Understanding the Role of Epithelial Cells in the Pathogenesis of Systemic Sclerosis
Abstract
1. Introduction
| Phenotype Change | Molecular Basis | Reference |
|---|---|---|
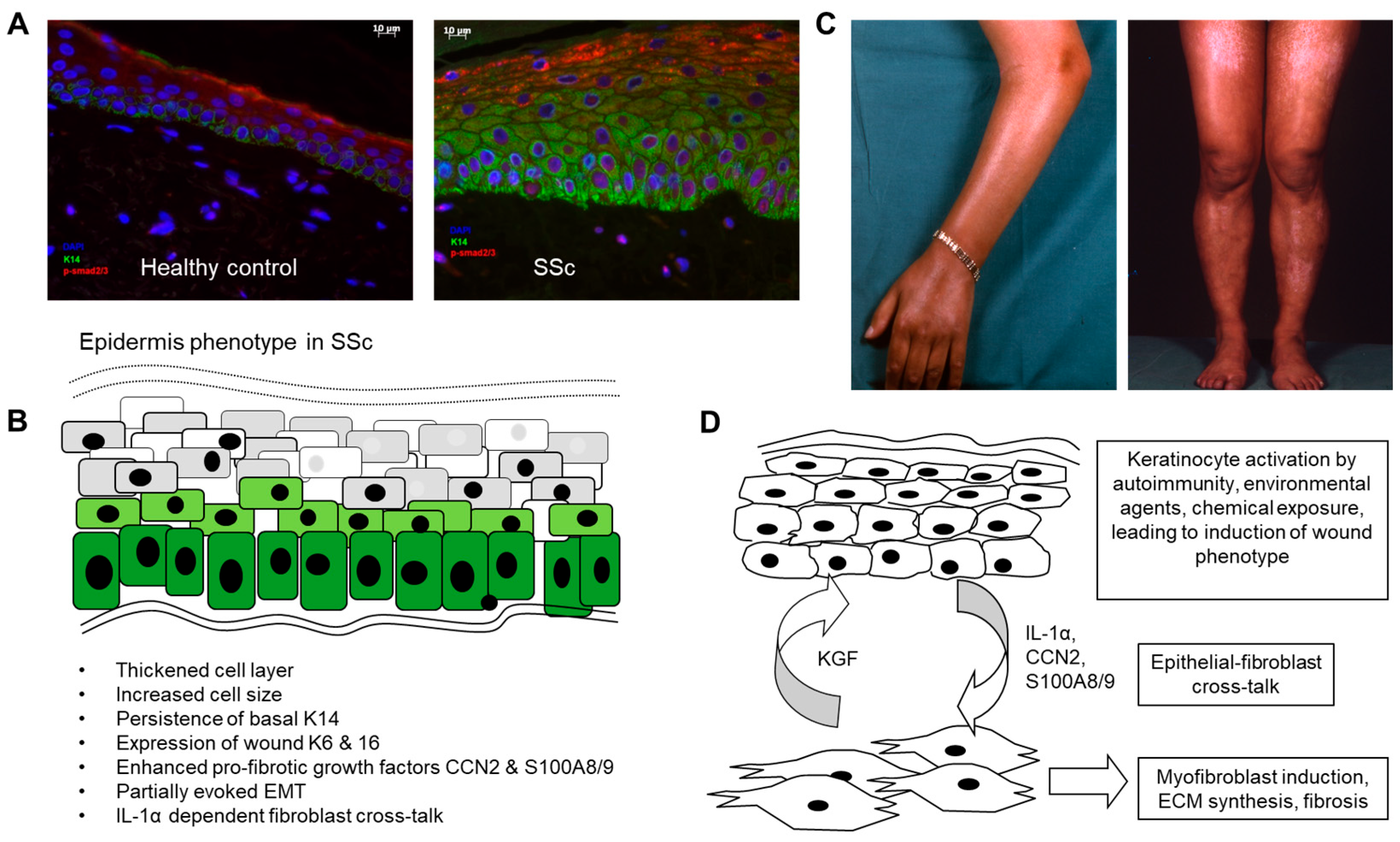
| Delayed differentiation of basal keratinocytes. | Continued expression of K5 and 14 into suprabasal and spinous layers. | [12] |
| EMT | Induction of SNAI and SFRP4. Altered Wnt signalling. | [11,23,24] |
| Wound healing phenotype. | Expression of K6 and K16 usually seen in wound epidermis. | [13,15,16] |
| Thickening of epidermis. | Increased number of keratinocyte cell layers. Increase size of keratinocytes. | [14] |
| Hyperproliferation. | Ki67 staining shows increased basal keratinocyte proliferation. | [14] |
| Altered gene expression signature. | Type I IFN signature responsive to JAK/STAT inhibitor. | [25] |
| Hyperpigmentation. | Increased melanocyte content. Altered CCN3 expression by melanocytes. Associations with early diffuse subset and race. | [26,27,28] |
| Altered barrier function. | Expansion of involucrin and loricrin-positive cell layers. | [14] |
| Pro-fibrotic activity | Activation of dermal fibroblasts in 3D co-culture. Media transfer activation of fibroblasts. | [13,16,29] |
2. Altered Differentiation of the Epidermis in Scleroderma: Activated Wound Healing Phenotype
3. The Epidermis as a Potential Site for Initiating Autoimmunity in SSc
4. Cross-Talk and Transition Between Epithelial Cells and Mesenchymal Cells in SSc
5. Possible Initiating Biomechanisms Leading to Epidermis Activation in SSc
6. Directing Therapies to the Epidermal Layer
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Prim. 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Altorok, N.; Wang, Y.; Kahaleh, B. Endothelial dysfunction in systemic sclerosis. Curr. Opin. Rheumatol. 2014, 26, 615–620. [Google Scholar] [CrossRef]
- Shiwen, X.; Stratton, R.; Nikitorowicz-Buniak, J.; Ahmed-Abdi, B.; Ponticos, M.; Denton, C.; Abraham, D.; Takahashi, A.; Suki, B.; Layne, M.D.; et al. A Role of Myocardin Related Transcription Factor-A (MRTF-A) in Scleroderma Related Fibrosis. PLoS ONE 2015, 10, e0126015. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Skaug, B.; Tabib, T.; Li, Y.N.; Tao, Y.; Matei, A.E.; Lyons, M.A.; Schett, G.; Lafyatis, R.; et al. Fibroblast Subpopulations in Systemic Sclerosis: Functional Implications of Individual Subpopulations and Correlations with Clinical Features. J. Investig. Dermatol. 2024, 144, 1251–1261e13. [Google Scholar] [CrossRef]
- Grieb, G.; Bucala, R. Fibrocytes in Fibrotic Diseases and Wound Healing. Adv. Wound Care 2012, 1, 36–40. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.D.; Wei, J.; Wood, T.A.; Graham, L.V.; Whitfield, M.L.; Scherer, P.E.; Tourtellotte, W.G.; Varga, J. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015, 67, 1062–1073. [Google Scholar] [CrossRef]
