Liver Regeneration as a Model for Studying Cellular Plasticity in Mammals: The Roles of Hepatocytes and Cholangiocytes
Abstract
1. Introduction
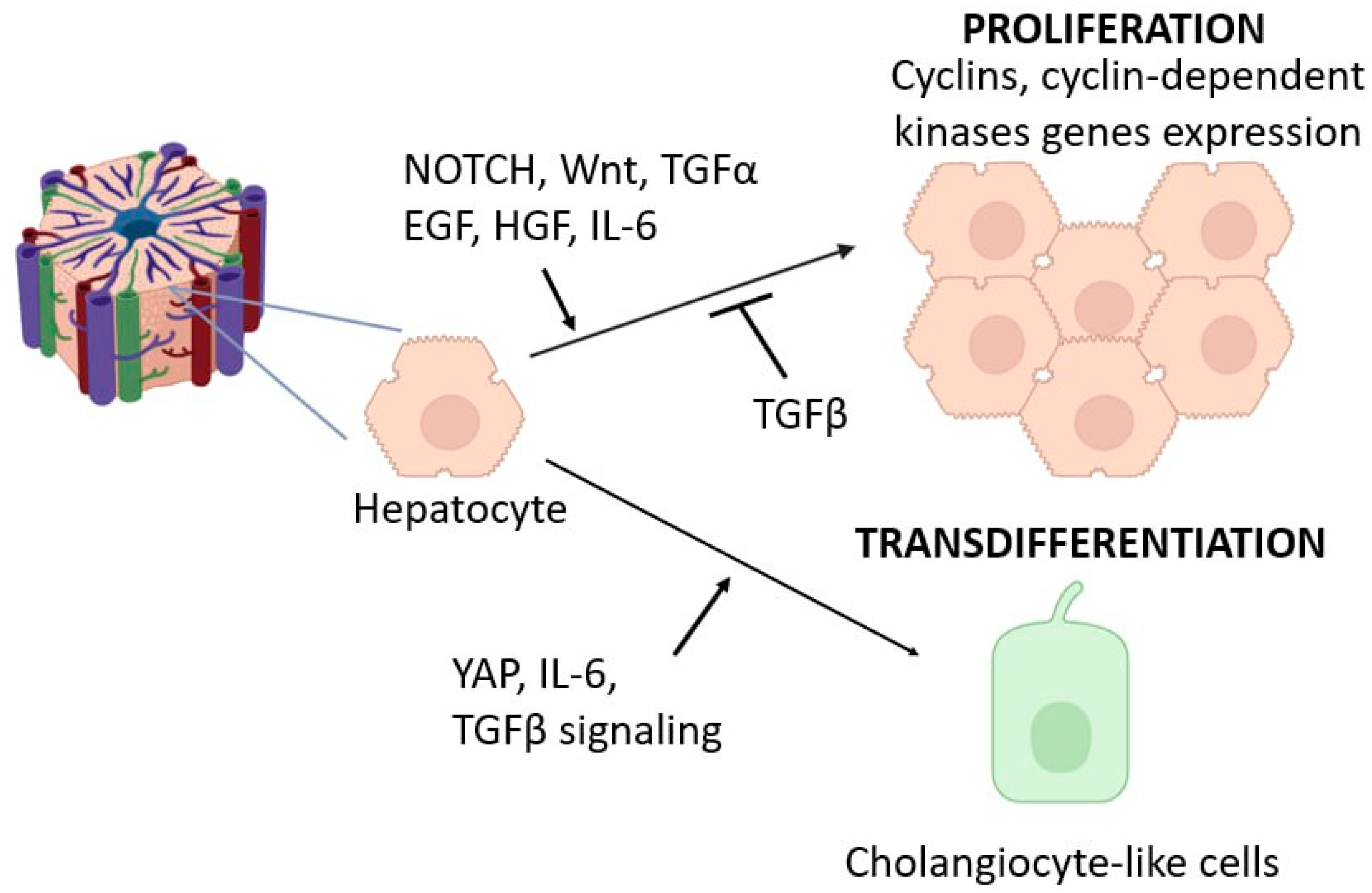
2. Hepatocytes
2.1. Major Signaling Cascades Activating Hepatocyte Proliferation
2.2. Dependence of Hepatocyte Proliferation on Localization
2.3. Factors Influencing Hepatocyte Proliferation
2.4. Transdifferentiation Capacity of Hepatocytes
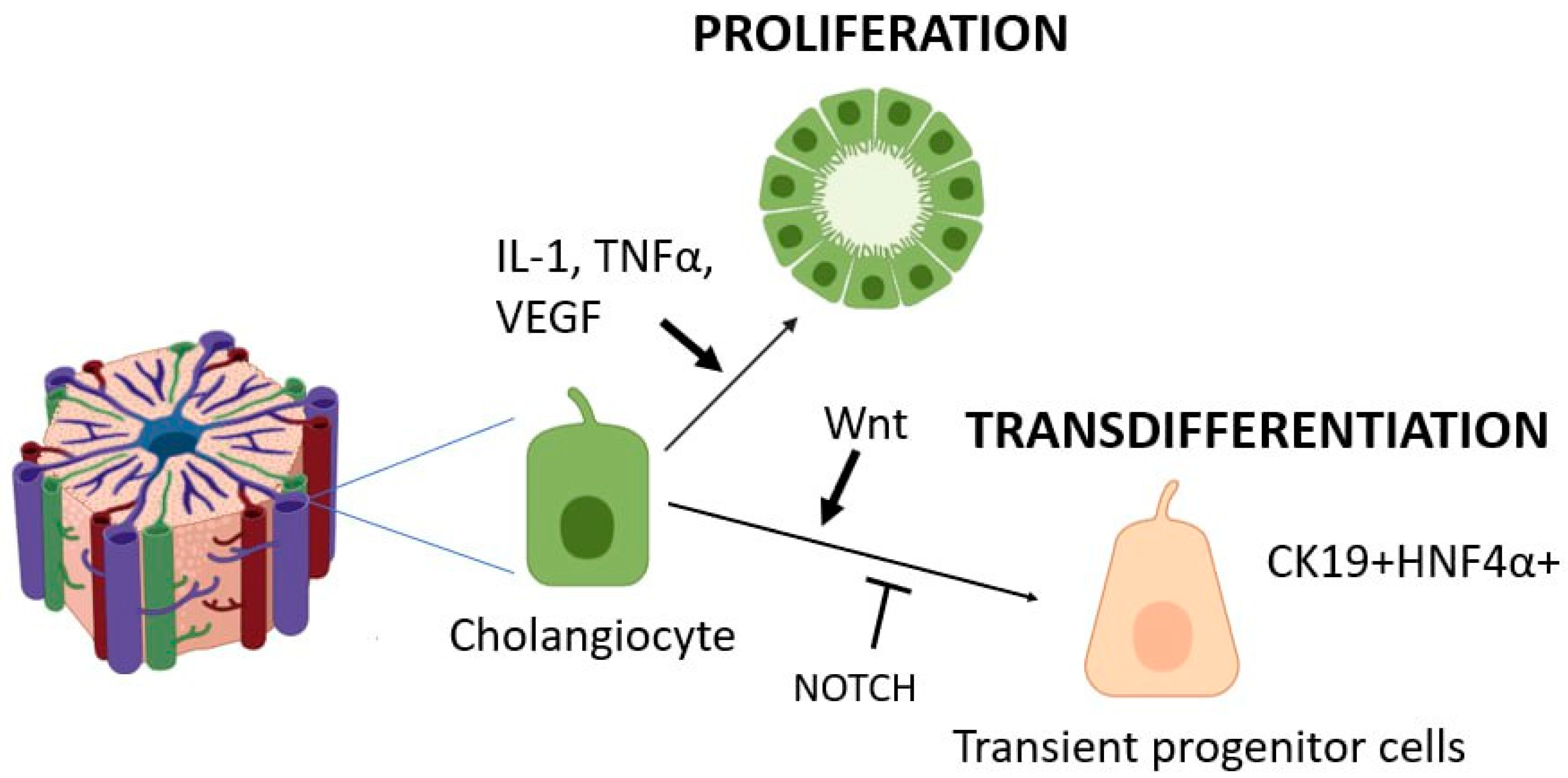
3. Cholangiocytes
3.1. Major Signaling Cascades Activating Cholangiocyte Proliferation
3.2. Transdifferentiation Capacity of Cholangiocytes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HGF | Hepatocyte growth factor |
| IL1 | Interleukin 1 |
| IL10 | Interleukin 10 |
| IL6 | Interleukin 6 |
| TGFb1 | Transforming growth factor β1 |
| TNFα | Tumor necrosis factor α |
| PDGFβ | Platelet-derived growth factor subunit B |
| VEGF | Vascular endothelial growth factor |
| MMP-9 | Matrix metallopeptidase 9 |
| MMP-13 | Matrix metallopeptidase 13 |
| iNOS | Inducible nitric oxide synthase |
| PH | Partial Hepatectomy |
| SR | Subtotal Resection |
References
- Galliot, B.; Ghila, L. Cell plasticity in homeostasis and regeneration. Mol. Reprod. Dev. 2010, 77, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Elchaninov, A.; Sukhikh, G.; Fatkhudinov, T. Evolution of Regeneration in Animals: A Tangled Story. Front. Ecol. Evol. 2021, 9, 621686. [Google Scholar] [CrossRef]
- Seifert, A.W.; Muneoka, K. The blastema and epimorphic regeneration in mammals. Dev. Biol. 2018, 433, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Monaghan, J.R.; Smith, M.D.; Pasch, B.; Stier, A.C.; Michonneau, F.; Maden, M. The influence of fundamental traits on mechanisms controlling appendage regeneration. Biol. Rev. 2012, 87, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, Y.; Kim, Y.S.; Ko, S. Cellular Plasticity in Gut and Liver Regeneration. Gut Liver 2024, 18, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Lokhonina, A.; Elchaninov, A.; Fatkhudinov, T.; Makarov, A.; Arutyunyan, I.; Grinberg, M.; Glinkina, V.; Surovtsev, V.; Bolshakova, G.; Goldshtein, D.; et al. Activated Macrophages of Monocytic Origin Predominantly Express Proinflammatory Cytokine Genes, Whereas Kupffer Cells Predominantly Express Anti-Inflammatory Cytokine Genes. Biomed Res. Int. 2019, 2019, 3912142. [Google Scholar] [CrossRef] [PubMed]
- Elchaninov, A.; Vishnyakova, P.; Menyailo, E.; Sukhikh, G.; Fatkhudinov, T. An Eye on Kupffer Cells: Development, Phenotype and the Macrophage Niche. Int. J. Mol. Sci. 2022, 23, 9868. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, J.; Cai, X.; Liou, Y.C.; Shen, H.M.; Hao, J.; Huang, C.; Luo, G.; He, W. Macrophage plasticity: Signaling pathways, tissue repair, and regeneration. MedComm 2024, 5, e658. [Google Scholar] [CrossRef] [PubMed]
- Elchaninov, A.; Lokhonina, A.; Vishnyakova, P.; Soboleva, A.; Poltavets, A.; Artemova, D.; Makarov, A.; Glinkina, V.; Goldshtein, D.; Bolshakova, G.; et al. Marco+ macrophage dynamics in regenerating liver after 70% liver resection in mice. Biomedicines 2021, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Novel insights into liver homeostasis and regeneration. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Bucher, N.L.; Swaffield, M.N.; Ditroia, J.F. The Influence of Age Upon the Incorporation of Thymidine-2-C14 Into The DNA of Regenerating Rat Liver. Cancer Res. 1964, 24, 509–512. [Google Scholar] [PubMed]
- Sidorova, V.F. Mitotic activity of the rat liver subjected to various surgical interferences. Bull. Exp. Biol. Med. 1965, 59, 438–441. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; DeFrances, M.C. Liver Regeneration. Science 1997, 276, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jimenez, R.J.; Sharma, K.; Luu, H.Y.; Hsu, B.Y.; Ravindranathan, A.; Stohr, B.A.; Willenbring, H. Broad Distribution of Hepatocyte Proliferation in Liver Homeostasis and Regeneration. Cell Stem Cell 2020, 26, 27–33.e4. [Google Scholar] [CrossRef] [PubMed]
