Enhanced Modulation of CaMKII in Mouse Hippocampus by an Antidepressant-like Dose of Melatonin/Ketamine Combination
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Pharmacological Treatments
2.2. Behavioral Assessment: Forced Swim Test
2.3. Sample Obtention for 2D Gel Electrophoresis Separation
2.4. Protein Separation by Two-Dimensional Electrophoresis (2-D EF)
2.5. Analysis of Proteins Separated by Two-Dimensional Electrophoresis
2.6. Western Blots
2.7. Immunohistochemical Analysis
2.8. Determination of CaMKII Activity
2.9. Statistical Analysis
3. Results
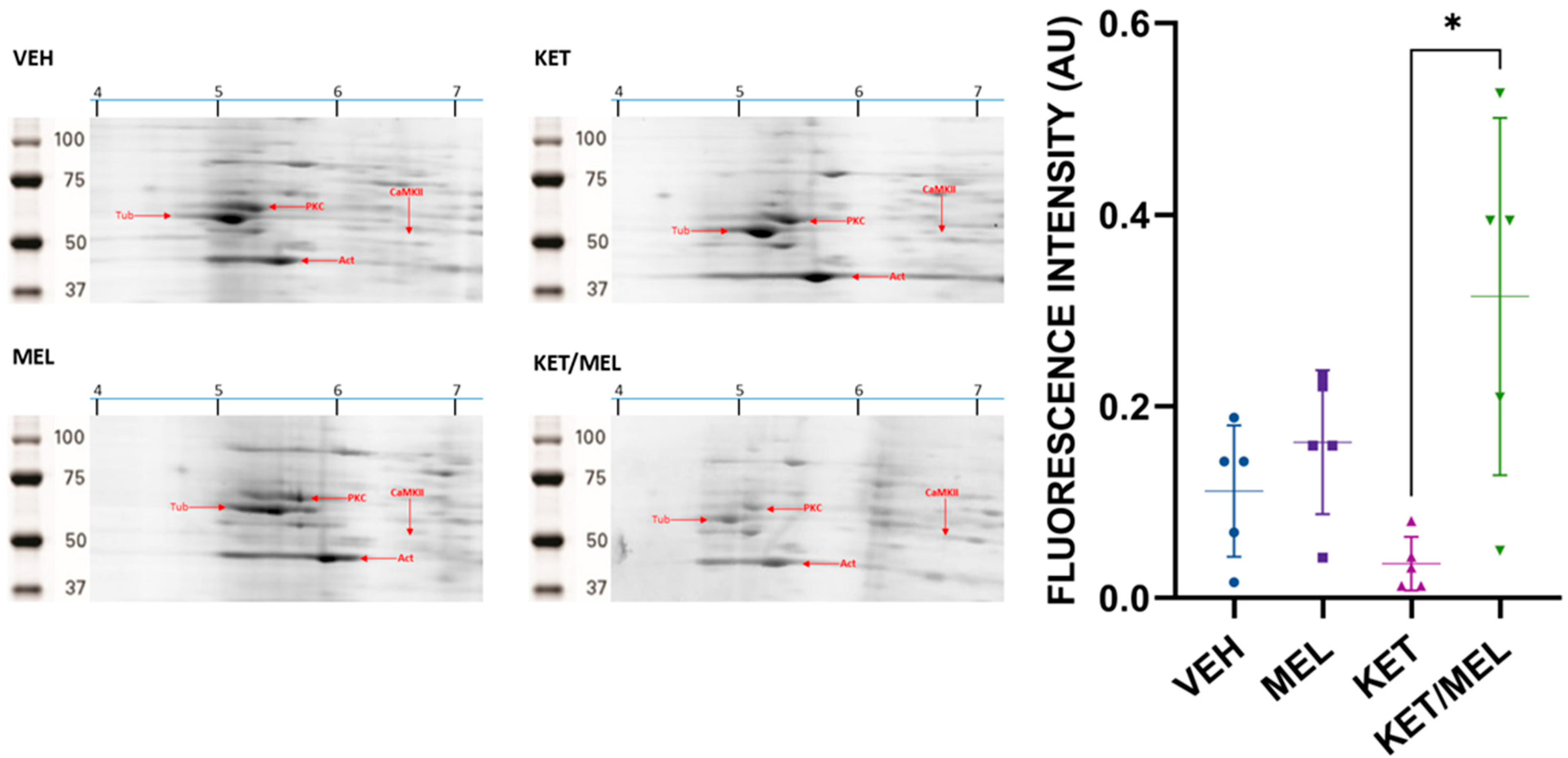
3.1. Identification of Hippocampal CaMKII by Isoelectric Point and Molecular Weight
3.2. The Relative Amount of α-CaMKII in Hippocampus Increases in Mice Treated with KET/MEL Combination
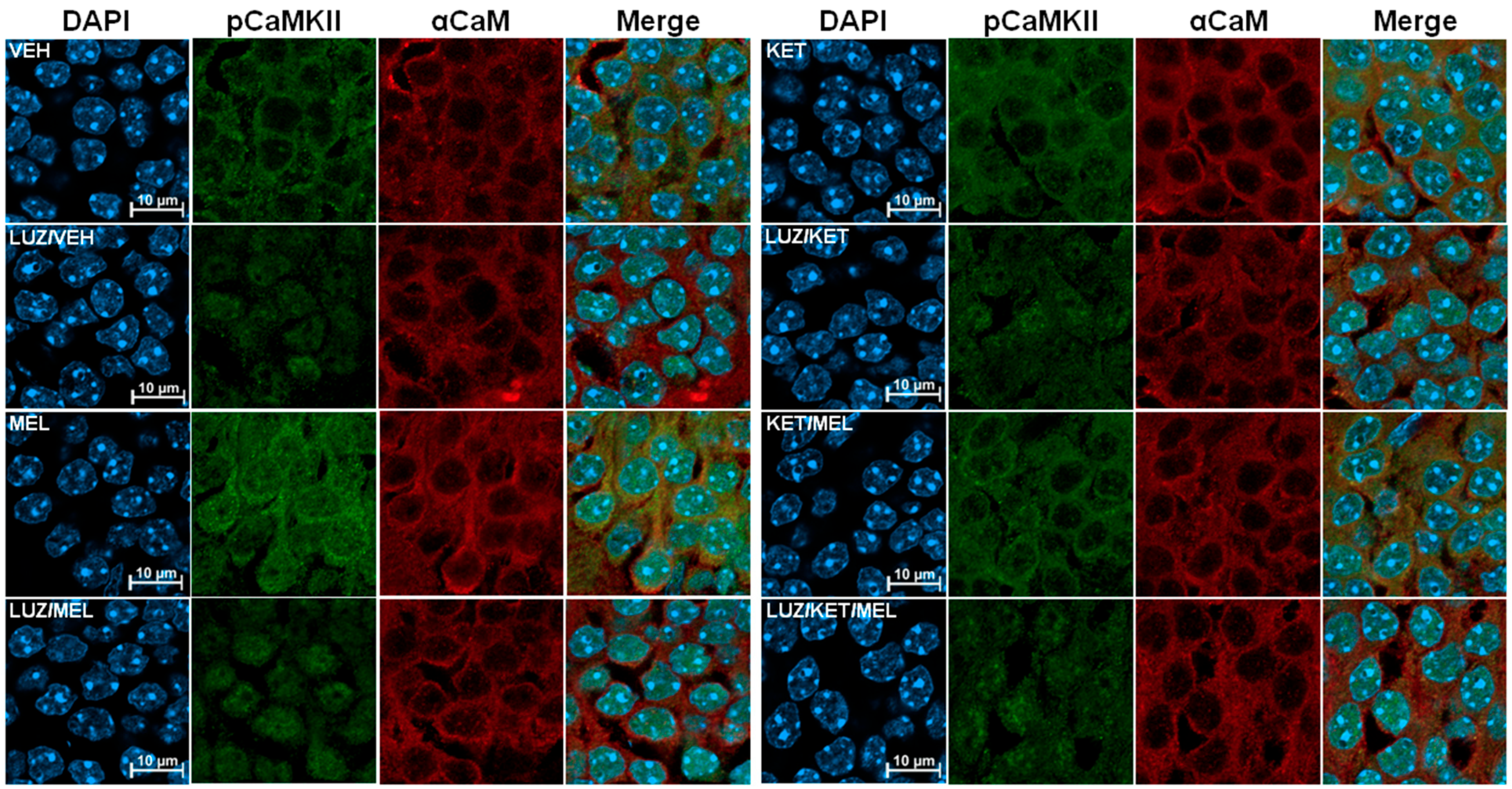
3.3. Differential Subcellular Distribution of p-CaMKII Is Observed in Cells of the Dentate Gyrus of Mice Treated with MEL or KET/MEL Combination
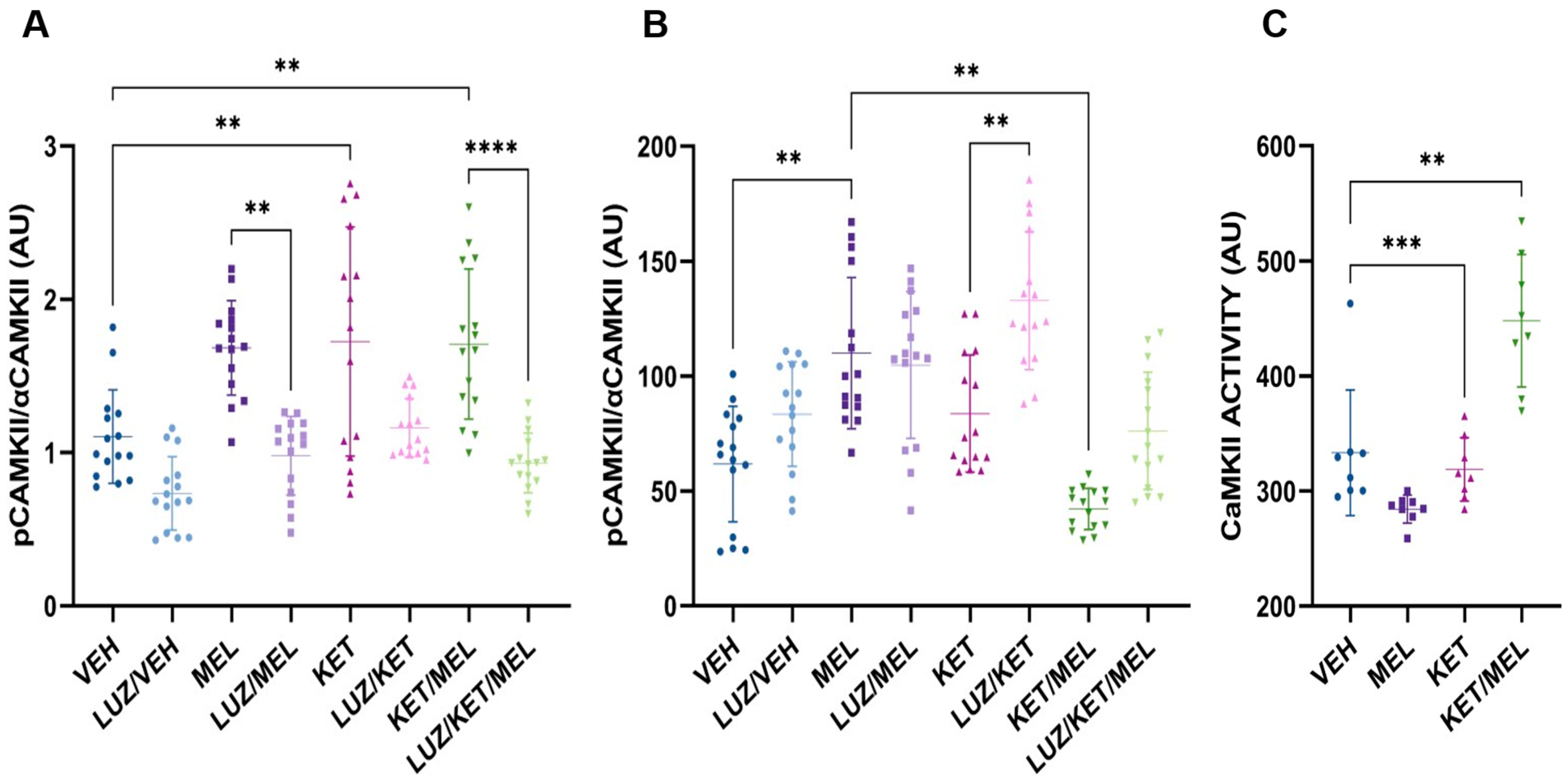
3.4. Relative Levels of Phosphorylated CaMKII in the Dentate Gyrus Cells in Mice Treated with the KET/MEL Combination
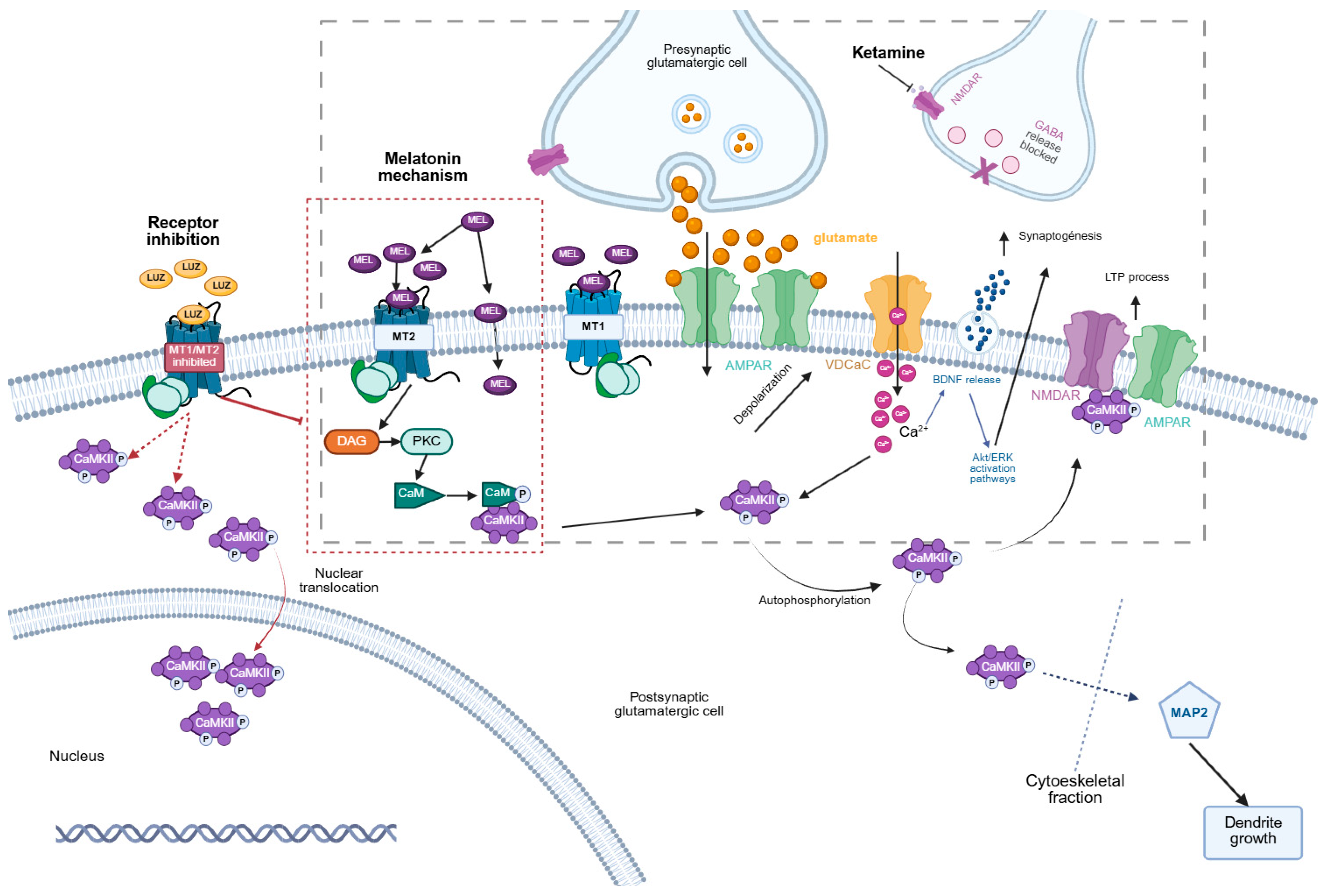
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-CaMKII | alpha calmodulin kinase II |
| AUC | area under curve |
| AMPA | a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| CaMKII | calcium/calmodulin-dependent protein kinase II |
| CaM | Calmodulin |
| CHAPS | 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate |
| CREB | cyclic AMP response-binding protein |
| Fd | freedom degree |
| HP | Hippocampus |
| IP | isoelectric point |
| ip | intraperitoneally |
| LUZ | luzindole |
| KET | ketamine |
| KET/MEL | ketamine/melatonin combination |
| LTP | long-term potentiation |
| MEL | melatonin |
| MW | molecular weight |
| NMDA | N-methyl-D-aspartate |
| NMDAR | N-methyl-D-aspartate receptor |
| MT1 | melatonin receptor T1 |
| MT2 | melatonin receptor T2 |
| p-CaM | phosphorated calmodulin |
| p-α-CaM | phosphorated calmodulin |
| PKC | protein kinase C |
| Ser332 | serine 332 |
| T238 | threonine 238 |
| VEH | vehicle |
| ZT | Zeitgeber time |
References
