Lidocaine Affects Collagen Breakdown Without Compromising Cell Viability in Cultured Human Tenocytes: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Primary Cell Cultures
2.3. Real-Time PCR
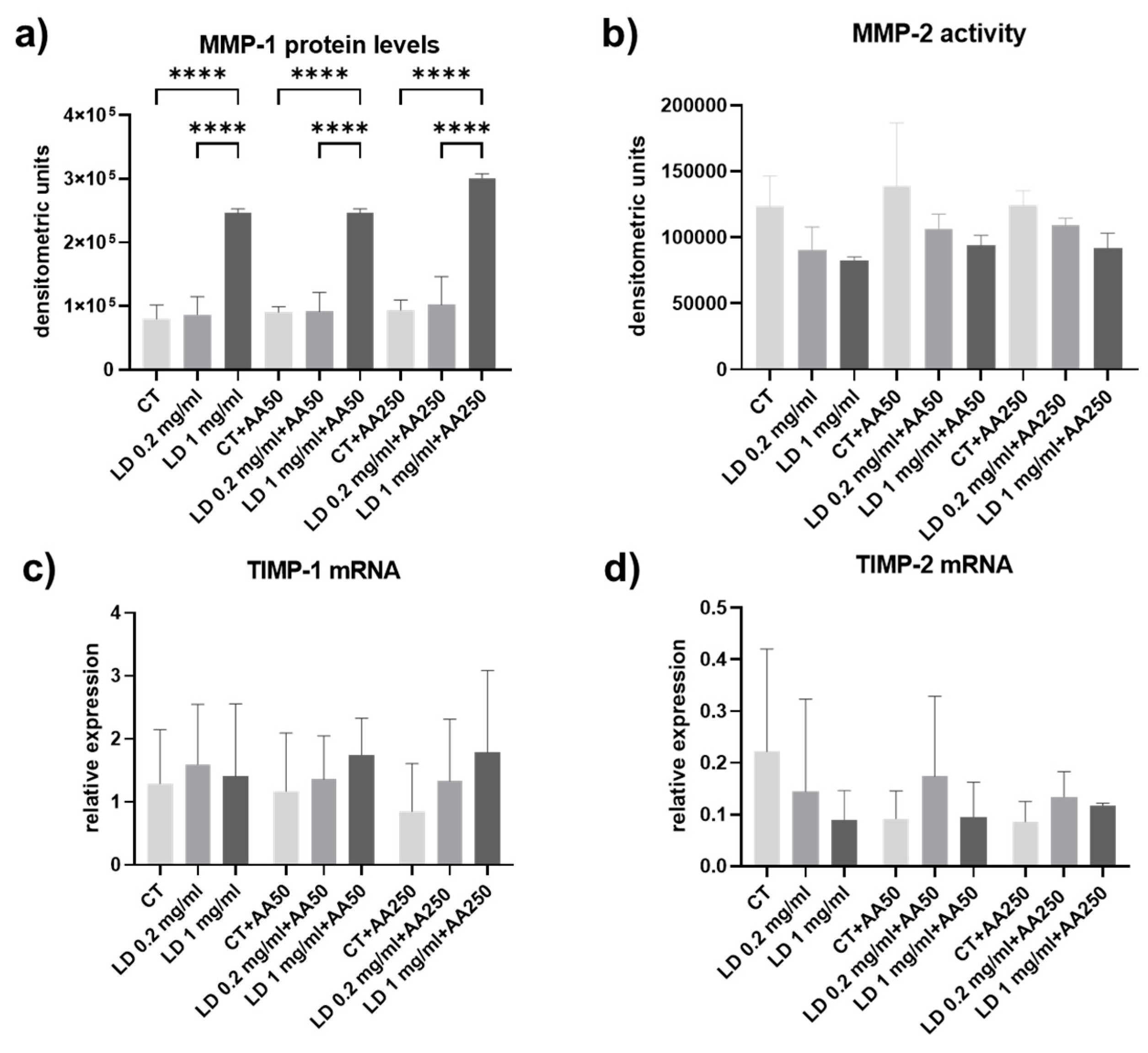
2.4. Slot Blot
2.5. SDS-Zymography
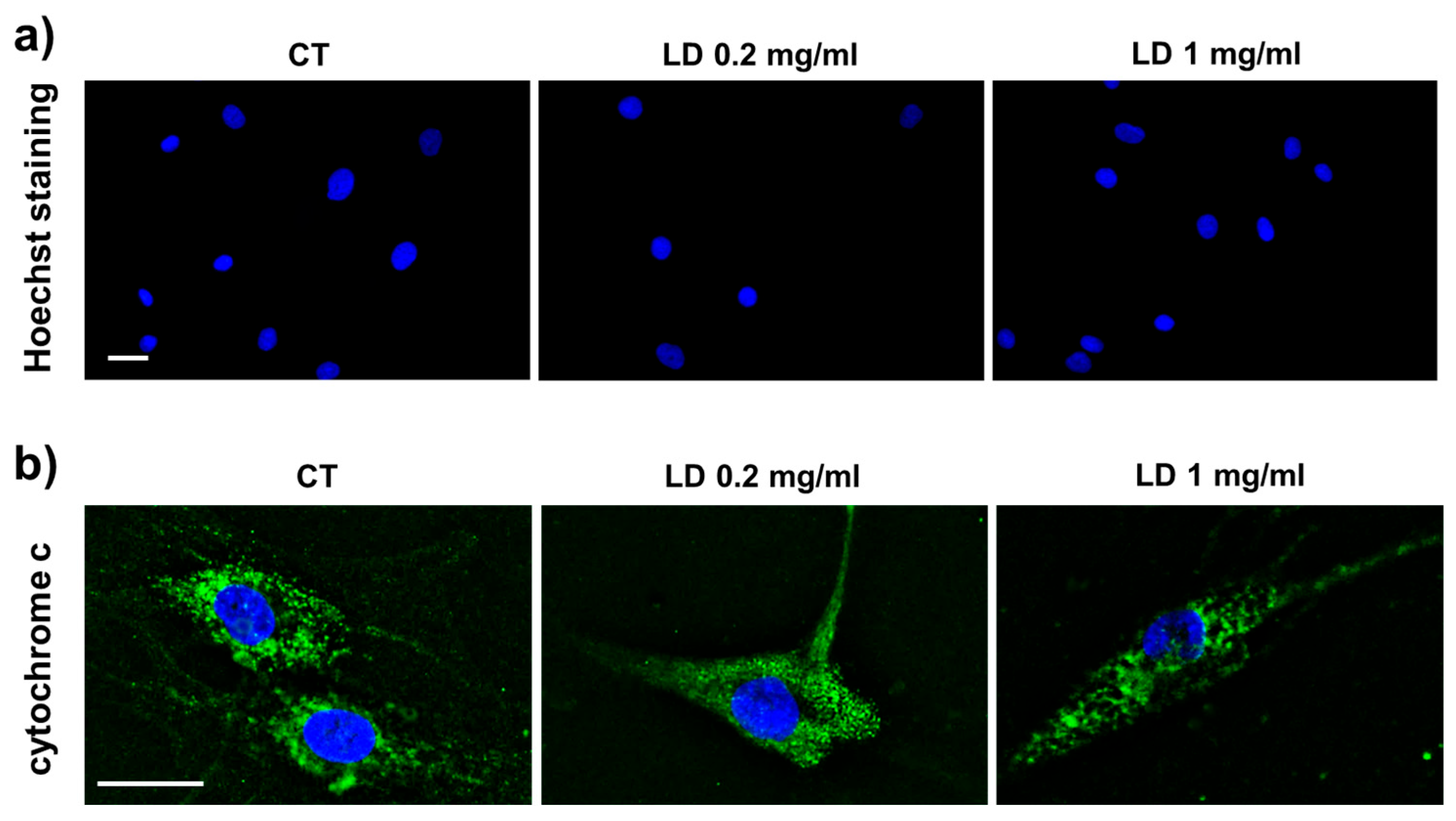
2.6. Hoechst Staining
2.7. Immunocytochemistry
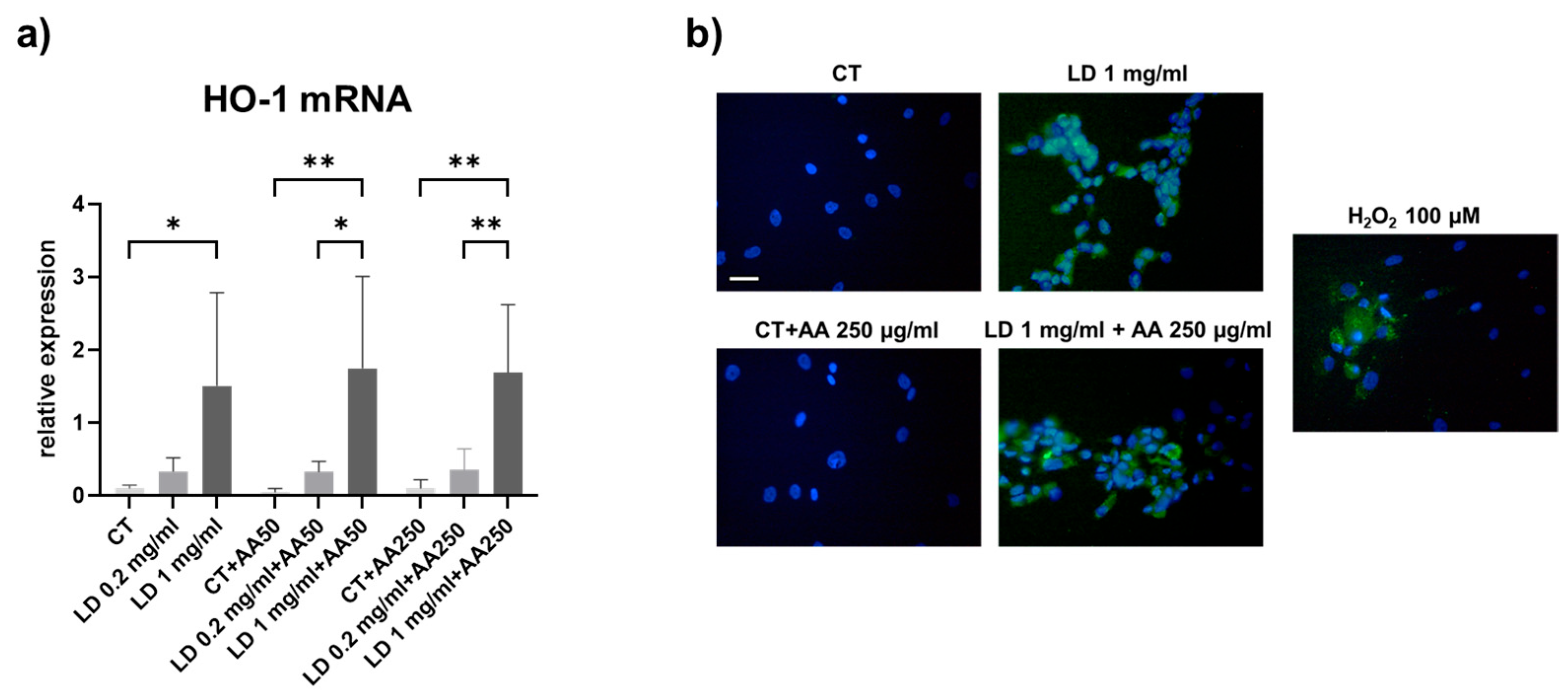
2.8. 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFDA) Assay
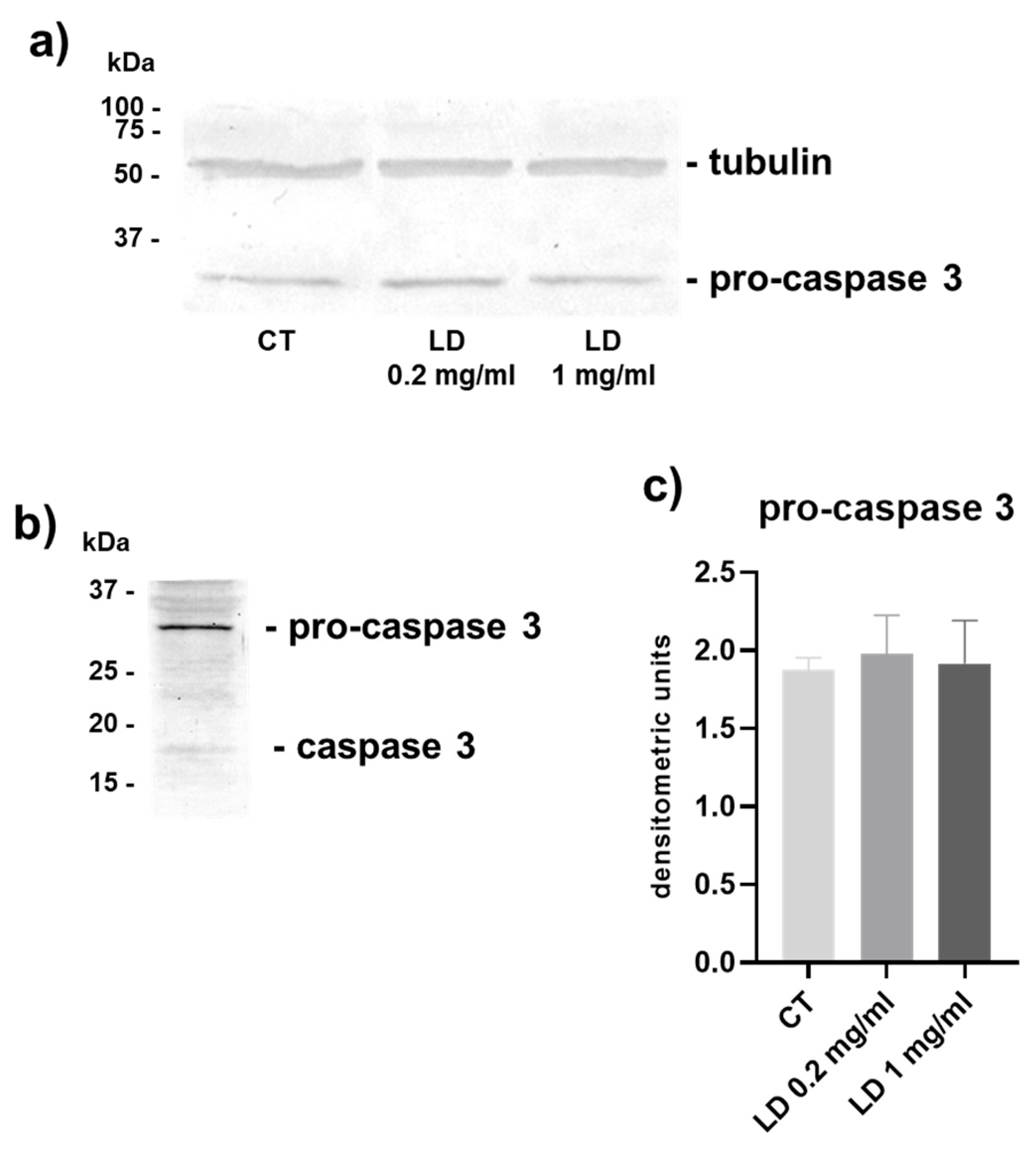
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Apoptosis
3.2. Oxidative Stress
3.3. Collagen Turnover Pathways: Synthesis and Maturation
3.4. Collagen Turnover Pathways: Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| DCFDA | 2′,7′-dichlorofluorescin diacetate |
| ECM | Extracellular matrix |
| COL-I | Collagen type I |
| GTPS | Greater trochanteric pain syndrome |
| HO-1 | Heme oxygenase 1 |
| LA | Local anesthetic |
| LD | Lidocaine |
| LH2b | Long lysyl hydroxylase 2 |
| LOX | Lysyl oxidase |
| MMP | Matrix metalloproteinase |
| ROS | Reactive oxygen species |
| TIMP | Tissue inhibitor of matrix metalloproteinase |
References
- Karpinski, M.R.; Piggott, H. Greater trochanteric pain syndrome. A report of 15 cases. J. Bone Jt. Surg. Br. 1985, 67, 762–763. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P. Gene expression and matrix turnover in overused and damaged tendons. Scand. J. Med. Sci. Sports 2005, 15, 241–251. [Google Scholar] [CrossRef]
- Ejnisman, L.; Safran, M.R. Biologics in hip preservation. Ann. Jt. 2018, 3, 50. [Google Scholar] [CrossRef]
- Williams, B.S.; Cohen, S.P. Greater trochanteric pain syndrome: A review of anatomy, diagnosis and treatment. Anesth. Analg. 2009, 108, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Aicale, R.; Tarantino, D.; Maffulli, N. Non-insertional Achilles Tendinopathy: State of the Art. In Sports Injuries of the Foot and Ankle; Canata, G.L., d’Hooghe, P., Hunt, K.J., Kerkhoffs, G.M., Longo, U.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 359–367. ISBN 978-3-662-58703-4. [Google Scholar]
