Yin Yang 1: Role in Leishmaniasis
Abstract
1. Introduction
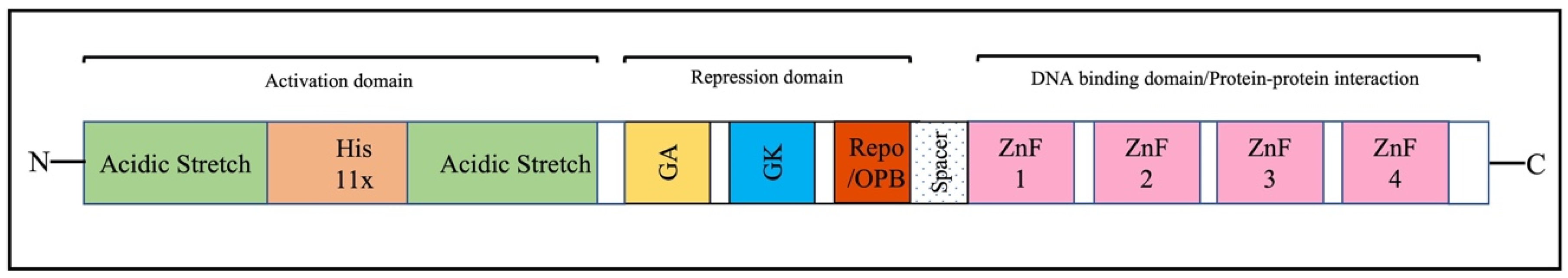
2. Overview of the Structure and Function of YY1
3. Regulation of YY1 Activity
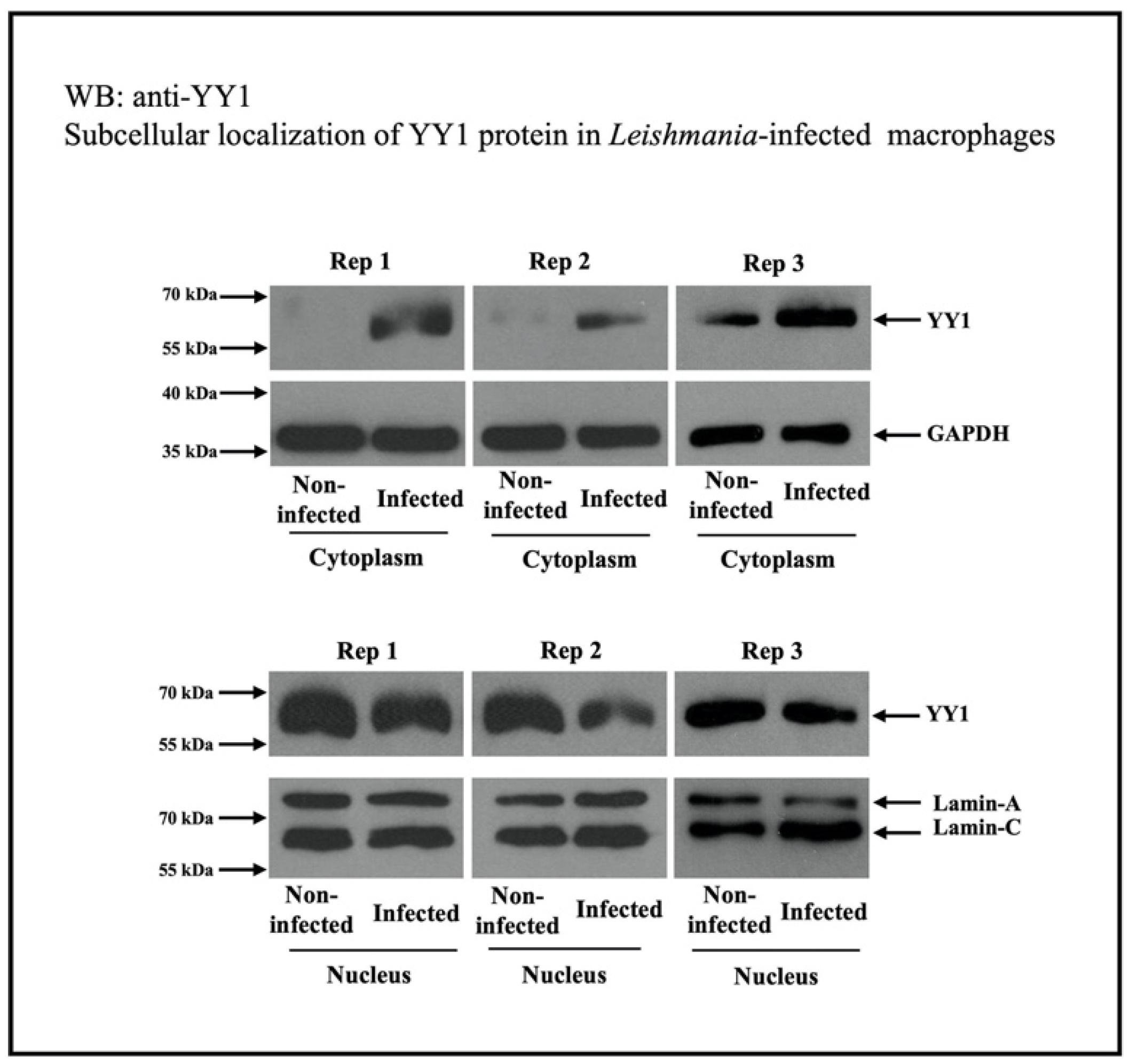
4. Subcellular Localization of YY1
5. YY1 Protein Relevance to Leishmaniasis
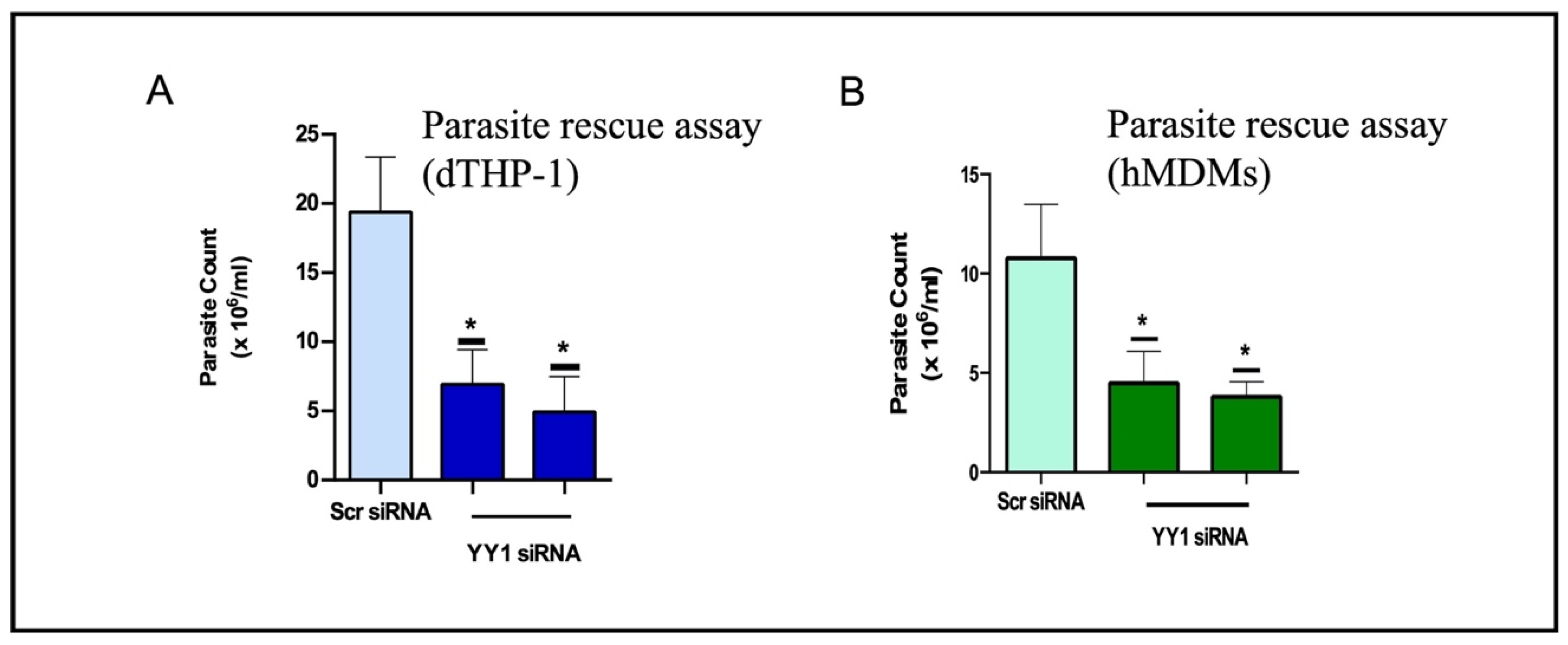
5.1. Role of YY1 in Leishmania Perseverance
5.2. Regulation of Macrophage YY1 Protein by Leishmania
5.3. Characterization of the Global Proteome Reveals Host Macrophage Proteins Modulated by Leishmania and Dependent on YY1
5.4. Comparative Proteome Analysis of YY1 Knockdown and Normal THP-1 Cells
6. Concluding Remarks
7. Unresolved Outstanding Questions
- -
- This study has evaluated the role of macrophage YY1 in Leishmania’s optimal survival. However, it is not known how Leishmania regulates YY1 to ensure its survival. As discussed in Section 3, post-translational modifications such as phosphorylation, acetylation, and others influence YY1 function. It would be interesting to examine the potential role of PTMs of YY1 during Leishmania infection.
- -
- Although it is clear that Leishmania induces the translocation of YY1 from its primary location in the nucleus to the cytoplasm of infected cells, the mechanism behind this process remains unknown, as does the question of whether Leishmania molecules contribute to triggering the translocation.
- -
- This study was conducted using monocytic THP-1 cells and a single species of Leishmania (L. donovani). It will be interesting to validate the key findings of this study using primary human macrophages. Using other Leishmania species, such as L. major and L. mexicana, which result in different disease manifestations, will shed light on whether the regulation of YY1 is a central strategy utilized by other Leishmania species.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gebremichael, D. Zoonotic Impact and Epidemiological Changes of Leishmaniasis in Ethiopia. Open Vet. J. 2018, 8, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, D.-A.; Rivero-Zambrano, L.; Martínez-Juárez, L.-A.; García-Rodríguez-Arana, R. Overcoming the Global Burden of Neglected Tropical Diseases. Ther. Adv. Infect. Dis. 2020, 7, 2049936120966449. [Google Scholar] [CrossRef] [PubMed]
