Regulation of Hindbrain Vascular Development by rps20 in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Bioinformatics Analysis
2.3. Evolutionary Conservation Analysis of RPS20
2.4. Whole-Mount In Situ Hybridization
2.5. Quantitative Real-Time PCR
2.6. O-Dianisidine Stain
2.7. Microinjection
2.8. Confocal Imaging
2.9. Measurement of Body Length
2.10. Quantification of Phenotype
2.11. Statistical Analysis
3. Results
3.1. RPS20 Is Conservatively Down-Regulated in Aged Brain Endothelial Cells
3.2. rps20 Protein Is Highly Evolutionarily Conserved in Vertebrates
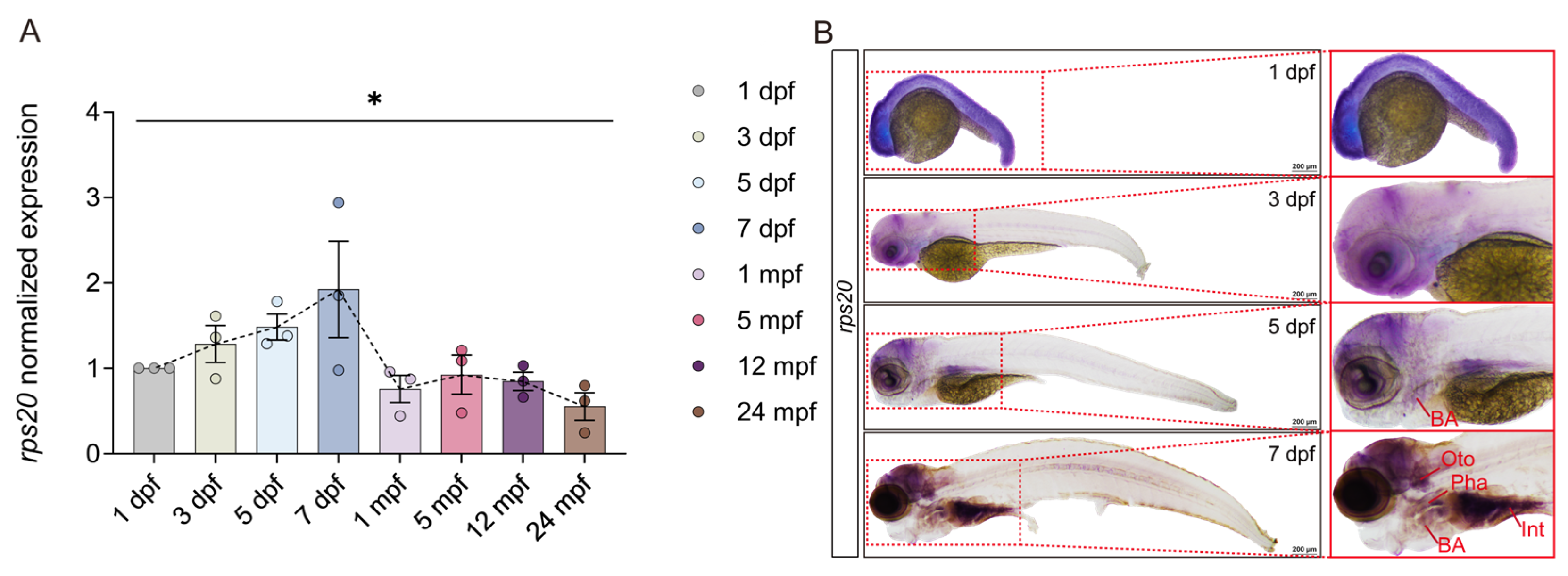
3.3. rps20 Is Highly Enriched in the Zebrafish Brain During Early Development
3.4. Knockout of rps20 Retards Hindbrain Vascular Development
3.5. Knockout of rps20 Causes Intracerebral Hemorrhage
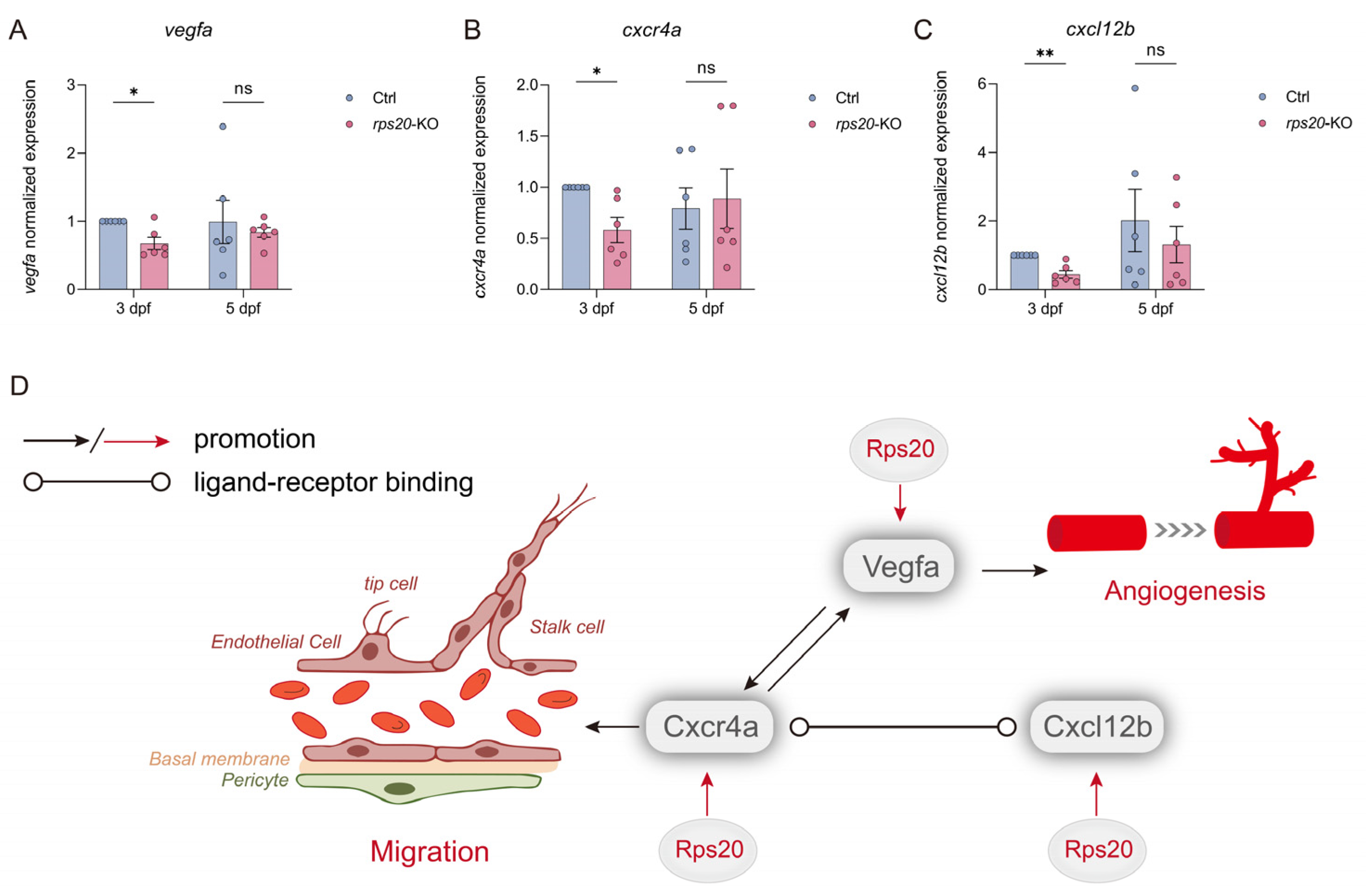
3.6. Knockout of rps20 Decreased the Angiogenesis-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACeV | anterior cerebral vein |
| BA | basilar artery |
| BBB | blood–brain barrier |
| ccRCC | clear cell renal cell carcinoma |
| CDS | coding sequence |
| CtA | central artery |
| DEG | Differentially Expressed Gene |
| DLAV | dorsal longitudinal anastomotic vessel |
| dpf | day post fertilization |
| GFP | Green Fluorescent Protein |
| GO | Gene Ontology |
| hpf | hour post-fertilization |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MCeV | middle cerebral vein |
| mpf | month post fertilization |
| PCS | posterior communicating segment |
| PFA | paraformaldehyde |
| PHBC | primordial hindbrain channel |
| RP | Ribosomal protein |
| WISH | whole-mount in situ hybridization |
References
- Nikolova, G.; Lammert, E. Interdependent development of blood vessels and organs. Cell Tissue Res. 2003, 314, 33–42. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. A systems view of the vascular endothelium in health and disease. Cell 2024, 187, 4833–4858. [Google Scholar] [CrossRef]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Global Burden of Cardiovascular, D.; Risks, C. Global Burden of Cardiovascular Diseases and Risks, 1990-2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Faria-Pereira, A.; Morais, V.A. Synapses: The Brain’s Energy-Demanding Sites. Int. J. Mol. Sci. 2022, 23, 3627. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef]
- Walchli, T.; Bisschop, J.; Carmeliet, P.; Zadeh, G.; Monnier, P.P.; De Bock, K.; Radovanovic, I. Shaping the brain vasculature in development and disease in the single-cell era. Nat. Rev. Neurosci. 2023, 24, 271–298. [Google Scholar] [CrossRef]
