Sexual Dimorphism of Synaptic Plasticity Changes in CA1 Hippocampal Networks in Hypergravity-Exposed Mice—New Insights for Cognition in Space
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Hypergravity Exposure
2.2. Brain Slice Preparation
2.3. Electrophysiological Measurements
2.3.1. Basal Synaptic Transmission
2.3.2. Paired-Pulse Facilitation
2.3.3. NMDA Receptor Activation
2.3.4. Functional Plasticity
2.4. Data Analysis
3. Results
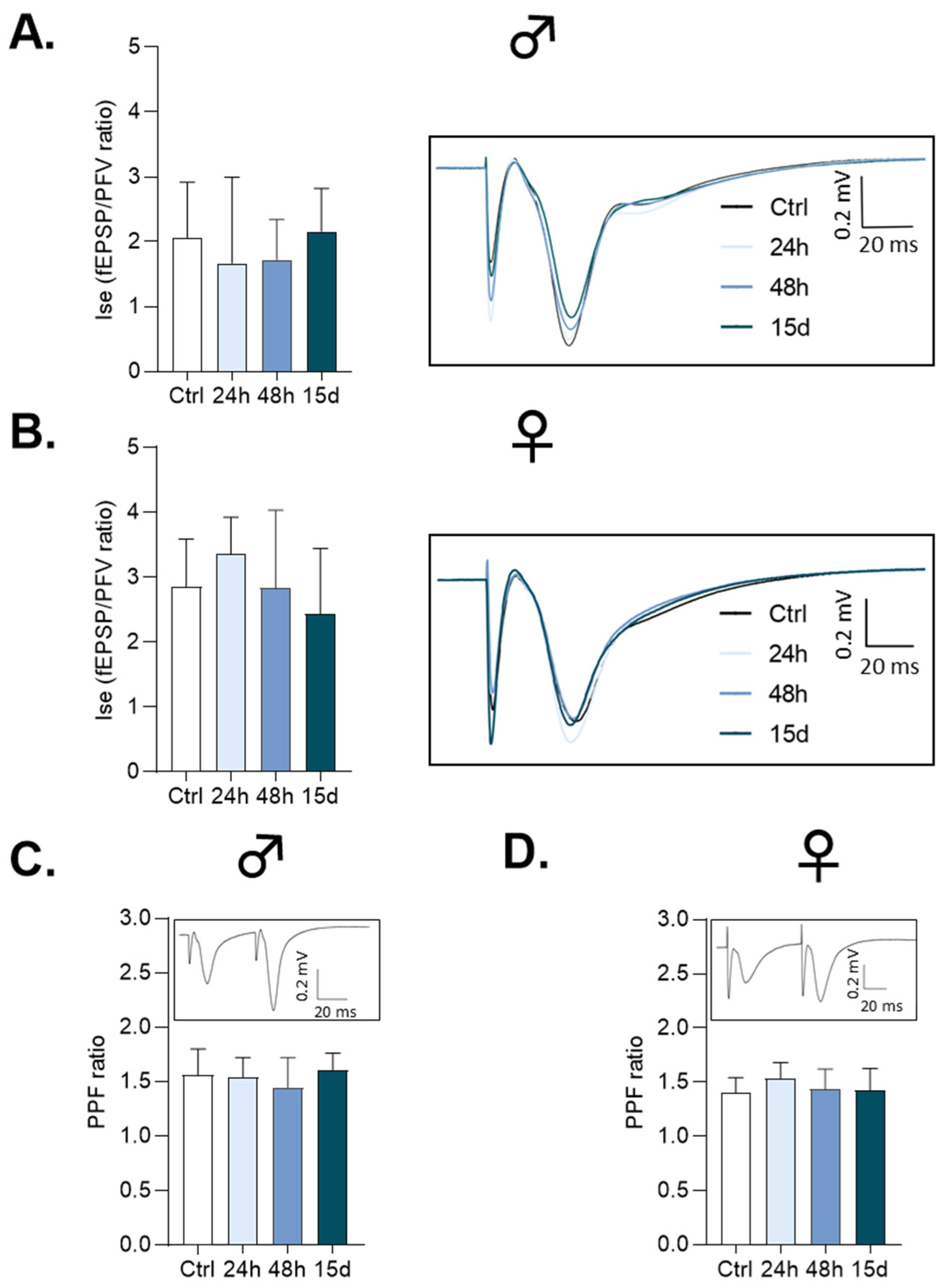
3.1. Basal Synaptic Transmission
3.2. Paired-Pulse Facilitation
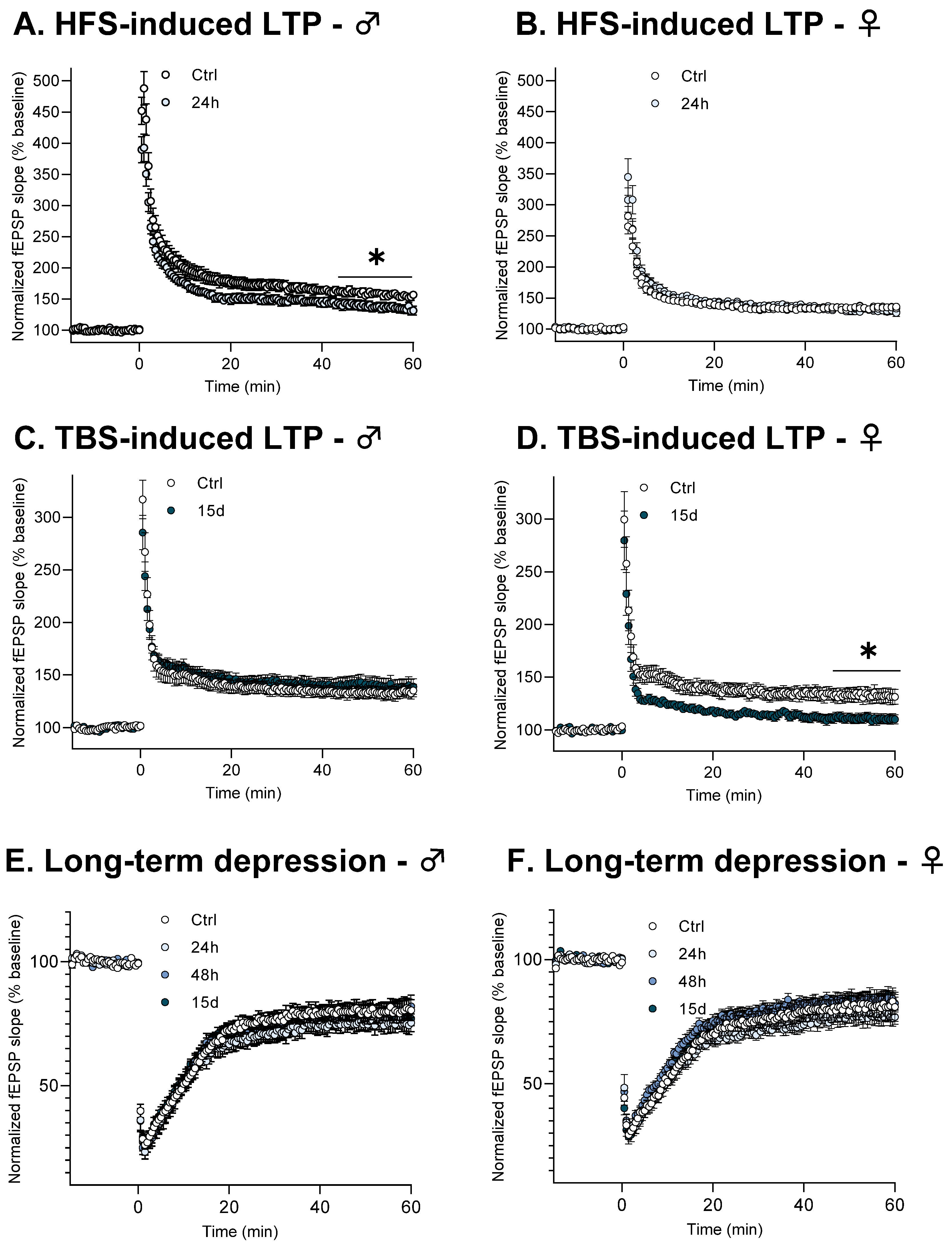
3.3. Functional Plasticity
3.4. NMDA Receptor Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCSF | Artificial cerebrospinal fluid |
| AMPA | α-amino-3-hydroxy-5methyl-4-isoxazolepropionic acid |
| AMPAr | α-amino-3-hydroxy-5methyl-4-isoxazolepropionic acid receptor |
| BBB | Blood–brain barrier |
| CNES | French National Center for Space Studies |
| CURB | Biological Resources University Center |
| HFS | High-frequency stimulation |
| HG | Hypergravity |
| ISE | Index of synaptic efficacy |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| MG | Microgravity |
| NMDA | N-methyl-D-aspartate |
| NMDAr | N-methyl-D-aspartate receptor |
| PFVs | Presynaptic fiber volleys |
| RPM | Revolution per minute |
| SR/KO | Serine racemase knock-out |
| TBS | Theta burst stimulation |
References
- Cauchoix, M.; Chaine, A.S.; Barragan-Jason, G. Cognition in Context: Plasticity in Cognitive Performance in Response to Ongoing Environmental Variables. Front. Ecol. Evol. 2020, 8, 106. [Google Scholar] [CrossRef]
- Kramer, A.F.; Bherer, L.; Colcombe, S.J.; Dong, W.; Greenough, W.T. Environmental Influences on Cognitive and Brain Plasticity during Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M940–M957. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red Risks for a Journey to the Red Planet: The Highest Priority Human Health Risks for a Mission to Mars. npj Microgravity 2020, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Carriot, J.; Mackrous, I.; Cullen, K.E. Challenges to the Vestibular System in Space: How the Brain Responds and Adapts to Microgravity. Front. Neural Circuits 2021, 15, 760313. [Google Scholar] [CrossRef]
- Strangman, G.E.; Sipes, W.; Beven, G. Human Cognitive Performance in Spaceflight and Analogue Environments. Aviat. Space Environ. Med. 2014, 85, 1033–1048. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A Multidimensional Analysis of a Year-Long Human Spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef]