- Ragguett, R.-M.; Tamura, J.K.; McIntyre, R.S. Keeping up with the Clinical Advances: Depression. CNS Spectr. 2019, 24, 25–37. [Google Scholar] [CrossRef]
- Shokri-Mashhadi, N.; Darand, M.; Rouhani, M.H.; Yahay, M.; Feltham, B.A.; Saraf-Bank, S. Effects of Melatonin Supplementation on BDNF Concentrations and Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Behav. Brain Res. 2023, 436, 114083. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Valdés-Tovar, M.; Arrieta-Baez, D.; Dorantes-Barrón, A.; Quero-Chávez, D.; Solís-Chagoyán, H.; Argueta, J.; Dubocovich, M.; Benítez-King, G. The Timing of Melatonin Administration Is Crucial for Its Antidepressant-Like Effect in Mice. Int. J. Mol. Sci. 2018, 19, 2278. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Picarel-Blanchot, F.; Kennedy, S.H. Efficacy of the Novel Antidepressant Agomelatine for Anxiety Symptoms in Major Depression. Hum. Psychopharmacol. Clin. Exp. 2013, 28, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal Melatonin: Analysis of Its Subcellular Distribution and Daily Fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dickson, E.J.; Jung, S.R.; Koh, D.S.; Hille, B. High Membrane Permeability for Melatonin. J. Gen. Physiol. 2016, 147, 63–76. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A Hormone, a Tissue Factor, an Autocoid, a Paracoid, and an Antioxidant Vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef]
- Yousefi, T.; Yousef Memar, M.; Ahmadi Jazi, A.; Zand, S.; Reiter, R.J.; Amirkhanlou, S.; Mostafa Mir, S. Molecular Pathways and Biological Roles of Melatonin and Vitamin D Effects on Immune System and Oxidative Stress. Int. Immunopharmacol. 2024, 143, 113548. [Google Scholar] [CrossRef]
- Said, A.; Shah, D.; Shah, P.; Singh, B.; Anamika, F.; Aggarwal, K.; Gupta, A.; Jain, R. Unlocking the Heart’s Guardian: Exploring Melatonin’s Impact on the Cardiovascular System. Cardiol. Rev. 2024, 2024, 10–97. [Google Scholar] [CrossRef]
- Sheibani, M.; Hosseinzadeh, A.; Fatemi, I.; Naeini, A.J.; Mehrzadi, S. Practical Application of Melatonin for Pancreas Disorders: Protective Roles against Inflammation, Malignancy, and Dysfunctions. Pharmacol. Rep. 2024, 77, 315–332. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, Mitochondria, and the Skin. Cell Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Riestra, A.; Estrada-Reyes, R.; Torres-Sanchez, E.D.; Carreño-García, S.; Ortiz, G.G.; Benítez-King, G. Melatonin: A Neurotrophic Factor? Molecules 2022, 27, 7742. [Google Scholar] [CrossRef] [PubMed]
