The Ac/N-Degron Domain of MARCHF6 E3 Ubiquitin Ligase and Its Role in Regulating Ferroptosis
Abstract
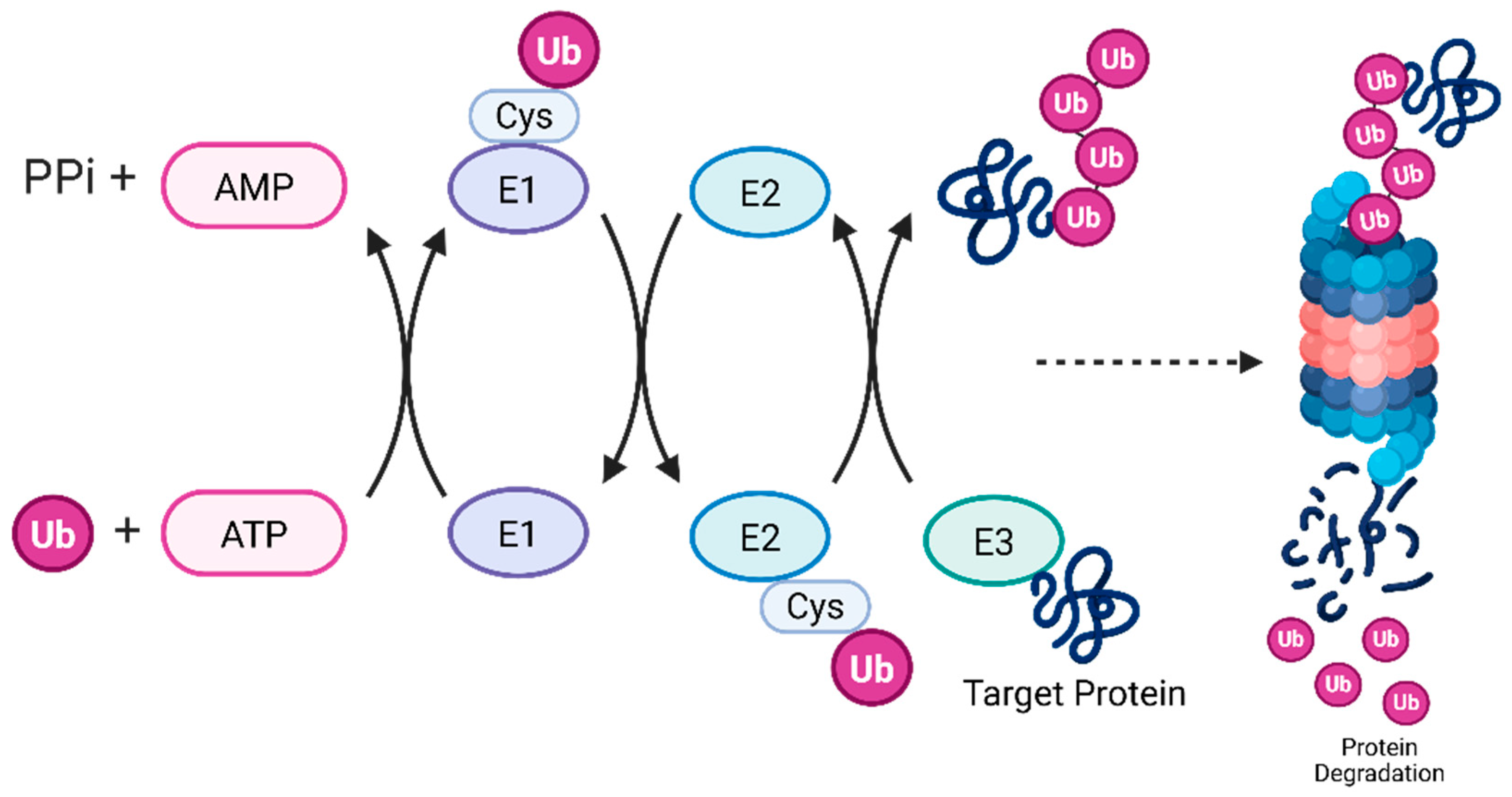
1. Introduction
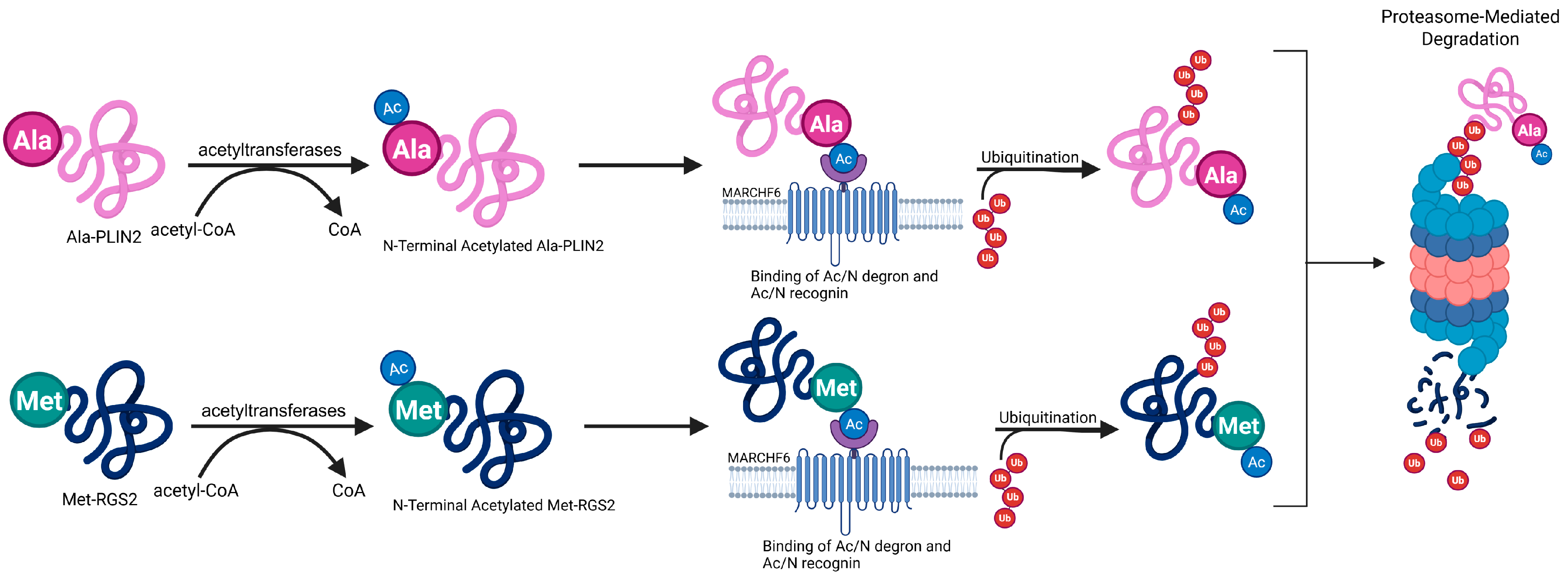
2. How Does MARCHF6 Interact with Ac/N Degrons to Facilitate Degradation?
3. The C5 Region of MARCHF6 Is Essential for Recognizing Ac/N Degrons
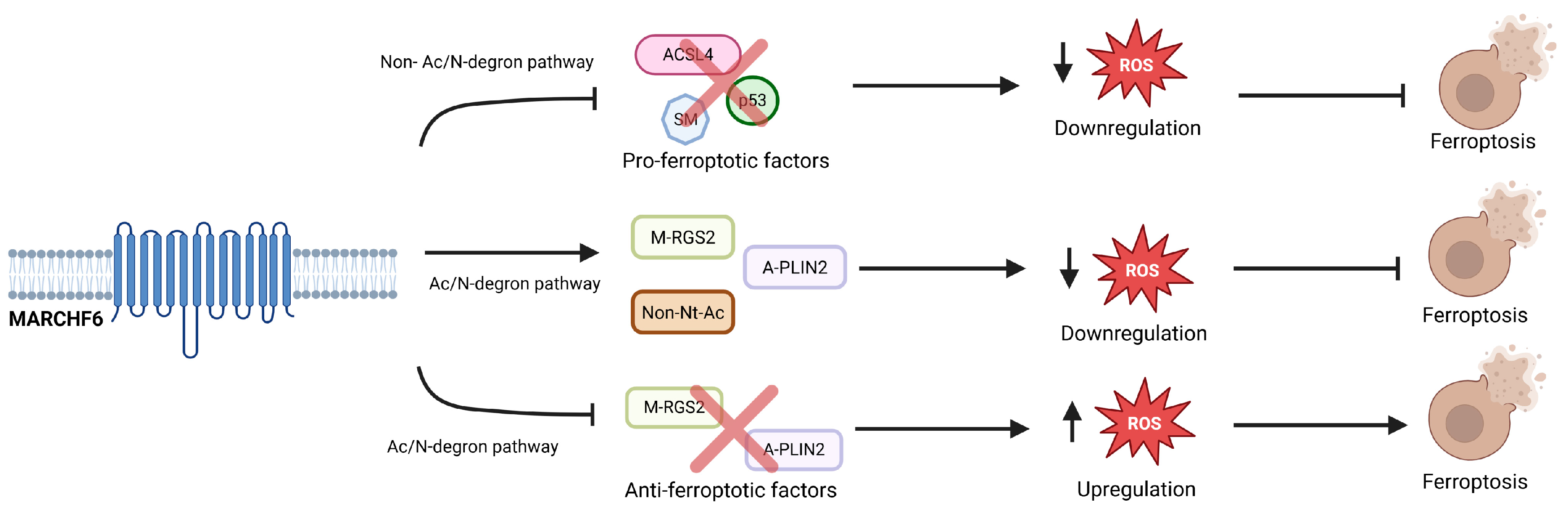
4. MARCHF6 Regulates Ferroptosis Through the Ac/N Degron Pathway
5. The Multifaceted Role of MARCHF6 in Regulating Ferroptosis
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cell Death: Types, Causes & Necrosis. Cleveland Clinic. 2024. Available online: https://my.clevelandclinic.org/health/articles/cell-death (accessed on 3 February 2025).