- Seidler, R.D.; Mao, X.W.; Tays, G.D.; Wang, T.; Zu Eulenburg, P. Effects of Spaceflight on the Brain. Lancet Neurol. 2024, 23, 826–835. [Google Scholar] [CrossRef]
- Bonnefoy, J.; Ghislin, S.; Beyrend, J.; Coste, F.; Calcagno, G.; Lartaud, I.; Gauquelin-Koch, G.; Poussier, S.; Frippiat, J.-P. Gravitational Experimental Platform for Animal Models, a New Platform at ESA’s Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models. Int. J. Mol. Sci. 2021, 22, 2961. [Google Scholar] [CrossRef]
- van Loon, J.J.W.A. Centrifuges for Microgravity Simulation. The Reduced Gravity Paradigm. Front. Astron. Space Sci. 2016, 3, 21. [Google Scholar] [CrossRef]
- Jones, S.M.; Warren, L.E.; Shukla, R.; Browning, A.; Fuller, C.A.; Jones, T.A. The Effects of Hypergravity and Substrate Vibration on Vestibular Function in Developing Chickens. J. Gravit. Physiol. 2000, 7, 31–44. [Google Scholar]
- Noh, W.; Lee, M.; Kim, H.J.; Kim, K.-S.; Yang, S. Hypergravity Induced Disruption of Cerebellar Motor Coordination. Sci. Rep. 2020, 10, 4452. [Google Scholar] [CrossRef]
- Bojados, M.; Jamon, M. The Long-Term Consequences of the Exposure to Increasing Gravity Levels on the Muscular, Vestibular and Cognitive Functions in Adult Mice. Behav. Brain Res. 2014, 264, 64–73. [Google Scholar] [CrossRef]
- Zennou-Azogui, Y.; Catz, N.; Xerri, C. Hypergravity within a Critical Period Impacts on the Maturation of Somatosensory Cortical Maps and Their Potential for Use-Dependent Plasticity in the Adult. J. Neurophysiol. 2016, 115, 2740–2760. [Google Scholar] [CrossRef] [PubMed]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular Pathways Involved in Cognition. Front. Integr. Neurosci. 2014, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Besnard, S.; Lopez, C.; Brandt, T.; Denise, P.; Smith, P.F. Editorial: The Vestibular System in Cognitive and Memory Processes in Mammalians. Front. Integr. Neurosci. 2015, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F. Vestibular-Hippocampal Interactions. Hippocampus 1997, 7, 465–471. [Google Scholar] [CrossRef]
- Ishii, M.; Tomizawa, K.; Matsushita, M.; Matsui, H. Exposure of Mouse to High Gravitation Forces Induces Long-Term Potentiation in the Hippocampus. Acta Med. Okayama 2004, 58, 143–149. [Google Scholar] [CrossRef]