- Horsley, V. Adipocyte plasticity in tissue regeneration, repair, and disease. Curr. Opin. Genet. Dev. 2022, 76, 101968. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, V.S.; Sundberg, C.; Abraham, D.J.; Rubin, K.; Black, C.M. Activation of microvascular pericytes in autoimmune Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 1999, 42, 930–941. [Google Scholar] [CrossRef]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef]
- Aden, N.; Shiwen, X.; Aden, D.; Black, C.; Nuttall, A.; Denton, C.P.; Leask, A.; Abraham, D.; Stratton, R. Proteomic analysis of scleroderma lesional skin reveals activated wound healing phenotype of epidermal cell layer. Rheumatology 2008, 47, 1754–1760. [Google Scholar] [CrossRef]
- Aden, N.; Nuttall, A.; Shiwen, X.; de Winter, P.; Leask, A.; Black, C.M.; Denton, C.P.; Abraham, D.J.; Stratton, R.J. Epithelial cells promote fibroblast activation via IL-1alpha in systemic sclerosis. J. Investig. Dermatol. 2010, 130, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Nikitorowicz-Buniak, J.; Shiwen, X.; Denton, C.P.; Abraham, D.; Stratton, R. Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S100A9. J. Investig. Dermatol. 2014, 134, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Nikitorowicz-Buniak, J.; Denton, C.P.; Abraham, D.; Stratton, R. Partially Evoked Epithelial-Mesenchymal Transition (EMT) Is Associated with Increased TGFbeta Signaling within Lesional Scleroderma Skin. PLoS ONE 2015, 10, e0134092. [Google Scholar] [CrossRef]
- Berkowitz, J.S.; Tabib, T.; Xiao, H.; Sadej, G.M.; Khanna, D.; Fuschiotti, P.; Lafyatis, R.A.; Das, J. Cell Type-Specific Biomarkers of Systemic Sclerosis Disease Severity Capture Cell-Intrinsic and Cell-Extrinsic Circuits. Arthritis Rheumatol. 2023, 75, 1819–1830. [Google Scholar] [CrossRef]
- Russo, B.; Borowczyk, J.; Boehncke, W.H.; Truchetet, M.E.; Modarressi, A.; Brembilla, N.C.; Chizzolini, C. Dysfunctional Keratinocytes Increase Dermal Inflammation in Systemic Sclerosis: Results From Studies Using Tissue-Engineered Scleroderma Epidermis. Arthritis Rheumatol. 2021, 73, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Blackwell, T.S.; Eickelberg, O.; Loyd, J.E.; Kaminski, N.; Jenkins, G.; Maher, T.M.; Molina-Molina, M.; Noble, P.W.; Raghu, G.; et al. Time for a change: Is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet. Respir. Med. 2018, 6, 154–160. [Google Scholar] [CrossRef]
- Parimon, T.; Yao, C.; Stripp, B.R.; Noble, P.W.; Chen, P. Alveolar Epithelial Type II Cells as Drivers of Lung Fibrosis in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 2269. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J. Mol. Med. 2004, 82, 175–181. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Fiorelli, R.F. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am. J. Surg. Pathol. 1994, 18, 136–147. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell. Signal. 2020, 66, 109482. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Bayle, J.; Fitch, J.; Jacobsen, K.; Kumar, R.; Lafyatis, R.; Lemaire, R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: Role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J. Investig. Dermatol. 2008, 128, 871–881. [Google Scholar] [CrossRef]
