Plasmodium falciparum Subtilisin-like Domain-Containing Protein (PfSDP), a Cross-Stage Antigen, Elicits Short-Lived Antibody Response Following Natural Infection with Plasmodium falciparum
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Selection
2.2. Genetic Diversity Assessment
2.3. Description and Source of Clinical Isolates and Lab Strains
Parasite Culture, Developmental Stage Production
2.4. RNA Extraction, RT q-PCR
2.5. Antibody Production
2.6. Immunoblotting
2.7. Full-Length Protein Expression and Purification
2.8. Protein Structure Prediction
2.9. Indirect Immunofluorescence Assay (IFA)
2.10. Growth Inhibition Assays (GIAs)
2.11. PfSDP Immunogenicity Assessment by Indirect-ELISA
Protein Immunogenicity Confirmation by Western and Dot Blots
2.12. Data Analysis
3. Results
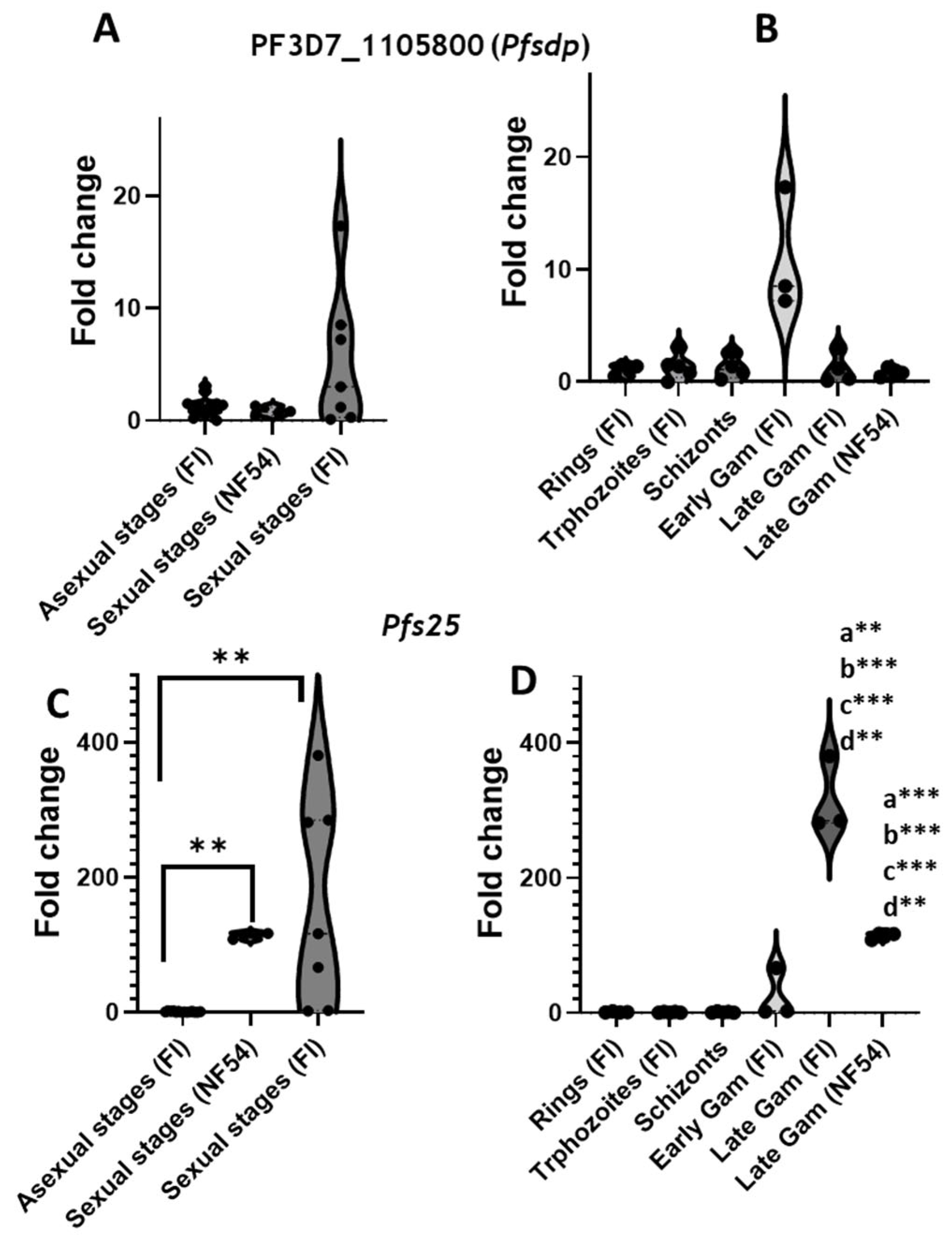
3.1. Expression Profiles of Selected Genes in Online Datasets (PlasmoDB)
3.2. Pfsdp May Be Under Directional Selection and Genetically Conserved Across Different Continents
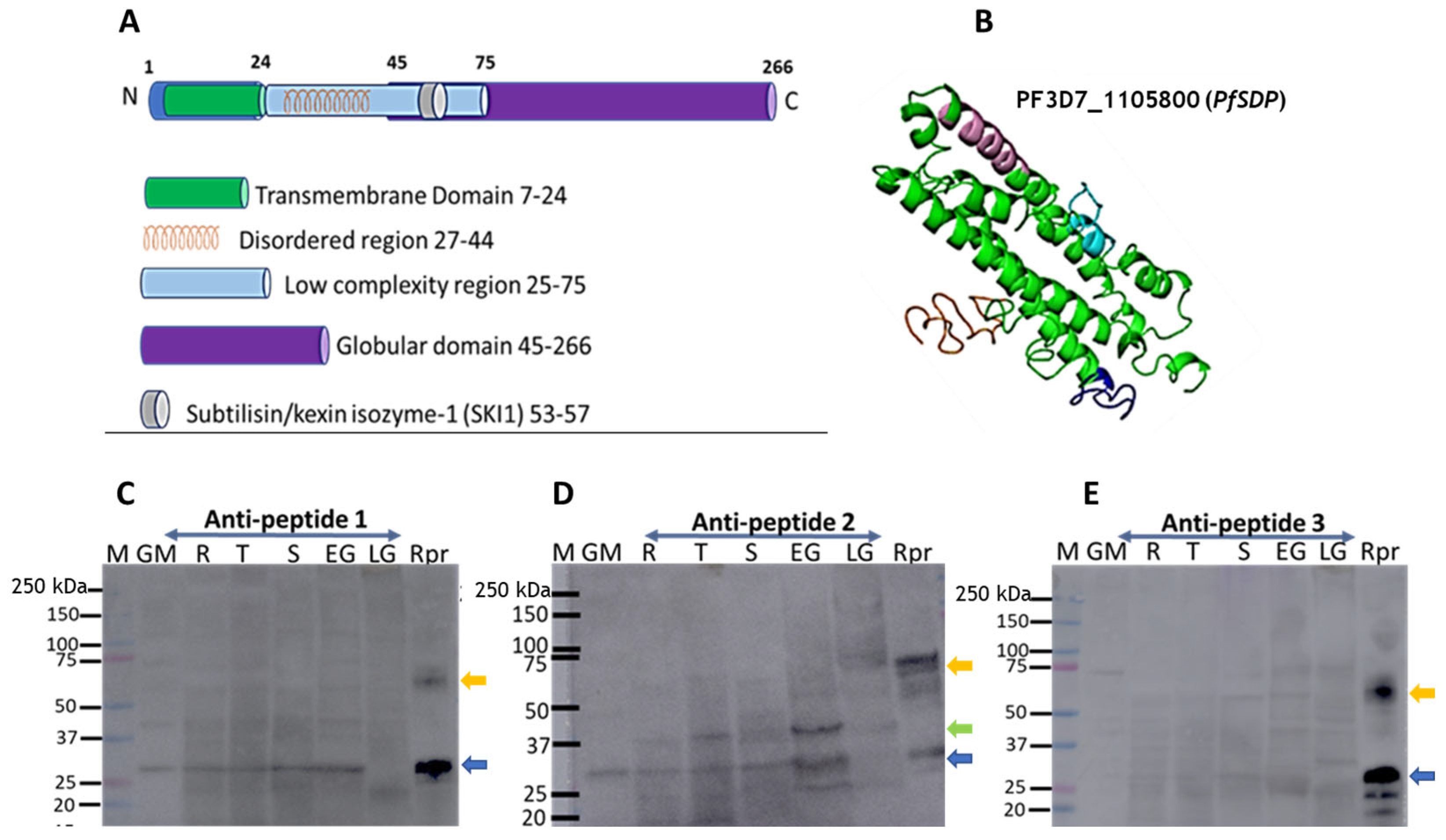
3.3. PfSDP Has Cross-Stage Expression at the Transcript and Protein Levels in Clinical Isolates
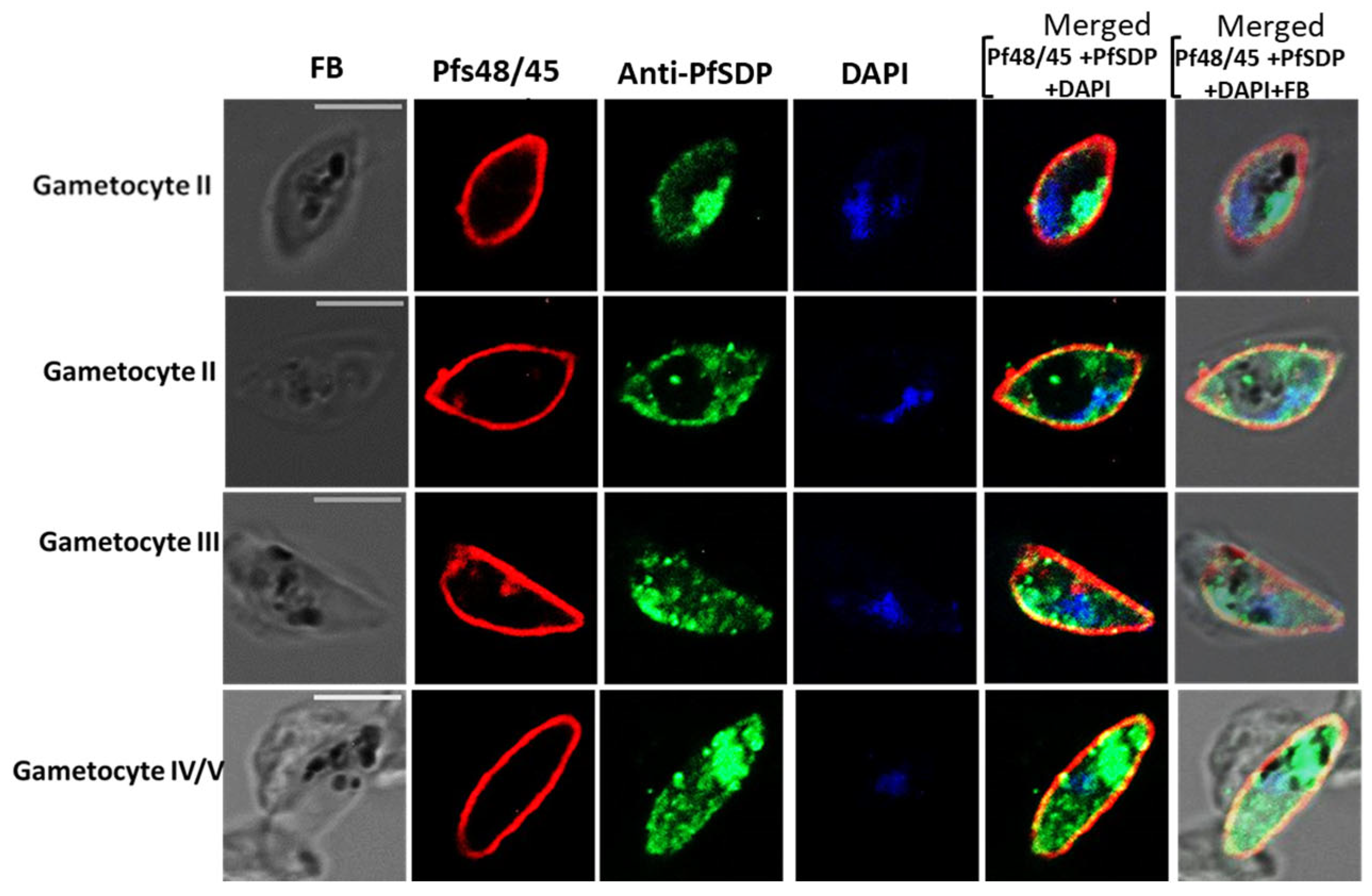
3.4. PfSDP Co-Localized with MSP-1, Partially with Pfs45/48, and Peptide-Specific Antibodies Showed Mild Impacts on Merozoite Invasion in a Concentration-Dependent Manner
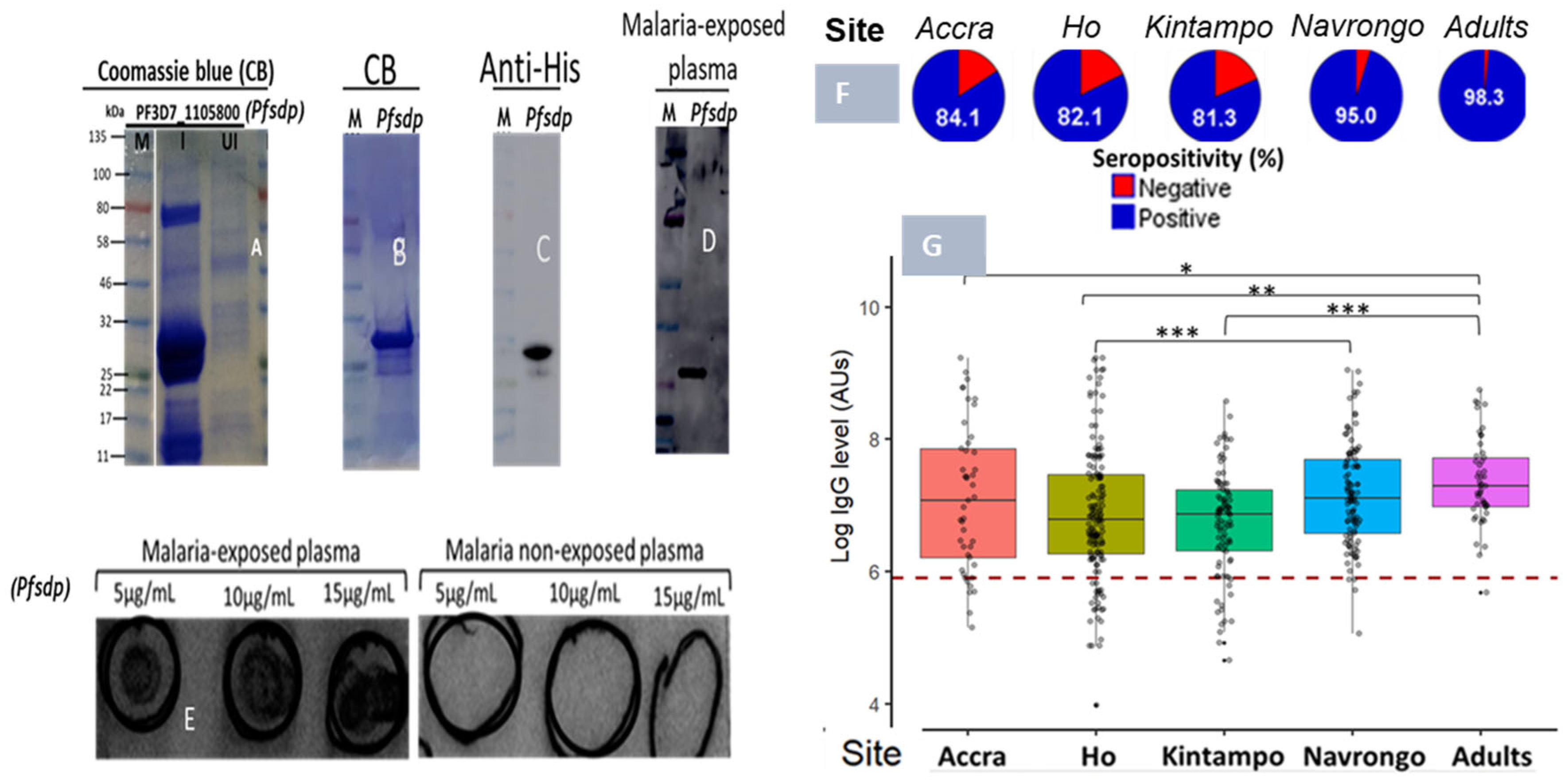
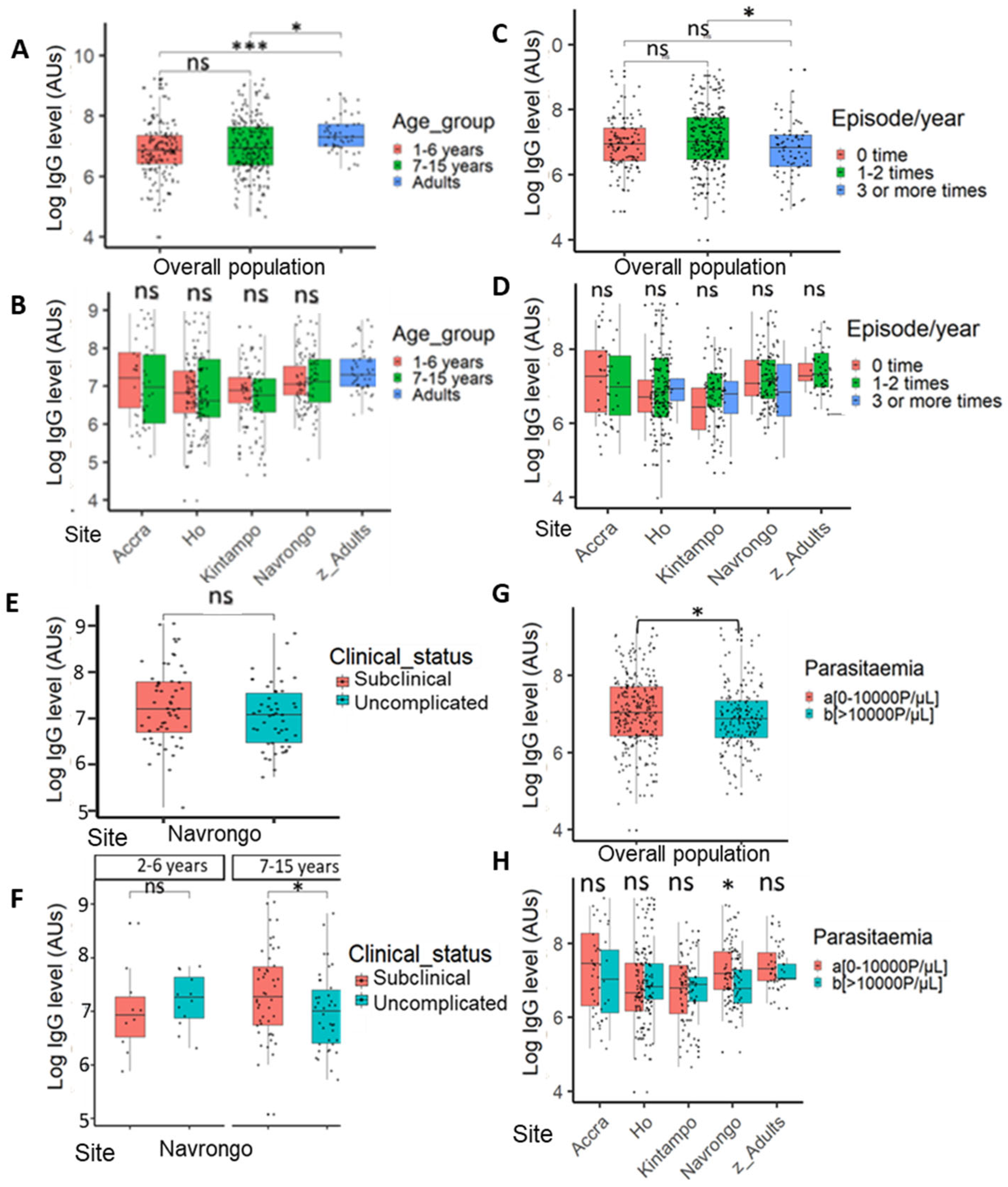
3.5. Plasma Samples from Children and Adults from Different Endemic Areas Reacted to PfSDP Recombinant Proteins
3.5.1. Associations Between Antibody Response Level, Age, Disease Episode, Parasite Density, and Exposure Among the Study Population
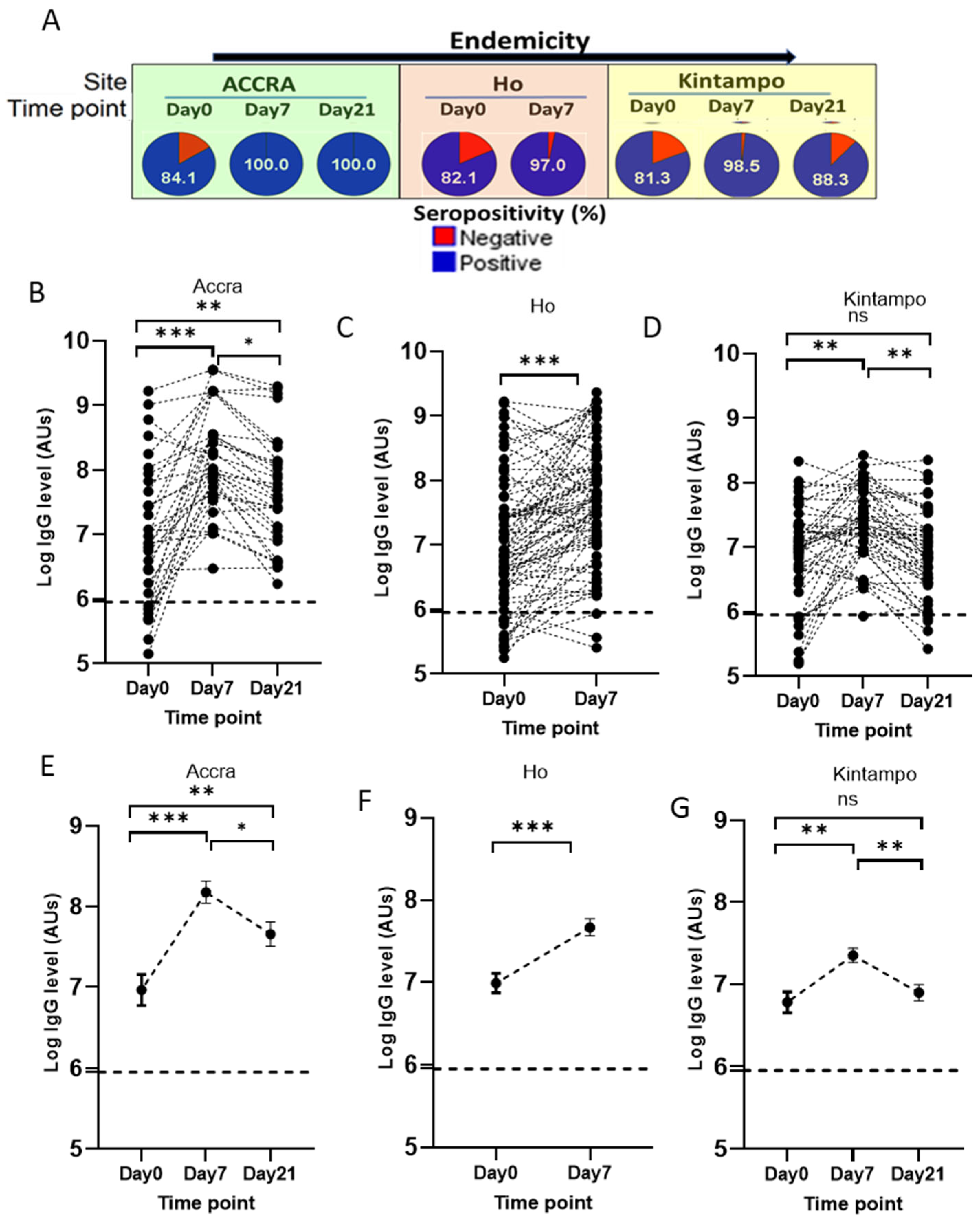
3.5.2. Time Point-Specific Variations in PfSDP Seropositivity and IgG Level in Malaria-Infected Children Were Observed Across Areas with Different Transmission Potentials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pfsdp | Plasmodium falciparum Subtilisin-like Domain-Containing Protein |
| PfMSP-1 | Plasmodium falciparum Merozoite Surface Protein 1 |
| SEA | South-East Asia |
| LEKMAH | Ledzokuku-Krowor Municipal Assembly Hospital |
| RBCs | Red Blood Cells |
| RPMI | Roswell Park Memorial Institute |
| PBS | Phosphate-Buffered Saline |
| RT q-PCR | Reverse Transcriptase Quantitative Polymerase Chain Reaction |
| cDNA | Complementary Deoxyribonucleic Acid |
| SDS-PAGE | Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis |
| RT | Room Temperature |
| DAPI | 4′, 6′-diamidino-2-phenylindole |
| CFDA-SE | Carboxyfluorescein Diacetate Succinimidyl Ester |
| GIAs | Growth Inhibition Assays |
| IgG | Immunoglobulin G |
| PCA | Principal Component Analysis |
| PfEBA181 | Plasmodium falciparum Erythrocyte-Binding Antigen 181 |
| PfCyRPA | Plasmodium falciparum Cysteine-Rich Protective Antigen |
| PfRAMA | Plasmodium falciparum Rhoptry-Associated Membrane Antigen |
| RH4.2 | Reticulocyte-Binding Protein Homologue 4.2 |
| MSP-7 | Merozoite Surface Protein 1 |
| MSP-119 | Merozoite Surface Protein 1 (C-terminal 42 kDa fragment) |
| MSP-2 | Merozoite Surface Protein 2 |