- Tancheva, L.; Lazarova, M.; Saso, L.; Kalfin, R.; Stefanova, M.; Uzunova, D.; Atanasov, A.G. Beneficial Effect of Melatonin on Motor and Memory Disturbances in 6-OHDA-Lesioned Rats. J. Mol. Neurosci. 2021, 71, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin Is a Potent Modulator of Dopamine Release in the Retina. Nature 1983, 306, 782–784. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, Classification, and Pharmacology of G Protein-Coupled Melatonin Receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic Treatment with Melatonin Stimulates Dendrite Maturation and Complexity in Adult Hippocampal Neurogenesis of Mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Dubocovich, M.L. Role of the MT1 and MT2 Melatonin Receptors in Mediating Depressive- and Anxiety-like Behaviors in C3H/HeN Mice. Genes. Brain Behav. 2017, 16, 546–553. [Google Scholar] [CrossRef]
- Benítez-King, G.; Argueta, J.; Miranda-Riestra, A.; Muñoz-Delgado, J.; Estrada-Reyes, R. Interaction of the Melatonin/Ca21-CaM Complex with Calmodulin Kinase II: Physiological Importance. Mol. Pharmacol. 2024, 106, 3–12. [Google Scholar] [CrossRef]
- Argueta, J.; Solís-Chagoyán, H.; Estrada-Reyes, R.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Velázquez-Moctezuma, J.; Benítez-King, G. Further Evidence of the Melatonin Calmodulin Interaction: Effect on CaMKII Activity. Int. J. Mol. Sci. 2022, 23, 2479. [Google Scholar] [CrossRef]
- Wang, L.M.; Suthana, N.A.; Chaudhury, D.; Weaver, D.R.; Colwell, C.S. Melatonin Inhibits Hippocampal Long-Term Potentiation. Eur. J. Neurosci. 2005, 22, 2231–2237. [Google Scholar] [CrossRef]
- Domínguez-Alonso, A.; Valdés-Tovar, M.; Solís-Chagoyán, H.; Benítez-King, G. Melatonin Stimulates Dendrite Formation and Complexity in the Hilar Zone of the Rat Hippocampus: Participation of the Ca++/Calmodulin Complex. Int. J. Mol. Sci. 2015, 16, 1907–1927. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Quero-Chávez, D.B.; Alarcón-Elizalde, S.; Cercós, M.G.; Trueta, C.