- Tinazzi, I.; Mulipa, P.; Colato, C.; Abignano, G.; Ballarin, A.; Biasi, D.; Emery, P.; Ross, R.L.; Del Galdo, F. SFRP4 Expression Is Linked to Immune-Driven Fibrotic Conditions, Correlates with Skin and Lung Fibrosis in SSc and a Potential EMT Biomarker. J. Clin. Med. 2021, 10, 5820. [Google Scholar] [CrossRef]
- Khanna, D.; Padilla, C.; Tsoi, L.C.; Nagaraja, V.; Khanna, P.P.; Tabib, T.; Kahlenberg, J.M.; Young, A.; Huang, S.; Gudjonsson, J.E.; et al. Tofacitinib blocks IFN-regulated biomarker genes in skin fibroblasts and keratinocytes in a systemic sclerosis trial. JCI Insight 2022, 7, e159566. [Google Scholar] [CrossRef] [PubMed]
- Tabata, H.; Hara, N.; Otsuka, S.; Yamakage, A.; Yamazaki, S.; Koibuchi, N. Correlation between diffuse pigmentation and keratinocyte-derived endothelin-1 in systemic sclerosis. Int. J. Dermatol. 2000, 39, 899–902. [Google Scholar] [CrossRef]
- Henrot, P.; Pain, C.; Taieb, A.; Truchetet, M.E.; Cario, M. Dysregulation of CCN3 (NOV) expression in the epidermis of systemic sclerosis patients with pigmentary changes. Pigment. Cell Melanoma Res. 2020, 33, 895–898. [Google Scholar] [CrossRef]
- Nusbaum, J.S.; Gordon, J.K.; Steen, V.D. African American race associated with body image dissatisfaction among patients with systemic sclerosis. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 100), 70–73. [Google Scholar] [PubMed]
- McCoy, S.S.; Reed, T.J.; Berthier, C.C.; Tsou, P.S.; Liu, J.; Gudjonsson, J.E.; Khanna, D.; Kahlenberg, J.M. Scleroderma keratinocytes promote fibroblast activation independent of transforming growth factor beta. Rheumatology 2017, 56, 1970–1981. [Google Scholar] [CrossRef]
- Watt, F.M. Mammalian skin cell biology: At the interface between laboratory and clinic. Science 2014, 346, 937–940. [Google Scholar] [CrossRef]
- Louis, B.; Tewary, M.; Bremer, A.W.; Philippeos, C.; Negri, V.A.; Zijl, S.; Gartner, Z.J.; Schaffer, D.V.; Watt, F.M. A reductionist approach to determine the effect of cell-cell contact on human epidermal stem cell differentiation. Acta Biomater. 2022, 150, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Green, H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 1980, 19, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, M.; Zhang, L.J. Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Snyder, R.J.; Lantis, J.; Kirsner, R.S.; Shah, V.; Molyneaux, M.; Carter, M.J. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair. Regen. 2016, 24, 613–629. [Google Scholar] [CrossRef]
- Watanabe, S.; Osumi, M.; Ohnishi, T.; Ichikawa, E.; Takahashi, H. Changes in cytokeratin expression in epidermal keratinocytes during wound healing. Histochem. Cell Biol. 1995, 103, 425–433. [Google Scholar] [CrossRef]