- Lee, J.; Jang, D.; Jeong, H.; Kim, K.-S.; Yang, S. Impairment of Synaptic Plasticity and Novel Object Recognition in the Hypergravity-Exposed Rats. Sci. Rep. 2020, 10, 15813. [Google Scholar] [CrossRef]
- Mark, S.; Scott, G.B.I.; Donoviel, D.B.; Leveton, L.B.; Mahoney, E.; Charles, J.B.; Siegel, B. The Impact of Sex and Gender on Adaptation to Space: Executive Summary. J. Women’s Health 2014, 23, 941–947. [Google Scholar] [CrossRef]
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of Sex and Gender on Adaptations to Space: Reproductive Health. J. Women’s Health 2014, 23, 967. [Google Scholar] [CrossRef]
- Ploutz-Snyder, L.; Bloomfield, S.; Smith, S.M.; Hunter, S.K.; Templeton, K.; Bemben, D. Effects of Sex and Gender on Adaptation to Space: Musculoskeletal Health. J. Women’s Health 2014, 23, 963–966. [Google Scholar] [CrossRef]
- Goel, N.; Bale, T.L.; Epperson, C.N.; Kornstein, S.G.; Leon, G.R.; Palinkas, L.A.; Stuster, J.W.; Dinges, D.F. Effects of Sex and Gender on Adaptation to Space: Behavioral Health. J. Women’s Health 2014, 23, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Platts, S.H.; Bairey Merz, C.N.; Barr, Y.; Fu, Q.; Gulati, M.; Hughson, R.; Levine, B.D.; Mehran, R.; Stachenfeld, N.; Wenger, N.K. Effects of Sex and Gender on Adaptation to Space: Cardiovascular Alterations. J. Women’s Health 2014, 23, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Reschke, M.F.; Cohen, H.S.; Cerisano, J.M.; Clayton, J.A.; Cromwell, R.; Danielson, R.W.; Hwang, E.Y.; Tingen, C.; Allen, J.R.; Tomko, D.L. Effects of Sex and Gender on Adaptation to Space: Neurosensory Systems. J. Women’s Health 2014, 23, 959–962. [Google Scholar] [CrossRef]
- Anderson, W.W.; Collingridge, G.L. The LTP Program: A Data Acquisition Program for on-Line Analysis of Long-Term Potentiation and Other Synaptic Events. J. Neurosci. Methods 2001, 108, 71–83. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A Synaptic Model of Memory: Long-Term Potentiation in the Hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Bliss, T.V. Memories of NMDA Receptors and LTP. Trends Neurosci. 1995, 18, 54–56. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nicoll, R.A. NMDA-Receptor-Dependent Synaptic Plasticity: Multiple Forms and Mechanisms. Trends Neurosci. 1993, 16, 521–527. [Google Scholar] [CrossRef]
- Junjaud, G.; Rouaud, E.; Turpin, F.; Mothet, J.-P.; Billard, J.-M. Age-Related Effects of the Neuromodulator D-Serine on Neurotransmission and Synaptic Potentiation in the CA1 Hippocampal Area of the Rat. J. Neurochem. 2006, 98, 1159–1166. [Google Scholar] [CrossRef]
