Characteristics of Scar Formation After Intracerebral Hemorrhage in Aged Rats: Effects of Deferoxamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and ICH Model
2.2. Experimental Groups
2.3. Hematoxylin and Eosin Staining and Perls’ Prussian Blue Staining
2.4. Immunofluorescence
2.5. Immunohistochemistry
2.6. Cell Counting
2.7. Statistical Analysis
3. Results
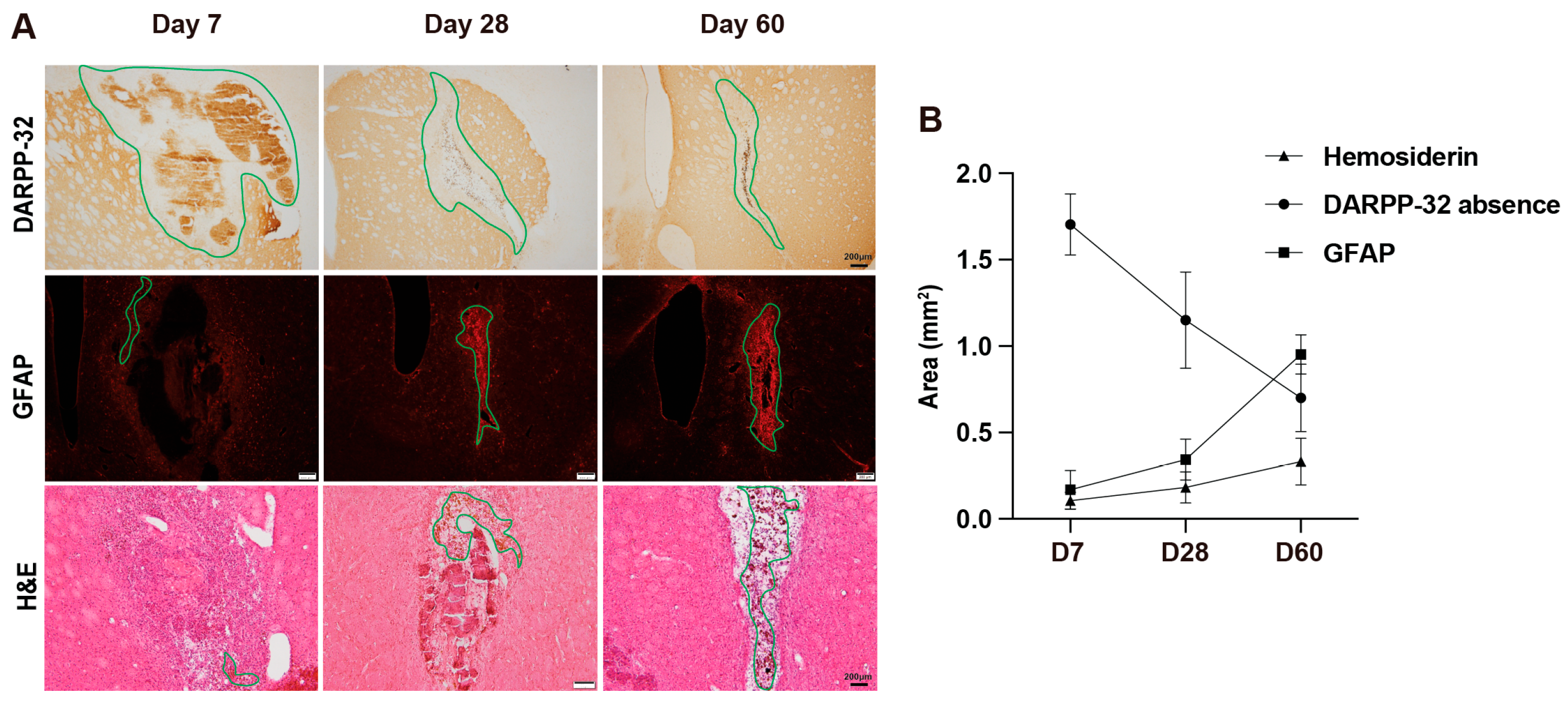
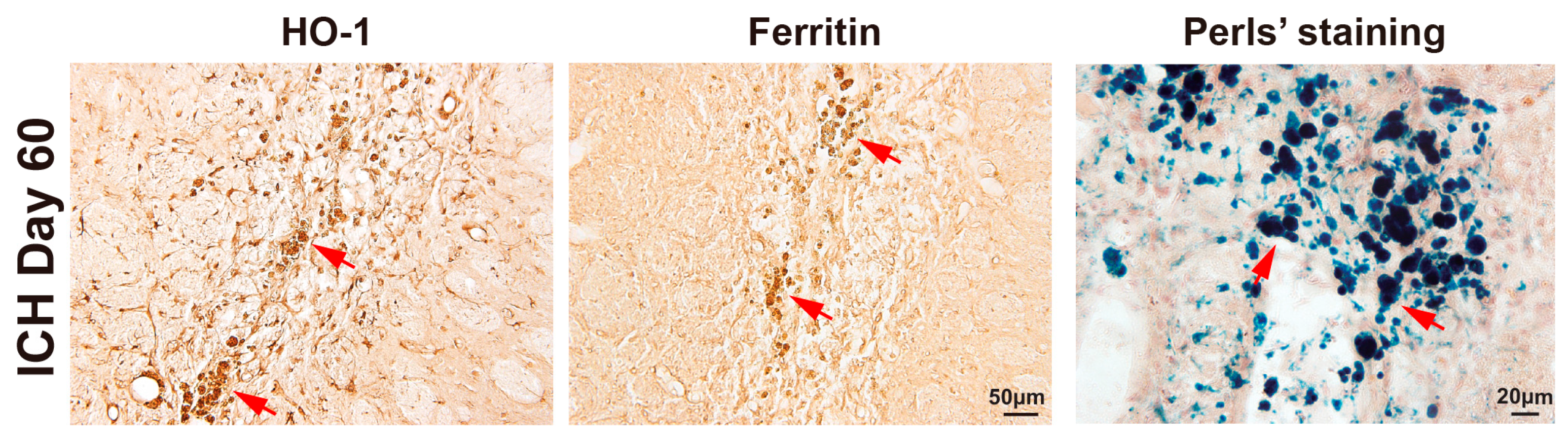
3.1. Evolution of Tissue Injury and Scar Formation from Day 7 to Day 60 After ICH
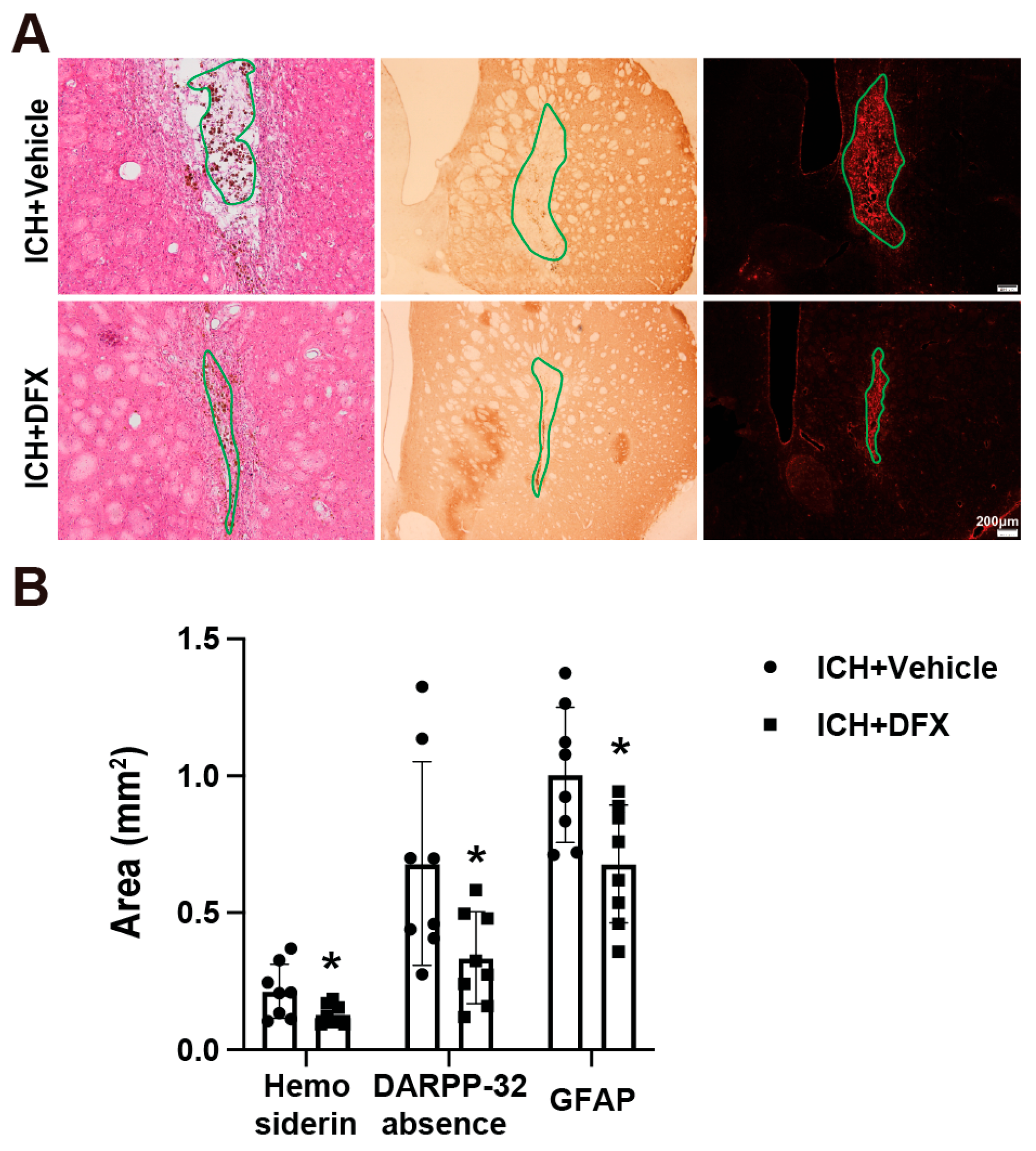
3.2. DFX Treatment Significantly Reduces Hemosiderin Deposition, Astrogliosis, and DARPP-32 Absence Area on Day 60 Post-ICH
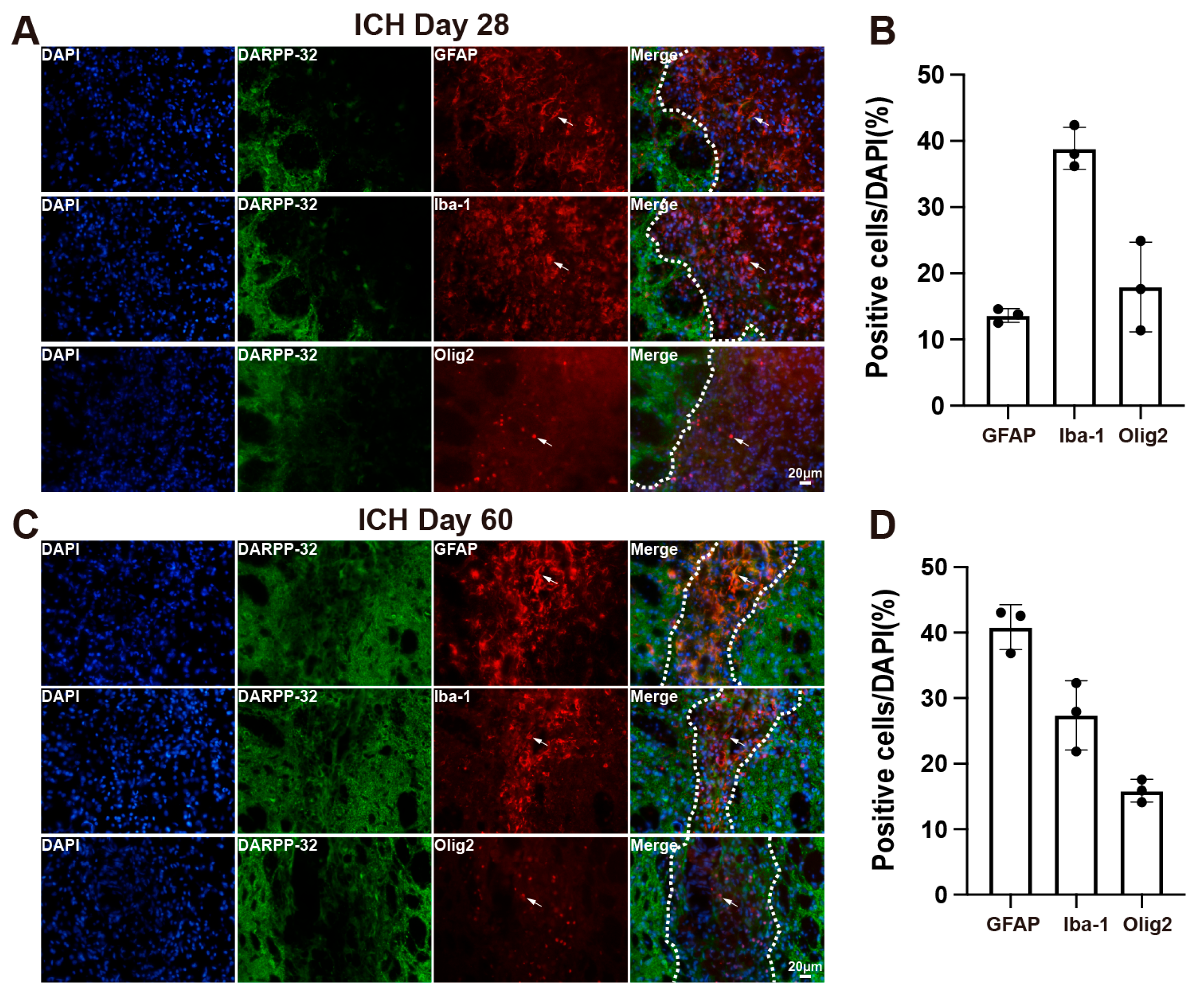
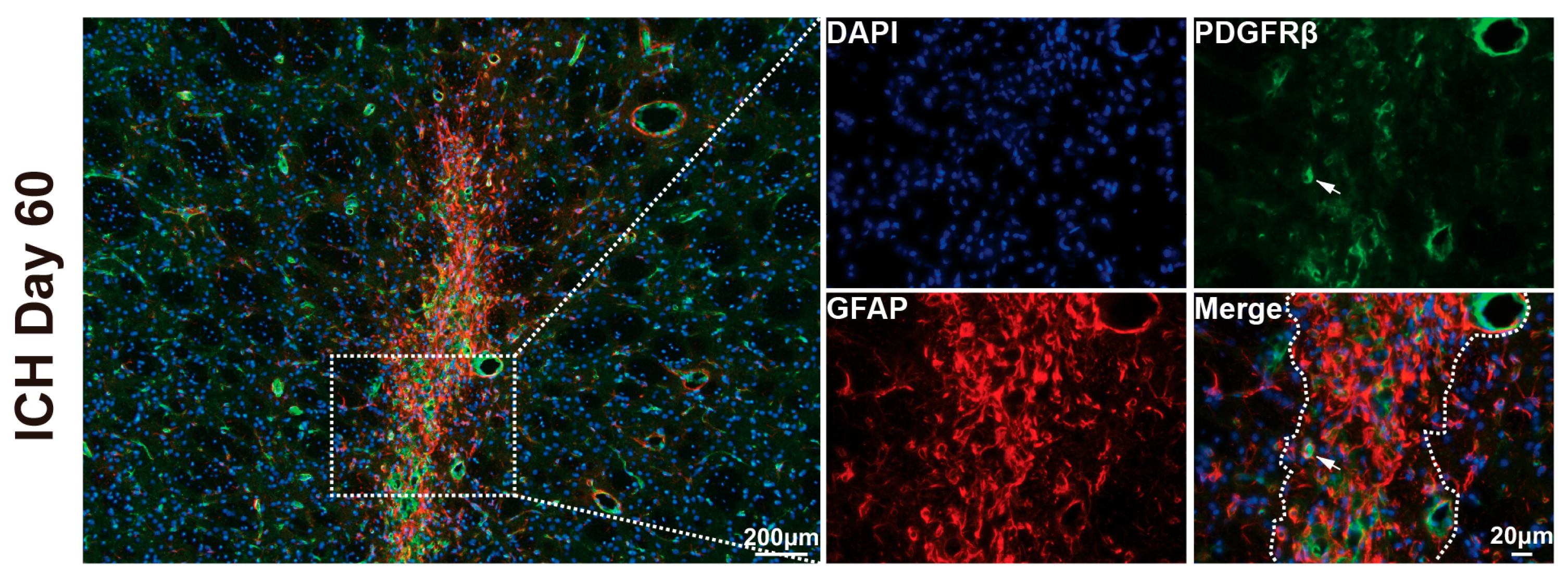
3.3. Cellular Composition of the Scar in Aged Rats After ICH
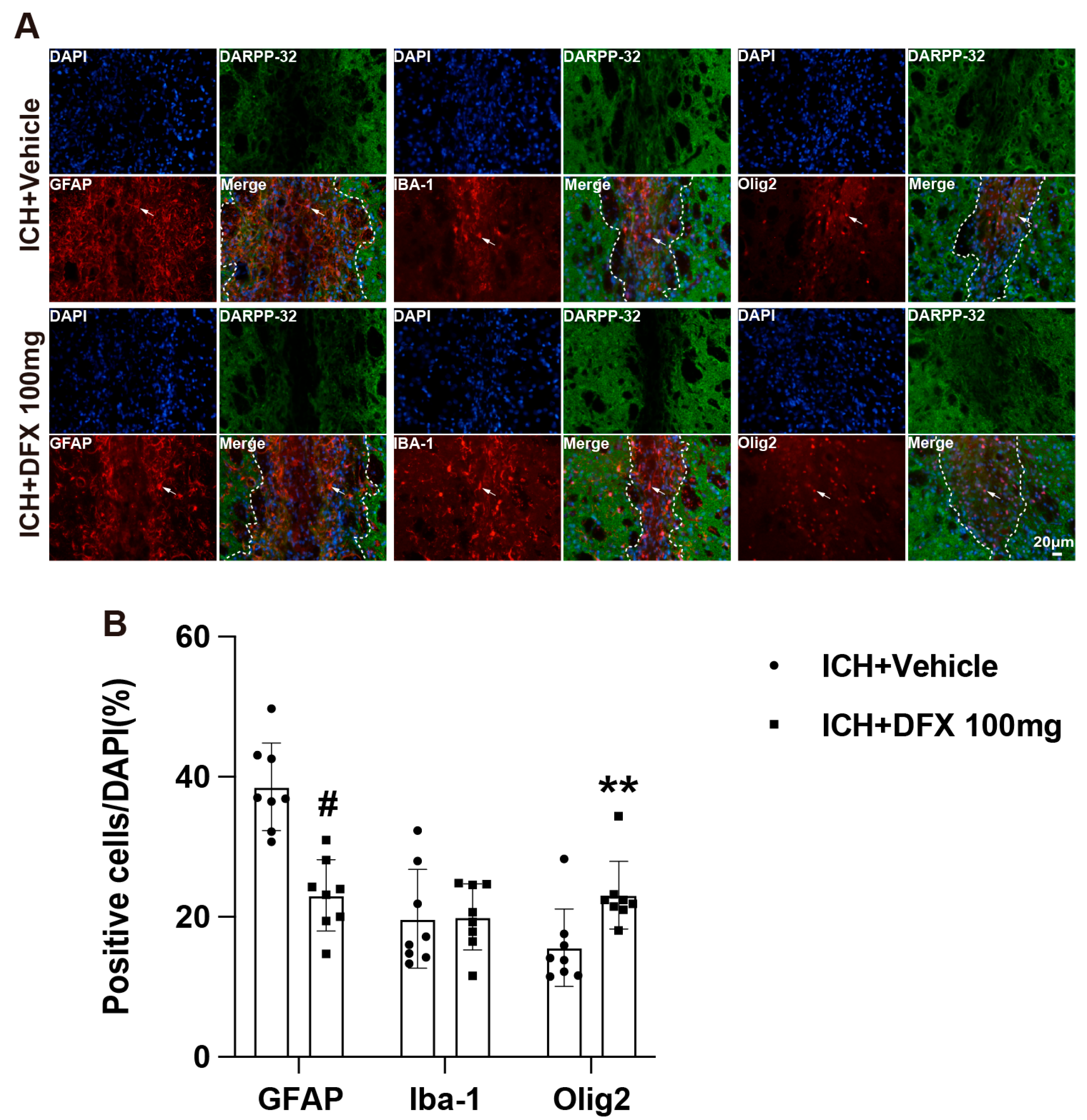
3.4. DFX Treatment Modulates Glial Composition Within the Scar Following ICH in Aged Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICH | Intracerebral Hemorrhage |
| Hb | Hemoglobin |
| HO-1 | Heme Oxygenase-1 |
| Iba-1 | Ionized Calcium-Binding Adapter Molecule 1 |
| Olig2 | Oligodendrocyte Transcription Factor 2 |
| PDGFRβ | Platelet-Derived Growth Factor Receptor Beta |