- Klochendler, A.; Weinberg-Corem, N.; Moran, M.; Swisa, A.; Pochet, N.; Savova, V.; Vikeså, J.; Van de Peer, Y.; Brandeis, M.; Regev, A.; et al. A transgenic mouse marking live replicating cells reveals in vivo transcriptional program of proliferation. Dev. Cell 2012, 23, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Dong, H.H.; Liang, H.F.; He, S.Q.; Zhang, W.; Li, C.H.; Zhang, B.X.H.; Zhang, B.X.H.; Jing, K.; Tomlinson, S.; et al. Oval cell response is attenuated by depletion of liver resident macrophages in the 2-AAF/partial hepatectomy rat. PLoS ONE 2012, 7, e35180. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.S.; Jiang, Y.; Zhang, L.X.; Chang, C.F.; Wang, G.P.; Shi, R.J.; Yang, Y.J. The role of kupffer cells in rat liver regeneration revealed by cell-specific microarray analysis. J. Cell. Biochem. 2012, 113, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Liver Regeneration after Partial Hepatectomy. Am. J. Pathol. 2010, 176, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N. Liver regeneration. J. Hepatol. 2000, 32, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. J. Hepatol. 2011, 57, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.M.; Lee, F.Y.J.; Rosen, A.; Yang, S.Q.; Lin, H.Z.; Koteish, A.; Liew, F.Y.; Zaragoza, C.; Lowenstein, C.; Diehl, A.M. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13829–13834. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, H.; Park, O.; Wei, W.; Shen, J.; Gao, B. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3. Am. J. Pathol. 2011, 178, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Advances in liver regeneration. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.M.; Kizy, S.; Steer, C.J. Liver Regeneration in the Acute Liver Failure Patient. Clin. Liver Dis. 2018, 22, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Shanmukhappa, K.; Matte, U.; Degen, J.L.; Bezerra, J.A. Plasmin-mediated proteolysis is required for hepatocyte growth factor activation during liver repair. J. Biol. Chem. 2009, 284, 12917–12923. [Google Scholar] [CrossRef] [PubMed]
- Knittel, T.; Mehde, M.; Grundmann, A.; Saile, B.; Scharf, J.G.; Ramadori, G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem. Cell Biol. 2000, 113, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Olle, E.W.; Ren, X.; McClintock, S.D.; Warner, R.L.; Deogracias, M.P.; Johnson, K.J.; Colletti, L.M. Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology 2006, 44, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Tomiya, T.; Ogata, I.; Yamaoka, M.; Yanase, M.; Inoue, Y.; Fujiwara, K. The mitogenic activity of hepatocyte growth factor on rat hepatocytes is dependent upon endogenous transforming growth factor-α. Am. J. Pathol. 2000, 157, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Song, G.; Roll, G.R.; Frandsen, N.M.; Willenbring, H. A microRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration. J. Clin. Investig. 2012, 122, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Z.; Huang, Y.; Ju, W.; Wang, D.; Zhu, X.; He, X. MicroRNA-26a targets the mdm2/p53 loop directly in response to liver regeneration. Int. J. Mol. Med. 2019, 44, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Delgado, E.R.; Otero, P.A.; Teng, K.-Y.; Kutay, H.; Meehan, K.M.; Moroney, J.B.; Monga, J.K.; Hand, N.J.; Friedman, J.R.; et al. MicroRNA-122 regulates polyploidization in the murine liver. Hepatology 2016, 64, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, M.; Chen, E.; Tang, H. Liver Regeneration: Analysis of the Main Relevant Signaling Molecules. Mediat. Inflamm. 2017, 2017, 4256352. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, M.; Okabe, H.; Poddar, M.; Singh, S.; Monga, S.P. Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: A sequel to the Wnt-Wnt situation. Hepatol. Commun. 2018, 2, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.O.; Monga, S.P. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Ortica, S.; Tarantino, N.; Aulner, N.; Israël, A.; Gupta-Rossi, N. The 4 Notch receptors play distinct and antagonistic roles in the proliferation and hepatocytic differentiation of liver progenitors. FASEB J. 2014, 28, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Morell, C.M.; Fiorotto, R.; Fabris, L.; Strazzabosco, M. Notch signalling beyond liver development: Emerging concepts in liver repair and oncogenesis. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.; Bell, A.W.; Bowen, W.C.; Monga, S.P.; Fleig, W.; Michalopoulos, G.K. Expression of Notch-1 and its Ligand Jagged-1 in Rat Liver during Liver Regeneration. Hepatology 2004, 39, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Croquelois, A.; Blindenbacher, A.; Terracciano, L.; Wang, X.; Langer, I.; Radtke, F.; Heim, M.H. Inducible inactivation of Notch1 causes nodular regenerative hyperplasia in mice. Hepatology 2005, 41, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, A.M. The microcirculatory hepatic unit. Microvasc. Res. 1973, 6, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Gilgenkrantz, H.; Collin de l’Hortet, A. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am. J. Pathol. 2018, 188, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Volk, A.; Michalopoulos, G.; Weidner, M.; Gebhardt, R. Different proliferative responses of periportal and pericentral rat hepatocytes to hepatocyte growth factor. Biochem. Biophys. Res. Commun. 1995, 207, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, S.; Bian, Y.; Poddar, M.; Singh, S.; Cao, C.; McGaughey, J.; Bell, A.; Blazer, L.L.; Adams, J.J.; et al. Single-cell spatial transcriptomics reveals a dynamic control of metabolic zonation and liver regeneration by endothelial cell Wnt2 and Wnt9b. Cell Rep. Med. 2022, 3, 100754. [Google Scholar] [CrossRef] [PubMed]
- Manco, R.; Leclercq, I.A.; Clerbaux, L.-A. Liver Regeneration: Different Sub-Populations of Parenchymal Cells at Play Choreographed by an Injury-Specific Microenvironment. Int. J. Mol. Sci. 2018, 19, 4115. [Google Scholar] [CrossRef] [PubMed]
- Karaca, G.; Swiderska-Syn, M.; Xie, G.; Syn, W.K.; Krüger, L.; Machado, M.V.; Garman, K.; Choi, S.S.; Michelotti, G.A.; Burkly, L.C.; et al. TWEAK/Fn14 signaling is required for liver regeneration after partial hepatectomy in mice. PLoS ONE 2014, 9, e83987. [Google Scholar] [CrossRef] [PubMed]
- Passman, A.M.; Haughey, M.J.; Carlotti, E.; Williams, M.J.; Cereser, B.; Lin, M.L.; Devkumar, S.; Gabriel, J.P.; Gringeri, E.; Cillo, U.; et al. Hepatocytes undergo punctuated expansion dynamics from a periportal stem cell niche in normal human liver. J. Hepatol. 2023, 79, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Elchaninov, A.; Fatkhudinov, T.; Usman, N.; Kananykhina, E.; Arutyunyan, I.; Makarov, A.; Bolshakova, G.; Goldshtein, D.; Sukhikh, G. Molecular survey of cell source usage during subtotal hepatectomy-induced liver regeneration in rats. PLoS ONE 2016, 11, e0162613. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, K.; Kawaguchi, Y.; Akiyama, H.; Horiguchi, M.; Kodama, S.; Kuhara, T.; Hosokawa, S.; Elbahrawy, A.; Soeda, T.; Koizumi, M.; et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 2011, 43, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Wang, X.; Xie, G.; Wang, L.L.; Hill, C.K.; DeLeve, L.D. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J. Clin. Investig. 2012, 122, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Pikiolek, M.; Orsini, V.; Bergling, S.; Holwerda, S.; Morelli, L.; Hoppe, P.S.; Planas-Paz, L.; Yang, Y.; Ruffner, H.; et al. AXIN2+ Pericentral Hepatocytes Have Limited Contributions to Liver Homeostasis and Regeneration. Cell Stem Cell 2020, 26, 97–107.e6. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Y.G.; Jia, Y.; Li, L.; Yoon, J.; Zhang, S.; Wang, Z.; Zhang, Y.; Zhu, M.; Sharma, T.; et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science 2021, 371, eabb1625. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pu, W.; Liu, X.; Zhang, Z.; Han, M.; Li, Y.; Huang, X.; Han, X.; Li, Y.; Liu, K.; et al. Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science 2021, 371, eabc4346. [Google Scholar] [CrossRef] [PubMed]