- Bennett, H.C.; Zhang, Q.; Wu, Y.T.; Manjila, S.B.; Chon, U.; Shin, D.; Vanselow, D.J.; Pi, H.J.; Drew, P.J.; Kim, Y. Aging drives cerebrovascular network remodeling and functional changes in the mouse brain. Nat. Commun. 2024, 15, 6398. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, A.H.; Markus, H.S.; Schneider, J.A. Cerebral Small Vessel Disease, Hypertension, and Vascular Contributions to Cognitive Impairment and Dementia. Hypertension 2024, 81, 75–86. [Google Scholar] [CrossRef]
- Iadecola, C.; Gottesman, R.F. Cerebrovascular Alterations in Alzheimer Disease. Circ. Res. 2018, 123, 406–408. [Google Scholar] [CrossRef]
- Abdellatif, M.; Rainer, P.P.; Sedej, S.; Kroemer, G. Hallmarks of cardiovascular ageing. Nat. Rev. Cardiol. 2023, 20, 754–777. [Google Scholar] [CrossRef]
- de la Cruz, J.; Karbstein, K.; Woolford, J.L., Jr. Functions of ribosomal proteins in assembly of eukaryotic ribosomes in vivo. Annu. Rev. Biochem. 2015, 84, 93–129. [Google Scholar] [CrossRef]
- Daftuar, L.; Zhu, Y.; Jacq, X.; Prives, C. Ribosomal proteins RPL37, RPS15 and RPS20 regulate the Mdm2-p53-MdmX network. PLoS ONE 2013, 8, e68667. [Google Scholar] [CrossRef] [PubMed]
- Deisenroth, C.; Franklin, D.A.; Zhang, Y. The Evolution of the Ribosomal Protein-MDM2-p53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026138. [Google Scholar] [CrossRef]
- Shen, C.; Chen, Z.; Zhang, Y.; Xu, W.; Peng, R.; Jiang, J.; Zuo, W.; Fan, Y.; Zheng, B. Biochemical and clinical effects of RPS20 expression in renal clear cell carcinoma. Oncol. Rep. 2023, 49, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H. Signaling to p53: Ribosomal proteins find their way. Cancer Cell 2009, 16, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef]
- Winkler, E.A.; Kim, C.N.; Ross, J.M.; Garcia, J.H.; Gil, E.; Oh, I.; Chen, L.Q.; Wu, D.; Catapano, J.S.; Raygor, K.; et al. A single-cell atlas of the normal and malformed human brain vasculature. Science 2022, 375, eabi7377. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Dieguez-Hurtado, R.; Arf, H.; Song, J.; Park, H.; Kruse, K.; Sorokin, L.; Adams, R.H. Single-cell transcriptomics reveals functionally specialized vascular endothelium in brain. Elife 2022, 11, e57520. [Google Scholar] [CrossRef]
- Detrich, H.W., 3rd; Kieran, M.W.; Chan, F.Y.; Barone, L.M.; Yee, K.; Rundstadler, J.A.; Pratt, S.; Ransom, D.; Zon, L.I. Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl. Acad. Sci. USA 1995, 92, 10713–10717. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Yan, R.; Xu, D.; Yang, J.; Walker, S.; Zhang, Y. A comparative assessment and analysis of 20 representative sequence alignment methods for protein structure prediction. Sci. Rep. 2013, 3, 2619. [Google Scholar] [CrossRef]