| DARPP-32 | Dopamine- and cAMP-Regulated Phosphoprotein of 32 kDa |
| CNS | Central Nervous System |
| DFX | Deferoxamine |
| PFA | Paraformaldehyde |
| OTC | Optimal Cutting Temperature |
| H&E | Hematoxylin and Eosin |
| NIH | National Institutes of Health |
| SD | Standard Deviation |
| DAB | Diaminobenzidine |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| PBS | Phosphate-Buffered Saline |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| GFAP | Glial Fibrillary Acidic Protein |
References
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Pradilla, G.; Ratcliff, J.J.; Hall, A.J.; Saville, B.R.; Allen, J.W.; Paulon, G.; McGlothlin, A.; Lewis, R.J.; Fitzgerald, M.; Caveney, A.F.; et al. Trial of Early Minimally Invasive Removal of Intracerebral Hemorrhage. N. Engl. J. Med. 2024, 390, 1277–1289. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Puy, L.; Parry-Jones, A.R.; Sandset, E.C.; Dowlatshahi, D.; Ziai, W.; Cordonnier, C. Intracerebral haemorrhage. Nat. Rev. Dis. Primers 2023, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 2010, 92, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.L.; Viaene, A.N. What are activated and reactive glia and what is their role in neurodegeneration? Neurobiol. Dis. 2021, 148, 105172. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.O.; Kim, H.; Holl, D.; Werne Solnestam, B.; Lundeberg, J.; Carlén, M.; Göritz, C.; Frisén, J. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 2018, 173, 153–165.e122. [Google Scholar] [CrossRef]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Balkaya, M.; Kim, I.D.; Shakil, F.; Cho, S. CD36 deficiency reduces chronic BBB dysfunction and scar formation and improves activity, hedonic and memory deficits in ischemic stroke. J. Cereb. Blood Flow Metab. 2021, 41, 486–501. [Google Scholar] [CrossRef]
- Schiweck, J.; Murk, K.; Ledderose, J.; Münster-Wandowski, A.; Ornaghi, M.; Vida, I.; Eickholt, B.J. Drebrin controls scar formation and astrocyte reactivity upon traumatic brain injury by regulating membrane trafficking. Nat. Commun. 2021, 12, 1490. [Google Scholar] [CrossRef]
- Dias, D.O.; Kalkitsas, J.; Kelahmetoglu, Y.; Estrada, C.P.; Tatarishvili, J.; Holl, D.; Jansson, L.; Banitalebi, S.; Amiry-Moghaddam, M.; Ernst, A.; et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 2021, 12, 5501. [Google Scholar] [CrossRef]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef]
- Raposo, C.; Schwartz, M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia 2014, 62, 1895–1904. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wei, R.; Khan, S.; Zhang, R.; Zhang, Y.; Yong, V.W.; Xue, M. Iron Neurotoxicity and Protection by Deferoxamine in Intracerebral Hemorrhage. Front. Mol. Neurosci. 2022, 15, 927334. [Google Scholar] [CrossRef]
- Okauchi, M.; Hua, Y.; Keep, R.F.; Morgenstern, L.B.; Xi, G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke 2009, 40, 1858–1863. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Okauchi, M.; Hua, Y.; Keep, R.F.; Morgenstern, L.B.; Schallert, T.; Xi, G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: Therapeutic time window and optimal duration. Stroke 2010, 41, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.A.; Allan, S.M.; Parry-Jones, A.R. Experimental intracerebral hemorrhage: Avoiding pitfalls in translational research. J. Cereb. Blood Flow Metab. 2011, 31, 2135–2151. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef] [PubMed]
- Camand, E.; Morel, M.P.; Faissner, A.; Sotelo, C.; Dusart, I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur. J. Neurosci. 2004, 20, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; Lin, J.; Liang, R.; He, M.; Wang, Y.; Tan, H. Porous Three-Dimensional Polyurethane Scaffolds Promote Scar-Free Endogenous Regeneration