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Argueta, J.; Cruz-Garduño, R.; Dubocovich, M.L.; et al. Antidepressant Low Doses of Ketamine and Melatonin in Combination Produce Additive Neurogenesis in Human Olfactory Neuronal Precursors. Molecules 2022, 27, 5650. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Hayashi, Y.; Hell, J.W. CaMKII: A Central Molecular Organizer of Synaptic Plasticity, Learning and Memory. Nat. Rev. Neurosci. 2022, 23, 666–682. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Salvadore, G.; DiazGranados, N.; Zarate, C.A. Ketamine and the next Generation of Antidepressants with a Rapid Onset of Action. Pharmacol. Ther. 2009, 123, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Li, N.; Liu, R.J.; Duric, V.; Aghajanian, G. Signaling Pathways Underlying the Rapid Antidepressant Actions of Ketamine. Neuropharmacology 2012, 62, 35–41. [Google Scholar] [CrossRef]
- Li, Y.-D.; Luo, Y.-J.; Su, W.-K.; Ge, J.; Crowther, A.; Chen, Z.-K.; Wang, L.; Lazarus, M.; Liu, Z.-L.; Qu, W.-M.; et al. Anterior Cingulate Cortex Projections to the Dorsal Medial Striatum Underlie Insomnia Associated with Chronic Pain. Neuron 2024, 112, 1328–1341.e4. [Google Scholar] [CrossRef]
- Cooper, M.D.; Rosenblat, J.D.; Cha, D.S.; Lee, Y.; Kakar, R.; McIntyre, R.S. Strategies to Mitigate Dissociative and Psychotomimetic Effects of Ketamine in the Treatment of Major Depressive Episodes: A Narrative Review. World J. Biol. Psychiatry 2017, 18, 410–423. [Google Scholar] [CrossRef]
- Bustos, F.J.; Jury, N.; Martinez, P.; Ampuero, E.; Campos, M.; Abarzúa, S.; Jaramillo, K.; Ibing, S.; Mardones, M.D.; Haensgen, H.; et al. NMDA Receptor Subunit Composition Controls Dendritogenesis of Hippocampal Neurons through CAMKII, CREB-P, and H3K27ac. J. Cell Physiol. 2017, 232, 3677–3692. [Google Scholar] [CrossRef]
- Weigand, A.; Horn, A.; Caballero, R.; Cooke, D.; Stern, A.P.; Taylor, S.F.; Press, D.; Pascual-Leone, A.; Fox, M.D. Prospective Validation That Subgenual Connectivity Predicts Antidepressant Efficacy of Transcranial Magnetic Stimulation Sites. Biol. Psychiatry 2018, 84, 28–37. [Google Scholar] [CrossRef]