| MSP-3 | Merozoite Surface Protein 3 |
| RH5 | Reticulocyte-Binding Protein Homologue 5 |
| AMA-1 | Apical Membrane Antigen 1 |
References
- Neher, R.A.; Amato, R.; Miotto, O.; Woodrow, C.J.; Almagro-Garcia, J.; Sinha, I.; Campino, S.; Mead, D.; Drury, E.; Kekre, M. Genomic epidemiology of artemisinin resistant malaria. eLife 2016, 5, e08714. [Google Scholar] [CrossRef] [PubMed]
- Mbacham, W.F.; Evehe, M.-S.B.; Netongo, P.M.; Ateh, I.A.; Mimche, P.N.; Ajua, A.; Nji, A.M.; Irenee, D.; Echouffo-Tcheugui, J.B.; Tawe, B. Efficacy of amodiaquine, sulphadoxine-pyrimethamine and their combination for the treatment of uncomplicated Plasmodium falciparum malaria in children in Cameroon at the time of policy change to artemisinin-based combination therapy. Malar. J. 2010, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Current status of two adjuvanted malaria vaccines and the World Health Organization (WHO) strategy to eradicate malaria by 2030. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 28, e939357-1. [Google Scholar] [CrossRef] [PubMed]
- Ogieuhi, I.J.; Ajekiigbe, V.O.; Kolo-Manma, K.; Akingbola, A.; Odeniyi, T.A.; Soyemi, T.S.; Ayomide, J.H.; Thiyagarajan, B.; Awolola, B.D. A narrative review of the RTS S AS01 malaria vaccine and its implementation in Africa to reduce the global malaria burden. Discov. Public Health 2024, 21, 152. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Richie, T.L.; Church, L.P.; Murshedkar, T.; Billingsley, P.F.; James, E.R.; Chen, M.C.; Abebe, Y.; KC, N.; Chakravarty, S.; Dolberg, D.; et al. Sporozoite immunization: Innovative translational science to support the fight against malaria. Expert Rev. Vaccines 2023, 22, 964–1007. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Bharti, P.K.; Bansal, D.; Raman, R.K.; Mohapatra, P.K.; Sehgal, R.; Mahanta, J.; Sultan, A.A.; Singh, N. Genetic diversity and antibody responses against Plasmodium falciparum vaccine candidate genes from Chhattisgarh, Central India: Implication for vaccine development. PLoS ONE 2017, 12, e0182674. [Google Scholar] [CrossRef] [PubMed]
- Smalley, M.; Abdalla, S.; Brown, J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.; Magallon-Tejada, A.; Achtman, A.H.; Moraleda, C.; Joice, R.; Cisteró, P.; Suen, C.S.L.W.; Nhabomba, A.; Macete, E.; Mueller, I. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood 2014, 123, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Joice, R.; Nilsson, S.K.; Montgomery, J.; Dankwa, S.; Egan, E.; Morahan, B.; Seydel, K.B.; Bertuccini, L.; Alano, P.; Williamson, K.C. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014, 6, 244re5. [Google Scholar] [CrossRef] [PubMed]
- Farfour, E.; Charlotte, F.; Settegrana, C.; Miyara, M.; Buffet, P. The extravascular compartment of the bone marrow: A niche for Plasmodium falciparum gametocyte maturation? Malar. J. 2012, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Lourenco, P.; Carter, R.; Walliker, D.; Ranford-Cartwright, L. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology 2000, 121, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Peatey, C.L.; Skinner-Adams, T.S.; Dixon, M.W.A.; McCarthy, J.S.; Gardiner, D.L.; Trenholme, K.R. Effect of Antimalarial Drugs on Plasmodium falciparum Gametocytes. J. Infect. Dis. 2009, 200, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Vega-Rodríguez, J.; Jacobs-Lorena, M. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Memórias Do Inst. Oswaldo Cruz 2014, 109, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V.S. Vaccines against malaria. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011, 366, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.; Good, M.F. A case for whole-parasite malaria vaccines. Int. J. Parasitol. 2007, 37, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Doumbo, O.K.; Niaré, K.; Healy, S.A.; Sagara, I.; Duffy, P.E. Malaria transmission-blocking vaccines: Present status and future perspectives. In Towards Malaria Elimination-A Leap Forward; IntechOpen: London, UK, 2018. [Google Scholar]
- Conway, D.J. Paths to a malaria vaccine illuminated by parasite genomics. Trends Genet. 2015, 31, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Amlabu, E.; Mensah-Brown, H.; Nyarko, P.B.; Akuh, O.-a.; Opoku, G.; Ilani, P.; Oyagbenro, R.; Asiedu, K.; Aniweh, Y.; Awandare, G.A. Functional Characterization of Plasmodium falciparum Surface Related Antigen (PfSRA) as a Potential Blood-Stage Vaccine Target. J. Infect. Dis. 2018, 218, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Kengne-Ouafo, J.A.; Sutherland, C.J.; Binka, F.N.; Awandare, G.A.; Urban, B.C.; Dinko, B. Immune Responses to the Sexual Stages of Plasmodium falciparum Parasites. Front. Immunol. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, A.; Kusi, K.A.; Ofori, M.F.; Ndifon, W. Novel strategies for malaria vaccine design. Front. Immunol. 2018, 9, 2769. [Google Scholar] [CrossRef] [PubMed]
- Krishnarjuna, B.; Andrew, D.; MacRaild, C.A.; Morales, R.A.V.; Beeson, J.G.; Anders, R.F.; Richards, J.S.; Norton, R.S. Strain-transcending immune response generated by chimeras of the malaria vaccine candidate merozoite surface protein 2. Sci. Rep. 2016, 6, 20613. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Stern, P.L.; Cunningham, A.L. Understanding Modern Vaccines: Perspectives in Vaccinology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, B.; Wittelsbürger, U.; Ramos-Onsins, S.E.; Lercher, M.J. PopGenome: An efficient Swiss army knife for population genomic analyses in R. Mol. Biol. Evol. 2014, 31, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Moll, K.; Kaneko, A.; Scherf, A.; Wahlgren, M. Methods in Malaria Research; Humana Press: Towaco, NJ, USA, 2013. [Google Scholar]
- Fivelman, Q.L.; McRobert, L.; Sharp, S.; Taylor, C.J.; Saeed, M.; Swales, C.A.; Sutherland, C.J.; Baker, D.A. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 2007, 154, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Beshir, K.B.; Hallett, R.L.; Eziefula, A.C.; Bailey, R.; Watson, J.; Wright, S.G.; Chiodini, P.L.; Polley, S.D.; Sutherland, C.J. Measuring the efficacy of anti-malarial drugs in vivo: Quantitative PCR measurement of parasite clearance. Malar. J. 2010, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Ayanful-Torgby, R.; Oppong, A.; Abankwa, J.; Acquah, F.; Williamson, K.C.; Amoah, L.E. Plasmodium falciparum genotype and gametocyte prevalence in children with uncomplicated malaria in coastal Ghana. Malar. J. 2016, 15, 592. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Staalsoe, T.; Lavstsen, T.; Jensen, A.T.; Sowa, M.P.; Arnot, D.E.; Hviid, L.; Theander, T.G. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003, 49, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Heiber, A.; Spielmann, T. Preparation of parasite protein extracts and western blot analysis. Electrophoresis 2014, 115, 035–062. [Google Scholar] [CrossRef]
- Schreiber, N.; Khattab, A.; Petter, M.; Marks, F.; Adjei, S.; Kobbe, R.; May, J.; Klinkert, M.-Q. Expression of Plasmodium falciparum 3D7 STEVOR proteins for evaluation of antibody responses following malaria infections in naive infants. Parasitology 2008, 135, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Brown, H.E.; Amoako, N.; Abugri, J.; Stewart, L.B.; Agongo, G.; Dickson, E.K.; Ofori, M.F.; Stoute, J.A.; Conway, D.J.; Awandare, G.A. Analysis of Erythrocyte Invasion Mechanisms of Plasmodium falciparum Clinical Isolates Across 3 Malaria-Endemic Areas in Ghana. J. Infect. Dis. 2015, 212, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Zanghì, G.; Vembar, S.S.; Baumgarten, S.; Ding, S.; Guizetti, J.; Bryant, J.M.; Mattei, D.; Jensen, A.T.R.; Rénia, L.; Goh, Y.S.; et al. A Specific PfEMP1 Is Expressed in P. falciparum Sporozoites and Plays a Role in Hepatocyte Infection. Cell Rep. 2018, 22, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- López-Barragán, M.J.; Lemieux, J.; Quiñones, M.; Williamson, K.C.; Molina-Cruz, A.; Cui, K.; Barillas-Mury, C.; Zhao, K.; Su, X.-z. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genom. 2011, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- LaCount, D.J.; Vignali, M.; Chettier, R.; Phansalkar, A.; Bell, R.; Hesselberth, J.R.; Schoenfeld, L.W.; Ota, I.; Sahasrabudhe, S.; Kurschner, C.; et al. A protein interaction network of the malaria parasite Plasmodium falciparum. Nature 2005, 438, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.J.; Carruthers, V.B.; Niles, J.C.; et al. A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 2016, 166, 1423–1435.e1412. [Google Scholar] [CrossRef] [PubMed]
- Polley, S.D.; Tetteh, K.K.; Cavanagh, D.R.; Pearce, R.J.; Lloyd, J.M.; Bojang, K.A.; Okenu, D.M.; Greenwood, B.M.; McBride, J.S.; Conway, D.J. Repeat sequences in block 2 of Plasmodium falciparum merozoite surface protein 1 are targets of antibodies associated with protection from malaria. Infect. Immun. 2003, 71, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Mukherjee, P.; Yazdani, S.S.; Jain, S.K.; Mohmmed, A.; Chauhan, V.S. Plasmodium falciparum Merozoite Surface Protein 1 (MSP-1)-MSP-3 Chimeric Protein: Immunogenicity Determined with Human-Compatible Adjuvants and Induction of Protective Immune Response. Infect. Immun. 