- Kmetiuk, L.B.; Tirado, T.C.; Biondo, L.M.; Biondo, A.W.; Figueiredo, F.B. Leishmania Spp. in Indigenous Populations: A Mini-Review. Front. Public Health 2022, 10, 1033803. [Google Scholar] [CrossRef] [PubMed]
- Herricks, J.R.; Hotez, P.J.; Wanga, V.; Coffeng, L.E.; Haagsma, J.A.; Basáñez, M.-G.; Buckle, G.; Budke, C.M.; Carabin, H.; Fèvre, E.M.; et al. The Global Burden of Disease Study 2013: What Does It Mean for the NTDs? PLoS Negl. Trop. Dis. 2017, 11, e0005424. [Google Scholar] [CrossRef]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. Soc. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef]
- Steverding, D. The History of Leishmaniasis. Parasit. Vectors 2017, 10, 82. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional Regulation of Macrophage Polarization: Enabling Diversity with Identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Ostareck, D.H.; Ostareck-Lederer, A. RNA-Binding Proteins in the Control of LPS-Induced Macrophage Response. Front. Genet. 2019, 10, 31. [Google Scholar] [CrossRef]
- Sprenkle, N.T.; Serezani, C.H.; Pua, H.H. MicroRNAs in Macrophages: Regulators of Activation and Function. J. Immunol. 2023, 210, 359–368. [Google Scholar] [CrossRef]
- Han, Z.; Shen, Y.; Yan, Y.; Bin, P.; Zhang, M.; Gan, Z. Metabolic Reprogramming Shapes Post-Translational Modification in Macrophages. Mol. Asp. Med. 2025, 102, 101338. [Google Scholar] [CrossRef]
- Bichiou, H.; Bouabid, C.; Rabhi, I.; Guizani-Tabbane, L. Transcription Factors Interplay Orchestrates the Immune-Metabolic Response of Leishmania Infected Macrophages. Front. Cell. Infect. Microbiol. 2021, 11, 660415. [Google Scholar] [CrossRef]
- Colineau, L.; Lambertz, U.; Fornes, O.; Wasserman, W.W.; Reiner, N.E. C-Myc Is a Novel Leishmania Virulence Factor by Proxy That Targets the Host miRNA System and Is Essential for Survival in Human Macrophages. J. Biol. Chem. 2018, 293, 12805–12819. [Google Scholar] [CrossRef] [PubMed]
- Brar, H.K.; Chen, E.; Chang, F.; Lu, S.A.; Longowal, D.K.; Moon, K.-M.; Foster, L.J.; Reiner, N.; Nandan, D. Leishmania Regulates Host YY1: Comparative Proteomic Analysis Identifies Infection Modulated YY1 Dependent Proteins. PLoS ONE 2025, 20, e0323227. [Google Scholar] [CrossRef] [PubMed]