After Acute Brain Hemorrhage. Transl. Stroke Res. 2023, 16, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, H.; Wang, X.; Zhang, G.; Lu, J.; Xu, W.; Xu, S.; Fang, Y.; Zhang, A.; Shao, A.; et al. Temporal dynamics of microglia-astrocyte interaction in neuroprotective glial scar formation after intracerebral hemorrhage. J. Pharm. Anal. 2023, 13, 862–879. [Google Scholar] [CrossRef]
- Germonpré, C.; Proesmans, S.; Bouckaert, C.; Sprengers, M.; Boon, P.; Raedt, R.; De Herdt, V. Seizures and Interictal Epileptiform Activity in the Rat Collagenase Model for Intracerebral Hemorrhage. Front. Neurosci. 2021, 15, 682036. [Google Scholar] [CrossRef]
- Love, C.J.; Kirschenbaum, D.; Selim, M.; Lo, E.H.; Rushing, E.; Spector, M.; Aguzzi, A. Observation of Collagen-Containing Lesions After Hematoma Resolution in Intracerebral Hemorrhage. Stroke 2021, 52, 1856–1860. [Google Scholar] [CrossRef]
- Farr, A.C.; Xiong, M.P. Challenges and Opportunities of Deferoxamine Delivery for Treatment of Alzheimer’s Disease, Parkinson’s Disease, and Intracerebral Hemorrhage. Mol. Pharm. 2021, 18, 593–609. [Google Scholar] [CrossRef]
- Nakamura, T.; Keep, R.F.; Hua, Y.; Schallert, T.; Hoff, J.T.; Xi, G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J. Neurosurg. 2004, 100, 672–678. [Google Scholar] [CrossRef]