- Tao-Cheng, J.H.; Yang, Y.; Bayer, K.U.; Reese, T.S.; Dosemeci, A. Effects of CaMKII Inhibitor TatCN21 on Activity-Dependent Redistribution of CaMKII in Hippocampal Neurons. Neuroscience 2013, 244, 188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Estrada-Reyes, R.; Quero-Chávez, D.B.; Trueta, C.; Miranda, A.; Valdés-Tovar, M.; Alarcón-Elizalde, S.; Oikawa-Sala, J.; Argueta, J.; Constantino-Jonapa, L.A.; Muñoz-Estrada, J.; et al. Low Doses of Ketamine and Melatonin in Combination Produce Additive Antidepressant-like Effects in Mice. Int. J. Mol. Sci. 2021, 22, 9225. [Google Scholar] [CrossRef] [PubMed]
- Forest, A.; Swulius, M.T.; Tse, J.K.Y.; Bradshaw, J.M.; Gaertner, T.; Waxham, M.N. Role of the N- and C-Lobes of Calmodulin in the Activation of Ca2+/Calmodulin-Dependent Protein Kinase II. Biochemistry 2008, 47, 10587–10599. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, T.R.; Putkey, J.A.; Waxham, M.N. RC3/Neurogranin and Ca2+/Calmodulin-Dependent Protein Kinase II Produce Opposing Effects on the Affinity of Calmodulin for Calcium. J. Biol. Chem. 2004, 279, 39374–39382. [Google Scholar] [CrossRef]
- Hanson, P.I.; Meyer, T.; Stryer, L.; Schulman, H. Dual Role of Calmodulin in Autophosphorylation of Multifunctional Cam Kinase May Underlie Decoding of Calcium Signals. Neuron 1994, 12, 943–956. [Google Scholar] [CrossRef]
- Zalcman, G.; Federman, N.; Romano, A. CaMKII Isoforms in Learning and Memory: Localization and Function. Front. Mol. Neurosci. 2018, 11, 445. [Google Scholar] [CrossRef]
- Chapman, P.F.; Frenguelli, B.G.; Smith, A.; Chen, C.-M.; Silva, A.J. The α-Ca2+/Calmodulin Kinase II: A Bidirectional Modulator of Presynaptic Plasticity. Neuron 1995, 14, 591–597. [Google Scholar] [CrossRef]
- Caffino, L.; Piva, A.; Mottarlini, F.; Di Chio, M.; Giannotti, G.; Chiamulera, C.; Fumagalli, F. Ketamine Self-Administration Elevates ACaMKII Autophosphorylation in Mood and Reward-Related Brain Regions in Rats. Mol. Neurobiol. 2018, 55, 5453–5461. [Google Scholar] [CrossRef]
- Madison, D.V.; Edson, E.B. Preparation of Hippocampal Brain Slices. Curr. Protoc. Neurosci. 1997, 6.4.1–6.4.7. [Google Scholar] [CrossRef]