2010, 78, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Kadekoppala, M.; O’Donnell, R.A.; Grainger, M.; Crabb, B.S.; Holder, A.A. Deletion of the Plasmodium falciparum Merozoite Surface Protein 7 Gene Impairs Parasite Invasion of Erythrocytes. Eukaryot. Cell 2008, 7, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Bousema, T.; Drakeley, C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 2011, 24, 377–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 1987, 9, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Rener, J.; Graves, P.M.; Carter, R.; Williams, J.L.; Burkot, T.R. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J. Exp. Med. 1983, 158, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Quakyi, I.A.; Carter, R.; Rener, J.; Kumar, N.; Good, M.F.; Miller, L.H. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 1987, 139, 4213–4217. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.W.; Assefa, S.A.; Abugri, J.; Amoako, N.; Owusu-Agyei, S.; Anyorigiya, T.; MacInnis, B.; Kwiatkowski, D.P.; Conway, D.J.; Awandare, G.A. Comparison of genomic signatures of selection on Plasmodium falciparum between different regions of a country with high malaria endemicity. BMC Genom. 2015, 16, 527. [Google Scholar] [CrossRef] [PubMed]
- Ahouidi, A.D.; Bei, A.K.; Neafsey, D.E.; Sarr, O.; Volkman, S.; Milner, D.; Cox-Singh, J.; Ferreira, M.U.; Ndir, O.; Premji, Z.; et al. Population genetic analysis of large sequence polymorphisms in Plasmodium falciparum blood-stage antigens. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2010, 10, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ochola, L.I.; Tetteh, K.K.; Stewart, L.B.; Riitho, V.; Marsh, K.; Conway, D.J. Allele frequency-based and polymorphism-versus-divergence indices of balancing selection in a new filtered set of polymorphic genes in Plasmodium falciparum. Mol. Biol. Evol. 2010, 27, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.V.; Ahouidi, A.D.; Zilversmit, M.; Bei, A.K.; Rayner, J.; Sarr, O.; Ndir, O.; Wirth, D.F.; Mboup, S.; Duraisingh, M.T. Molecular analysis of erythrocyte invasion in Plasmodium falciparum isolates from Senegal. Infect. Immun. 2007, 75, 3531–3538. [Google Scholar] [CrossRef] [PubMed]
- Kassegne, K.; Komi Koukoura, K.; Shen, H.-M.; Chen, S.-B.; Fu, H.-T.; Chen, Y.-Q.; Zhou, X.-N.; Chen, J.-H.; Cheng, Y. Genome-Wide Analysis of the Malaria Parasite Plasmodium falciparum Isolates From Togo Reveals Selective Signals in Immune Selection-Related Antigen Genes. Front. Immunol. 2020, 11, 552698. [Google Scholar] [CrossRef] [PubMed]
- Benavente, E.D.; Oresegun, D.R.; de Sessions, P.F.; Walker, E.M.; Roper, C.; Dombrowski, J.G.; de Souza, R.M.; Marinho, C.R.F.; Sutherland, C.J.; Hibberd, M.L.; et al. Global genetic diversity of var2csa in Plasmodium falciparum with implications for malaria in pregnancy and vaccine development. Sci. Rep. 2018, 8, 15429. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.S.; Ali Albsheer, M.M.; Abdelbagi, H.; Siddig, E.E.; Mohamed, M.A.; Ahmed, A.E.; Omer, R.A.; Muneer, M.S.; Ahmed, A.; Osman, H.A.; et al. Genetic polymorphism of the N-terminal region in circumsporozoite surface protein of Plasmodium falciparum field isolates from Sudan. Malar. J. 2019, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Pringle, J.C.; Carpi, G.; Almagro-Garcia, J.; Zhu, S.J.; Kobayashi, T.; Mulenga, M.; Bobanga, T.; Chaponda, M.; Moss, W.J.; Norris, D.E. RTS,S/AS01 malaria vaccine mismatch observed among Plasmodium falciparum isolates from southern and central Africa and globally. Sci. Rep. 2018, 8, 6622. [Google Scholar] [CrossRef] [PubMed]
