The Mechanical Properties of Erythrocytes Are Influenced by the Conformational State of Albumin
Abstract
1. Introduction
1.1. Albumin
1.2. Albumin Unfolding
1.3. Serum Albumin Physiological Role
- It may guide the development of biomarkers based on albumin structural modifications to predict erythrocyte-related complications in disease.
- It could inform therapeutic strategies aimed at preserving albumin’s native conformation or supplementing functional albumin in patients with low or dysfunctional albumin levels.
- It may influence decisions regarding transfusion practices or blood storage, particularly in patients at risk for erythrocyte mechanical fragility.
1.4. Role of Shear Stress
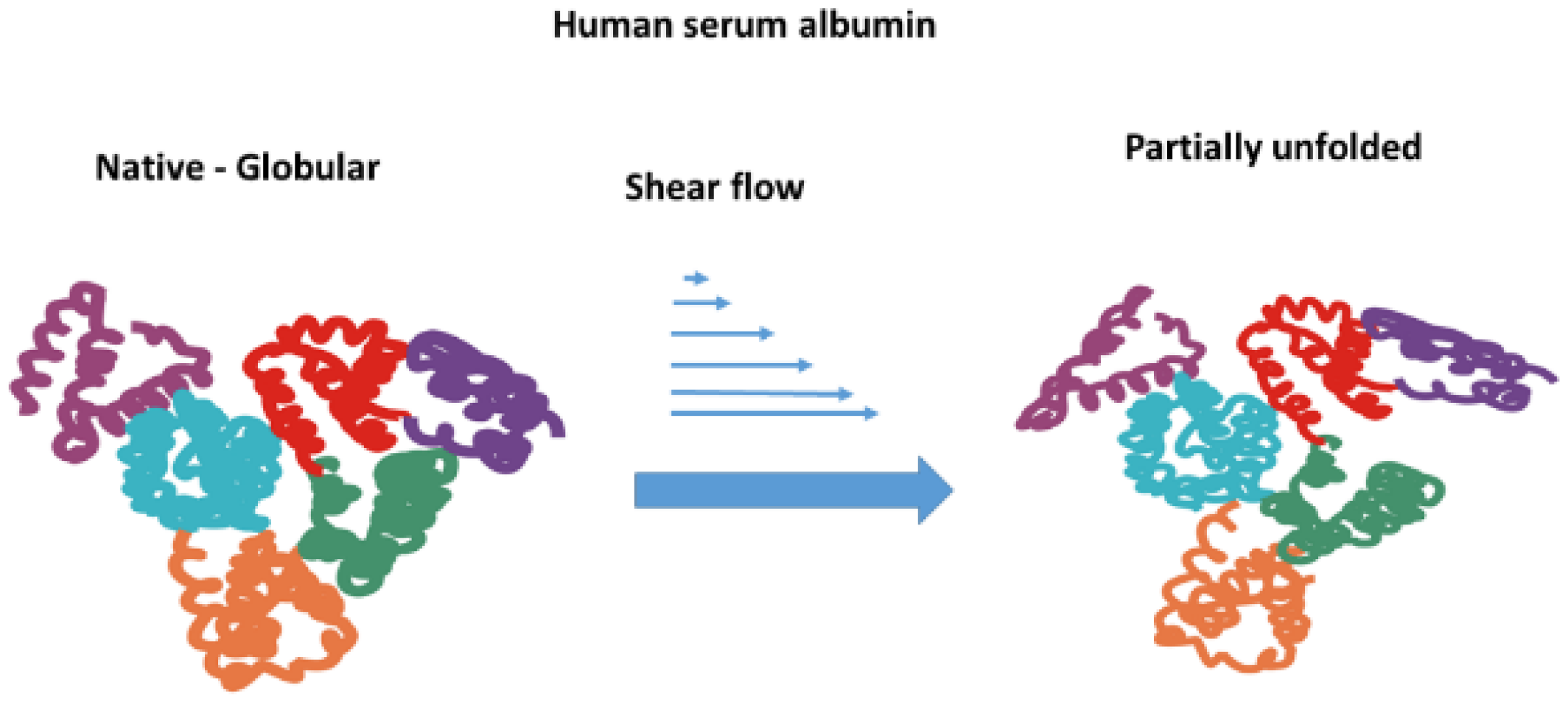
1.5. Unfolding of Albumin Under Shear Flow
1.6. Shear Stress Sensitivity of Erythrocytes
1.7. Factors That Dictate Erythrocyte Mechanical Stability
2. Effect of Albumin on Erythrocyte Mechanical Stability
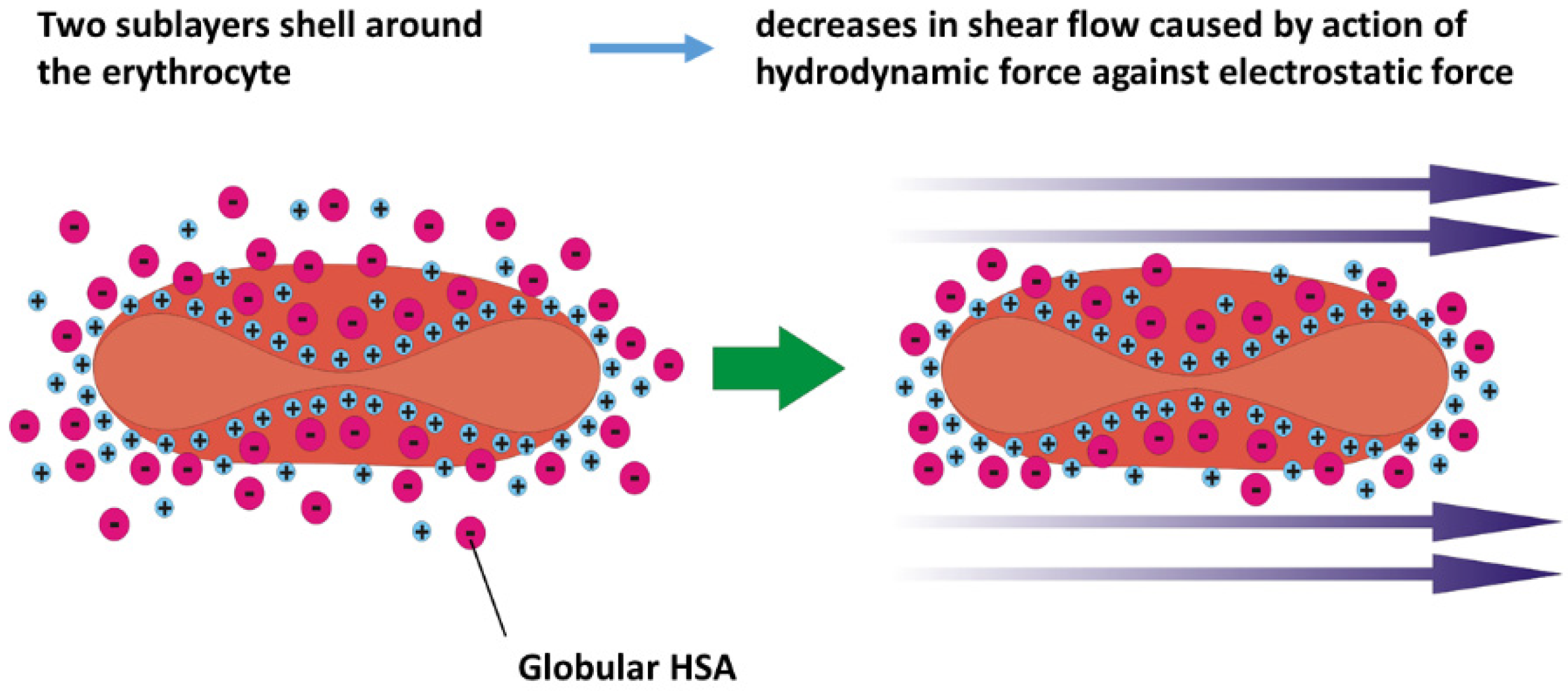
2.1. Scenario 1 of Cell Response
2.2. Scenario 2 of Cell Response
3. Conclusions
- Two possible scenarios of cell response under higher shear stress in the presence of HSA were considered, depending on the ability of albumin to unfold in shear flow. One scenario discusses the electrostatic and hydrodynamic interactions between albumin in its native state and the erythrocyte membrane. The other scenario describes the consequences of hydrophobic interactions between unfolded albumin and the RBC membrane.
- Electrostatic interactions between albumin in its native state and the RBC membrane under isotonic conditions influence the zeta potential of the membrane and may lead to mechanical stabilization of membranes. An increase in shear rate during the flow of erythrocytes through capillaries causes an increase in hydrodynamic interactions between various ions and the membrane, resulting in a decrease in zeta potential. This decrease in zeta potential can destabilize erythrocytes.