- Ahmed Abdi, B.; Lopez, H.; Karrar, S.; Renzoni, E.; Wells, A.; Tam, A.; Etomi, O.; Hsuan, J.J.; Martin, G.R.; Shiwen, X.; et al. Use of Patterned Collagen Coated Slides to Study Normal and Scleroderma Lung Fibroblast Migration. Sci. Rep. 2017, 7, 2628. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landen, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Kim, Y.C.; Quan, F.S.; Yoo, D.G.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine 2009, 27, 6932–6938. [Google Scholar] [CrossRef]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells—The Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef]
- Kitashima, D.Y.; Kobayashi, T.; Woodring, T.; Idouchi, K.; Doebel, T.; Voisin, B.; Adachi, T.; Ouchi, T.; Takahashi, H.; Nishifuji, K.; et al. Langerhans Cells Prevent Autoimmunity via Expansion of Keratinocyte Antigen-Specific Regulatory T Cells. EBioMedicine 2018, 27, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hasegawa, T.; Lee, T.; Oliver-Garcia, V.S.; Mortaja, M.; Azin, M.; Horiba, S.; Smith, S.S.; Khattab, S.; Trerice, K.E.; et al. Langerhans Cells Directly Interact with Resident T Cells in the Human Epidermis. JID Innov. 2025, 5, 100324. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.S.; Friou, G.J.; Barr, R.J.; Mirick, G.R.; Berman, M.; Sandborg, C.; Ross, P.A. Loss of epidermal Langerhans’ cells and endothelial cell HLA-DR antigens in the skin in progressive systemic sclerosis. J. Rheumatol. 1986, 13, 341–348. [Google Scholar]
- Goobar, J.P.; Fang, M.; Weisman, M.H.; Zvaifler, N.; Gigli, I. Langerhans cells in connective tissue diseases. Scand. J. Rheumatol. 1987, 16, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Chong, B.; Mirchandani, N.; Brinster, N.K.; Yamanaka, K.; Dowgiert, R.K.; Kupper, T.S. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 2006, 176, 4431–4439. [Google Scholar] [CrossRef]
- Watanabe, R.; Gehad, A.; Yang, C.; Scott, L.L.; Teague, J.E.; Schlapbach, C.; Elco, C.P.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015, 7, 279ra239. [Google Scholar] [CrossRef]
- Simmons, J.; Gallo, R.L. The Central Roles of Keratinocytes in Coordinating Skin Immunity. J. Investig. Dermatol. 2024, 144, 2377–2398. [Google Scholar] [CrossRef]
- Werner, S.; Smola, H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 2001, 11, 143–146. [Google Scholar] [CrossRef]
- Liu, S.; Shi-wen, X.; Abraham, D.J.; Leask, A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011, 63, 239–246. [Google Scholar] [CrossRef]
- Lafyatis, R. Transforming growth factor beta–at the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tochimoto, A.; Ichikawa, N.; Harigai, M.; Hara, M.; Kotake, S.; Kitamura, Y.; Kamatani, N. Association of IL1A gene polymorphisms with susceptibility to and severity of systemic sclerosis in the Japanese population. Arthritis Rheum. 2003, 48, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Harigai, M.; Suzuki, K.; Hara, M.; Kobayashi, K.; Ishizuka, T.; Matsuki, Y.; Tanaka, N.; Nakamura, H. Interleukin 1 receptor on fibroblasts from systemic sclerosis patients induces excessive functional responses to interleukin 1 beta. Biochem. Biophys. Res. Commun. 1993, 190, 154–161. [Google Scholar] [CrossRef]