- Gu, Y.; Hua, Y.; Keep, R.F.; Morgenstern, L.B.; Xi, G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke 2009, 40, 2241–2243. [Google Scholar] [CrossRef] [PubMed]
- Auriat, A.M.; Silasi, G.; Wei, Z.; Paquette, R.; Paterson, P.; Nichol, H.; Colbourne, F. Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome. Exp. Neurol. 2012, 234, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Kim, D.W.; Yi, H.J.; Kim, Y.S.; Kim, E.H.; Hwang, S.J.; Jwa, C.S.; Lee, Y.K.; Ryou, H. Effects of statin and deferoxamine administration on neurological outcomes in a rat model of intracerebral hemorrhage. Neurol. Sci. 2012, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Okauchi, M.; Hua, Y.; Keep, R.F.; Xi, G. Deferoxamine reduces cavity size in the brain after intracerebral hemorrhage in aged rats. Acta Neurochir. Suppl. 2011, 111, 185–190. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Jin, H.; Xi, G.; Keep, R.F.; Wu, J.; Hua, Y. DARPP-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Transl. Stroke Res. 2013, 4, 130–134. [Google Scholar] [CrossRef]
- Arasaratnam, C.J.; Song, J.J.; Yoshida, T.; Curtis, M.A.; Graybiel, A.M.; Faull, R.L.M.; Waldvogel, H.J. DARPP-32 cells and neuropil define striosomal system and isolated matrix cells in human striatum. J. Comp. Neurol. 2023, 531, 888–920. [Google Scholar] [CrossRef]
