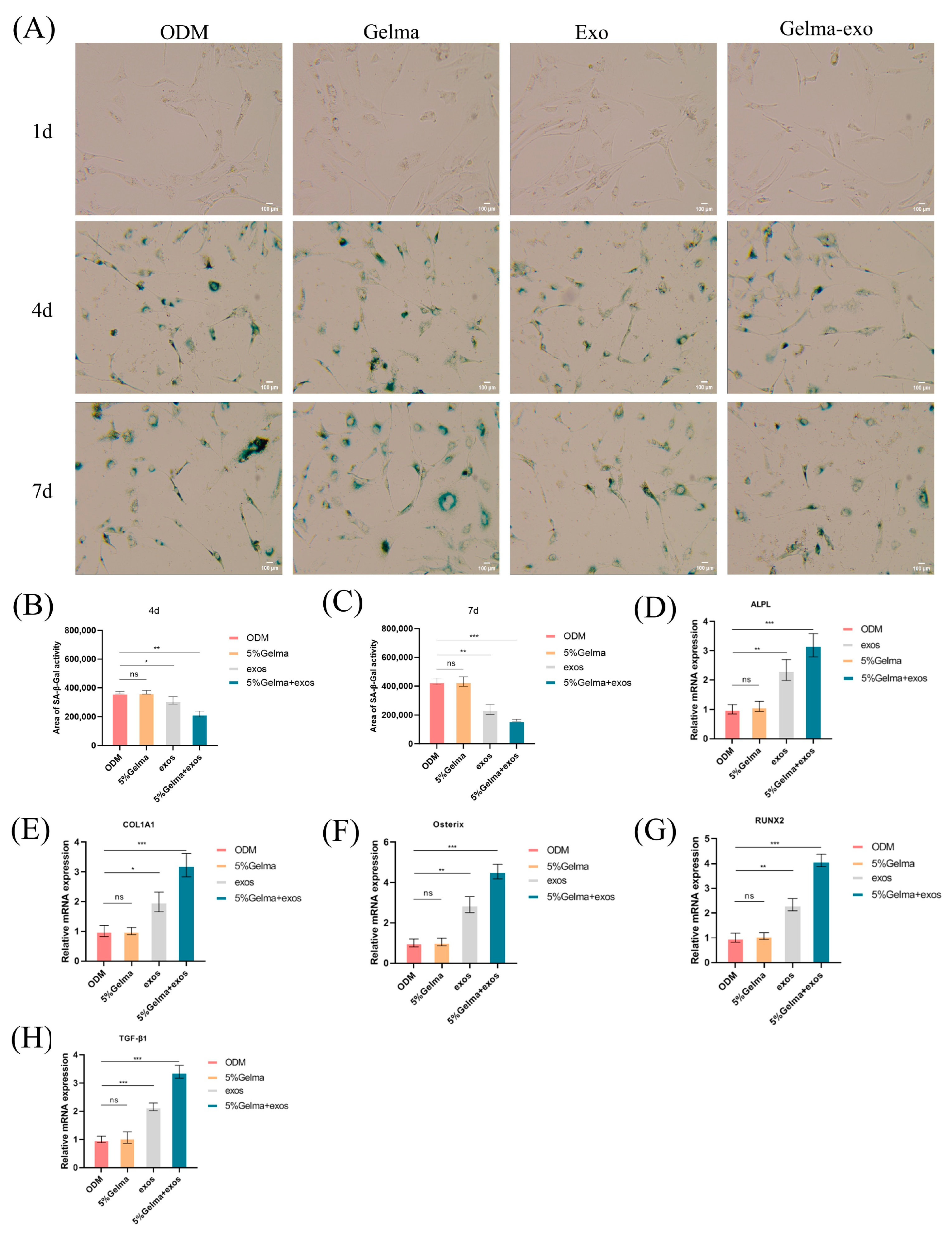
4.6. GelMA@Exo Attenuates Cellular Senescence and Upregulates Osteogenic Gene Expression
4.7. GelMA@Exo Synergistically Upregulates Osteogenic Protein Expression
4.8. Transcriptomic Profiling of GelMA@Exo-Treated Cells
4.9. Activation of PI3K/Akt Signaling Mediates GelMA@Exo’s Osteogenic Effect
5. Conclusions
- (1)
- Epimedium-derived exosomes were roughly 90–130 nm in size with intact RNA and protein cargo, indicating high purity and stability. GelMA hydrogels (5–12.5% w/v) formed porous 3D networks with tunable pore size, swelling ratio, and degradation rate, making them suitable and controllable scaffolds for delivering bioactive factors.
