Neuro-Cells Mitigate Amyloid Plaque Formation and Behavioral Deficits in the APPswe/PS1dE9 Model of Alzheimer Disease While Also Reducing IL-6 Production in Human Monocytes
Abstract
1. Introduction
2. Materials and Methods
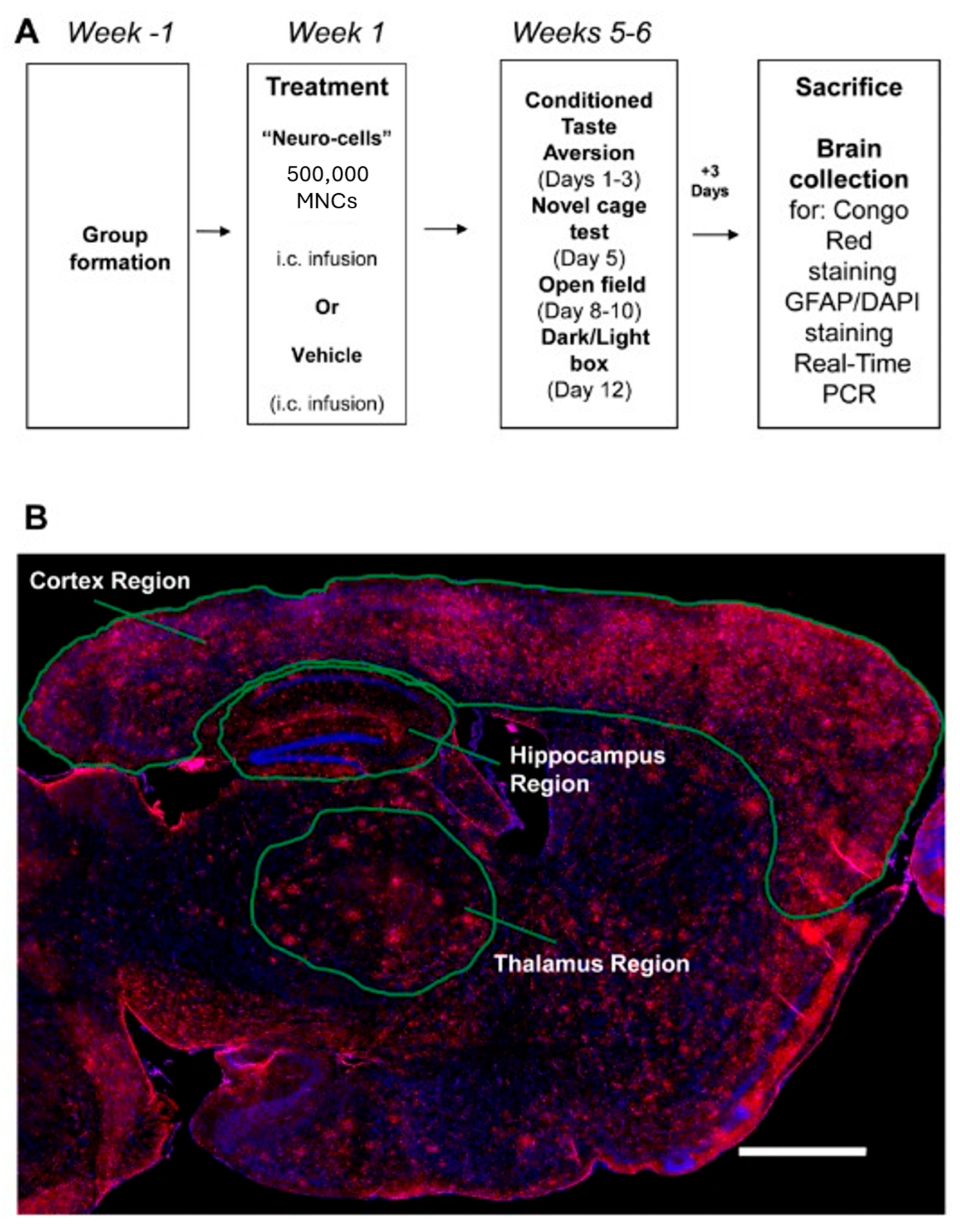
2.1. Animals and Study Design
2.2. Neuro-Cells Preparation
2.3. Stereotaxic Surgery and Administration of Neuro-Cells
2.4. Behavioral Assays for Learning and Emotionality
2.4.1. Conditioned Taste Aversion Learning
2.4.2. Novel Cage
2.4.3. Open Field Paradigm
2.4.4. Dark–Light Box
2.5. Tissue Collection and Brain Histology
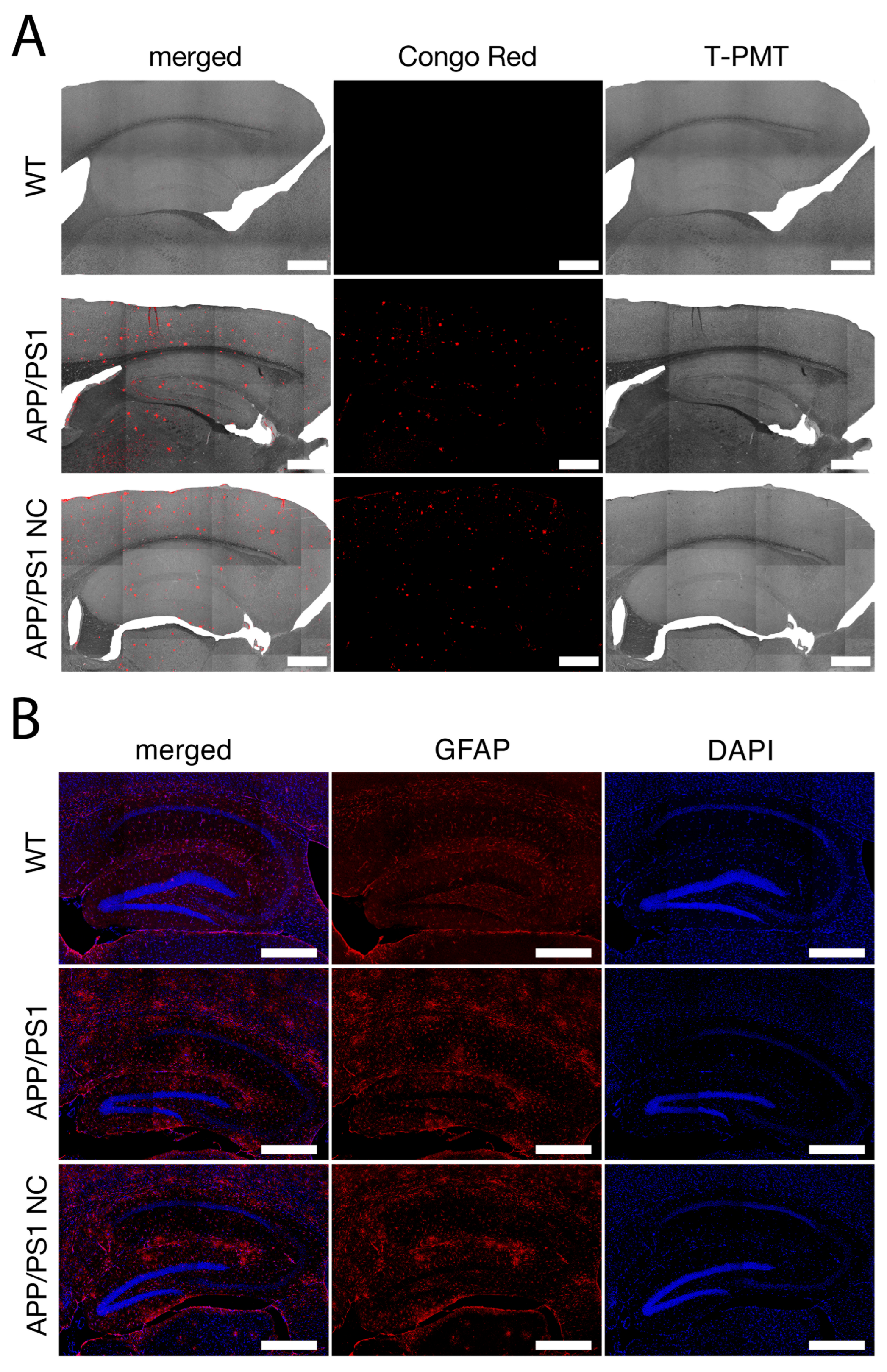
2.5.1. Congo Red Staining, Plaque Microscopy and Scoring
2.5.2. Immunohistochemical Analysis of Astrocyte Activation
2.6. Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Cell Culture Assay of Endotoxin-Induced IL-6 Release of Human Monocytes
2.8. Statistical Analysis
3. Results
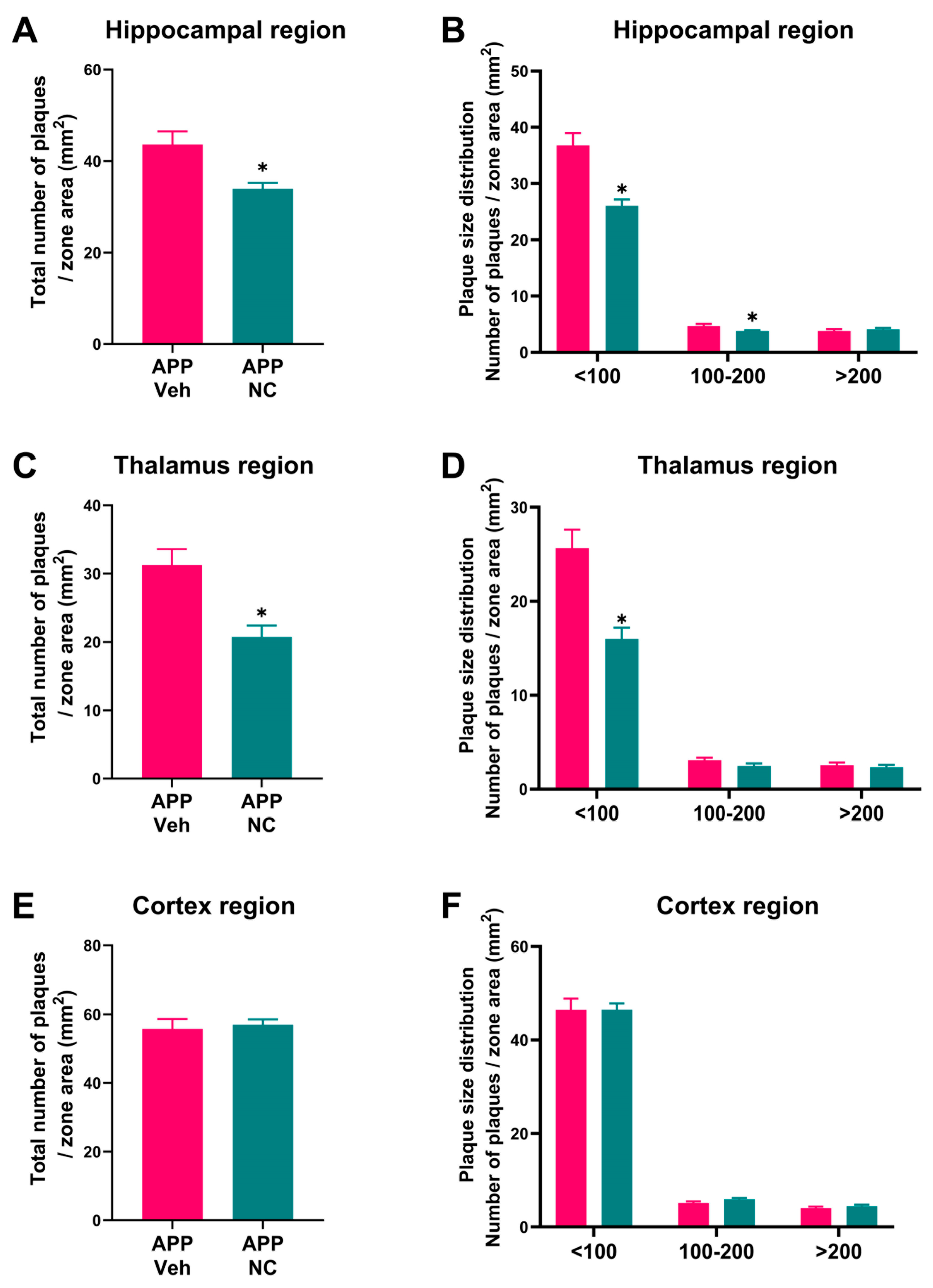
3.1. Reduced Amyloid Plaque Formation and Attenuated Astrogliosis in Neuro-Cells-Treated APP/PS1 Mice
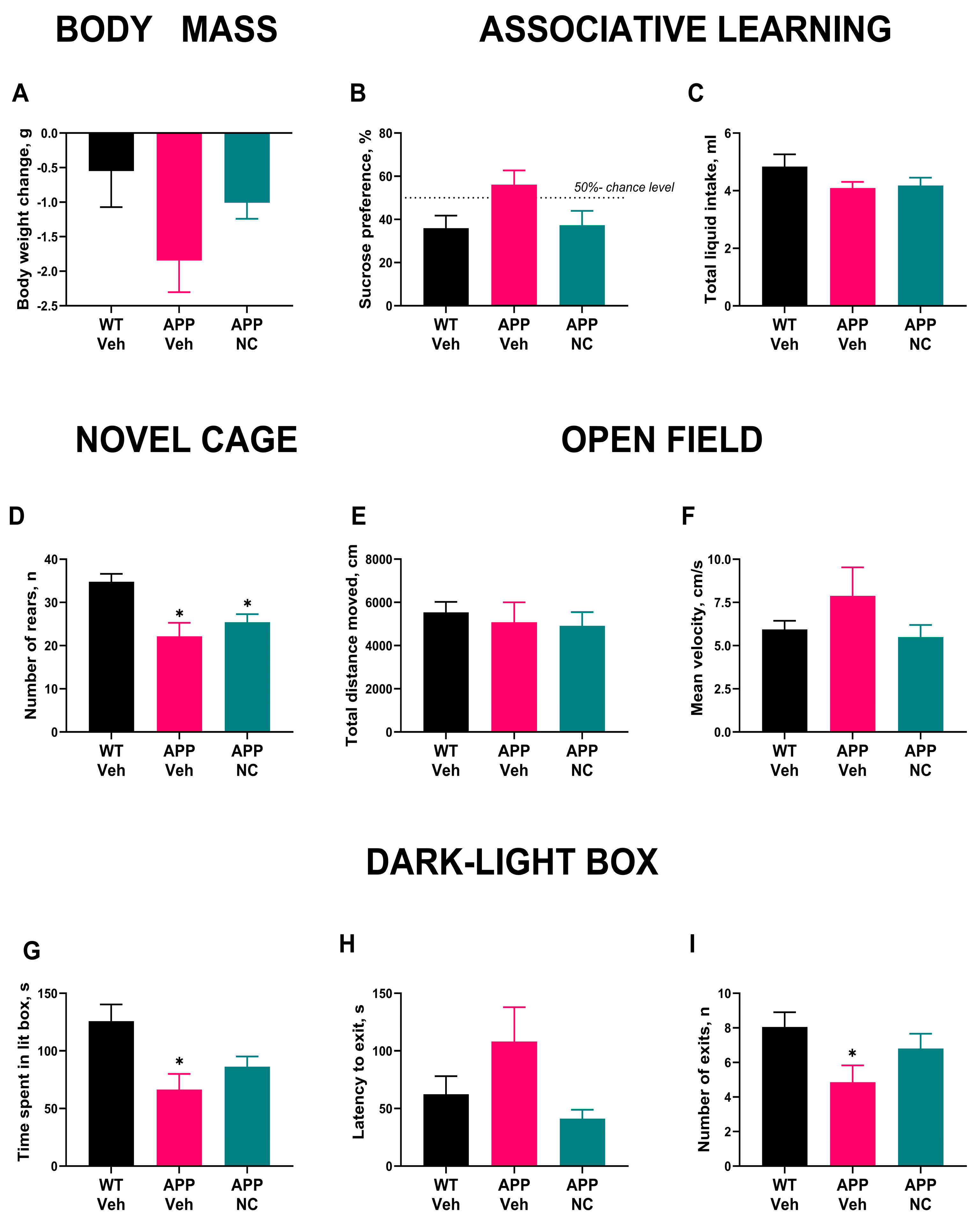
3.2. Administration of Neuro-Cells Improves Memory and Reduces Anxiety-like Behavior in APP/PS1 Mice
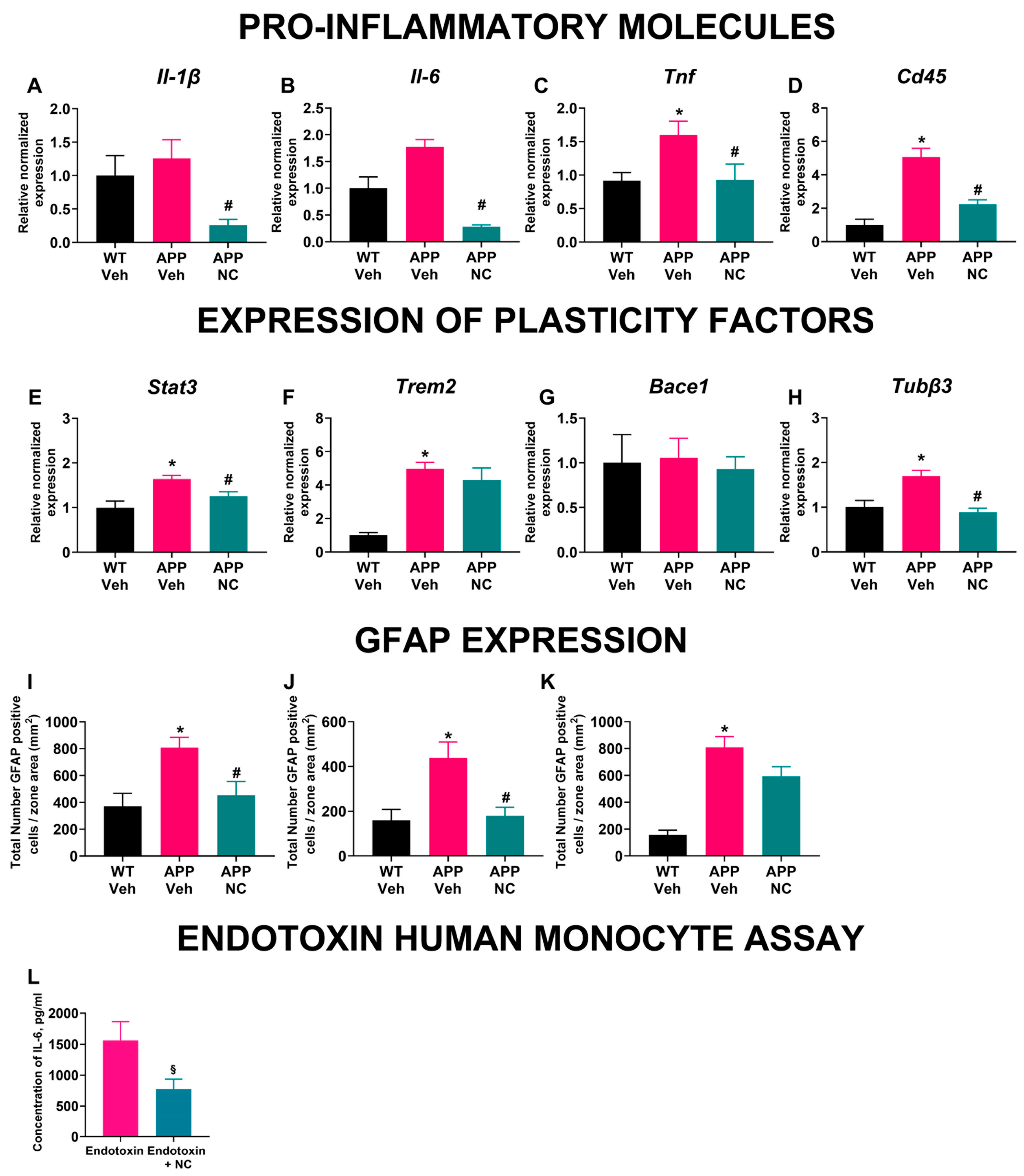
3.3. Neuro-Cells Treatment Ameliorates Inflammatory and Cellular Plasticity Marker Expression in the Prefrontal Cortex of APP/PS1 Mice
3.4. The Density of GFAP-Positive Cells Was Decreased in Neuro-Cells-Treated APP/PS1 Mice
3.5. Incubation with Neuro-Cells Diminished Endotoxin-Induced IL-6 Release of Human Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ANOVA | Analysis of Variance |
| APP | Amyloid precursor protein |
| Aβ | Amyloid beta |
| BACE1 | Beta-secretase 1 |
| BBB | Brain blood barrier |
| BDNF | Brain-derived neurotrophic factor |
| CD105+ | Cluster of Differentiation 105 positive |
| CD271+ | Cluster of Differentiation 271 positive |
| CD34+ | Cluster of Differentiation 34 positive |
| CD45 | Cluster of Differentiation 45 |
| CD73+ | Cluster of Differentiation 73 positive |
| CD90+ | Cluster of Differentiation 90 positive |
| cDNA | Complementary DNA |
| CNS | Central Nervous System |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DNA | Deoxyribonucleic acid |
| FACs | Fluorescence-activated cell sorting |
| FDTL | Fronto-temporal dementia |
| GFAP | Glial fibrillary acidic protein |
| HGF | Hepatocyte growth factor |
| HSCs | Hematopoietic stem cells |
| i.c. | Intracerebral |
| i.c.v. | Intracerebroventricular |
| IGF-1 | Insulin-like growth factor-1 |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1 beta |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| JAK | Janus kinases |
| MSCs | Mesenchymal stem cells |
| NC | Neuro-Cells |
| NGF | Neuronal growth factor |
| PBS | Phosphate-buffered saline |
| qRT-PCR | Real-time quantitative reverse transcription polymerase chain reaction |
| RNA | Ribonucleic acid |
| RRR | Replacement, Reduction and Refinement |
| RSE | Reference standard endotoxin |
| SEM | Standard error of the mean |
| STAT3 | Signal transducer of transcription factor-3 |
| TNF | Tumor necrosis factor |
| T-PMT | Transmitted light photomultiplier |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| Tubβ3 | Tubulin beta-3 chain |
| VEGF | Vascular endothelial growth factor |
| WT | Wild type |
References