- Le Bail, M.; Martineau, M.; Sacchi, S.; Yatsenko, N.; Radzishevsky, I.; Conrod, S.; Ait Ouares, K.; Wolosker, H.; Pollegioni, L.; Billard, J.-M.; et al. Identity of the NMDA Receptor Coagonist Is Synapse Specific and Developmentally Regulated in the Hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, E204–E213. [Google Scholar] [CrossRef]
- Izquierdo, I.; Medina, J.H. Correlation between the Pharmacology of Long-Term Potentiation and the Pharmacology of Memory. Neurobiol. Learn. Mem. 1995, 63, 19–32. [Google Scholar] [CrossRef]
- Kim, S.J.; Linden, D.J. Ubiquitous Plasticity and Memory Storage. Neuron 2007, 56, 582–592. [Google Scholar] [CrossRef]
- Lisman, J.E.; McIntyre, C.C. Synaptic Plasticity: A Molecular Memory Switch. Curr. Biol. 2001, 11, R788–R791. [Google Scholar] [CrossRef]
- Larson, J.; Munkácsy, E. Theta-Burst LTP. Brain Res. 2015, 1621, 38–50. [Google Scholar] [CrossRef]
- Nabavi, S.; Kessels, H.W.; Alfonso, S.; Aow, J.; Fox, R.; Malinow, R. Metabotropic NMDA Receptor Function Is Required for NMDA Receptor-Dependent Long-Term Depression. Proc. Natl. Acad. Sci. USA 2013, 110, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.C.; Tsai, G.E.; Ma, C.-L.; Ehmsen, J.T.; Mustafa, A.K.; Han, L.; Jiang, Z.I.; Benneyworth, M.A.; Froimowitz, M.P.; Lange, N.; et al. Targeted Disruption of Serine Racemase Affects Glutamatergic Neurotransmission and Behavior. Mol. Psychiatry 2009, 14, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Stahn, A.C.; Kühn, S. Brains in Space: The Importance of Understanding the Impact of Long-Duration Spaceflight on Spatial Cognition and Its Neural Circuitry. Cogn. Process 2021, 22, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Arshad, I.; Ferré, E.R. Cognition in Zero Gravity: Effects of Non-Terrestrial Gravity on Human Behaviour. Q. J. Exp. Psychol. 2022, 7, 979–994. [Google Scholar] [CrossRef]
- Reschke, M.F.; Bloomberg, J.J.; Harm, D.L.; Paloski, W.H.; Layne, C.; McDonald, V. Posture, Locomotion, Spatial Orientation, and Motion Sickness as a Function of Space Flight. Brain Res. Brain Res. Rev. 1998, 28, 102–117. [Google Scholar] [CrossRef]
- Clement, G.; Boyle, R.D.; Gunga, H.-C. Editorial: The Effects of Altered Gravity on Physiology. Front. Physiol. 2019, 10, 1447. [Google Scholar] [CrossRef]
- Mitani, K.; Horii, A.; Kubo, T. Impaired Spatial Learning after Hypergravity Exposure in Rats. Brain Res. Cogn. Brain Res. 2004, 22, 94–100. [Google Scholar] [CrossRef]