- Nandan, D.; Rath, C.T.; Reiner, N.E. Leishmania Regulates Host Macrophage miRNAs Expression by Engaging Transcription Factor C-Myc. J. Leukoc. Biol. 2021, 109, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Petkova, V.; Romanowski, M.J.; Sulijoadikusumo, I.; Rohne, D.; Kang, P.; Shenk, T.; Usheva, A. Interaction between YY1 and the Retinoblastoma Protein. Regulation of Cell Cycle Progression in Differentiated Cells. J. Biol. Chem. 2001, 276, 7932–7936. [Google Scholar] [CrossRef]
- Affar, E.B.; Gay, F.; Shi, Y.; Liu, H.; Huarte, M.; Wu, S.; Collins, T.; Li, E.; Shi, Y. Essential Dosage-Dependent Functions of the Transcription Factor Yin Yang 1 in Late Embryonic Development and Cell Cycle Progression. Mol. Cell. Biol. 2006, 26, 3565–3581. [Google Scholar] [CrossRef]
- Kurisaki, K.; Kurisaki, A.; Valcourt, U.; Terentiev, A.A.; Pardali, K.; Ten Dijke, P.; Heldin, C.-H.; Ericsson, J.; Moustakas, A. Nuclear Factor YY1 Inhibits Transforming Growth Factor Beta- and Bone Morphogenetic Protein-Induced Cell Differentiation. Mol. Cell. Biol. 2003, 23, 4494–4510. [Google Scholar] [CrossRef]
- Wu, S.; Shi, Y.; Mulligan, P.; Gay, F.; Landry, J.; Liu, H.; Lu, J.; Qi, H.H.; Wang, W.; Nickoloff, J.A.; et al. A YY1-INO80 Complex Regulates Genomic Stability through Homologous Recombination-Based Repair. Nat. Struct. Mol. Biol. 2007, 14, 1165–1172. [Google Scholar] [CrossRef]
- Sui, G.; Affar, E.B.; Shi, Y.; Brignone, C.; Wall, N.R.; Yin, P.; Donohoe, M.; Luke, M.P.; Calvo, D.; Grossman, S.R.; et al. Yin Yang 1 Is a Negative Regulator of P53. Cell 2004, 117, 859–872. [Google Scholar] [CrossRef]
- Sarvagalla, S.; Kolapalli, S.P.; Vallabhapurapu, S. The Two Sides of YY1 in Cancer: A Friend and a Foe. Front. Oncol. 2019, 9, 1230. [Google Scholar] [CrossRef]
- Khachigian, L.M. The Yin and Yang of YY1 in Tumor Growth and Suppression. Int. J. Cancer 2018, 143, 460–465. [Google Scholar] [CrossRef]
- Meliala, I.T.S.; Hosea, R.; Kasim, V.; Wu, S. The Biological Implications of Yin Yang 1 in the Hallmarks of Cancer. Theranostics 2020, 10, 4183–4200. [Google Scholar] [CrossRef]
- Shi, Y.; Seto, E.; Chang, L.S.; Shenk, T. Transcriptional Repression by YY1, a Human GLI-Krüppel-Related Protein, and Relief of Repression by Adenovirus E1A Protein. Cell 1991, 67, 377–388. [Google Scholar] [CrossRef]
- Park, K.; Atchison, M.L. Isolation of a Candidate Repressor/Activator, NF-E1 (YY-1, Delta), That Binds to the Immunoglobulin Kappa 3′ Enhancer and the Immunoglobulin Heavy-Chain Mu E1 Site. Proc. Natl. Acad. Sci. USA 1991, 88, 9804–9808. [Google Scholar] [CrossRef]