- Beata, B.-K.; Wojciech, J.; Johannes, K.; Piotr, L.; Barbara, M. Alzheimer’s Disease—Biochemical and Psychological Background for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1059. [Google Scholar] [CrossRef]
- Folch, J.; Ettcheto, M.; Petrov, D.; Abad, S.; Pedrós, I.; Marin, M.; Olloquequi, J.; Camins, A. Una revisión de los avances en la terapéutica de la enfermedad de Alzheimer: Estrategia frente a la proteína β-amiloide. Neurología 2018, 33, 47–58. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Toral-Rios, D.; Patiño-López, G.; Gómez-Lira, G.; Gutiérrez, R.; Becerril-Pérez, F.; Rosales-Córdova, A.; León-Contreras, J.C.; Hernández-Pando, R.; León-Rivera, I.; Soto-Cruz, I.; et al. Activation of STAT3 Regulates Reactive Astrogliosis and Neuronal Death Induced by AβO Neurotoxicity. Int. J. Mol. Sci. 2020, 21, 7458. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Karima, S.; Rajaei, S.; Aghamolaii, V.; Ghahremani, H.; Ataeia, R.; Sepasi Tehrani, H.; Mahmoodi Baram, S.; Tafakhori, A.; Safarpour Lima, B.; et al. Plasma Cytokines Profile in Subjects with Alzheimer’s Disease: Interleukin 1 Alpha as a Candidate for Target Therapy. Int. J. Mol. Sci. 2021, 10, e1974. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wu, Y.; Xie, M.; Tao, S.; Lv, Q.; Wang, Q. Plasma IL-6 Levels and Their Association with Brain Health and Dementia Risk: A Population-Based Cohort Study. Brain Behav. Immun. 2024, 120, 430–438. [Google Scholar] [CrossRef]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s Disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Shaftel, S.S.; Griffin, W.S.T.; O’Banion, M.K. The Role of Interleukin-1 in Neuroinflammation and Alzheimer Disease: An Evolving Perspective. J. Neuroinflamm. 2008, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Lyra E Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.D.P.F.; Vieira, E.L.M.; Teixeira, A.L.; De Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-Inflammatory Interleukin-6 Signaling Links Cognitive Impairments and Peripheral Metabolic Alterations in Alzheimer’s Disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- Sakata, H.; Narasimhan, P.; Niizuma, K.; Maier, C.M.; Wakai, T.; Chan, P.H. Interleukin 6-Preconditioned Neural Stem Cells Reduce Ischaemic Injury in Stroke Mice. Brain 2012, 135, 3298–3310. [Google Scholar] [CrossRef]
- Zhang, S.; Danchuk, S.D.; Bonvillain, R.W.; Xu, B.; Scruggs, B.A.; Strong, A.L.; Semon, J.A.; Gimble, J.M.; Betancourt, A.M.; Sullivan, D.E.; et al. Interleukin 6 Mediates the Therapeutic Effects of Adipose-Derived Stromal/Stem Cells in Lipopolysaccharide-Induced Acute Lung Injury. Stem Cells 2014, 32, 1616–1628. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Andrianto, A.; Cempaka Putri, D.K.S.; Al Farabi, M.J.; Yusrizal, T.; Hermawan, H.O. Mesenchymal Stem Cell Therapy Efficacy in COVID-19 Patients: A Systematic Review and Meta-Analysis. F1000Research 2021, 10, 956. [Google Scholar] [CrossRef]
- de Munter, J.P.J.M.; Shafarevich, I.; Liundup, A.; Pavlov, D.; Wolters, E.C.; Gorlova, A.; Veniaminova, E.; Umriukhin, A.; Kalueff, A.; Svistunov, A.; et al. Neuro-Cells Therapy Improves Motor Outcomes and Suppresses Inflammation during Experimental Syndrome of Amyotrophic Lateral Sclerosis in Mice. CNS Neurosci. Ther. 2020, 26, 504–517. [Google Scholar] [CrossRef]