- Truchet, B.; Benoit, A.; Chaillan, F.; Smith, P.F.; Philoxene, B.; Guillamin, M.; Poucet, B.; Coquerel, A.; Besnard, S. Hippocampal LTP Modulation and Glutamatergic Receptors Following Vestibular Loss. Brain Struct. Funct. 2019, 224, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Guinan, M.J.; Horowitz, J.M.; Fuller, C.A. Effects of Hyperdynamic Fields on Input-Output Relationships and Long-Term Potentiation in the Rat Hippocampus. J. Gravit. Physiol. 1998, 5, 31–40. [Google Scholar] [PubMed]
- Johnson, J.W.; Ascher, P. Glycine Potentiates the NMDA Response in Cultured Mouse Brain Neurons. Nature 1987, 325, 529–531. [Google Scholar] [CrossRef] [PubMed]
- McBain, C.J.; Kleckner, N.W.; Wyrick, S.; Dingledine, R. Structural Requirements for Activation of the Glycine Coagonist Site of N-Methyl-D-Aspartate Receptors Expressed in Xenopus Oocytes. Mol. Pharmacol. 1989, 36, 556–565. [Google Scholar] [CrossRef]
- Wolosker, H.; Blackshaw, S.; Snyder, S.H. Serine Racemase: A Glial Enzyme Synthesizing D-Serine to Regulate Glutamate-N-Methyl-D-Aspartate Neurotransmission. Proc. Natl. Acad. Sci. USA 1999, 96, 13409–13414. [Google Scholar] [CrossRef]
- Neame, S.; Safory, H.; Radzishevsky, I.; Touitou, A.; Marchesani, F.; Marchetti, M.; Kellner, S.; Berlin, S.; Foltyn, V.N.; Engelender, S.; et al. The NMDA Receptor Activation by D-Serine and Glycine Is Controlled by an Astrocytic Phgdh-Dependent Serine Shuttle. Proc. Natl. Acad. Sci. USA 2019, 116, 20736–20742. [Google Scholar] [CrossRef]
- Kaplan, E.; Zubedat, S.; Radzishevsky, I.; Valenta, A.C.; Rechnitz, O.; Sason, H.; Sajrawi, C.; Bodner, O.; Konno, K.; Esaki, K.; et al. ASCT1 (Slc1a4) Transporter Is a Physiologic Regulator of Brain d-Serine and Neurodevelopment. Proc. Natl. Acad. Sci. USA 2018, 115, 9628–9633. [Google Scholar] [CrossRef]
- Lichterfeld, Y.; Kalinski, L.; Schunk, S.; Schmakeit, T.; Feles, S.; Frett, T.; Herrmann, H.; Hemmersbach, R.; Liemersdorf, C. Hypergravity Attenuates Reactivity in Primary Murine Astrocytes. Biomedicines 2022, 10, 1966. [Google Scholar] [CrossRef]
- Osaki, A.; Aoyama, M.; Mita, M.; Hamase, K.; Yasui, M.; Sasabe, J. Endogenous D-Serine Exists in the Mammalian Brain Independent of Synthesis by Serine Racemase. Biochem. Biophys. Res. Commun. 2023, 641, 186–191. [Google Scholar] [CrossRef]