- Hariharan, N.; Kelley, D.E.; Perry, R.P. Delta, a Transcription Factor That Binds to Downstream Elements in Several Polymerase II Promoters, Is a Functionally Versatile Zinc Finger Protein. Proc. Natl. Acad. Sci. USA 1991, 88, 9799–9803. [Google Scholar] [CrossRef]
- Flanagan, J.R.; Becker, K.G.; Ennist, D.L.; Gleason, S.L.; Driggers, P.H.; Levi, B.Z.; Appella, E.; Ozato, K. Cloning of a Negative Transcription Factor That Binds to the Upstream Conserved Region of Moloney Murine Leukemia Virus. Mol. Cell. Biol. 1992, 12, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Odgren, P.R.; van Wijnen, A.J.; Last, T.J.; Nickerson, J.; Penman, S.; Lian, J.B.; Stein, J.L.; Stein, G.S. The Nuclear Matrix Protein NMP-1 Is the Transcription Factor YY1. Proc. Natl. Acad. Sci. USA 1995, 92, 10526–10530. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lee, A.S. YY1 as a Regulator of Replication-Dependent Hamster Histone H3.2 Promoter and an Interactive Partner of AP-2. J. Biol. Chem. 2001, 276, 28–34. [Google Scholar] [CrossRef]
- Last, T.J.; van Wijnen, A.J.; Birnbaum, M.J.; Stein, G.S.; Stein, J.L. Multiple Interactions of the Transcription Factor YY1 with Human Histone H4 Gene Regulatory Elements. J. Cell. Biochem. 1999, 72, 507–516. [Google Scholar] [CrossRef]
- He, Y.; Casaccia-Bonnefil, P. The Yin and Yang of YY1 in the Nervous System. J. Neurochem. 2008, 106, 1493–1502. [Google Scholar] [CrossRef]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription Factor YY1: Structure, Function, and Therapeutic Implications in Cancer Biology. Oncogene 2006, 25, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Salichs, E.; Ledda, A.; Mularoni, L.; Albà, M.M.; de la Luna, S. Genome-Wide Analysis of Histidine Repeats Reveals Their Role in the Localization of Human Proteins to the Nuclear Speckles Compartment. PLoS Genet. 2009, 5, e1000397. [Google Scholar] [CrossRef] [PubMed]
- Bushmeyer, S.; Park, K.; Atchison, M.L. Characterization of Functional Domains within the Multifunctional Transcription Factor, YY1. J. Biol. Chem. 1995, 270, 30213–30220. [Google Scholar] [CrossRef] [PubMed]
- Verheul, T.C.J.; van Hijfte, L.; Perenthaler, E.; Barakat, T.S. The Why of YY1: Mechanisms of Transcriptional Regulation by Yin Yang 1. Front. Cell Dev. Biol. 2020, 8, 592164. [Google Scholar] [CrossRef]