- The ability of albumin to unfold under higher shear rates depends on the anisotropic viscoelasticity of blood. Shear flow does not have the potential to directly induce the unfolding of albumin. However, the shear flow of complex anisotropic fluids, such as blood, causes the generation of extensional flow, quantified by the first normal stress difference, which can become significant at higher shear rates. Extensional flow can lead to the partial unfolding of albumin.
- The unfolding of albumin results in (i) an increase in osmotic stress and (ii) intensive hydrophobic interactions between albumin and the membrane of erythrocytes. These interactions can lead to the transition of discocytes into stomatocytes, a cell form that is smaller, stiffer, and more fragile.
- Stiffening of the membrane of erythrocytes depends on (i) the viscoelasticity of the bilayer and actin cortex and (ii) the coupling between them. The stiffness depends on the rearrangement of band 3 in response to membrane fluctuations induced by shear flow and the intracellular concentration of calcium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSA | Human serum albumin |
| BSA | Bovine serum albumin |
| RBC | Red blood cell |
| OUW | Osmotically unresponsive water |
| PS | Phosphatidylserine |
References
- Reinhart, W.H.; Piety, N.Z.; Goede, J.S.; Shevkoplyas, S.S. Effect of osmolality on erythrocyte rheology and perfusion of an artificial microvascular network. Microvasc. Res. 2015, 98, 102–107. [Google Scholar] [CrossRef]
- Reinhart, W.H.; Piety, N.Z.; Deuel, J.W.; Makhro, A.; Schulzki, T.; Bogdanov, N.; Goede, J.S.; Bogdanova, A.; Abidi, R.; Shevkoplyas, S.S. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion 2015, 55, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Kubota, K.; Yamada, N.; Tagami, U.; Takehana, K.; Sonaka, I.; Suzuki, E.; Hirayama, K. Identification and characterization of oxidized human serum albumin. FEBS J. 2006, 273, 3346–3357. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Ahmad, J.; Alam, K. Impact of glycation on structural and antioxidant function of human serum albumin: Relevance in diabetic complications. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef]
- Weaving, G.; Batstone, G.F.; Jones, R.G. Age and sex variation in serum albumin concentration: An observational study. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2015, 53, 106–111. [Google Scholar] [CrossRef]
- Żurawska-Płaksej, E.; Grzebyk, E.; Marciniak, D.; Szymańska-Chabowska, A.; Piwowar, A. Oxidatively modified forms of albumin in patients with risk factors of metabolic syndrome. J. Endocrinol. Investig. 2014, 37, 819–827. [Google Scholar] [CrossRef]
- Tenkorang, M.A.; Snyder, B.; Cunningham, R.L. Sex-related differences in oxidative stress and neurodegeneration. Steroids 2018, 133, 21–27. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2018, 43, 181–193. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Gao, M.-Q.; Jiang, X.-C.; Pan, X.; Zhang, X.-Y.; Li, Y.; Shen, Q.; Chen, Y.; Pang, B. Research progress and value of albumin-related inflammatory markers in the prognosis of non-small cell lung cancer: A review of clinical evidence. Ann. Med. 2023, 55, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Tarasev, M.; Chakraborty, S.; Light, L.; Davenport, R. Impact of environment on Red Blood Cell ability to withstand mechanical stress. Clin. Hemorheol. Microcirc. 2016, 64, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; A Bradley, C.; E Owensby, J. Plasma components protect erythrocytes against experimental haemolysis caused by mechanical trauma and by hypotonicity. Int. J. Exp. Pathol. 1992, 73, 27–33. [Google Scholar]
- Jay, A. Geometry of the human erythrocyte. I. Effect of albumin on cell geometry. Biophys. J. 1975, 15, 205–222. [Google Scholar] [CrossRef]