- Frizziero, A.; Vittadini, F.; Pignataro, A.; Gasparre, G.; Biz, C.; Ruggieri, P.; Masiero, S. Conservative management of tendinopathies around hip. Muscles Ligaments Tendons J. 2016, 6, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Oderuth, E.; Atchia, I.; Malviya, A. The use of platelet-rich plasma in the treatment of greater trochanteric pain syndrome: A systematic literature review. J. Hip Preserv. Surg. 2018, 5, 209–219. [Google Scholar] [CrossRef]
- Grimaldi, A.; Mellor, R.; Hodges, P.; Bennell, K.; Wajswelner, H.; Vicenzino, B. Gluteal Tendinopathy: A Review of Mechanisms, Assessment and Management. Sports Med. 2015, 45, 1107–1119. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Bulsara, M.K.; O’Donnell, J.; McCrory, P.R.; Zheng, M.H. The Effectiveness of Platelet-Rich Plasma Injections in Gluteal Tendinopathy: A Randomized, Double-Blind Controlled Trial Comparing a Single Platelet-Rich Plasma Injection with a Single Corticosteroid Injection. Am. J. Sports Med. 2018, 46, 933–939. [Google Scholar] [CrossRef]
- Reid, D. The management of greater trochanteric pain syndrome: A systematic literature review. J. Orthop. 2016, 13, 15–28. [Google Scholar] [CrossRef]
- Bedi, A.; Trinh, T.Q.; Olszewski, A.M.; Maerz, T.; Ramme, A.J. Nonbiologic Injections in Sports Medicine. JBJS Rev. 2020, 8, e0052. [Google Scholar] [CrossRef]
- Coombes, B.K.; Bisset, L.; Vicenzino, B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: A systematic review of randomised controlled trials. Lancet 2010, 376, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Maab, H.; Mustafa, F.; Arshad Ali, S. Anti-inflammatory aspects of Lidocaine: A neglected therapeutic stance for COVID-19. Heart Lung 2020, 49, 877–878. [Google Scholar] [CrossRef]