- Niang, M.; Bei, A.K.; Madnani, K.G.; Pelly, S.; Dankwa, S.; Kanjee, U.; Gunalan, K.; Amaladoss, A.; Yeo, K.P.; Bob, N.S.; et al. STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host Microbe 2014, 16, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Tawk, L.; Lacroix, C.; Gueirard, P.; Kent, R.; Gorgette, O.; Thiberge, S.; Mercereau-Puijalon, O.; Ménard, R.; Barale, J.-C. A key role for Plasmodium subtilisin-like SUB1 protease in egress of malaria parasites from host hepatocytes. J. Biol. Chem. 2013, 288, 33336–33346. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Child, M.A.; Bogyo, M. Proteases as regulators of pathogenesis: Examples from the Apicomplexa. Biochim. Biophys. Acta 2012, 1824, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.J. Proteases in host cell invasion by the malaria parasite. Cell. Microbiol. 2004, 6, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, L.Y.; Powell, G.T.; Lin, Y.-C.; Macklin, M.D.; Cross, N.; Kemp, A.; Cawkill, P.; Sanderson, T.; Crosnier, C.; Muller-Sienerth, N.; et al. Synergistic malaria vaccine combinations identified by systematic antigen screening. Proc. Natl. Acad. Sci. USA 2017, 114, 12045–12050. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.O.; Silk, S.E.; Elias, S.C.; Miura, K.; Diouf, A.; Galaway, F.; de Graaf, H.; Brendish, N.J.; Poulton, I.D.; Griffiths, O.J.; et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight 2017, 2, e96381. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.S.; Björnsson, K.H.; Bassi, M.R.; Walker, M.R.; Kok, A.; Cristinoi, B.; Jensen, A.R.; Barfod, L. Strain-dependent inhibition of erythrocyte invasion by monoclonal antibodies against Plasmodium falciparum CyRPA. Front. Immunol. 2021, 12, 716305. [Google Scholar] [CrossRef] [PubMed]
- Graves, P.M.; Wirtz, R.A.; Carter, R.; Burkot, T.R.; Looker, M.; Targett, G.A. Naturally occurring antibodies to an epitope on Plasmodium falciparum gametes detected by monoclonal antibody-based competitive enzyme-linked immunosorbent assay. Infect. Immun. 1988, 56, 2818–2821. [Google Scholar] [CrossRef] [PubMed]
- Kapulu, M.; Da, D.; Miura, K.; Li, Y.; Blagborough, A.; Churcher, T.; Nikolaeva, D.; Williams, A.R.; Goodman, A.; Sangare, I. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci. Rep. 2015, 5, 11193. [Google Scholar] [CrossRef] [PubMed]
- Gebru, T.; Ajua, A.; Theisen, M.; Esen, M.; Ngoa, U.A.; Issifou, S.; Adegnika, A.A.; Kremsner, P.G.; Mordmuller, B.; Held, J. Recognition of Plasmodium falciparum mature gametocyte-infected erythrocytes by antibodies of semi-immune adults and malaria-exposed children from Gabon. Malar. J. 2017, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, M.; de Vlas, S.J.; Sauerwein, R.W. Reduction and enhancement of Plasmodium falciparum transmission by endemic human sera. Int. J. Parasitol. 