- Wiig, H.; Kolmannskog, O.; Tenstad, O.; Bert, J.L. Effect of charge on interstitial distribution of albumin in rat dermis in vitro. J. Physiol. 2003, 550, 505–514. [Google Scholar] [CrossRef]
- Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Taheri-Araghi, S.; Ha, B.-Y. Electrostatic Bending of Lipid Membranes: How Are Lipid and Electrostatic Properties Interrelated? Langmuir 2010, 26, 14737–14746. [Google Scholar] [CrossRef]
- McLaughlin, S. The Electrostatic Properties of Membranes. Annu. Rev. Biophys. 1989, 18, 113–136. [Google Scholar] [CrossRef]
- Skalak, R.; Chien, S. Handbook of Bioengineering; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Chen, X.-Y.; Huang, Y.-X.; Liu, W.-J.; Yuan, Z.-J. Membrane surface charge and morphological and mechanical properties of young and old erythrocytes. Curr. Appl. Phys. 2007, 7, e94–e96. [Google Scholar] [CrossRef]
- Sutera, S.; Mehrjardi, M. Deformation and fragmentation of human red blood cells in turbulent shear flow. Biophys. J. 1975, 15, 1–10. [Google Scholar] [CrossRef]
- Reinhart, S.A.; Schulzki, T.; Reinhart, W.H. Albumin reverses the echinocytic shape transformation of stored erythrocytes. Clin. Hemorheol. Microcirc. 2015, 60, 437–449. [Google Scholar] [CrossRef]
- Reinhart, W.H.; Chien, S. Echinocyte-stomatocyte transformation and shape control of human red blood cells: Morphological aspects. Am. J. Hematol. 1987, 24, 1–14. [Google Scholar] [CrossRef]
- Selvan, R.; Parthasarathi, P.; Iyengar, S.S.; Ananthamurthy, S.; Bhattacharya, S.; Tian, F.-B. Estimation of membrane bending modulus of stiffness tuned human red blood cells from micropore filtration studies. PLOS ONE 2019, 14, e0226640. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Anguizola, J.; Matsuda, R.; Barnaby, O.S.; Hoy, K.S.; Wa, C.; DeBolt, E.; Koke, M.; Hage, D.S. Review: Glycation of human serum albumin. Clin. Chim. Acta 2013, 425, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Barshtein, G.; Mardi, T.; Deutch, V.; Elkayam, O.; Yedgar, S.; Berliner, S. A synergistic effect of albumin and fibrinogen on immunoglobulin-induced red blood cell aggregation. Am. J. Physiol. Circ. Physiol. 2003, 285, H2663–H2669. [Google Scholar] [CrossRef] [PubMed]
- Groisman, A.; Steinberg, V. Elastic turbulence in a polymer solution flow. Nature 2000, 405, 53–55. [Google Scholar] [CrossRef]
- Guerin-Dubourg, A.; Catan, A.; Bourdon, E.; Rondeau, P. Structural modifications of human albumin in diabetes. Diabetes Metab. 2012, 38, 171–178. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood Rheology and Hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef]
- Vinik, A.I.; Ziegler, D. Diabetic Cardiovascular Autonomic Neuropathy. Circulation 2007, 115, 387–397. [Google Scholar] [CrossRef]
- Oettl, K.; E Stauber, R. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Marrone, G.; Fernández-Iglesias, A. Hepatic microcirculation and mechanisms of portal hypertension. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y. Association between red blood cell distribution width-to-albumin ratio and prognosis of patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2023, 23, 1–12. [Google Scholar] [CrossRef]
- Lanzaro, A. A microfluidic approach to studying the injection flow of concentrated albumin solutions. SN Appl. Sci. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Gural, A.; Pajić-Lijaković, I.; Barshtein, G. Mechanical Stimulation of Red Blood Cells Aging: Focusing on the Microfluidics Application. Micromachines 2025, 16, 259. [Google Scholar] [CrossRef]
- Saito, G.E.; Werff, T.J.V. The importance of viscoelasticity in arterial blood flow models. J. Biomech. 1975, 8, 237–245. [Google Scholar] [CrossRef]
- Armstrong, M.; Horner, J.; Clark, M.; Deegan, M.; Hill, T.; Keith, C.; Mooradian, L. Evaluating rheological models for human blood using steady state, transient, and oscillatory shear predictions. Rheol. Acta 2018, 57, 705–728. [Google Scholar] [CrossRef]