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Berndt, C.; Alborzinia, H.; Amen, V.S.; Ayton, S.; Barayeu, U.; Bartelt, A.; Bayir, H.; Bebber, C.M.; Birsoy, K.; Böttcher, J.P.; et al. Ferroptosis in health and disease. Redox Biol. 2024, 75, 103211. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Ding, Y. The role of ferroptosis in neurodegenerative diseases. Front. Cell. Neurosci. 2024, 18, 1475934. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, S.-Y.; Hwang, C.-S. Delineation of the substrate recognition domain of MARCHF6 E3 ubiquitin ligase in the Ac/N-degron pathway and its regulatory role in ferroptosis. J. Biol. Chem. 2024, 300, 107731. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, Y.; Hwang, C.-S. The ubiquitin-proteasome system links NADPH metabolism to ferroptosis. Trends Cell Biol. 2023, 33, 1088–1103. [Google Scholar] [CrossRef]
- Mun, S.-H.; Hwang, C.-S. Marchf6: A guardian against cytosol-spilled POMC-induced ferroptosis. Mol. Cells 2024, 47, 100008. [Google Scholar] [CrossRef]
- Goossens, A.; Chen, Y.-T.; Hsia, M.M.; Gryffory, L.; Beathard, C.; Hua, Z.; Li, Y.; Hoecker, U.; Lourenço, T.F.; Mooney, S.; et al. Structure, Function, and Evolution of E3 Ligases and Targets. Frontiers 2020. Available online: https://www.frontiersin.org/research-topics/14852/structure-function-and-evolution-of-e3-ligases-and-targets (accessed on 5 February 2025).