2006, 36, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Bousema, T.; Okell, L.; Shekalaghe, S.; Griffin, J.T.; Omar, S.; Sawa, P.; Sutherland, C.; Sauerwein, R.; Ghani, A.C.; Drakeley, C. Revisiting the circulation time of Plasmodium falciparum gametocytes: Molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 2010, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Bousema, T.; Sutherland, C.J.; Churcher, T.S.; Mulder, B.; Gouagna, L.C.; Riley, E.M.; Targett, G.A.; Drakeley, C.J. Human immune responses that reduce the transmission of Plasmodium falciparum in African populations. Int. J. Parasitol. 2011, 41, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.S.; Yazdani, S.S.; Gaur, D. Malaria vaccine development based on merozoite surface proteins of Plasmodium falciparum. Hum. Vaccines 2010, 6, 757–762. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.S.; Marjason, J.; Elliott, S.; Fahey, P.; Bang, G.; Malkin, E.; Tierney, E.; Aked-Hurditch, H.; Adda, C.; Cross, N.; et al. A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide® ISA 720. PLoS ONE 2011, 6, e24413. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Fürle, K.; Jäschke, A.; Mikus, G.; Lehmann, M.; Hüsing, J.; Heiss, K.; Giese, T.; Carter, D.; Böhnlein, E.; et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. Npj Vaccines 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Dery, D.B.; Brown, C.; Asante, K.P.; Adams, M.; Dosoo, D.; Amenga-Etego, S.; Wilson, M.; Chandramohan, D.; Greenwood, B.; Owusu-Agyei, S. Patterns and seasonality of malaria transmission in the forest-savannah transitional zones of Ghana. Malar. J. 2010, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyei, S.; Asante, K.P.; Adjuik, M.; Adjei, G.; Awini, E.; Adams, M.; Newton, S.; Dosoo, D.; Dery, D.; Agyeman-Budu, A.; et al. Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malar. J. 2009, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Kweku, M.; Liu, D.; Adjuik, M.; Binka, F.; Seidu, M.; Greenwood, B.; Chandramohan, D. Seasonal intermittent preventive treatment for the prevention of anaemia and malaria in Ghanaian children: A randomized, placebo controlled trial. PLoS ONE 2008, 3, e4000. [Google Scholar] [CrossRef] [PubMed]
- Baffoe-Wilmot, A.; Koram, K.; Appawu, M.; Dunyo, S.; Nkrumah, F.; Afari, E. Malaria vector studies in two ecological zones in southern Ghana. Afr. Entomol. 2001, 9, 59–65. [Google Scholar]
- Amlabu, E.; Ilani, P.; Opoku, G.; Nyarko, P.B.; Quansah, E.; Thiam, L.G.; Anim, M.; Ayivor-Djanie, R.; Akuh, O.-A.; Mensah-Brown, H.; et al. Molecular Characterization and Immuno-Reactivity Patterns of a Novel Plasmodium falciparum Armadillo-Type Repeat Protein, PfATRP. Front. Cell. Infect. Microbiol. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Brown, H.E.; Aspeling-Jones, H.; Delimini, R.K.; Asante, K.P.; Amlabu, E.; Bah, S.Y.; Beeson, J.G.; Wright, G.J.; Conway, D.J.; Awandare, G.A. Antibody Reactivity to Merozoite Antigens in Ghanaian Adults Correlates With Growth Inhibitory Activity Against Plasmodium falciparum in Culture. Open Forum Infect. Dis. 2019, 6, ofz254. [Google Scholar] [CrossRef] [PubMed]