- Li, X.K.; Luo, Y.; Qi, Y.; Zhang, R. On non-Newtonian lubrication with the upper convected Maxwell model. Appl. Math. Model. 2011, 35, 2309–2323. [Google Scholar] [CrossRef]
- Saengow, C.; Giacomin, A.J.; Dimitrov, A.S. Normal Stress Differences of Human Blood in Unidirectional Large-Amplitude Oscillatory Shear Flow. J. Fluids Eng. 2020, 142. [Google Scholar] [CrossRef]
- Spyridakis, A.; Moschopoulos, P.; Varchanis, S.; Dimakopoulos, Y.; Tsamopoulos, J. Thixo-elastoviscoplastic modeling of human blood. J. Rheol. 2023, 68, 1–23. [Google Scholar] [CrossRef]
- Lipowsky, H.H. Microvascular Rheology and Hemodynamics. Microcirculation 2005, 12, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.; Kumar, A.; Willis, L.F.; Tuma, R.; Higazi, D.R.; Turner, R.; Lowe, D.C.; Ashcroft, A.E.; Radford, S.E.; Kapur, N.; et al. Inducing protein aggregation by extensional flow. Proc. Natl. Acad. Sci. USA 2017, 114, 4673–4678. [Google Scholar] [CrossRef]
- Jaspe, J.; Hagen, S.J. Do Protein Molecules Unfold in a Simple Shear Flow? Biophys. J. 2006, 91, 3415–3424. [Google Scholar] [CrossRef]
- Moino, C.; Artusio, F.; Pisano, R. Shear stress as a driver of degradation for protein-based therapeutics: More accomplice than culprit. Int. J. Pharm. 2023, 650, 123679. [Google Scholar] [CrossRef]
- Brückl, L.; Schröder, T.; Scheler, S.; Hahn, R.; Sonderegger, C. The Effect of Shear on the Structural Conformation of rhGH and IgG1 in Free Solution. J. Pharm. Sci. 2016, 105, 1810–1818. [Google Scholar] [CrossRef]
- Bekard, I.B.; Asimakis, P.; Teoh, C.L.; Ryan, T.; Howlett, G.J.; Bertolini, J.; Dunstan, D.E. Bovine serum albumin unfolds in Couette flow. Soft Matter 2012, 8, 385–389. [Google Scholar] [CrossRef]
- Zocchi, G. Proteins unfold in steps. Proc. Natl. Acad. Sci. USA 1997, 94, 10647–10651. [Google Scholar] [CrossRef]
- Kiese, S.; Papppenberger, A.; Friess, W.; Mahler, H.-C. Shaken, Not Stirred: Mechanical Stress Testing of an IgG1 Antibody. J. Pharm. Sci. 2008, 97, 4347–4366. [Google Scholar] [CrossRef]
- Nevaril, C.; Lynch, E.; Alfrey, C.; Hellums, J. Erythrocyte damage and destruction induced by shearing stress. J. Lab. Clin. Med. 1968, 71, 784–790. [Google Scholar]
- Simmonds, M.J.; Meiselman, H.J. Prediction of the level and duration of shear stress exposure that induces subhemolytic damage to erythrocytes. Biorheology 2017, 53, 237–249. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, X.; Zhang, C.; Dao, M.; Gong, X. Evolution of surface area and membrane shear modulus of matured human red blood cells during mechanical fatigue. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Herreros, A.; Yeh, Y.; Peng, Z.; del Álamo, J.C. Cyclic Mechanical Stresses Alter Erythrocyte Membrane Composition and Microstructure and Trigger Macrophage Phagocytosis. Adv. Sci. 2022, 9, e2201481. [Google Scholar] [CrossRef] [PubMed]
- Kameneva, M.V.; Antaki, J.F.; Borovetz, H.S.; Griffith, B.P.; Butler, K.C.; Yeleswarapu, K.K.; Watach, M.J.; Kormos, R.L. Mechanisms of red blood cell trauma in assisted circulation. Rheologic similarities of red blood cell transformations due to natural aging and mechanical stress. ASAIO J. 1995, 41, M457–M460. [Google Scholar] [CrossRef] [PubMed]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red Blood Cells: Chasing Interactions. Front. Physiol. 2019, 10, 945. [Google Scholar] [CrossRef]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef]
- Orbach, A.; Zelig, O.; Yedgar, S.; Barshtein, G. Biophysical and Biochemical Markers of Red Blood Cell Fragility. Transfus. Med. Hemotherapy 2017, 44, 183–187. [Google Scholar] [CrossRef]
- Olia, S.E.; Maul, T.M.; Antaki, J.F.; Kameneva, M.V. Mechanical Blood Trauma in Assisted Circulation: Sublethal RBC Damage Preceding Hemolysis. Int. J. Artif. Organs 2016, 39, 150–159. [Google Scholar] [CrossRef]