- de Munter, J.P.J.M.; Mey, J.; Strekalova, T.; Kramer, B.W.; Wolters, E.C. Why Do Anti-Inflammatory Signals of Bone Marrow-Derived Stromal Cells Improve Neurodegenerative Conditions Where Anti-Inflammatory Drugs Fail? J. Neural Transm. 2020, 127, 715–727. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.; Babaevskaya, D.; Wolters, E.C.; Pavlov, D.; Lysikova, E.; Kalueff, A.V.; Gorlova, A.; Oplatchikova, M.; Pomytkin, I.A.; Proshin, A.; et al. Molecular and Behavioural Abnormalities in the FUS-Tg Mice Mimic Frontotemporal Lobar Degeneration: Effects of Old and New Anti-Inflammatory Therapies. J. Cell. Mol. Med. 2020, 24, 10251–10257. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Sig. Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-mediated Astrogliosis Ameliorates Pathology in an Alzheimer’s Disease Model. EMBO Mol. Med. 2019, 11, e9665. [Google Scholar] [CrossRef]
- Rusek, M.; Smith, J.; El-Khatib, K.; Aikins, K.; Czuczwar, S.J.; Pluta, R. The Role of the JAK/STAT Signaling Pathway in the Pathogenesis of Alzheimer’s Disease: New Potential Treatment Target. Int. J. Mol. Sci. 2023, 24, 864. [Google Scholar] [CrossRef]
- Duly, A.M.P.; Kao, F.C.L.; Teo, W.S.; Kavallaris, M. βIII-Tubulin Gene Regulation in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 851542. [Google Scholar] [CrossRef]
- Yan, B.; Xie, S.; Liu, Z.; Luo, Y.; Zhou, J.; Li, D.; Liu, M. STAT3 Association with Microtubules and Its Activation Are Independent of HDAC6 Activity. DNA Cell Biol. 2015, 34, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Palencia, L.; Ramon-Duaso, C.; González-Parra, J.A.; Busquets-Garcia, A. Gene Expression Analysis of the Endocannabinoid System in Presymptomatic APP/PS1 Mice. Front. Pharmacol. 2022, 13, 864591. [Google Scholar] [CrossRef]
- Bivona, G.; Iemmolo, M.; Agnello, L.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V.; Scazzone, C.; Ghersi, G.; Ciaccio, M. Microglial Activation and Priming in Alzheimer’s Disease: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 884. [Google Scholar] [CrossRef]
- Varma, V.R.; Desai, R.J.; Navakkode, S.; Wong, L.-W.; Anerillas, C.; Loeffler, T.; Schilcher, I.; Mahesri, M.; Chin, K.; Horton, D.B.; et al. Hydroxychloroquine Lowers Alzheimer’s Disease and Related Dementias Risk and Rescues Molecular Phenotypes Related to Alzheimer’s Disease. Mol. Psychiatry 2023, 28, 1312–1326. [Google Scholar] [CrossRef]
- Cicognola, C.; Janelidze, S.; Hertze, J.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Plasma Glial Fibrillary Acidic Protein Detects Alzheimer Pathology and Predicts Future Conversion to Alzheimer Dementia in Patients with Mild Cognitive Impairment. Alzheimer’s Res. Ther. 2021, 13, 68. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.-A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yang, X.; Lan, Y.; Chen, G. The Neuro-Inflammatory Microenvironment: An Important Regulator of Stem Cell Survival in Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 98, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Chun, H.; Im, H.; Kang, Y.J.; Kim, Y.; Shin, J.H.; Won, W.; Lim, J.; Ju, Y.; Park, Y.M.; Kim, S.; et al. Severe Reactive Astrocytes Precipitate Pathological Hallmarks of Alzheimer’s Disease via H2O2− Production. Nat. Neurosci. 2020, 23, 1555–1566. [Google Scholar] [CrossRef]