- (2)
- GelMA hydrogels enabled sustained exosome release for up to 7 days in a concentration-dependent manner. The released exosomes were uniformly distributed throughout the hydrogel and efficiently internalized by MC3T3-E1 cells, demonstrating that the GelMA@Exo system has high delivery efficiency and excellent biocompatibility.
- (3)
- GelMA@Exo synergistically enhanced multiple cellular functions. MC3T3-E1 proliferation increased with exosome dose (peaking at 195 μg/mL), and the combination of 5% GelMA + 195 μg/mL exosomes produced a strong synergistic proliferative effect (whereas GelMA alone had minimal impact). Notably, the GelMA@Exo composite Compared with other groups significantly improved angiogenesis (tube formation by HUVECs) and cell migration (scratch wound closure), highlighting its promise for enhancing tissue regeneration.
- (4)
- GelMA@Exo promoted osteogenic differentiation. It significantly elevated early ALP activity and later matrix mineralization (Alizarin Red staining) in MC3T3-E1 cells. Molecular analyses corroborated these results: GelMA alone did not upregulate osteogenic markers, exosomes alone did to some extent, and GelMA@Exo further amplified the expression of key osteogenic genes/proteins (RUNX2, Osterix, COL1A1, ALPL, TGF-β1). contributing to the composite’s enhanced osteogenic effect and fostering a more regenerative microenvironment.
- (5)
- Anti-senescence: GelMA@Exo exhibited a potent anti-senescent effect—treated osteoblasts had markedly fewer SA-β-Gal-positive senescent cells compared to ODM. Mechanistically, transcriptomic profiling showed broad upregulation of osteogenesis-related genes in GelMA@Exo-treated cells. Western blot analysis revealed elevated phosphorylation of PI3K and Akt following GelMA@Exo treatment, indicating activation of the PI3K/Akt signaling pathway. Moreover, treatment with the specific PI3K inhibitor LY294002 significantly suppressed Akt phosphorylation, confirming the involvement of the PI3K/Akt pathway in this process. Collectively, these results suggest that GelMA@Exo may facilitate the formation of an osteogenic microenvironment via activation of the PI3K/Akt signaling cascade.
- (6)
- Overall: We developed a GelMA@Exo composite hydrogel that synergistically provides structural support and osteoinductive stimulation, creating a robust pro-osteogenic microenvironment. This system enhanced the osteogenic differentiation of MC3T3-E1 cells by acting as a supportive 3D scaffold while concurrently delivering bioactive exosomes that modulate gene expression. Plant-derived exosome-loaded hydrogels could be a viable translational strategy for bone tissue engineering and regenerative medicine, possibly through activation of the PI3K/Akt signaling pathway.