- Wang, G.Z.; Goff, S.P. Regulation of Yin Yang 1 by Tyrosine Phosphorylation. J. Biol. Chem. 2015, 290, 21890–21900. [Google Scholar] [CrossRef]
- Brayer, K.J.; Segal, D.J. Keep Your Fingers off My DNA: Protein-Protein Interactions Mediated by C2H2 Zinc Finger Domains. Cell Biochem. Biophys. 2008, 50, 111–131. [Google Scholar] [CrossRef]
- Wilkinson, F.; Pratt, H.; Atchison, M.L. PcG Recruitment by the YY1 REPO Domain Can Be Mediated by Yaf2. J. Cell. Biochem. 2010, 109, 478–486. [Google Scholar] [CrossRef]
- Wilkinson, F.H.; Park, K.; Atchison, M.L. Polycomb Recruitment to DNA in Vivo by the YY1 REPO Domain. Proc. Natl. Acad. Sci. USA 2006, 103, 19296–19301. [Google Scholar] [CrossRef]
- Qiao, S.; Wang, W.; Yi, C.; Xu, Q.; Wang, W.; Shi, J.; Stovall, D.B.; Li, D.; Sui, G. YY1 Oligomerization Is Regulated by Its OPB Domain and Competes with Its Regulation of Oncoproteins. Cancers 2022, 14, 1611. [Google Scholar] [CrossRef]
- Zhu, W.; Olson, S.Y.; Garban, H. Transcription Regulator Yin-Yang 1: From Silence to Cancer. Crit. Rev. Oncog. 2011, 16, 227–238. [Google Scholar] [CrossRef]
- Che, F.; Ye, X.; Wang, Y.; Wang, X.; Ma, S.; Tan, Y.; Mao, Y.; Luo, Z. METTL3 Facilitates Multiple Myeloma Tumorigenesis by Enhancing YY1 Stability and Pri-microRNA-27 Maturation in m6A-Dependent Manner. Cell Biol. Toxicol. 2023, 39, 2033–2050. [Google Scholar] [CrossRef]
- Wang, S.; Nie, J.; Xu, K.; Liu, Y.; Tong, W.; Li, A.; Zuo, W.; Liu, Z.; Yang, F. YY1 Is Regulated by ALKBH5-Mediated m6A Modification and Promotes Autophagy and Cancer Progression through Targeting ATG4B. Aging 2023, 15, 9590–9613. [Google Scholar] [CrossRef]
- Pan, S.; Chen, R. Pathological Implication of Protein Post-Translational Modifications in Cancer. Mol. Asp. Med. 2022, 86, 101097. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Liu, M.; Luo, J. Protein Post-Translational Modifications in the Regulation of Cancer Hallmarks. Cancer Gene Ther. 2023, 30, 529–547. [Google Scholar] [CrossRef]