- Piper, S.L.; Kim, H.T. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J. Bone Jt. Surg. Am. 2008, 90, 986–991. [Google Scholar] [CrossRef]
- Grishko, V.; Xu, M.; Wilson, G.; Pearsall, A.W. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J. Bone Jt. Surg. Am. 2010, 92, 609–618. [Google Scholar] [CrossRef]
- Piper, S.L.; Kramer, J.D.; Kim, H.T.; Feeley, B.T. Effects of local anesthetics on articular cartilage. Am. J. Sports Med. 2011, 39, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, R.; Wang, M.; Dang, A.C.; Kim, H.T.; Kuo, A.C. Cytotoxicity of local anesthetics on human mesenchymal stem cells. J. Bone Jt. Surg. Am. 2013, 95, 132–137. [Google Scholar] [CrossRef]
- Fedder, C.; Beck-Schimmer, B.; Aguirre, J.; Hasler, M.; Roth-Z’graggen, B.; Urner, M.; Kalberer, S.; Schlicker, A.; Votta-Velis, G.; Bonvini, J.M.; et al. In vitro exposure of human fibroblasts to local anaesthetics impairs cell growth. Clin. Exp. Immunol. 2010, 162, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ficklscherer, A.; Sievers, B.; Redeker, J.; Gülecyüz, M.F.; Paulus, A.; Pietschmann, M.F.; Müller, P.E. Comparison of ropivacaine and fentanyl toxicity in human fibroblasts. Arch. Med. Sci. 2013, 9, 576–580. [Google Scholar] [CrossRef]
- Haasters, F.; Polzer, H.; Prall, W.C.; Saller, M.M.; Kohler, J.; Grote, S.; Mutschler, W.; Docheva, D.; Schieker, M. Bupivacaine, ropivacaine, and morphine: Comparison of toxicity on human hamstring-derived stem/progenitor cells. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2138–2144. [Google Scholar] [CrossRef]
- Nouette-Gaulain, K.; Jose, C.; Capdevila, X.; Rossignol, R. From analgesia to myopathy: When local anesthetics impair the mitochondrion. Int. J. Biochem. Cell Biol. 2011, 43, 14–19. [Google Scholar] [CrossRef]
- Honda, H.; Gotoh, M.; Kanazawa, T.; Nakamura, H.; Ohta, K.; Nakamura, K.-I.; Shiba, N. Effects of lidocaine on torn rotator cuff tendons. J. Orthop. Res. 2016, 34, 1620–1627. [Google Scholar] [CrossRef]