- Morales, I.; Guzmán-Martà nez, L.; Cerda-Troncoso, C.; Farà as, G.A.; Maccioni, R.B. Neuroinflammation in the Pathogenesis of Alzheimer’s Disease. A Rational Framework for the Search of Novel Therapeutic Approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Orellana, D.I.; González-Billault, C.; Maccioni, R.B. Interleukin-6 Induces Alzheimer-Type Phosphorylation of Tau Protein by Deregulating the Cdk5/P35 Pathway. Exp. Cell Res. 2004, 295, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Oddo, S.; Yamasaki, T.R.; Green, K.N.; LaFerla, F.M. Lipopolysaccharide-Induced Inflammation Exacerbates Tau Pathology by a Cyclin-Dependent Kinase 5-Mediated Pathway in a Transgenic Model of Alzheimer’s Disease. J. Neurosci. 2005, 25, 8843–8853. [Google Scholar] [CrossRef]
- Li, S.; Mallory, M.; Alford, M.; Tanaka, S.; Masliah, E. Glutamate Transporter Alterations in Alzheimer Disease Are Possibly Associated with Abnormal APP Expression. J. Neuropathol. Exp. Neurol. 1997, 56, 901–911. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, Y.R.; Ong, L.; Gold, M.; Kalali, A.; Sarkar, J. Alzheimer’s Disease: Key Insights from Two Decades of Clinical Trial Failures. J. Alzheimer’s Dis. 2022, 87, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-Inflammatories in Alzheimer’s Disease—Potential Therapy or Spurious Correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-N.; Kim, J.-H.; Lim, T.S.; Park, S.H.; Kim, T.-G.; Yoon, B.S.; Son, K.S.; Yoon, J.-K.; An, Y.-S. Therapeutic Effect of Mesenchymal Stem Cells in an Animal Model of Alzheimer’s Disease Evaluated by β-Amyloid Positron Emission Tomography Imaging. Aust. New Zealand J. Psychiatry 2020, 54, 883–891. [Google Scholar] [CrossRef]
- Oliva, A.A.; McClain-Moss, L.; Pena, A.; Drouillard, A.; Hare, J.M. Allogeneic Mesenchymal Stem Cell Therapy: A Regenerative Medicine Approach to Geroscience. Aging Med. 2019, 2, 142–146. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Skok, M. Mesenchymal Stem Cells as a Potential Therapeutic Tool to Cure Cognitive Impairment Caused by Neuroinflammation. World J. Stem Cells 2021, 13, 1072–1083. [Google Scholar] [CrossRef]
- Brody, M.; Agronin, M.; Herskowitz, B.J.; Bookheimer, S.Y.; Small, G.W.; Hitchinson, B.; Ramdas, K.; Wishard, T.; McInerney, K.F.; Vellas, B.; et al. Results and Insights from a Phase I Clinical Trial of Lomecel-B for Alzheimer’s Disease. Alzheimer’s Dement. 2023, 19, 261–273. [Google Scholar] [CrossRef]
- Kshitiz; Ellison, D.D.; Suhail, Y.; Afzal, J.; Woo, L.; Kilic, O.; Spees, J.; Levchenko, A. Dynamic Secretome of Bone Marrow-Derived Stromal Cells Reveals a Cardioprotective Biochemical Cocktail. Proc. Natl. Acad. Sci. USA 2019, 116, 14374–14383. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 Primes Human Mesenchymal Stem Cells towards an Anti-Inflammatory and pro-Trophic Phenotype in Vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef]
- Baumheter, S.; Singer, M.S.; Henzel, W.; Hemmerich, S.; Renz, M.; Rosen, S.D.; Lasky, L.A. Binding of L-Selectin to the Vascular Sialomucin CD34. Science 1993, 262, 436–438. [Google Scholar] [CrossRef]
- Krizanac-Bengez, L.; Mayberg, M.R.; Janigro, D. The Cerebral Vasculature as a Therapeutic Target for Neurological Disorders and the Role of Shear Stress in Vascular Homeostatis and Pathophysiology. Neurol. Res. 2004, 26, 846–853. [Google Scholar] [CrossRef]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Jablonska, A.; Seta, M.; Dabrowska, S.; Walczak, P.; Janowski, M.; Lukomska, B. Labeling of Human Mesenchymal Stem Cells with Different Classes of Vital Stains: Robustness and Toxicity. Stem Cell Res. Ther. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Munter, J.P.D.; Beugels, J.; Munter, S.; Jansen, L.; Cillero-Pastor, B.; Moskvin, O.; Brook, G.; Pavlov, D.; Strekalova, T.; Kramer, B.W.; et al. Standardized Human Bone Marrow-Derived Stem Cells Infusion Improves Survival and Recovery in a Rat Model of Spinal Cord Injury. J. Neurol. Sci. 2019, 402, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Barisone, G.A.; Diaz, E.; Jin, L.; DeCarli, C.; Despa, F. Amylin Deposition in the Brain: A Second Amyloid in Alzheimer Disease? Ann. Neurol. 2013, 74, 517–526. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of Class 1 Histone Deacetylases Reverse Contextual Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef]
- Onos, K.D.; Uyar, A.; Keezer, K.J.; Jackson, H.M.; Preuss, C.; Acklin, C.J.; O’Rourke, R.; Buchanan, R.; Cossette, T.L.; Sukoff Rizzo, S.J.; et al. Enhancing Face Validity of Mouse Models of Alzheimer’s Disease with Natural Genetic Variation. PLoS Genet. 2019, 15, e1008155. [Google Scholar] [CrossRef]
- Von Linstow, C.U.; Waider, J.; Bergh, M.S.-S.; Anzalone, M.; Madsen, C.; Nicolau, A.B.; Wirenfeldt, M.; Lesch, K.-P.; Finsen, B. The Combined Effects of Amyloidosis and Serotonin Deficiency by Tryptophan Hydroxylase-2 Knockout Impacts Viability of the APP/PS1 Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 85, 1283–1300. [Google Scholar] [CrossRef]
- Carroll, J.C.; Rosario, E.R.; Kreimer, S.; Villamagna, A.; Gentzschein, E.; Stanczyk, F.Z.; Pike, C.J. Sex Differences in β-Amyloid Accumulation in 3xTg-AD Mice: Role of Neonatal Sex Steroid Hormone Exposure. Brain Res. 2010, 1366, 233–245. [Google Scholar] [CrossRef]
- Zhao, L.; Woody, S.K.; Chhibber, A. Estrogen Receptor β in Alzheimer’s Disease: From Mechanisms to Therapeutics. Ageing Res. Rev. 2015, 24, 178–190. [Google Scholar] [CrossRef]
- Jiao, S.-S.; Bu, X.-L.; Liu, Y.-H.; Zhu, C.; Wang, Q.-H.; Shen, L.-L.; Liu, C.-H.; Wang, Y.-R.; Yao, X.-Q.; Wang, Y.-J. Sex Dimorphism Profile of Alzheimer’s Disease-Type Pathologies in an APP/PS1 Mouse Model. Neurotox. Res. 2016, 29, 256–266. [Google Scholar] [CrossRef]
- Strekalova, T.; Steinbusch, H.W.M. Measuring Behavior in Mice with Chronic Stress Depression Paradigm. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef]
- Strekalova, T. Optimization of the Chronic Stress Depression Model in C57 BL/6 Mice: Evidences for Improved Validity. In Behavioral Models in Stress Research; Kalueff, A.V., LaPorte, J.L., Eds.; Nova Biomedical Books: New York, NY, USA, 2008; pp. 111–157. ISBN 1-60456-361-3. [Google Scholar]
- Vignisse, J.; Sambon, M.; Gorlova, A.; Pavlov, D.; Caron, N.; Malgrange, B.; Shevtsova, E.; Svistunov, A.; Anthony, D.C.; Markova, N.; et al. Thiamine and Benfotiamine Prevent Stress-Induced Suppression of Hippocampal Neurogenesis in Mice Exposed to Predation without Affecting Brain Thiamine Diphosphate Levels. Mol. Cell. Neurosci. 2017, 82, 126–136. [Google Scholar] [CrossRef]
- Vignisse, J.; Steinbusch, H.W.M.; Grigoriev, V.; Bolkunov, A.; Proshin, A.; Bettendorff, L.; Bachurin, S.; Strekalova, T. Concomitant Manipulation of Murine NMDA- and AMPA-Receptors to Produce pro-Cognitive Drug Effects in Mice. Eur. Neuropsychopharmacol. 2014, 24, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Veniaminova, E.; Cespuglio, R.; Cheung, C.W.; Umriukhin, A.; Markova, N.; Shevtsova, E.; Lesch, K.-P.; Anthony, D.C.; Strekalova, T. Autism-Like Behaviours and Memory Deficits Result from a Western Diet in Mice. Neural Plast. 2017, 2017, 9498247. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Bahzenova, N.; Trofimov, A.; Schmitt-Böhrer, A.G.; Markova, N.; Grigoriev, V.; Zamoyski, V.; Serkova, T.; Redkozubova, O.; Vinogradova, D.; et al. Pro-Neurogenic, Memory-Enhancing and Anti-Stress Effects of DF302, a Novel Fluorine Gamma-Carboline Derivative with Multi-Target Mechanism of Action. Mol. Neurobiol. 2018, 55, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial Activation, Increased TNF and SERT Expression in the Prefrontal Cortex Define Stress-Altered Behaviour in Mice Susceptible to Anhedonia. Brain Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Semakov, A.; Tsypyshev, D.; Chaprov, K.; Klochkov, S.; Neganova, M. Neuroprotective Effects and Cognitive Enhancement of Allomargaritarine in 5xFAD Alzheimer’s Disease Mice Model. OBM Neurobiol. 2024, 8, 207. [Google Scholar] [CrossRef]
- Bély, M.; Makovitzky, J. Sensitivity and Specificity of Congo Red Staining According to Romhányi. Comparison with Puchtler’s or Bennhold’s Methods. Acta Histochem. 2006, 108, 175–180. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- De Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.V.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased Oxidative Stress in the Prefrontal Cortex as a Shared Feature of Depressive- and PTSD-Like Syndromes: Effects of a Standardized Herbal Antioxidant. Front. Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef]
- Schapovalova, O.; Gorlova, A.; De Munter, J.; Sheveleva, E.; Eropkin, M.; Gorbunov, N.; Sicker, M.; Umriukhin, A.; Lyubchyk, S.; Lesch, K.-P.; et al. Immunomodulatory Effects of New Phytotherapy on Human Macrophages and TLR4- and TLR7/8-Mediated Viral-like Inflammation in Mice. Front. Med. 2022, 9, 952977. [Google Scholar] [CrossRef] [PubMed]
- Van Der Poll, T.; Van De Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The Immunopathology of Sepsis and Potential Therapeutic Targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Jaimes, Y.; Naaldijk, Y.; Wenk, K.; Leovsky, C.; Emmrich, F. Mesenchymal Stem Cell-Derived Microvesicles Modulate Lipopolysaccharides-Induced Inflammatory Responses to Microglia Cells. Stem Cells 2017, 35, 812–823. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in Sepsis: Potent Immunoregulators and Potential Therapeutic Targets—An Updated View. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 Is an Antiinflammatory Cytokine Required for Controlling Local or Systemic Acute Inflammatory Responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, I.; Reversé, D.; Caluwaerts, N.; Ris, L.; Kuipéri, C.; Van Den Haute, C.; Spittaels, K.; Umans, L.; Serneels, L.; Thiry, E.; et al. Neuronal Deficiency of Presenilin 1 Inhibits Amyloid Plaque Formation and Corrects Hippocampal Long-Term Potentiation But Not a Cognitive Defect of Amyloid Precursor Protein [V717I] Transgenic Mice. J. Neurosci. 2002, 22, 3445–3453. [Google Scholar] [CrossRef]
- Cacquevel, M.; Lebeurrier, N.; Cheenne, S.; Vivien, D. Cytokines in Neuroinflammation and Alzheimers Disease. Curr. Drug Targets 2004, 5, 529–534. [Google Scholar] [CrossRef]