- Zhong, Q.; Xiao, X.; Qiu, Y.; Xu, Z.; Chen, C.; Chong, B.; Zhao, X.; Hai, S.; Li, S.; An, Z.; et al. Protein Posttranslational Modifications in Health and Diseases: Functions, Regulatory Mechanisms, and Therapeutic Implications. MedComm 2023, 4, e261. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.; Lin, S.; Deng, W.; Peng, D.; Cui, Q.; Xue, Y. PTMD: A Database of Human Disease-Associated Post-Translational Modifications. Genom. Proteom. Bioinform. 2018, 16, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-Y.; Lee, T.-Y.; Kao, H.-J.; Ma, C.-T.; Lee, C.-C.; Lin, T.-H.; Chang, W.-C.; Huang, H.-D. dbPTM in 2019: Exploring Disease Association and Cross-Talk of Post-Translational Modifications. Nucleic Acids Res. 2019, 47, D298–D308. [Google Scholar] [CrossRef]
- Hiromura, M.; Choi, C.H.; Sabourin, N.A.; Jones, H.; Bachvarov, D.; Usheva, A. YY1 Is Regulated by O-LinkedN-Acetylglucosaminylation (O-GlcNAcylation). J. Biol. Chem. 2003, 278, 14046–14052. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Methylation-Sensitive Binding of Transcription Factor YY1 to an Insulator Sequence within the Paternally Expressed Imprinted Gene, Peg3. Hum. Mol. Genet. 2003, 12, 233–245. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, S.H.; Yum, J.; Yeo, C.-Y.; Lee, K.Y. Smurf2 Regulates the Degradation of YY1. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2014, 1843, 2005–2011. [Google Scholar] [CrossRef]
- Deng, Z.; Wan, M.; Sui, G. PIASy-Mediated Sumoylation of Yin Yang 1 Depends on Their Interaction but Not the RING Finger. Mol. Cell. Biol. 2007, 27, 3780–3792. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-L.; Yang, W.-M.; Seto, E. Regulation of Transcription Factor YY1 by Acetylation and Deacetylation. Mol. Cell. Biol. 2001, 21, 5979–5991. [Google Scholar] [CrossRef] [PubMed]
- Dillen, A.; Bui, I.; Jung, M.; Agioti, S.; Zaravinos, A.; Bonavida, B. Regulation of PD-L1 Expression by YY1 in Cancer: Therapeutic Efficacy of Targeting YY1. Cancers 2024, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing Multi-Omics Data within and across 32 Cancer Types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Montaner, J.; Ramiro, L.; Simats, A.; Tiedt, S.; Makris, K.; Jickling, G.C.; Debette, S.; Sanchez, J.-C.; Bustamante, A. Multilevel Omics for the Discovery of Biomarkers and Therapeutic Targets for Stroke. Nat. Rev. Neurol. 2020, 16, 247–264. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Cao, Y. Crosstalk between YY1 and lncRNAs in Cancer: A Review. Medicine 2022, 101, e31990. [Google Scholar] [CrossRef]