- Gonzalez-Magana, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C. PCNA: A silent housekeeper or a potential therapeutic target? Trends Pharmacol. Sci. 2014, 35, 178–186. [Google Scholar] [CrossRef]
- Tsihlias, J.; Kapusta, L.; Slingerland, J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu. Rev. Med. 1999, 50, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lee, Y.K.; Park, S.H.; Lim, S.B.; Choi, Y.W.; Shin, J.S.; Kim, Y.H.; Kim, J.H.; Park, T.J. p15(INK4B) is an alternative marker of senescent tumor cells in colorectal cancer. Heliyon 2023, 9, e13170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Ulrich, F.; Ma, L.H.; Baker, R.G.; Torres-Vazquez, J. Neurovascular development in the embryonic zebrafish hindbrain. Dev. Biol. 2011, 357, 134–151. [Google Scholar] [CrossRef]
- Fujita, M.; Cha, Y.R.; Pham, V.N.; Sakurai, A.; Roman, B.L.; Gutkind, J.S.; Weinstein, B.M. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development 2011, 138, 1705–1715. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Woodson, S.A. Global stabilization of rRNA structure by ribosomal proteins S4, S17, and S20. J. Mol. Biol. 2009, 392, 666–677. [Google Scholar] [CrossRef]
- Ben-Shem, A.; Garreau de Loubresse, N.; Melnikov, S.; Jenner, L.; Yusupova, G.; Yusupov, M. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 2011, 334, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Strunk, B.S.; Loucks, C.R.; Su, M.; Vashisth, H.; Cheng, S.; Schilling, J.; Brooks, C.L., 3rd; Karbstein, K.; Skiniotis, G. Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates. Science 2011, 333, 1449–1453. [Google Scholar] [CrossRef]
- Sung, M.K.; Porras-Yakushi, T.R.; Reitsma, J.M.; Huber, F.M.; Sweredoski, M.J.; Hoelz, A.; Hess, S.; Deshaies, R.J. A conserved quality-control pathway that mediates degradation of unassembled ribosomal proteins. Elife 2016, 5, e19105. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.T.; O’Donohue, M.F.; Wu, Y.; Lohi, H.; Scherer, S.W.; Paterson, A.D.; Ellonen, P.; Abdel-Rahman, W.M.; Valo, S.; Mecklin, J.P.; et al. Germline mutation of RPS20, encoding a ribosomal protein, causes predisposition to hereditary nonpolyposis colorectal carcinoma without DNA mismatch repair deficiency. Gastroenterology 2014, 147, 595–598.e5. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.H.; Shabihkhani, M.; Telesca, D.; Yang, S.; Tso, J.L.; Menjivar, J.C.; Wei, B.; Lucey, G.M.; Mareninov, S.; Chen, Z.; et al. Ribosomal Proteins RPS11 and RPS20, Two Stress-Response Markers of Glioblastoma Stem Cells, Are Novel Predictors of Poor Prognosis in Glioblastoma Patients. PLoS ONE 2015, 10, e0141334. [Google Scholar] [CrossRef]