- Piper, S.L.; Laron, D.; Manzano, G.; Pattnaik, T.; Liu, X.; Kim, H.T.; Feeley, B.T. A comparison of lidocaine, ropivacaine and dexamethasone toxicity on bovine tenocytes in culture. J. Bone Jt. Surg. Br. 2012, 94, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Jiang, Z.; Li, Z.; Jiang, D. Lidocaine potentiates the deleterious effects of triamcinolone acetonide on tenocytes. Med. Sci. Monit. 2014, 20, 2478–2483. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.-M.; Hah, Y.-S.; Kim, J.-S.; Nam, J.-B.; Kim, R.J.; Lee, S.-J.; Park, H.B. Cytotoxic effects of ropivacaine, bupivacaine, and lidocaine on rotator cuff tenofibroblasts. Am. J. Sports Med. 2014, 42, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Lehner, C.; Gehwolf, R.; Hirzinger, C.; Stephan, D.; Augat, P.; Tauber, M.; Resch, H.; Bauer, H.-C.; Bauer, H.; Tempfer, H. Bupivacaine induces short-term alterations and impairment in rat tendons. Am. J. Sports Med. 2013, 41, 1411–1418. [Google Scholar] [CrossRef]
- Zhang, A.Z.; Ficklscherer, A.; Gülecyüz, M.F.; Paulus, A.C.; Niethammer, T.R.; Jansson, V.; Müller, P.E. Cell Toxicity in Fibroblasts, Tenocytes, and Human Mesenchymal Stem Cells-A Comparison of Necrosis and Apoptosis-Inducing Ability in Ropivacaine, Bupivacaine, and Triamcinolone. Arthroscopy 2017, 33, 840–848. [Google Scholar] [CrossRef]
- Nuelle, C.W.; Cook, C.R.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. In vitro toxicity of local anaesthetics and corticosteroids on supraspinatus tenocyte viability and metabolism. J. Orthop. Translat. 2017, 8, 20–24. [Google Scholar] [CrossRef]
- Scholz, C.; Wieder, T.; Stärck, L.; Essmann, F.; Schulze-Osthoff, K.; Dörken, B.; Daniel, P.T. Arsenic trioxide triggers a regulated form of caspase-independent necrotic cell death via the mitochondrial death pathway. Oncogene 2005, 24, 1904–1913. [Google Scholar] [CrossRef]