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munoz, M.; Robinson, K.; Shibli-Rahhal, A. Bone health and osteoporosis prevention and treatment. Clin. Obstet. Gynecol. 2020, 63, 770–787. [Google Scholar] [CrossRef] [PubMed]
- Ramchand, S.K.; Leder, B.Z. Sequential therapy for the long-term treatment of postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 2024, 109, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P. Long-term treatment of postmenopausal osteoporosis. Endocrinol. Metab. 2021, 36, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lv, B.; Li, Z.; Ma, C.; Gui, Z.; Geng, Y.; Liu, G.; Sang, L.; Xu, C.; Min, Q. Bone-Targeted Biomimetic Nanogels Re-Establish Osteoblast/Osteoclast Balance to Treat Postmenopausal Osteoporosis. Small 2024, 20, 2303494. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Park, Y.-S.; Kim, H.-S.; Kim, D.-H.; Lee, S.-H.; Lee, C.-H.; Lee, S.-H.; Kim, J.-E.; Lee, S.; Kim, H.M. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J. Control. Release 2023, 355, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, X.; Zheng, K.; Ge, G.; Chen, X.; Xu, Y.; Bai, J.; Pan, G.; Geng, D. Horizon of exosome-mediated bone tissue regeneration: The all-rounder role in biomaterial engineering. Mater. Today Bio 2022, 16, 100355. [Google Scholar] [CrossRef] [PubMed]
- Sisso, A.M.; Boit, M.O.; DeForest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. Part A 2020, 108, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yue, Y.; Zhang, Y.; Liang, J.; Liu, L.; Wang, Q.; Feng, Q.; Zhao, H. Plant-derived exosome-like nanoparticles: Emerging nanosystems for enhanced tissue engineering. Int. J. Nanomed. 2024, 19, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Diao, N.; Cai, X.; Chen, X.; Xiao, Y.; Guo, C.; Chen, D.; Zhang, X. Plant exosome nanovesicles (PENs): Green delivery platforms. Mater. Horiz. 2023, 10, 3879–3894. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 2023, 18, 100522. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Barzin, M.; Bagheri, A.M.; Ohadi, M.; Abhaji, A.M.; Salarpour, S.; Dehghannoudeh, G. Application of plant-derived exosome-like nanoparticles in drug delivery. Pharm. Dev. Technol. 2023, 28, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Lin, Q.; Shi, T.; Liu, G. Exosome-loaded hydrogels for craniofacial bone tissue regeneration. Biomed. Mater. 2024, 19, 052002. [Google Scholar] [CrossRef] [PubMed]
- Mohanto, S.; Narayana, S.; Merai, K.P.; Kumar, J.A.; Bhunia, A.; Hani, U.; Al Fatease, A.; Gowda, B.J.; Nag, S.; Ahmed, M.G. Advancements in gelatin-based hydrogel systems for biomedical applications: A state-of-the-art review. Int. J. Biol. Macromol. 2023, 253, 127143. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Chen, H. Functional modification of gelatin-based biodegradable composite films: A review. Food Addit. Contam. Part A 2023, 40, 928–949. [Google Scholar] [CrossRef] [PubMed]
- Nii, T. Strategies using gelatin microparticles for regenerative therapy and drug screening applications. Molecules 2021, 26, 6795. [Google Scholar] [CrossRef] [PubMed]
- Abar, E.S.; Vandghanooni, S.; Torab, A.; Jaymand, M.; Eskandani, M. A comprehensive review on nanocomposite biomaterials based on gelatin for bone tissue engineering. Int. J. Biol. Macromol. 2024, 254, 127556. [Google Scholar]
- Baruah, H.; Sarma, A.; Basak, D.; Das, M. Exosome: From biology to drug delivery. Drug Deliv. Transl. Res. 2024, 14, 1480–1516. [Google Scholar] [CrossRef] [PubMed]
- Bova, L.; Maggiotto, F.; Micheli, S.; Giomo, M.; Sgarbossa, P.; Gagliano, O.; Falcone, D.; Cimetta, E. A Porous Gelatin Methacrylate-Based Material for 3D Cell-Laden Constructs. Macromol. Biosci. 2023, 23, 2200357. [Google Scholar] [CrossRef]