- De Bortoli, M.; Castellino, R.C.; Lu, X.Y.; Deyo, J.; Sturla, L.M.; Adesina, A.M.; Perlaky, L.; Pomeroy, S.L.; Lau, C.C.; Man, T.K.; et al. Medulloblastoma outcome is adversely associated with overexpression of EEF1D, RPL30, and RPS20 on the long arm of chromosome 8. BMC Cancer 2006, 6, 223. [Google Scholar] [CrossRef]
- Suzuki, M.; Tezuka, K.; Handa, T.; Sato, R.; Takeuchi, H.; Takao, M.; Tano, M.; Uchida, Y. Upregulation of ribosome complexes at the blood-brain barrier in Alzheimer’s disease patients. J. Cereb. Blood Flow. Metab. 2022, 42, 2134–2150. [Google Scholar] [CrossRef]
- Dubois, B.; von Arnim, C.A.F.; Burnie, N.; Bozeat, S.; Cummings, J. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res. Ther. 2023, 15, 175. [Google Scholar] [CrossRef]
- Song, Q.; Liu, G.; Hu, S.; Zhang, Y.; Tao, Y.; Han, Y.; Zeng, H.; Huang, W.; Li, F.; Chen, P.; et al. Novel autoimmune hepatitis-specific autoantigens identified using protein microarray technology. J. Proteome Res. 2010, 9, 30–39. [Google Scholar] [CrossRef]
- Sundaramoorthy, E.; Ryan, A.P.; Fulzele, A.; Leonard, M.; Daugherty, M.D.; Bennett, E.J. Ribosome quality control activity potentiates vaccinia virus protein synthesis during infection. J. Cell Sci. 2021, 134, jcs257188. [Google Scholar] [CrossRef]
- DiGiuseppe, S.; Rollins, M.G.; Bartom, E.T.; Walsh, D. ZNF598 Plays Distinct Roles in Interferon-Stimulated Gene Expression and Poxvirus Protein Synthesis. Cell Rep. 2018, 23, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, Z.; Chen, Y.; Huang, X.; Tian, B. Potential core genes associated with COVID-19 identified via weighted gene co-expression network analysis. Swiss Med. Wkly. 2022, 152, 40033. [Google Scholar] [CrossRef]
- Graifer, D.; Malygin, A.; Zharkov, D.O.; Karpova, G. Eukaryotic ribosomal protein S3: A constituent of translational machinery and an extraribosomal player in various cellular processes. Biochimie 2014, 99, 8–18. [Google Scholar] [CrossRef]
- Landry-Voyer, A.M.; Mir Hassani, Z.; Avino, M.; Bachand, F. Ribosomal Protein uS5 and Friends: Protein-Protein Interactions Involved in Ribosome Assembly and Beyond. Biomolecules 2023, 13, 853. [Google Scholar] [CrossRef]