- Daniel, P.T.; Schulze-Osthoff, K.; Belka, C.; Güner, D. Guardians of cell death: The Bcl-2 family proteins. Essays Biochem. 2003, 39, 73–88. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Yuan, J.; Murrell, G.A.C.; Wei, A.-Q.; Wang, M.-X. Apoptosis in rotator cuff tendonopathy. J. Orthop. Res. 2002, 20, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.Y.; Zhang, X.; Yao, S.; Sun, H.; Huang, C. Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy-A Target for Intervention. Int. J. Mol. Sci. 2022, 23, 3571. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Battaglia, S.; Raimondi, M.; Fontana, F.; Cozzi, M.; Ranieri, F.R.; Sacchi, R.; Curti, V.; Limonta, P. Anti-Inflammatory and Antioxidant Properties of a New Mixture of Vitamin C, Collagen Peptides, Resveratrol, and Astaxanthin in Tenocytes: Molecular Basis for Future Applications in Tendinopathies. Mediat. Inflamm. 2024, 2024, 5273198. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Carr, A.J.; Hulley, P.A. Protection against glucocorticoid-induced damage in human tenocytes by modulation of ERK, Akt, and forkhead signaling. Endocrinology 2011, 152, 503–514. [Google Scholar] [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar]
- Hakimi, O.; Poulson, R.; Thakkar, D.; Yapp, C.; Carr, A. Ascorbic acid is essential for significant collagen deposition by human tenocytes in vitro. Oxid. Antioxid. Med. Sci. 2014, 3, 119. [Google Scholar] [CrossRef]
- Randelli, F.; Menon, A.; Giai Via, A.; Mazzoleni, M.G.; Sciancalepore, F.; Brioschi, M.; Gagliano, N. Effect of a Collagen-Based Compound on Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2018, 7, 246. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Lucero, H.A.; Kagan, H.M. Lysyl oxidase: An oxidative enzyme and effector of cell function. Cell. Mol. Life Sci. 2006, 63, 2304–2316. [Google Scholar] [CrossRef]