- Barshtein, G.; Gural, A.; Arbell, D.; Barkan, R.; Livshits, L.; Pajic-Lijakovic, I.; Yedgar, S. Red Blood Cell Deformability Is Expressed by a Set of Interrelated Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 12755. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M. Modeling analysis of the lipid bilayer–cytoskeleton coupling in erythrocyte membrane. Biomech. Model. Mechanobiol. 2014, 13, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Pajic-Lijakovic, I.; Milivojevic, M. Actin Cortex Rearrangement Caused by Coupling with the Lipid Bilayer-Modeling Considerations. J. Membr. Biol. 2015, 248, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Williamson, P.; Schlegel, R.A. Bilayer/cytoskeleton interactions in lipid-symmetric erythrocytes assessed by a photoactivable phospholipid analog. Biochemistry 1991, 30, 7754–7758. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L. The Calcium Homeostasis of Human Red Blood Cells in Health and Disease: Interactions of PIEZO1, the Plasma Membrane Calcium Pump, and Gardos Channels. Annu. Rev. Physiol. 2024. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Milivojevic, M.; Martinac, B.; McClintock, P.V.E. Targeted elimination of mesenchymal-like cancer cells through cyclic stretch activation of Piezo1 channels: The physical aspects. Biophys. Rev. 2025. [Google Scholar] [CrossRef]
- Wieschhaus, A.; Khan, A.; Zaidi, A.; Rogalin, H.; Hanada, T.; Liu, F.; De Franceschi, L.; Brugnara, C.; Rivera, A.; Chishti, A.H. Calpain-1 knockout reveals broad effects on erythrocyte deformability and physiology. Biochem. J. 2012, 448, 141–152. [Google Scholar] [CrossRef]
- Kamada, T.; McMillan, D.E.; Sternlieb, J.J.; Björk, V.O.; Otsuji, S. Albumin prevents erythrocyte crenation in patients undergoing extracorporeal circulation. Scand. J. Thorac. Cardiovasc. Surg. 1988, 22, 155–158. [Google Scholar] [CrossRef]
- Williams, A.R. The effect of bovine and human serum albumins on the mechanical properties of human erythrocyte membranes. Biochim. Et Biophys. Acta (BBA)—Biomembr. 1973, 307, 58–64. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Antaki, J.F.; Yeleswarapu, K.K.; Watach, M.J.; Griffith, B.P.; Borovetz, H.S. Plasma protective effect on red blood cells exposed to mechanical stress. ASAIO J. 1997, 43, M571–M575. [Google Scholar] [CrossRef]
- Sümpelmann, R.; Schürholz, T.; Marx, G.; Zander, R. Protective effects of plasma replacement fluids on erythrocytes exposed to mechanical stress. Anaesthesia 2000, 55, 976–979. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Repko, B.M.; Krasik, E.F.; Perricelli, B.C.; Borovetz, H.S. Polyethylene Glycol Additives Reduce Hemolysis in Red Blood Cell Suspensions Exposed to Mechanical Stress. Asaio J. 2003, 49, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kamal, J.K.A.; Zhao, L.; Zewail, A.H. Ultrafast hydration dynamics in protein unfolding: Human serum albumin. Proc. Natl. Acad. Sci. USA 2004, 101, 13411–13416. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, G.; Kanal, K.; Cameron, I. Osmotically unresponsive water fraction on proteins: Non-ideal osmotic pressure of bovine serum albumin as a function of pH and salt concentration. Cell Biol. Int. 2006, 30, 86–92. [Google Scholar] [CrossRef]
- Cloos, A.-S.; Ghodsi, M.; Stommen, A.; Vanderroost, J.; Dauguet, N.; Pollet, H.; D’auria, L.; Mignolet, E.; Larondelle, Y.; Terrasi, R.; et al. Interplay Between Plasma Membrane Lipid Alteration, Oxidative Stress and Calcium-Based Mechanism for Extracellular Vesicle Biogenesis From Erythrocytes During Blood Storage. Front. Physiol. 2020, 11, 712. [Google Scholar] [CrossRef]
- Cameron, I.; Kanal, K.; Fullerton, G. Role of protein conformation and aggregation in pumping water in and out of a cell. Cell Biol. Int. 2006, 30, 78–85. [Google Scholar] [CrossRef]
- Son, M.; Lee, Y.S.; Lee, M.J.; Park, Y.; Bae, H.-R.; Lee, S.Y.; Shin, M.-G.; Yang, S.; Chalmers, J. Effects of osmolality and solutes on the morphology of red blood cells according to three-dimensional refractive index tomography. PLoS ONE 2021, 16, e0262106. [Google Scholar] [CrossRef]