- Nguyen, K.T.; Mun, S.-H.; Lee, C.-S.; Hwang, C.-S. Control of protein degradation by N-terminal acetylation and the N-end rule pathway. Exp. Mol. Med. 2018, 50, 1–8. [Google Scholar] [CrossRef]
- Damgaard, R.B. The ubiquitin system: From cell signalling to disease biology and New Therapeutic Opportunities. Cell Death Differ. 2021, 28, 423–426. [Google Scholar] [CrossRef]
- Arnesen, T.; Kjosås, I.; McTiernan, N. Protein N-terminal acetylation is entering the degradation end game. Nat. Rev. Mol. Cell Biol. 2024, 25, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, O.H.M.H.; Mascarenhas, B.; Cheng, H.-Y.M. Death of a Protein: The Role of E3 Ubiquitin Ligases in Circadian Rhythms of Mice and Flies. Int. J. Mol. Sci. 2022, 23, 10569. [Google Scholar] [CrossRef]
- Park, S.-E.; Kim, J.-M.; Seok, O.-H.; Cho, H.; Wadas, B.; Kim, S.-Y.; Varshavsky, A.; Hwang, C.-S. Control of mammalian G protein signaling by N-terminal acetylation and the N-end rule pathway. Science 2015, 347, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Lee, C.-S.; Mun, S.-H.; Truong, N.T.; Park, S.K.; Hwang, C.-S. N-terminal acetylation and the N-end rule pathway control degradation of the lipid droplet protein PLIN2. J. Biol. Chem. 2019, 294, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-H.; Lee, C.-S.; Kim, H.J.; Kim, J.; Lee, H.; Yang, J.; Im, S.-H.; Kim, J.-H.; Seong, J.K.; Hwang, C.-S. Marchf6 E3 ubiquitin ligase critically regulates endoplasmic reticulum stress, ferroptosis, and metabolic homeostasis in POMC neurons. Cell Rep. 2023, 42, 112746. [Google Scholar] [CrossRef]
- Chua, N.K.; Coates, H.W.; Brown, A.J. Squalene monooxygenase: A journey to the heart of cholesterol synthesis. Prog. Lipid Res. 2020, 79, 101033. [Google Scholar] [CrossRef]
- Zelcer, N.; Sharpe, L.J.; Loregger, A.; Kristiana, I.; Cook, E.C.L.; Phan, L.; Stevenson, J.; Brown, A.J. The E3 Ubiquitin Ligase MARCH6 Degrades Squalene Monooxygenase and Affects 3-Hydroxy-3-Methyl-Glutaryl Coenzyme A Reductase and the Cholesterol Synthesis Pathway. Mol. Cell. Biol. 2014, 34, 1262–1270. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. Ros Induced Lipid Peroxidation and Their Role in Ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Mun, S.-H.; Yang, J.; Lee, J.; Seok, O.-H.; Kim, E.; Kim, D.; An, S.Y.; Seo, D.-Y.; Suh, J.-Y.; et al. The MARCHF6 E3 ubiquitin ligase acts as an NADPH sensor for the regulation of ferroptosis. Nat. Cell Biol. 2022, 24, 1239–1251. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, Y.; Niu, Y.; Wang, Y.; Liu, L.; Tang, Y.; Huang, J.; Leung, E.L.-H. Investigating the interaction between calcium signaling and ferroptosis for novel cancer treatment. Phytomedicine 2025, 137, 156377. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoniyi, H.; Hohman, G.; Eldeeb, M. The Ac/N-Degron Domain of MARCHF6 E3 Ubiquitin Ligase and Its Role in Regulating Ferroptosis. Cells 2025, 14, 954. https://doi.org/10.3390/cells14130954
Omoniyi H, Hohman G, Eldeeb M. The Ac/N-Degron Domain of MARCHF6 E3 Ubiquitin Ligase and Its Role in Regulating Ferroptosis. Cells. 2025; 14(13):954. https://doi.org/10.3390/cells14130954
Chicago/Turabian StyleOmoniyi, Hope, Grace Hohman, and Mohamed Eldeeb. 2025. "The Ac/N-Degron Domain of MARCHF6 E3 Ubiquitin Ligase and Its Role in Regulating Ferroptosis" Cells 14, no. 13: 954. https://doi.org/10.3390/cells14130954
APA StyleOmoniyi, H., Hohman, G., & Eldeeb, M. (2025). The Ac/N-Degron Domain of MARCHF6 E3 Ubiquitin Ligase and Its Role in Regulating Ferroptosis. Cells, 14(13), 954. https://doi.org/10.3390/cells14130954