- Walker, L.C.; Overstreet, M.A.; Yeowell, H.N. Tissue-specific expression and regulation of the alternatively-spliced forms of lysyl hydroxylase 2 (LH2) in human kidney cells and skin fibroblasts. Matrix Biol. 2005, 23, 515–523. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, H.-N.; Pang, J.-H.S.; Lin, L.-P.; Chen, J.-M.; Yu, T.-Y.; Tsai, W.-C. Lidocaine Inhibited Tendon Cell Proliferation and Extracellular Matrix Production by Down Regulation of Cyclin A, CDK2, Type I and Type III Collagen Expression. Int. J. Mol. Sci. 2022, 23, 8787. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Chen, P.; Chen, A.C.-Y.; Chan, Y.-S.; Hsu, K.-Y.; Rei, H.; Lei, K.F. Real-Time Monitoring of Ascorbic Acid-Mediated Reduction of Cytotoxic Effects of Analgesics and NSAIDs on Tenocytes Proliferation. Dose Response 2019, 17, 1559325819832143. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Scimè, A. Mitochondrial Function in Muscle Stem Cell Fates. Front. Cell Dev. Biol. 2020, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Morikawa, D.; Nojiri, H.; Itoigawa, Y.; Ozawa, Y.; Kaneko, K.; Shimizu, T. Antioxidant treatment with vitamin C attenuated rotator cuff degeneration caused by oxidative stress in Sod1-deficient mice. JSES Open Access 2018, 2, 91–96. [Google Scholar] [CrossRef]
- Yamaura, K.; Mifune, Y.; Inui, A.; Nishimoto, H.; Kurosawa, T.; Mukohara, S.; Hoshino, Y.; Niikura, T.; Kuroda, R. Antioxidant effect of nicotinamide mononucleotide in tendinopathy. BMC Musculoskelet. Disord. 2022, 23, 249. [Google Scholar] [CrossRef]
- Morikawa, D.; Itoigawa, Y.; Nojiri, H.; Sano, H.; Itoi, E.; Saijo, Y.; Kaneko, K.; Shimizu, T. Contribution of oxidative stress to the degeneration of rotator cuff entheses. J. Shoulder Elbow Surg. 2014, 23, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Siow, R.C.; Sato, H.; Mann, G.E. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: Anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc. Res. 1999, 41, 385–394. [Google Scholar] [CrossRef]