- Palko, L.; Bass, H.W.; Beyrouthy, M.J.; Hurt, M.M. The Yin Yang-1 (YY1) Protein Undergoes a DNA-Replication-Associated Switch in Localization from the Cytoplasm to the Nucleus at the Onset of S Phase. J. Cell Sci. 2004, 117 Pt 3, 465–476. [Google Scholar] [CrossRef]
- Krippner-Heidenreich, A.; Walsemann, G.; Beyrouthy, M.J.; Speckgens, S.; Kraft, R.; Thole, H.; Talanian, R.V.; Hurt, M.M.; Lüscher, B. Caspase-Dependent Regulation and Subcellular Redistribution of the Transcriptional Modulator YY1 during Apoptosis. Mol. Cell. Biol. 2005, 25, 3704–3714. [Google Scholar] [CrossRef]
- Broyles, S.S.; Liu, X.; Zhu, M.; Kremer, M. Transcription Factor YY1 Is a Vaccinia Virus Late Promoter Activator. J. Biol. Chem. 1999, 274, 35662–35667. [Google Scholar] [CrossRef]
- Slezak, K.; Michalik, M.; Kowalczyk, A.; Rokita, H. YY1 Is Recruited to the Cytoplasm of Vaccinia Virus-Infected Human Macrophages by the Crm1 System. Virus Res. 2004, 102, 177–184. [Google Scholar] [CrossRef]
- Na, Y.-R.; Yoon, Y.-N.; Son, D.-I.; Seok, S.-H. Cyclooxygenase-2 Inhibition Blocks M2 Macrophage Differentiation and Suppresses Metastasis in Murine Breast Cancer Model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; da Silva Bortoleti, B.T.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef] [PubMed]
- Emerson, L.E.; Gioseffi, A.; Barker, H.; Sheppe, A.; Morrill, J.K.; Edelmann, M.J.; Kima, P.E. Leishmania Infection-Derived Extracellular Vesicles Drive Transcription of Genes Involved in M2 Polarization. Front. Cell. Infect. Microbiol. 2022, 12, 934611. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-S.; Xu, Y.; Wang, Q.-S. YY1 Promotes Microglia M2 Polarization Through the MIR-130A-3P/TREM-2 Axis tO Alleviate Sepsis-Associated Encephalopathy. Shock 2022, 58, 128–136. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, M.; Shi, J.; Horita, D.A.; Miller, L.D.; Kute, T.E.; Kridel, S.J.; Kulik, G.; Sui, G. Yin Yang 1 Promotes mTORC2-Mediated AKT Phosphorylation. J. Mol. Cell Biol. 2016, 8, 232–243. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastav, S.; Saha, S.; Das, P.K.; Ukil, A. Leishmania Donovani Inhibits Macrophage Apoptosis and Pro-Inflammatory Response through AKT-Mediated Regulation of β-Catenin and FOXO-1. Cell Death Differ. 2016, 23, 1815–1826. [Google Scholar] [CrossRef]