- Anderson, K.D.; Reiner, A. Immunohistochemical localization of DARPP-32 in striatal projection neurons and striatal interneurons: Implications for the localization of D1-like dopamine receptors on different types of striatal neurons. Brain Res. 1991, 568, 235–243. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Deng, P.; Zhang, Y.; Xu, Z.C. Inhibition of Ih in striatal cholinergic interneurons early after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 939–947. [Google Scholar] [CrossRef]
- Wan, J.; Ren, H.; Wang, J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc. Neurol. 2019, 4, 93–95. [Google Scholar] [CrossRef]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme oxygenase-1: A metabolic nike. Antioxid. Redox Signal 2014, 20, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Cairo, G.; Bou-Abdallah, F. A Brief History of Ferritin, an Ancient and Versatile Protein. Int. J. Mol. Sci. 2024, 26, 206. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Hesp, Z.C.; Goldstein, E.Z.; Miranda, C.J.; Kaspar, B.K.; McTigue, D.M. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J. Neurosci. 2015, 35, 1274–1290. [Google Scholar] [CrossRef]
- Selim, M.; Foster, L.D.; Moy, C.S.; Xi, G.; Hill, M.D.; Morgenstern, L.B.; Greenberg, S.M.; James, M.L.; Singh, V.; Clark, W.M.; et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): A multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019, 18, 428–438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Wan, Y.; Hua, Y.; Xi, G.; Keep, R.F. Characteristics of Scar Formation After Intracerebral Hemorrhage in Aged Rats: Effects of Deferoxamine. Cells 2025, 14, 1127. https://doi.org/10.3390/cells14151127
Fu X, Wan Y, Hua Y, Xi G, Keep RF. Characteristics of Scar Formation After Intracerebral Hemorrhage in Aged Rats: Effects of Deferoxamine. Cells. 2025; 14(15):1127. https://doi.org/10.3390/cells14151127
Chicago/Turabian StyleFu, Xiongjie, Yingfeng Wan, Ya Hua, Guohua Xi, and Richard F. Keep. 2025. "Characteristics of Scar Formation After Intracerebral Hemorrhage in Aged Rats: Effects of Deferoxamine" Cells 14, no. 15: 1127. https://doi.org/10.3390/cells14151127
APA StyleFu, X., Wan, Y., Hua, Y., Xi, G., & Keep, R. F. (2025). Characteristics of Scar Formation After Intracerebral Hemorrhage in Aged Rats: Effects of Deferoxamine. Cells, 14(15), 1127. https://doi.org/10.3390/cells14151127