- Ademolue, T.W.; Aniweh, Y.; Kusi, K.A.; Awandare, G.A. Patterns of inflammatory responses and parasite tolerance vary with malaria transmission intensity. Malar. J. 2017, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Graves, P.M.; Carter, R.; Burkot, T.R.; Quakyi, I.A.; Kumar, N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988, 10, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.E.; Traore, B.; Kayentao, K.; Ongoiba, A.; Doumbo, S.; Doumtabe, D.; Kone, Y.; Dia, S.; Guindo, A.; Traore, A.; et al. The Plasmodium falciparum-Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections. PLoS Pathog. 2010, 6, e1000912. [Google Scholar] [CrossRef] [PubMed]
- Partey, F.D.; Castberg, F.C.; Sarbah, E.W.; Silk, S.E.; Awandare, G.A.; Draper, S.J.; Opoku, N.; Kweku, M.; Ofori, M.F.; Hviid, L.; et al. Kinetics of antibody responses to PfRH5-complex antigens in Ghanaian children with Plasmodium falciparum malaria. PLoS ONE 2018, 13, e0198371. [Google Scholar]
- Yman, V.; White, M.T.; Asghar, M.; Sundling, C.; Sondén, K.; Draper, S.J.; Osier, F.H.A.; Färnert, A. Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: Kinetics and longevity in absence of re-exposure. BMC Med. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Kinyanjui, S.M.; Conway, D.J.; Lanar, D.E.; Marsh, K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar. J. 2007, 6, 82. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kengne-Ouafo, J.A.; Morang’a, C.M.; Nyakoe, N.K.; Dosoo, D.; Tackie, R.; Mutungi, J.K.; Bah, S.Y.; Amenga-Etego, L.N.; Urban, B.; Awandare, G.A.; et al. Plasmodium falciparum Subtilisin-like Domain-Containing Protein (PfSDP), a Cross-Stage Antigen, Elicits Short-Lived Antibody Response Following Natural Infection with Plasmodium falciparum. Cells 2025, 14, 1184. https://doi.org/10.3390/cells14151184
Kengne-Ouafo JA, Morang’a CM, Nyakoe NK, Dosoo D, Tackie R, Mutungi JK, Bah SY, Amenga-Etego LN, Urban B, Awandare GA, et al. Plasmodium falciparum Subtilisin-like Domain-Containing Protein (PfSDP), a Cross-Stage Antigen, Elicits Short-Lived Antibody Response Following Natural Infection with Plasmodium falciparum. Cells. 2025; 14(15):1184. https://doi.org/10.3390/cells14151184
Chicago/Turabian StyleKengne-Ouafo, Jonas A., Collins M. Morang’a, Nancy K. Nyakoe, Daniel Dosoo, Richmond Tackie, Joe K. Mutungi, Saikou Y. Bah, Lucas N. Amenga-Etego, Britta Urban, Gordon A. Awandare, and et al. 2025. "Plasmodium falciparum Subtilisin-like Domain-Containing Protein (PfSDP), a Cross-Stage Antigen, Elicits Short-Lived Antibody Response Following Natural Infection with Plasmodium falciparum" Cells 14, no. 15: 1184. https://doi.org/10.3390/cells14151184
APA StyleKengne-Ouafo, J. A., Morang’a, C. M., Nyakoe, N. K., Dosoo, D., Tackie, R., Mutungi, J. K., Bah, S. Y., Amenga-Etego, L. N., Urban, B., Awandare, G. A., Dinko, B., & Aniweh, Y. (2025). Plasmodium falciparum Subtilisin-like Domain-Containing Protein (PfSDP), a Cross-Stage Antigen, Elicits Short-Lived Antibody Response Following Natural Infection with Plasmodium falciparum. Cells, 14(15), 1184. https://doi.org/10.3390/cells14151184