- Radzishevsky, I.; Odeh, M.; Bodner, O.; Zubedat, S.; Shaulov, L.; Litvak, M.; Esaki, K.; Yoshikawa, T.; Agranovich, B.; Li, W.-H.; et al. Impairment of Serine Transport across the Blood-Brain Barrier by Deletion of Slc38a5 Causes Developmental Delay and Motor Dysfunction. Proc. Natl. Acad. Sci. USA 2023, 120, e2302780120. [Google Scholar] [CrossRef]
- Dubayle, D.; Vanden-Bossche, A.; Beraneck, M.; Vico, L.; Morel, J.-L. Effects of Centrifugation and Whole-Body Vibrations on Blood-Brain Barrier Permeability in Mice. npj Microgravity 2020, 6, 1. [Google Scholar] [CrossRef]
- Dubayle, D.; Vanden-Bossche, A.; Peixoto, T.; Morel, J.-L. Hypergravity Increases Blood–Brain Barrier Permeability to Fluorescent Dextran and Antisense Oligonucleotide in Mice. Cells 2023, 12, 734. [Google Scholar] [CrossRef]
- Babiec, W.E.; Guglietta, R.; Jami, S.A.; Morishita, W.; Malenka, R.C.; O’Dell, T.J. Ionotropic NMDA Receptor Signaling Is Required for the Induction of Long-Term Depression in the Mouse Hippocampal CA1 Region. J. Neurosci. 2014, 34, 5285–5290. [Google Scholar] [CrossRef] [PubMed]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-Term Potentiation and the Role of N-Methyl-D-Aspartate Receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Park, D.K.; Stein, I.S.; Zito, K. Ion Flux-Independent NMDA Receptor Signaling. Neuropharmacology 2022, 210, 109019. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wullen, M.; Bouet, V.; Freret, T.; Billard, J.-M. Sexual Dimorphism of Synaptic Plasticity Changes in CA1 Hippocampal Networks in Hypergravity-Exposed Mice—New Insights for Cognition in Space. Cells 2025, 14, 1186. https://doi.org/10.3390/cells14151186
Wullen M, Bouet V, Freret T, Billard J-M. Sexual Dimorphism of Synaptic Plasticity Changes in CA1 Hippocampal Networks in Hypergravity-Exposed Mice—New Insights for Cognition in Space. Cells. 2025; 14(15):1186. https://doi.org/10.3390/cells14151186
Chicago/Turabian StyleWullen, Mathilde, Valentine Bouet, Thomas Freret, and Jean-Marie Billard. 2025. "Sexual Dimorphism of Synaptic Plasticity Changes in CA1 Hippocampal Networks in Hypergravity-Exposed Mice—New Insights for Cognition in Space" Cells 14, no. 15: 1186. https://doi.org/10.3390/cells14151186
APA StyleWullen, M., Bouet, V., Freret, T., & Billard, J.-M. (2025). Sexual Dimorphism of Synaptic Plasticity Changes in CA1 Hippocampal Networks in Hypergravity-Exposed Mice—New Insights for Cognition in Space. Cells, 14(15), 1186. https://doi.org/10.3390/cells14151186