- Towbin, H.; Özbey, Ö.; Zingel, O. An Immunoblotting Method for High-Resolution Isoelectric Focusing of Protein Isoforms on Immobilized PH Gradients. Electrophoresis 2001, 22, 1887–1893. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Brauner, J.M.; Groemer, T.W.; Stroebel, A.; Grosse-Holz, S.; Oberstein, T.; Wiltfang, J.; Kornhuber, J.; Maler, J.M. Spot Quantification in Two Dimensional Gel Electrophoresis Image Analysis: Comparison of Different Approaches and Presentation of a Novel Compound Fitting Algorithm. BMC Bioinform. 2014, 15, 181. [Google Scholar] [CrossRef][Green Version]
- Natale, M.; Maresca, B.; Abrescia, P.; Bucci, E.M. Image Analysis Workflow for 2-D Electrophoresis Gels Based on ImageJ. Proteom. Insights 2011, 4, PRI-S7971. [Google Scholar] [CrossRef]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole Animal Perfusion Fixation for Rodents. J. Vis. Exp. 2012, 65, e3564. [Google Scholar] [CrossRef] [PubMed]
- Awalsh, D.; Glass, D.B. Utilization of the Inhibitor Protein of Adenosine Cyclic Monophosphate-Dependent Protein Kinase, and Peptides Derived from It, as Tools to Study Adenosine Cyclic Monophosphate-Mediated Cellular Processes. In Methods Enzymol; Academic Press: Cambridge, MA, USA, 1991; Volume 201, pp. 304–316. [Google Scholar]
- Scholz, W.K.; Baitinger, C.; Schulman, H.; Kelly, P.T. Developmental Changes in Ca2+/Calmodulin-Dependent Protein Kinase II in Cultures of Hippocampal Pyramidal Neurons and Astrocytes. J. Neurosci. 1988, 8, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Benítez-King, G.; Cazares, F.; Meza, I. Synthesis and Phosphorylation of Cytoskeletal Proteins during the in Vitro Biogenesis of MDCK Cell Monolayers. J. Cell Sci. 1989, 93, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.N.; Bossinger, J.; Hayashi, M. Tubulin and Actin Synthesis during Brain Development. Dev. Biol. 1981, 81, 349–355. [Google Scholar] [CrossRef]
- Adaikkan, C.; Taha, E.; Barrera, I.; David, O.; Rosenblum, K. Calcium/Calmodulin-Dependent Protein Kinase II and Eukaryotic Elongation Factor 2 Kinase Pathways Mediate the Antidepressant Action of Ketamine. Biol. Psychiatry 2018, 84, 65–75. [Google Scholar] [CrossRef]
- Tzortzopoulos, A.; Best, S.L.; Kalamida, D.; Török, K. Ca2+/Calmodulin-Dependent Activation and Inactivation Mechanisms of AlphaCaMKII and Phospho-Thr286-AlphaCaMKII. Biochemistry 2004, 43, 6270–6280. [Google Scholar] [CrossRef]