- Bucher, N.L.R.; Glinos, A.D. The effect of age on regeneration of rat liver. Cancer Res. 1950, 10, 324–332. [Google Scholar] [PubMed]
- Timchenko, N.A.; Wilde, M.; Darlington, G.J. C/EBPα Regulates Formation of S-Phase-Specific E2F-p107 Complexes in Livers of Newborn Mice. Mol. Cell. Biol. 1999, 19, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, N.A. Aging and liver regeneration. Trends Endocrinol. Metab. 2009, 20, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Lambotte, L.; Saliez, A.; Triest, S.; Tagliaferri, E.M.; Barker, A.P.; Baranski, A.G. Control of rate and extent of the proliferative response after partial hepatectomy. Am. J. Physiol. Liver Physiol. 1997, 273, G905–G912. [Google Scholar] [CrossRef] [PubMed]
- Gadd, V.L.; Aleksieva, N.; Forbes, S.J. Epithelial Plasticity during Liver Injury and Regeneration. Cell Stem Cell 2020, 27, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Yanger, K.; Zong, Y.; Maggs, L.R.; Shapira, S.N.; Maddipati, R.; Aiello, N.M.; Thung, S.N.; Wells, R.G.; Greenbaum, L.E.; Stanger, B.Z. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013, 27, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Sone, M.; Chen, X.; Okada, Y.; Yamamoto, M.; Xin, B.; Matsuo, Y.; Komatsu, M.; Suzuki, A.; Enomoto, K.; et al. Contributions of Hepatocytes and Bile Ductular Cells in Ductular Reactions and Remodeling of the Biliary System after Chronic Liver Injury. Am. J. Pathol. 2014, 184, 3001–3012. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G. Microenvironmental control of the ductular reaction: Balancing repair and disease progression. Cell Death Dis. 2025, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Tarlow, B.D.; Pelz, C.; Naugler, W.E.; Wakefield, L.; Wilson, E.M.; Finegold, M.J.; Grompe, M. Bipotential Adult Liver Progenitors Are Derived from Chronically Injured Mature Hepatocytes. Cell Stem Cell 2014, 15, 605–618. [Google Scholar] [CrossRef]
- Lan, T.; Tai, Y.; Zhao, C.; Xiao, Y.; Yang, Z.; Zhang, L.; Gan, C.; Dai, W.; Tong, H.; Tang, C.; et al. Atypical cholangiocytes derived from hepatocyte-cholangiocyte transdifferentiation mediated by COX-2: A kind of misguided liver regeneration. Inflamm. Regen. 2023, 43, 37. [Google Scholar] [CrossRef] [PubMed]
- Planas-Paz, L.; Sun, T.; Pikiolek, M.; Cochran, N.R.; Bergling, S.; Orsini, V.; Yang, Z.; Sigoillot, F.; Jetzer, J.; Syed, M.; et al. YAP, but Not RSPO-LGR4/5, Signaling in Biliary Epithelial Cells Promotes a Ductular Reaction in Response to Liver Injury. Cell Stem Cell 2019, 25, 39–53.e10. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Sussman, J.H.; Ito, K.; Katznelson, A.; Yuan, S.; Takenaka, N.; Li, J.; Merrell, A.J.; Cure, H.; Li, Q.; et al. Cellular reprogramming in vivo initiated by SOX4 pioneer factor activity. Nat. Commun. 2024, 15, 1761. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, K.; Tanimizu, N. Lineage plasticity and reprogramming of epithelial cells during tissue injury and regeneration-lessons from the lineage plasticity of hepatocytes and cholangiocytes induced by liver injury. Regen. Ther. 2025, 29, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.M.; Zhu, J.; Li, Q.; Pradhan-Sundd, T.; Krutsenko, Y.; Sayed, K.; Jenkins, N.; Vats, R.; Bhushan, B.; Ko, S.; et al. Compensatory hepatic adaptation accompanies permanent absence of intrahepatic biliary network due to YAP1 loss in liver progenitors. Cell Rep. 2021, 36, 109310. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Jiang, T.; Cui, X.; Pan, Y.; Feng, X.; Dong, L.; Wang, H. Diverse functions of SOX9 in liver development and homeostasis and hepatobiliary diseases. Genes Dis. 2024, 11, 100996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhuo, S.; Zhou, Y.; Ma, L.; Sun, Z.; Wu, X.; Wang, X.W.; Gao, B.; Yang, Y. Yap-Sox9 signaling determines hepatocyte plasticity and lineage-specific hepatocarcinogenesis. J. Hepatol. 2022, 76, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, L.; Lin, P.; Liu, Z.; Bao, S.; Ma, X.; Nan, H.; Zhu, W.; Cen, J.; Mao, Y.; et al. Kupffer-cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers. Cell Stem Cell 2023, 30, 283–299.e9. [Google Scholar] [CrossRef] [PubMed]