- Kutty, R.K.; Kutty, G.; Rodriguez, I.R.; Chader, G.J.; Wiggert, B. Chromosomal localization of the human heme oxygenase genes: Heme oxygenase-1 (HMOX1) maps to chromosome 22q12 and heme oxygenase-2 (HMOX2) maps to chromosome 16p13.3. Genomics 1994, 20, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Gross, J. Some properties of the products of reaction of tadpole collagenase with collagen. Biochemistry 1967, 6, 518–528. [Google Scholar] [CrossRef]
- Woessner, J.F. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991, 5, 2145–2154. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Tian, T.; Lavagnino, M.; Gardner, K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J. Orthop. Res. 2004, 22, 328–333. [Google Scholar] [CrossRef]
- Prasetia, R.; Purwana, S.Z.B.; Lesmana, R.; Herman, H.; Chernchujit, B.; Rasyid, H.N. The pathology of oxidative stress-induced autophagy in a chronic rotator cuff enthesis tear. Front. Physiol. 2023, 14, 1222099. [Google Scholar] [CrossRef]
- Wang, F.; Murrell, G.A.C.; Wang, M.-X. Oxidative stress-induced c-Jun N-terminal kinase (JNK) activation in tendon cells upregulates MMP1 mRNA and protein expression. J. Orthop. Res. 2007, 25, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.E.; Reed, K.L. Local anesthetics: Review of pharmacological considerations. Anesth. Prog. 2012, 59, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.M.T.; Jørgensen, E.; Cornett, C. The concentration of lidocaine and mepivacaine measured in synovial fluid of different joints of horses after single intra-articular injection. Front. Vet. Sci. 2022, 9, 1007399. [Google Scholar] [CrossRef]