- Oh, J.Y.; Ko, J.H.; Lee, H.J.; Yu, J.M.; Choi, H.; Kim, M.K.; Wee, W.R.; Prockop, D.J. Mesenchymal Stem/Stromal Cells Inhibit the NLRP3 Inflammasome by Decreasing Mitochondrial Reactive Oxygen Species. Stem Cells 2014, 32, 1553–1563. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Kim, H.O.; Choi, S.-M.; Kim, H.-S. Mesenchymal Stem Cell-Derived Secretome and Microvesicles as a Cell-Free Therapeutics for Neurodegenerative Disorders. Tissue Eng. Regen. Med. 2013, 10, 93–101. [Google Scholar] [CrossRef]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.B.; et al. Transplantation of Mesenchymal Stem Cells Promotes an Alternative Pathway of Macrophage Activation and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Kim, H.-S.; Choi, D.-Y.; Yun, S.J.; Choi, S.-M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.-W. Proteomic Analysis of Microvesicles Derived from Human Mesenchymal Stem Cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef]
- Schindler, C.; Levy, D.E.; Decker, T. JAK-STAT Signaling: From Interferons to Cytokines. J. Biol. Chem. 2007, 282, 20059–20063. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional Control and Biological Impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Naaldijk, Y.; Jäger, C.; Fabian, C.; Leovsky, C.; Blüher, A.; Rudolph, L.; Hinze, A.; Stolzing, A. Effect of Systemic Transplantation of Bone Marrow-derived Mesenchymal Stem Cells on Neuropathology Markers in APP/PS 1 Alzheimer Mice. Neuropathol. Appl. Neurobiol. 2017, 43, 299–314. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Morgan, D.; Zhao, L.-R. Reparative Effects of Stem Cell Factor and Granulocyte Colony-Stimulating Factor in Aged APP/PS1 Mice. Aging Dis. 2020, 11, 1423. [Google Scholar] [CrossRef]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. STAT3 Is a Critical Regulator of Astrogliosis and Scar Formation after Spinal Cord Injury. J. Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef]
- Zhu, Y.; Hou, H.; Rezai-Zadeh, K.; Giunta, B.; Ruscin, A.; Gemma, C.; Jin, J.; Dragicevic, N.; Bradshaw, P.; Rasool, S.; et al. CD45 Deficiency Drives Amyloid-β Peptide Oligomers and Neuronal Loss in Alzheimer’s Disease Mice. J. Neurosci. 2011, 31, 1355–1365. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.-L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Piña-Crespo, J.C.; Zhang, M.; et al. TREM2 Is a Receptor for β-Amyloid That Mediates Microglial Function. Neuron 2018, 97, 1023–1031. [Google Scholar] [CrossRef]
- Li, R.-Y.; Qin, Q.; Yang, H.-C.; Wang, Y.-Y.; Mi, Y.-X.; Yin, Y.-S.; Wang, M.; Yu, C.-J.; Tang, Y. TREM2 in the Pathogenesis of AD: A Lipid Metabolism Regulator and Potential Metabolic Therapeutic Target. Mol. Neurodegener. 2022, 17, 40. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The Alzheimer’s Disease Beta-Secretase Enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef]
- Li, J.Z.; Ramalingam, N.; Li, S. Targeting Epigenetic Mechanisms in Amyloid-β–Mediated Alzheimer’s Pathophysiology: Unveiling Therapeutic Potential. Neural Regen. Res. 2025, 20, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Suelves, N.; Perrin, F.; Vadukul, D.M.; Vrancx, C.; Constantinescu, S.N.; Kienlen-Campard, P. Structural Determinant of β-Amyloid Formation: From Transmembrane Protein Dimerization to β-Amyloid Aggregates. Biomedicines 2022, 10, 2753. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Janus, C.; Welzl, H.; Hanna, A.; Lovasic, L.; Lane, N.; St George-Hyslop, P.; Westaway, D. Impaired Conditioned Taste Aversion Learning in APP Transgenic Mice. Neurobiol. Aging 2004, 25, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Rattoni, F. The Forgotten Insular Cortex: Its Role on Recognition Memory Formation. Neurobiol. Learn. Mem. 2014, 109, 207–216. [Google Scholar] [CrossRef]