- Merrell, A.J.; Peng, T.; Li, J.; Sun, K.; Li, B.; Katsuda, T.; Grompe, M.; Tan, K.; Stanger, B.Z. Dynamic Transcriptional and Epigenetic Changes Drive Cellular Plasticity in the Liver. Hepatology 2021, 74, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, P.; Hao, S.; Shangguan, S.; Shi, Q.; Volpe, G.; Huang, K.; Zuo, J.; An, J.; Yuan, Y.; et al. A spatiotemporal atlas of mouse liver homeostasis and regeneration. Nat. Genet. 2024, 56, 953–969. [Google Scholar] [CrossRef] [PubMed]
- El’chaninov, A.V.; Fatkhudinov, T.K.; Kananykhina, E.Y.; Usman, N.Y.; Arutyunyan, I.V.; Makarov, A.V.; Bykov, A.V.; Bolshakova, G.V.; Sukhikh, G.T. Role of Progenitor Cells in Liver Regeneration after Subtotal Resection. Bull. Exp. Biol. Med. 2016, 161, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.H.; Cure, H.W.; Yuan, S.; Ito, K.; Asangani, I.A.; Garcia, B.A.; Stanger, B.Z.; Katsuda, T. In vivo CRISPR screening reveals epigenetic regulators of hepatobiliary plasticity. Genes Dev. 2025, 39, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.; Eccleston, J.; Sabag, O.; Marcus, H.; Sussman, J.; Ouro, A.; Rahamim, M.; Azagury, M.; Azria, B.; Stanger, B.Z.; et al. Transdifferentiation occurs without resetting development-specific DNA methylation, a key determinant of full-function cell identity. Proc. Natl. Acad. Sci. USA 2024, 121, e2411352121. [Google Scholar] [CrossRef] [PubMed]
- Ruzittu, S.; Willnow, D.; Spagnoli, F.M. Direct Lineage Reprogramming: Harnessing Cell Plasticity between Liver and Pancreas. Cold Spring Harb. Perspect. Biol. 2020, 12, a035626. [Google Scholar] [CrossRef] [PubMed]
- Shanmukhappa, K.; Mourya, R.; Sabla, G.E.; Degen, J.L.; Bezerra, J.A. Hepatic to pancreatic switch defines a role for hemostatic factors in cellular plasticity in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 10182–10187. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.; Halkin, A.; Cohen, H.; Ber, I.; Einav, Y.; Goldberg, I.; Barshack, I.; Seijffers, R.; Kopolovic, J.; Kaiser, N.; et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 2000, 6, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Esteban, N.; Naumann, H.; Ruzittu, S.; Mah, N.; Pongrac, I.M.; Cozzitorto, C.; Hommel, A.; Andrade-Navarro, M.A.; Bonifacio, E.; Spagnoli, F.M. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat. Commun. 2017, 8, 14127. [Google Scholar] [CrossRef]
- Yechoor, V.; Liu, V.; Espiritu, C.; Paul, A.; Oka, K.; Kojima, H.; Chan, L. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev. Cell 2009, 16, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Gribben, C.; Galanakis, V.; Calderwood, A.; Williams, E.C.; Chazarra-Gil, R.; Larraz, M.; Frau, C.; Puengel, T.; Guillot, A.; Rouhani, F.J.; et al. Acquisition of epithelial plasticity in human chronic liver disease. Nature 2024, 630, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Glaser, S.S.; Ueno, Y.; Pham, L.; Podila, P.V.; Caligiuri, A.; LeSage, G.; LaRusso, N.F. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am. J. Physiol.-Gastrointest. Liver Physiol. 1998, 274, G767–G775. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.; Glaser, S.; Alpini, G. Regulation of cholangiocyte proliferation. Liver 2001, 21, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Ulrich, C.; Roberts, S.; Phillips, J.O.; Ueno, Y.; Podila, P.V.; Colegio, O.; LeSage, G.D.; Miller, L.J.; LaRusso, N.F. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am. J. Physiol. 1997, 272, G289–G297. [Google Scholar] [CrossRef]