- Gu, Y.; Miao, F.; Liu, K.; Su, Y.; Wei, Y.; Hu, Y.; Lian, X.; Han, W.; Chen, W.; Huang, D. Fabrication of gelatin methacryloyl/graphene oxide conductive hydrogel for bone repair. J. Biomater. Sci. Polym. Ed. 2023, 34, 2076–2090. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, J.; An, R. Hyaluronic acid-based hydrogels: As an exosome delivery system in bone regeneration. Front. Pharmacol. 2023, 14, 1131001. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zaro, J.; Shen, Y. Advances in exosome-based drug delivery and tumor targeting: From tissue distribution to intracellular fate. Int. J. Nanomed. 2020, 15, 9355–9371. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, Y.; Gao, Q.; He, C.; Yao, K.; Wang, T.; Xie, M.; Yu, K.; Nie, J.; Chen, Y. Gelatin methacryloyl hydrogel, from standardization, performance, to biomedical application. Adv. Healthc. Mater. 2023, 12, 2300395. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Park, K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D printing of microenvironment-specific bioinspired and exosome-reinforced hydrogel scaffolds for efficient cartilage and subchondral bone regeneration. Adv. Sci. 2023, 10, 2303650. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.-A.; Sigurjonsson, O.E.; Gulei, D.; Tomuleasa, C. The malignant role of exosomes as nanocarriers of rare RNA species. Int. J. Mol. Sci. 2020, 21, 5866. [Google Scholar] [CrossRef] [PubMed]
- Nail, H.M.; Chiu, C.-C.; Leung, C.-H.; Ahmed, M.M.; Wang, H.-M.D. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J. Biomed. Sci. 2023, 30, 69. [Google Scholar] [CrossRef] [PubMed]
- Hillege, M.M.; Galli Caro, R.A.; Offringa, C.; de Wit, G.M.; Jaspers, R.T.; Hoogaars, W.M. TGF-β regulates collagen type I expression in myoblasts and myotubes via transient Ctgf and Fgf-2 expression. Cells 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Aasebø, E.; Birkeland, E.; Selheim, F.; Berven, F.; Brenner, A.K.; Bruserud, Ø. The extracellular bone marrow microenvironment—A proteomic comparison of constitutive protein release by in vitro cultured osteoblasts and mesenchymal stem cells. Cancers 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Luo, X.; Li, Y.; Shao, L.; Yang, F.; Pang, Q.; Zhu, Y.; Hou, R. Advanced Hybrid Strategies of GelMA Composite Hydrogels in Bone Defect Repair. Polymers 2024, 16, 3039. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jiang, X.; Zhou, X.; Tan, W.; Luo, H.; Lei, S.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Filidou, E.; Kolios, G. Special Issue “Gut Microbiota, Inflammatory Bowel Diseases, and Therapeutic Targets”. Pharmaceuticals 2023, 16, 714. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhao, P.; Xing, J.; Wang, Z.; Xu, Y.; Yan, Y.; Zhang, H.; Qu, J. GelMA encapsulating BMSCs-exosomes combined with interference screw or suture anchor promotes tendon-bone healing in a rabbit model. Sci. Rep. 2024, 14, 28212. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Exosome-Integrated Hydrogels for Bone Tissue Engineering. Gels 2024, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, S.; Wang, S.; Xie, Y.; Xia, Y.; Wang, P.; Hao, Z.; Xu, S.; Zhang, Y. Hypoxia preconditioning of adipose stem cell-derived exosomes loaded in gelatin methacryloyl (GelMA) promote type H angiogenesis and osteoporotic fracture repair. J. Nanobiotechnol. 2024, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, X.; Hu, H.; Ma, X.; Zhao, Z.; Deng, S.; Wang, J.; Zhang, L.; Wu, C.; Liu, Z. Synergetic osteogenesis of extracellular vesicles and loading RGD colonized on 3D-printed titanium implants. Biomater. Sci. 2022, 10, 4773–4784. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, L.; Lin, X.; Yu, Y.; Zhao, Y. Pearl powder hybrid bioactive scaffolds from microfluidic 3D printing for bone regeneration. Adv. Sci. 2023, 10, 2304190. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-derived exosome-like nanovesicles: Current progress and prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.M. Current advances in stimuli-responsive hydrogels as smart drug delivery carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Tsai, T.H.; Lee, C.H. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Xie, X.; Xu, J. Epimedium-Derived Exosome-Loaded GelMA Hydrogel Enhances MC3T3-E1 Osteogenesis via PI3K/Akt Pathway. Cells 2025, 14, 1214. https://doi.org/10.3390/cells14151214
Hu W, Xie X, Xu J. Epimedium-Derived Exosome-Loaded GelMA Hydrogel Enhances MC3T3-E1 Osteogenesis via PI3K/Akt Pathway. Cells. 2025; 14(15):1214. https://doi.org/10.3390/cells14151214
Chicago/Turabian StyleHu, Weijian, Xin Xie, and Jiabin Xu. 2025. "Epimedium-Derived Exosome-Loaded GelMA Hydrogel Enhances MC3T3-E1 Osteogenesis via PI3K/Akt Pathway" Cells 14, no. 15: 1214. https://doi.org/10.3390/cells14151214
APA StyleHu, W., Xie, X., & Xu, J. (2025). Epimedium-Derived Exosome-Loaded GelMA Hydrogel Enhances MC3T3-E1 Osteogenesis via PI3K/Akt Pathway. Cells, 14(15), 1214. https://doi.org/10.3390/cells14151214