- Hudmon, A.; Schulman, H. Neuronal Ca2+/Calmodulin-Dependent Protein Kinase II: The Role of Structure and Autoregulation in Cellular Function. Annu. Rev. Biochem. 2002, 71, 473–510. [Google Scholar] [CrossRef]
- Tiraboschi, E.; Giambelli, R.; D’Urso, G.; Galietta, A.; Barbon, A.; de Bartolomeis, A.; Gennarelli, M.; Barlati, S.; Racagni, G.; Popoli, M. Antidepressants Activate CaMKII in Neuron Cell Body by Thr286 Phosphorylation. Neuroreport 2004, 15, 2393–2396. [Google Scholar] [CrossRef]
- Abdoulaye, I.A.; Wu, S.S.; Chibaatar, E.; Yu, D.F.; Le, K.; Cao, X.J.; Guo, Y.J. Ketamine Induces Lasting Antidepressant Effects by Modulating the NMDAR/CaMKII-Mediated Synaptic Plasticity of the Hippocampal Dentate Gyrus in Depressive Stroke Model. Neural Plast. 2021, 2021, 6635084. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Gray, C.B.B.; Miyamoto, S.; Bers, D.M.; Brown, J.H. Location Matters: Clarifying the Concept of Nuclear and Cytosolic CaMKII Subtypes. Circ. Res. 2011, 109, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Sawai, M.; Ishizuka, Y.; Shirao, T.; Fukunaga, K. Nuclear Translocation of Calcium/Calmodulin-Dependent Protein Kinase IIδ3 Promoted by Protein Phosphatase-1 Enhances Brain-Derived Neurotrophic Factor Expression in Dopaminergic Neurons. J. Biol. Chem. 2015, 290, 21663–21675. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Mogilnicka, E.; Areso, P.M. Antidepressant-like Activity of the Melatonin Receptor Antagonist, Luzindole (N-0774), in the Mouse Behavioral Despair Test. Eur. J. Pharmacol. 1990, 182, 313–325. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Pérez-Beltran, C.; Ramírez-Rodríguez, G. Chronic Administration of a Melatonin Membrane Receptor Antagonist, Luzindole, Affects Hippocampal Neurogenesis without Changes in Hopelessness-like Behavior in Adult Mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef]
- Sakagami, H.; Kamata, A.; Nishimura, H.; Kasahara, J.; Owada, Y.; Takeuchi, Y.; Watanabe, M.; Fukunaga, K.; Kondo, H. Prominent Expression and Activity-Dependent Nuclear Translocation of Ca2+/Calmodulin-Dependent Protein Kinase Idelta in Hippocampal Neurons. Eur. J. Neurosci. 2005, 22, 2697–2707. [Google Scholar] [CrossRef]