- Lesage, G.; Glaser, S.S.; Gubba, S.; Robertson, W.E.; Phinizy, J.L.; Lasater, J.; Rodgers, R.E.D.; Alpini, G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 1996, 111, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- LeSage, G.D.; Benedetti, A.; Glaser, S.; Marucci, L.; Tretjak, Z.; Caligiuri, A.; Rodgers, R.; Lynne Phinizy, J.; Baiocchi, L.; Francis, H.; et al. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology 1999, 29, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.D.; Glaser, S.S.; Marucci, L.; Benedetti, A.; Phinizy, J.L.; Rodgers, R.; Caligiuri, A.; Papa, E.; Tretjak, Z.; Jezequel, A.M.; et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am. J. Physiol. 1999, 276, G1289–G1301. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Glaser, S.S.; Ueno, Y.; Rodgers, R.; Phinizy, J.L.; Francis, H.; Baiocchi, L.; Holcomb, L.A.; Caligiuri, A.; Lesage, G.D. Bile acid feeding induces cholangiocyte proliferation and secretion: Evidence for bile acid-regulated ductal secretion. Gastroenterology 1999, 116, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Roskams, T.A.; Theise, N.D.; Balabaud, C.; Bhagat, G.; Bhathal, P.S.; Bioulac-Sage, P.; Brunt, E.M.; Crawford, J.M.; Crosby, H.A.; Desmet, V.; et al. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004, 39, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.; Francis, H.; DeMorrow, S.; LeSage, G.; Fava, G.; Marzioni, M.; Venter, J.; Alpini, G. Heterogeneity of the intrahepatic biliary epithelium. World J. Gastroenterol. 2006, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Desmet, V.; Roskams, T.; Van Eyken, P. Ductular Reaction in the Livers. Pathol.-Res. Pract. 1995, 191, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Sirica, A.E.; Gainey, T.W.; Mumaw, V.R. Ductular hepatocytes. Evidence for a bile ductular cell origin in furan-treated rats. Am. J. Pathol. 1994, 145, 375–383. [Google Scholar] [PubMed]
- Sirica, A.E.; Mathis, G.A.; Sano, N.; Elmore, L.W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology 1990, 58, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.S.; Onori, P.; Wise, C.; Yang, F.; Marzioni, M.; Alvaro, D.; Franchitto, A.; Mancinelli, R.; Alpini, G.; Munshi, M.K.; et al. Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis. Dig. Liver Dis. 2010, 42, 245. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Meng, F.; Giang, T.; Glaser, S.; Alpini, G. Mechanisms of cholangiocyte responses to injury. Biochim. Biophys. Acta 2018, 1864, 1262. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, E.; Onori, P.; Pannarale, L.; Alvaro, D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: A morphological study. Gastroenterology 1996, 111, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Rosmorduc, O.; Wendum, D.; Corpechot, C.; Galy, B.; Sebbagh, N.; Raleigh, J.; Housset, C.; Poupon, R. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am. J. Pathol. 1999, 155, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.; Alvaro, D.; Benedetti, A.; Glaser, S.; Marucci, L.; Baiocchi, L.; Eisel, W.; Caligiuri, A.; Phinizy, J.L.; Rodgers, R.; et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 1999, 117, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.J.; Jones, J.M.; Sacaan, A.I.; Lloyd, G.K.; Corey-Naeve, J. 6-hydroxydopamine lesion of rat nigrostriatal dopaminergic neurons differentially affects nicotinic acetylcholine receptor subunit mRNA expression. J. Mol. Neurosci. 1998, 10, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.F.; Tector, A.J.; Goerke, M.E.; Kitchen, S.; Lagunoff, D. Somatostatin analogue (octreotide) inhibits bile duct epithelial cell proliferation and fibrosis after extrahepatic biliary obstruction. Am. J. Pathol. 1993, 143, 1574. [Google Scholar] [PubMed]