- Iglič, A.; Kralj-Iglič, V.; Hägerstrand, H. Amphiphile induced echinocyte-spheroechinocyte transformation of red blood cell shape. Eur. Biophys. J. 1998, 27, 335–339. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Lim, H.G.; Wortis, M. Echinocyte Shapes: Bending, Stretching, and Shear Determine Spicule Shape and Spacing. Biophys. J. 2002, 82, 1756–1772. [Google Scholar] [CrossRef]
- Rudenko, S.V. Erythrocyte morphological states, phases, transitions and trajectories. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2010, 1798, 1767–1778. [Google Scholar] [CrossRef]
- Geekiyanage, N.M.; Sauret, E.; Saha, S.C.; Flower, R.L.; Gu, Y.T. Deformation behaviour of stomatocyte, discocyte and echinocyte red blood cell morphologies during optical tweezers stretching. Biomech. Model. Mechanobiol. 2020, 19, 1827–1843. [Google Scholar] [CrossRef]
- Park, Y.; Best, C.A.; Badizadegan, K.; Dasari, R.R.; Feld, M.S.; Kuriabova, T.; Henle, M.L.; Levine, A.J.; Popescu, G. Measurement of red blood cell mechanics during morphological changes. Proc. Natl. Acad. Sci. USA 2010, 107, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Livshits, L.; Gural, A.; Arbell, D.; Barkan, R.; Pajic-Lijakovic, I.; Yedgar, S. Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability. Int. J. Mol. Sci. 2024, 25, 5814. [Google Scholar] [CrossRef] [PubMed]
- Durocher, J.R.; Payne, R.C.; Conrad, M.E. Role of sialic acid in erythrocyte survival. Blood 1975, 45, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro, M.d.L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. De Hematol. E Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef]
- Tarasev, M.; Chakraborty, S.; Alfano, K. RBC Mechanical Fragility as a Direct Blood Quality Metric to Supplement Storage Time. Mil. Med. 2015, 180, 150–157. [Google Scholar] [CrossRef]
- Gaikwad, S.; Avari, J.G. Effect on Morphology, Osmotic Fragility and Electro Kinetic Potential of Erythrocytes in Hypertension. Curr. Hypertens. Rev. 2018, 13, 132–137. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tateishi, N.; Maeda, N. Electrostatic repulsion among erythrocytes in tube flow, demonstrated by the thickness of marginal cell-free layer. Biorheology 1998, 35, 155–170. [Google Scholar] [CrossRef]

| Role of Albumin | Mechanics of Erythrocyte Membrane | References |
|---|---|---|
| Folding | Stabilizing effect caused by electrostatic interactions under wide range of shear rates | [49] |
| Folding | Stabilizing effect caused by electrostatic interactions under moderate shear rates | [48,50] |
| Albumin-induced | Discocyte-to-stomatocyte transition caused by a change in the membrane stiffness and bending modulus of the bilayer | [11,12] |
| Albumin-induced | Stiffening of the erythrocyte membrane is pronounced with an increase in albumin concentration, which can enhance the fragility of cells | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajic-Lijakovic, I.; Milivojevic, M.; Barshtein, G.; Gural, A. The Mechanical Properties of Erythrocytes Are Influenced by the Conformational State of Albumin. Cells 2025, 14, 1139. https://doi.org/10.3390/cells14151139
Pajic-Lijakovic I, Milivojevic M, Barshtein G, Gural A. The Mechanical Properties of Erythrocytes Are Influenced by the Conformational State of Albumin. Cells. 2025; 14(15):1139. https://doi.org/10.3390/cells14151139
Chicago/Turabian StylePajic-Lijakovic, Ivana, Milan Milivojevic, Gregory Barshtein, and Alexander Gural. 2025. "The Mechanical Properties of Erythrocytes Are Influenced by the Conformational State of Albumin" Cells 14, no. 15: 1139. https://doi.org/10.3390/cells14151139
APA StylePajic-Lijakovic, I., Milivojevic, M., Barshtein, G., & Gural, A. (2025). The Mechanical Properties of Erythrocytes Are Influenced by the Conformational State of Albumin. Cells, 14(15), 1139. https://doi.org/10.3390/cells14151139