- Lessard, F.; Igelmann, S.; Trahan, C.; Huot, G.; Saint-Germain, E.; Mignacca, L.; Del Toro, N.; Lopes-Paciencia, S.; Le Calve, B.; Montero, M.; et al. Senescence-associated ribosome biogenesis defects contributes to cell cycle arrest through the Rb pathway. Nat. Cell Biol. 2018, 20, 789–799. [Google Scholar] [CrossRef]
- McGowan, K.A.; Li, J.Z.; Park, C.Y.; Beaudry, V.; Tabor, H.K.; Sabnis, A.J.; Zhang, W.; Fuchs, H.; de Angelis, M.H.; Myers, R.M.; et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat. Genet. 2008, 40, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Taye, M.; Yoon, J.; Dessie, T.; Cho, S.; Oh, S.J.; Lee, H.K.; Kim, H. Deciphering signature of selection affecting beef quality traits in Angus cattle. Genes. Genom. 2018, 40, 63–75. [Google Scholar] [CrossRef]
- Quinonez-Silvero, C.; Hubner, K.; Herzog, W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev. Biol. 2020, 457, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bassler, J.; Hurt, E. Eukaryotic Ribosome Assembly. Annu. Rev. Biochem. 2019, 88, 281–306. [Google Scholar] [CrossRef]
- Hu, H.; Li, X. Transcriptional regulation in eukaryotic ribosomal protein genes. Genomics 2007, 90, 421–423. [Google Scholar] [CrossRef]
- Rattner, A.; Wang, Y.; Nathans, J. Signaling Pathways in Neurovascular Development. Annu. Rev. Neurosci. 2022, 45, 87–108. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Chiba, A.; Fukumoto, M.; Morooka, N.; Mochizuki, N. Zebrafish Vascular Development: General and Tissue-Specific Regulation. J. Lipid Atheroscler. 2021, 10, 145–159. [Google Scholar] [CrossRef]
- Bussmann, J.; Wolfe, S.A.; Siekmann, A.F. Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development 2011, 138, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, Y.; Liu, J.; Mok, S.C.; Xue, F.; Zhang, W. CXCL12/CXCR4: A symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 2016, 35, 816–826. [Google Scholar] [CrossRef]
- Li, W.; Kohara, H.; Uchida, Y.; James, J.M.; Soneji, K.; Cronshaw, D.G.; Zou, Y.R.; Nagasawa, T.; Mukouyama, Y.S. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev. Cell 2013, 24, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, X.; Deng, S.; Chen, Q.; Xu, B. Neural Regulation of Vascular Development: Molecular Mechanisms and Interactions. Biomolecules 2024, 14, 966. [Google Scholar] [CrossRef]
- Kryczek, I.; Lange, A.; Mottram, P.; Alvarez, X.; Cheng, P.; Hogan, M.; Moons, L.; Wei, S.; Zou, L.; Machelon, V.; et al. CXCL12 and Vascular Endothelial Growth Factor Synergistically Induce Neoangiogenesis in Human Ovarian Cancers. Cancer Res. Res. 2005, 65, 465–472. [Google Scholar] [CrossRef]