- Glaser, S.; Benedetti, A.; Marucci, L.; Alvaro, D.; Baiocchi, L.; Kanno, N.; Caligiuri, A.; Phinizy, J.L.; Chowdury, U.; Papa, E.; et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/gastrin receptors via D-myo-Inositol 1,4,5-triphosphate-, Ca2+-, and protein kinase C α-dependent mechanisms. Hepatology 2000, 32, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Glaser, S.S.; Rodgers, R.; Phinizy, J.L.; Robertson, W.E.; Lasater, J.; Caligiuri, A.; Tretjak, Z.; LeSage, G.D. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology 1997, 113, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Glaser, S.; Alvaro, D.; Ueno, Y.; Marzioni, M.; Francis, H.; Baiocchi, L.; Stati, T.; Barbaro, B.; Phinizy, J.L.; et al. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology 2002, 123, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Alpini, G.; Onori, P.; Perego, L.; Baroni, G.S.; Franchitto, A.; Baiocchi, L.; Glaser, S.S.; Le Sage, G.; Folli, F.; et al. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology 2000, 119, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.K.; Ludwig, J.; LaRusso, N.F. The pathobiology of biliary epithelia. Gastroenterology 1997, 112, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.; Glaser, S.; Ueno, Y.; Lesage, G.; Marucci, L.; Benedetti, A.; Taffetani, S.; Marzioni, M.; Alvaro, D.; Venter, J.; et al. CAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J. Hepatol. 2004, 41, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, L.; Lenci, I.; Milana, M.; Kennedy, L.; Sato, K.; Zhang, W.; Ekser, B.; Ceci, L.; Meadows, V.; Glaser, S.; et al. Cyclic AMP Signaling in Biliary Proliferation: A Possible Target for Cholangiocarcinoma Treatment? Cells 2021, 10, 1692. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Meng, F.; Venter, J.; Giang, T.; Glaser, S.; Alpini, G. The role of the secretin/secretin receptor axis in inflammatory cholangiocyte communication via extracellular vesicles. Sci. Rep. 2017, 7, 11183. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Ulrich, C.D.; Phillips, J.O.; Pham, L.D.; Miller, L.J.; LaRusso, N.F. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am. J. Physiol. 1994, 266, G922–G928. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gores, G.J.; Patel, T. Lipopolysaccharide induces cholangiocyte proliferation via an interleukin-6-mediated activation of p44/p42 mitogen-activated protein kinase. Hepatology 1999, 29, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.; Prentice, D.; Niinami, C.; Bishop, G.A.; Desmond, P.; Mccaughan, G.W. Sequential increases in the intrahepatic expression of epidermal growth factor, basic fibroblast growth factor, and transforming growth factor β in a bile duct ligated rat model of cirrhosis. Hepatology 1997, 26, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Zhu, H.; Zhang, M.; Pikiolek, M.; Ercan, C.; Li, J.; Huang, X.; Han, X.; Zhang, Z.; Lv, Z.; et al. Bipotent transitional liver progenitor cells contribute to liver regeneration. Nat. Genet. 2023, 55, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, A.; Tanaka, M.; Itoh, T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell 2014, 14, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Mavila, N.; Siraganahalli Eshwaraiah, M.; Kennedy, J. Ductular Reactions in Liver Injury, Regeneration, and Disease Progression—An Overview. Cells 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.O.; Lu, W.Y.; Okabe, H.; Abrams, M.; Oertel, M.; Poddar, M.; Singh, S.; Forbes, S.J.; Monga, S.P. Hepatocyte-Specific β-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes. Hepatology 2019, 69, 742–759. [Google Scholar] [CrossRef] [PubMed]