- Nandan, D.; Camargo de Oliveira, C.; Moeenrezakhanlou, A.; Lopez, M.; Silverman, J.M.; Subek, J.; Reiner, N.E. Myeloid Cell IL-10 Production in Response to Leishmania Involves Inactivation of Glycogen Synthase Kinase-3β Downstream of Phosphatidylinositol-3 Kinase. J. Immunol. Baltim. Md 1950 2012, 188, 367–378. [Google Scholar] [CrossRef]
- Thomas, S.A.; Nandan, D.; Kass, J.; Reiner, N.E. Countervailing, Time-Dependent Effects on Host Autophagy Promotes Intracellular Survival of Leishmania. J. Biol. Chem. 2018, 293, 2617–2630. [Google Scholar] [CrossRef]
- Zan, J.; Zhang, H.; Gu, A.-P.; Zhong, K.-L.; Lu, M.-Y.; Bai, X.-X.; Zhang, J.-Y.; Cai, J. Yin Yang 1 Dynamically Regulates Antiviral Innate Immune Responses During Viral Infection. Cell. Physiol. Biochem. 2017, 44, 607–617. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Rosas, L.E.; Lafuse, W.P.; Satoskar, A.R. Leishmania Inhibits STAT1-Mediated IFN-Gamma Signaling in Macrophages: Increased Tyrosine Phosphorylation of Dominant Negative STAT1beta by Leishmania Mexicana. Int. J. Parasitol. 2005, 35, 75–82. [Google Scholar] [CrossRef]
- Chaparro, V.; Graber, T.E.; Alain, T.; Jaramillo, M. Transcriptional Profiling of Macrophages Reveals Distinct Parasite Stage-Driven Signatures during Early Infection by Leishmania Donovani. Sci. Rep. 2022, 12, 6369. [Google Scholar] [CrossRef]
- Azmi, A.S.; Uddin, M.H.; Mohammad, R.M. The Nuclear Export Protein XPO1—From Biology to Targeted Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 152–169. [Google Scholar] [CrossRef]
- Sun, Q.; Carrasco, Y.P.; Hu, Y.; Guo, X.; Mirzaei, H.; Macmillan, J.; Chook, Y.M. Nuclear Export Inhibition through Covalent Conjugation and Hydrolysis of Leptomycin B by CRM1. Proc. Natl. Acad. Sci. USA 2013, 110, 1303–1308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandan, D.; Longowal, D.K.; Reiner, N. Yin Yang 1: Role in Leishmaniasis. Cells 2025, 14, 1149. https://doi.org/10.3390/cells14151149
Nandan D, Longowal DK, Reiner N. Yin Yang 1: Role in Leishmaniasis. Cells. 2025; 14(15):1149. https://doi.org/10.3390/cells14151149
Chicago/Turabian StyleNandan, Devki, Dilraj Kaur Longowal, and Neil Reiner. 2025. "Yin Yang 1: Role in Leishmaniasis" Cells 14, no. 15: 1149. https://doi.org/10.3390/cells14151149
APA StyleNandan, D., Longowal, D. K., & Reiner, N. (2025). Yin Yang 1: Role in Leishmaniasis. Cells, 14(15), 1149. https://doi.org/10.3390/cells14151149