- Liang, Z.; Brooks, J.; Willard, M.; Liang, K.; Yoon, Y.; Kang, S.; Shim, H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Biophys. Res. Commun. 2007, 359, 716–722. [Google Scholar] [CrossRef]
- Wagner, K.D.; Safwan-Zaiter, H.; Wagner, N. A Dual Role of the Senescence Marker P16Ink4a in Liver Endothelial Cell Function. Cells 2024, 13, 1929. [Google Scholar] [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Michiels, J.F.; Wagner, K.D. Dynamic Spatiotemporal Expression Pattern of the Senescence-Associated Factor p16Ink4a in Development and Aging. Cells 2022, 11, 541. [Google Scholar] [CrossRef] [PubMed]




| R-Packages | Version |
|---|---|
| Seurat | 5.0.1 |
| clusterProfiler | 4.10.0 |
| EnhancedVolcano | 1.20.0 |
| ggplot2 | 3.4.4 |
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | CGAGCAGGAGATGGGAACC | CAACGGAAACGCTCATTGC |
| rps20 | CTGACCAGCCGTAACGTCAA | GATGCGGAGGGTCTTGGTAG |
| pcna | AGGAGGATGAAGCGGTAACAA | GTCTTGGACAGAGGAGTGGC |
| p15 | GAGGATGAACTGACCACAGCA | CAAGAGCCAAAGGTGCGTTAC |
| p53 | AGGTCTTTTGAGGTGCGTGT | AGAAGATTCTTTCACCAAACTACG |
| cxcl12b | CCCAGAGACTGACGCAAAGC | TTGGGTTGATGCAGACCTCTC |
| cxcr4a | TGGCTTATTACGGACACATCGT | CCGTACACCGTTGGGAGAAA |
| vegfa | TCAAAGCAAAGAAAGAAAACCACTG | ATTTGCAGGAGCATTTACAGGTG |
| Component | Dosage |
|---|---|
| O-Dianisidine (Sigma-Aldrich, Darmstadt, Germany) | 3 mg |
| 3M NaAc, PH 5.2 (Beyotime, Shanghai, China) | 16.5 μL |
| H2O2 | 108.5 μL |
| Ethanol | 2 mL |
| H2O | To 5 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Wen, Z.; Deng, S.; Qiu, Y.; Ma, W.; Dong, X.; Gong, J.; Zhang, Y.; Liu, D.; Xu, B. Regulation of Hindbrain Vascular Development by rps20 in Zebrafish. Cells 2025, 14, 1070. https://doi.org/10.3390/cells14141070
Shen X, Wen Z, Deng S, Qiu Y, Ma W, Dong X, Gong J, Zhang Y, Liu D, Xu B. Regulation of Hindbrain Vascular Development by rps20 in Zebrafish. Cells. 2025; 14(14):1070. https://doi.org/10.3390/cells14141070
Chicago/Turabian StyleShen, Xinyu, Zhaozhi Wen, Shunze Deng, Yuxuan Qiu, Weijie Ma, Xinyue Dong, Jie Gong, Yu Zhang, Dong Liu, and Bing Xu. 2025. "Regulation of Hindbrain Vascular Development by rps20 in Zebrafish" Cells 14, no. 14: 1070. https://doi.org/10.3390/cells14141070
APA StyleShen, X., Wen, Z., Deng, S., Qiu, Y., Ma, W., Dong, X., Gong, J., Zhang, Y., Liu, D., & Xu, B. (2025). Regulation of Hindbrain Vascular Development by rps20 in Zebrafish. Cells, 14(14), 1070. https://doi.org/10.3390/cells14141070