- Dahleh, M.M.M.; Mello, C.F.; Ferreira, J.; Rubin, M.A.; Prigol, M.; Guerra, G.P. CaMKIIα Mediates Spermidine-Induced Memory Enhancement in Rats: A Potential Involvement of PKA/CREB Pathway. Pharmacol. Biochem. Behav. 2024, 240, 173774. [Google Scholar] [CrossRef]
- Tsui, J.; Inagaki, M.; Schulmann, H. Calcium/Calmodulin-Dependent Protein Kinase II (CaMKII) Localization Acts in Concert with Substrate Targeting to Create Spatial Restriction for Phosphorylation. J. Biol. Chem. 2005, 280, 9210–9216. [Google Scholar] [CrossRef]
- Valdés-Tovar, M.; Estrada-Reyes, R.; Solís-Chagoyán, H.; Argueta, J.; Dorantes-Barrón, A.M.; Quero-Chávez, D.; Cruz-Garduño, R.; Cercós, M.G.; Trueta, C.; Oikawa-Sala, J.; et al. Circadian Modulation of Neuroplasticity by Melatonin: A Target in the Treatment of Depression. Br. J. Pharmacol. 2018, 175, 3200–3208. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Riestra, A.; Estrada-Reyes, R.; Constantino-Jonapa, L.A.; Argueta, J.; Oikawa-Sala, J.; Reséndiz-Gachús, M.A.; Albarrán-Gaona, D.; Benítez-King, G. Enhanced Modulation of CaMKII in Mouse Hippocampus by an Antidepressant-like Dose of Melatonin/Ketamine Combination. Cells 2025, 14, 1187. https://doi.org/10.3390/cells14151187
Miranda-Riestra A, Estrada-Reyes R, Constantino-Jonapa LA, Argueta J, Oikawa-Sala J, Reséndiz-Gachús MA, Albarrán-Gaona D, Benítez-King G. Enhanced Modulation of CaMKII in Mouse Hippocampus by an Antidepressant-like Dose of Melatonin/Ketamine Combination. Cells. 2025; 14(15):1187. https://doi.org/10.3390/cells14151187
Chicago/Turabian StyleMiranda-Riestra, Armida, Rosa Estrada-Reyes, Luis A. Constantino-Jonapa, Jesús Argueta, Julián Oikawa-Sala, Miguel A. Reséndiz-Gachús, Daniel Albarrán-Gaona, and Gloria Benítez-King. 2025. "Enhanced Modulation of CaMKII in Mouse Hippocampus by an Antidepressant-like Dose of Melatonin/Ketamine Combination" Cells 14, no. 15: 1187. https://doi.org/10.3390/cells14151187
APA StyleMiranda-Riestra, A., Estrada-Reyes, R., Constantino-Jonapa, L. A., Argueta, J., Oikawa-Sala, J., Reséndiz-Gachús, M. A., Albarrán-Gaona, D., & Benítez-King, G. (2025). Enhanced Modulation of CaMKII in Mouse Hippocampus by an Antidepressant-like Dose of Melatonin/Ketamine Combination. Cells, 14(15), 1187. https://doi.org/10.3390/cells14151187