- Raven, A.; Lu, W.Y.; Man, T.Y.; Ferreira-Gonzalez, S.; O’Duibhir, E.; Dwyer, B.J.; Thomson, J.P.; Meehan, R.R.; Bogorad, R.; Koteliansky, V.; et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 2017, 547, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011, 458, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Clerbaux, L.A.; Manco, R.; Van Hul, N.; Bouzin, C.; Sciarra, A.; Sempoux, C.; Theise, N.D.; Leclercq, I.A. Invasive Ductular Reaction Operates Hepatobiliary Junctions upon Hepatocellular Injury in Rodents and Humans. Am. J. Pathol. 2019, 189, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Gouw, A.S.H.; Clouston, A.D.; Theise, N.D. Ductular reactions in human liver: Diversity at the interface. Hepatology 2011, 54, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Aizarani, N.; Saviano, A.; Sagar; Mailly, L.; Durand, S.; Herman, J.S.; Pessaux, P.; Baumert, T.F.; Grün, D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 2019, 572, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kamimoto, K.; Kaneko, K.; Kok, C.Y.Y.; Okada, H.; Miyajima, A.; Itoh, T. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. eLife 2016, 5, e15034. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, D.; Shimata, K.; Yokouchi, Y.; Komohara, Y.; Suda, H.; Honda, M.; Yamamura, K.; Hibi, T.; Inomata, Y. SOX9 contributes to the progression of ductular reaction for the protection from chronic liver injury. Hum. Cell 2022, 35, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Pepe-Mooney, B.J.; Dill, M.T.; Alemany, A.; Ordovas-Montanes, J.; Matsushita, Y.; Rao, A.; Sen, A.; Miyazaki, M.; Anakk, S.; Dawson, P.A.; et al. Single-Cell Analysis of the Liver Epithelium Reveals Dynamic Heterogeneity and an Essential Role for YAP in Homeostasis and Regeneration. Cell Stem Cell 2019, 25, 23–38.e8. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, F.; Lee, Y.R.; Diaz-Aragon, R.; Bawa, P.S.; So, J.; Florentino, R.M.; Wu, S.; Sarjoo, A.; Truong, E.; Smith, A.R.; et al. VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion in acute and chronic liver diseases and reverses steatosis and fibrosis. Cell Stem Cell 2023, 30, 1640–1657.e8. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; McCall, S.J.; Li, Y.X.; Diehl, A.M. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology 2007, 45, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, F.; Wang, J.; Wang, C.; Jiang, Y.; Liu, B.; He, J.; Yuan, K.; Pan, C.; Lin, M.; et al. Identification of a rare Gli1+ progenitor cell population contributing to liver regeneration during chronic injury. Cell Discov. 2022, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Tarlow, B.D.; Finegold, M.J.; Grompe, M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology 2014, 60, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, D.; Yan, Y.; Wu, J.; Meng, L.; Huang, W.; Wang, L.; Chen, X.; Zhang, L. Metabolic nuclear receptors coordinate energy metabolism to regulate Sox9+ hepatocyte fate. iScience 2021, 24, 103003. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.; Akinci, E.; Greder, L.V.; Dutton, J.R.; Slack, J.M.W. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc. Natl. Acad. Sci. USA 2012, 109, 15336–15341. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elchaninov, A.; Vishnyakova, P.; Glinkina, V.; Fatkhudinov, T.; Sukhikh, G. Liver Regeneration as a Model for Studying Cellular Plasticity in Mammals: The Roles of Hepatocytes and Cholangiocytes. Cells 2025, 14, 1129. https://doi.org/10.3390/cells14151129
Elchaninov A, Vishnyakova P, Glinkina V, Fatkhudinov T, Sukhikh G. Liver Regeneration as a Model for Studying Cellular Plasticity in Mammals: The Roles of Hepatocytes and Cholangiocytes. Cells. 2025; 14(15):1129. https://doi.org/10.3390/cells14151129
Chicago/Turabian StyleElchaninov, Andrey, Polina Vishnyakova, Valeria Glinkina, Timur Fatkhudinov, and Gennady Sukhikh. 2025. "Liver Regeneration as a Model for Studying Cellular Plasticity in Mammals: The Roles of Hepatocytes and Cholangiocytes" Cells 14, no. 15: 1129. https://doi.org/10.3390/cells14151129
APA StyleElchaninov, A., Vishnyakova, P., Glinkina, V., Fatkhudinov, T., & Sukhikh, G. (2025). Liver Regeneration as a Model for Studying Cellular Plasticity in Mammals: The Roles of Hepatocytes and Cholangiocytes. Cells, 14(15), 1129. https://doi.org/10.3390/cells14151129