- Di Salvo, A.; Chiaradia, E.; Della Rocca, G.; Mancini, F.; Galarini, R.; Giusepponi, D.; de Monte, V.; Cagnardi, P.; Marenzoni, M.L.; Bufalari, A. Intra-articular administration of lidocaine plus adrenaline in dogs: Pharmacokinetic profile and evaluation of toxicity in vivo and in vitro. Vet. J. 2016, 208, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Karnina, R.; Arif, S.K.; Hatta, M.; Bukhari, A. Molecular mechanisms of lidocaine. Ann. Med. Surg. 2021, 69, 102733. [Google Scholar] [CrossRef]
- Ali, Z.A.; El-Mallakh, R.S. Nebulized Lidocaine in COVID-19, an Hypothesis. Med. Hypotheses 2020, 144, 109947. [Google Scholar] [CrossRef]
- Segal, N.A.; Felson, D.T.; Torner, J.C.; Zhu, Y.; Curtis, J.R.; Niu, J.; Nevitt, M.C. Greater trochanteric pain syndrome: Epidemiology and associated factors. Arch. Phys. Med. Rehabil. 2007, 88, 988–992. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randelli, F.; Mazzoleni, M.G.; Menon, A.; Fioruzzi, A.; Henin, D.; Sommariva, M.; Gagliano, N. Lidocaine Affects Collagen Breakdown Without Compromising Cell Viability in Cultured Human Tenocytes: An In Vitro Study. Cells 2025, 14, 988. https://doi.org/10.3390/cells14130988
Randelli F, Mazzoleni MG, Menon A, Fioruzzi A, Henin D, Sommariva M, Gagliano N. Lidocaine Affects Collagen Breakdown Without Compromising Cell Viability in Cultured Human Tenocytes: An In Vitro Study. Cells. 2025; 14(13):988. https://doi.org/10.3390/cells14130988
Chicago/Turabian StyleRandelli, Filippo, Manuel G. Mazzoleni, Alessandra Menon, Alberto Fioruzzi, Dolaji Henin, Michele Sommariva, and Nicoletta Gagliano. 2025. "Lidocaine Affects Collagen Breakdown Without Compromising Cell Viability in Cultured Human Tenocytes: An In Vitro Study" Cells 14, no. 13: 988. https://doi.org/10.3390/cells14130988
APA StyleRandelli, F., Mazzoleni, M. G., Menon, A., Fioruzzi, A., Henin, D., Sommariva, M., & Gagliano, N. (2025). Lidocaine Affects Collagen Breakdown Without Compromising Cell Viability in Cultured Human Tenocytes: An In Vitro Study. Cells, 14(13), 988. https://doi.org/10.3390/cells14130988