- Gallo, M.; Cándido, A. Dorsal Hippocampal Lesions Impair Blocking but Not Latent Inhibition of Taste Aversion Learning in Rats. Behav. Neurosci. 1995, 109, 413–425. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Herrmann, F.R.; Bussière, T.; Bouras, C.; Kövari, E.; Perl, D.P.; Morrison, J.H.; Gold, G.; Hof, P.R. Tangle and Neuron Numbers, but Not Amyloid Load, Predict Cognitive Status in Alzheimer’s Disease. Neurology 2003, 60, 1495–1500. [Google Scholar] [CrossRef]
- Ingelsson, M.; Fukumoto, H.; Newell, K.L.; Growdon, J.H.; Hedley–Whyte, E.T.; Frosch, M.P.; Albert, M.S.; Hyman, B.T.; Irizarry, M.C. Early Aβ Accumulation and Progressive Synaptic Loss, Gliosis, and Tangle Formation in AD Brain. Neurology 2004, 62, 925–931. [Google Scholar] [CrossRef]
- Calhoon, G.G.; Tye, K.M. Resolving the Neural Circuits of Anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, Y.; Hao, Q.; Zhao, Y.; Dong, B. Effects of Mesenchymal Stem Cells Transplantation on Cognitive Deficits in Animal Models of Alzheimer’s Disease: A Systematic Review and Meta-analysis. Brain Behav. 2018, 8, e00982. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.F.; Camargo, C.; Premer, C.; Hare, J.M.; Baumel, B.S.; Pinto, M. Intravenous Administration of Mesenchymal Stem Cells Reduces Tau Phosphorylation and Inflammation in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Exp. Neurol. 2021, 341, 113706. [Google Scholar] [CrossRef]
- Qin, C.; Lu, Y.; Wang, K.; Bai, L.; Shi, G.; Huang, Y.; Li, Y. Transplantation of Bone Marrow Mesenchymal Stem Cells Improves Cognitive Deficits and Alleviates Neuropathology in Animal Models of Alzheimer’s Disease: A Meta-Analytic Review on Potential Mechanisms. Transl. Neurodegener. 2020, 9, 20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Munter, J.; Chaprov, K.; Lang, E.; Sitdikova, K.; Wolters, E.C.; Svirin, E.; Kassenova, A.; Tsoy, A.; Kramer, B.W.; Askarova, S.; et al. Neuro-Cells Mitigate Amyloid Plaque Formation and Behavioral Deficits in the APPswe/PS1dE9 Model of Alzheimer Disease While Also Reducing IL-6 Production in Human Monocytes. Cells 2025, 14, 1168. https://doi.org/10.3390/cells14151168
de Munter J, Chaprov K, Lang E, Sitdikova K, Wolters EC, Svirin E, Kassenova A, Tsoy A, Kramer BW, Askarova S, et al. Neuro-Cells Mitigate Amyloid Plaque Formation and Behavioral Deficits in the APPswe/PS1dE9 Model of Alzheimer Disease While Also Reducing IL-6 Production in Human Monocytes. Cells. 2025; 14(15):1168. https://doi.org/10.3390/cells14151168
Chicago/Turabian Stylede Munter, Johannes, Kirill Chaprov, Ekkehard Lang, Kseniia Sitdikova, Erik Ch. Wolters, Evgeniy Svirin, Aliya Kassenova, Andrey Tsoy, Boris W. Kramer, Sholpan Askarova, and et al. 2025. "Neuro-Cells Mitigate Amyloid Plaque Formation and Behavioral Deficits in the APPswe/PS1dE9 Model of Alzheimer Disease While Also Reducing IL-6 Production in Human Monocytes" Cells 14, no. 15: 1168. https://doi.org/10.3390/cells14151168
APA Stylede Munter, J., Chaprov, K., Lang, E., Sitdikova, K., Wolters, E. C., Svirin, E., Kassenova, A., Tsoy, A., Kramer, B. W., Askarova, S., Schroeter, C. A., Anthony, D. C., & Strekalova, T. (2025). Neuro-Cells Mitigate Amyloid Plaque Formation and Behavioral Deficits in the APPswe/PS1dE9 Model of Alzheimer Disease While Also Reducing IL-6 Production in Human Monocytes. Cells, 14(15), 1168. https://doi.org/10.3390/cells14151168