- Mantero, J.C.; Kishore, N.; Ziemek, J.; Stifano, G.; Zammitti, C.; Khanna, D.; Gordon, J.K.; Spiera, R.; Zhang, Y.; Simms, R.W.; et al. Randomised, double-blind, placebo-controlled trial of IL1-trap, rilonacept, in systemic sclerosis. A phase I/II biomarker trial. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 113), 146–149. [Google Scholar]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Maas-Szabowski, N.; Shimotoyodome, A.; Fusenig, N.E. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J. Cell Sci. 1999, 112 Pt 12, 1843–1853. [Google Scholar] [CrossRef]
- Canady, J.; Arndt, S.; Karrer, S.; Bosserhoff, A.K. Increased KGF expression promotes fibroblast activation in a double paracrine manner resulting in cutaneous fibrosis. J. Investig. Dermatol. 2013, 133, 647–657. [Google Scholar] [CrossRef]
- Moezinia, C.; Wong, V.; Watson, J.; Nagib, L.; Lopez Garces, S.; Zhang, S.; Ahmed Abdi, B.; Newton, F.; Abraham, D.; Stratton, R. Autoantibodies Which Bind to and Activate Keratinocytes in Systemic Sclerosis. Cells 2023, 12, 2490. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.; Savage, C.O.; Isenberg, D.; Pearson, J.D. IgG anti-endothelial cell autoantibodies from patients with systemic lupus erythematosus or systemic vasculitis stimulate the release of two endothelial cell-derived mediators, which enhance adhesion molecule expression and leukocyte adhesion in an autocrine manner. Arthritis Rheum. 1999, 42, 631–640. [Google Scholar] [CrossRef]
- Muntyanu, A.; Milan, R.; Rahme, E.; Baron, M.; Netchiporouk, E.; Canadian Scleroderma Research, G. Organic solvent exposure and systemic sclerosis: A retrospective cohort study based on the Canadian Scleroderma Research Group registry. J. Am. Acad. Dermatol. 2023, 90, 605–607. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, S.; Kwak, S.S.; Kim, J. Advancements in Skin-Mediated Drug Delivery: Mechanisms, Techniques, and Applications. Adv. Healthc. Mater. 2024, 13, e2302375. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Denton, C.P.; Jahreis, A.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet 2016, 387, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagib, L.; Kumar, A.S.; Stratton, R. Understanding the Role of Epithelial Cells in the Pathogenesis of Systemic Sclerosis. Cells 2025, 14, 962. https://doi.org/10.3390/cells14130962
Nagib L, Kumar AS, Stratton R. Understanding the Role of Epithelial Cells in the Pathogenesis of Systemic Sclerosis. Cells. 2025; 14(13):962. https://doi.org/10.3390/cells14130962
Chicago/Turabian StyleNagib, Lydia, Anshul Sheel Kumar, and Richard Stratton. 2025. "Understanding the Role of Epithelial Cells in the Pathogenesis of Systemic Sclerosis" Cells 14, no. 13: 962. https://doi.org/10.3390/cells14130962
APA StyleNagib, L., Kumar, A. S., & Stratton, R. (2025). Understanding the Role of Epithelial Cells in the Pathogenesis of Systemic Sclerosis. Cells, 14(13), 962. https://doi.org/10.3390/cells14130962






