Molecular Regulation of SASP in Cellular Senescence: Therapeutic Implications and Translational Challenges
Abstract
1. Introduction
2. Cellular Senescence: Mechanisms and Disease Implications
2.1. Accumulation of Senescent Cells and Their Role in Age-Related Diseases
2.2. Stress-Induced Premature Senescence (SIPS)
2.3. Molecular Triggers and Mechanisms
2.4. Heterogeneity of Senescent Phenotypes
2.5. Immunosenescence: Aging of the Immune System and Its Consequences
2.6. Dual Role of Senescence: Barrier and Risk Factor
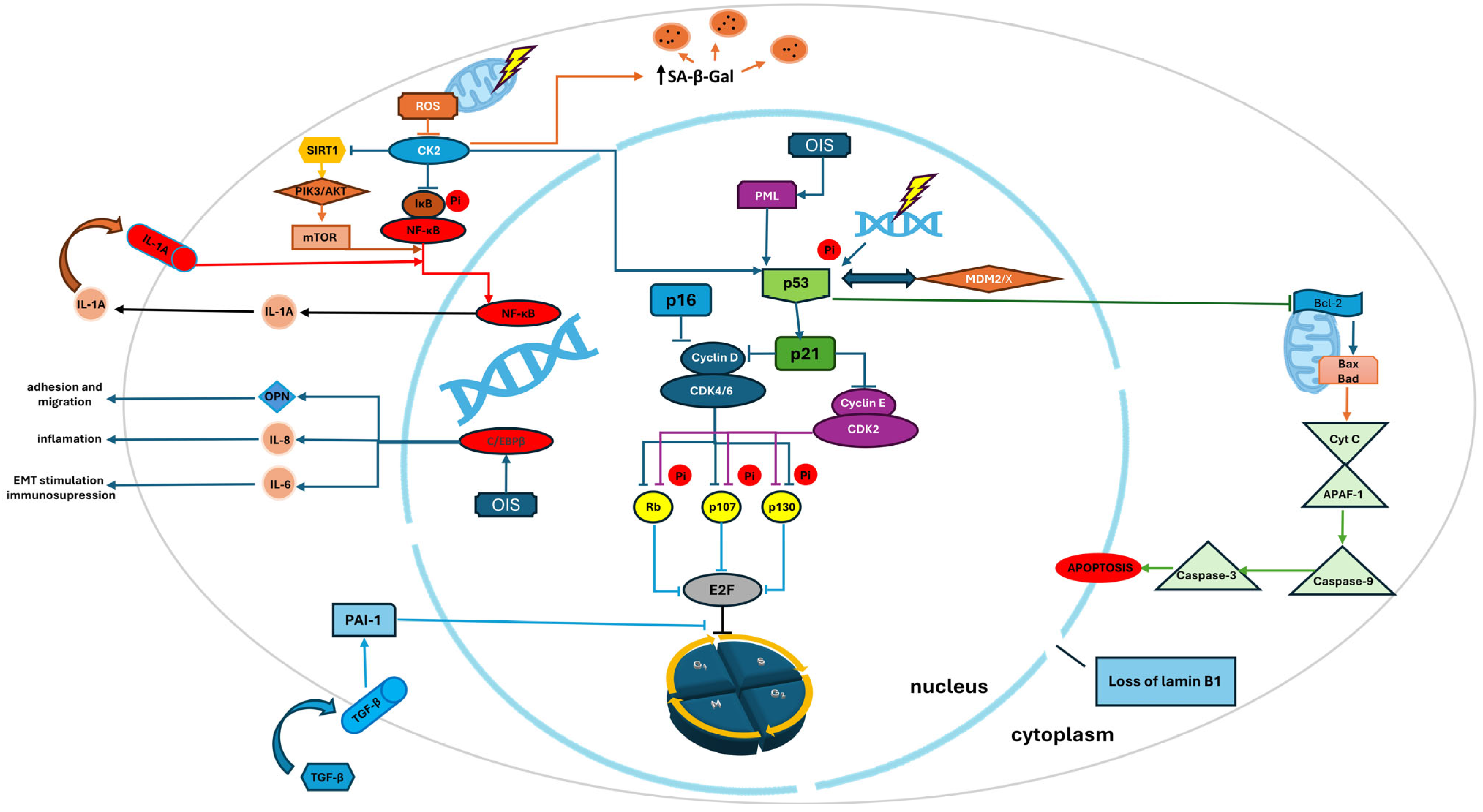
3. Selected Regulators of Senescence
3.1. p53/p21 Pathway: Guardian of the Genome
3.2. p16INK4a/Rb Pathway: Cell Cycle Arrest and Tumor Suppression
3.3. Autophagy and Mitophagy in the Regulation of Cellular Senescence
4. The Dual Role of Cellular Senescence and SASP in Tissue Homeostasis and Disease
4.1. The Physiological Role of SASP
4.2. Main Regulatory Pathways of SASP
4.3. IL-6—Between Senescence and Cancer
4.4. IL-8 and TNFα in Cancer Progression and Inflammation
4.5. Pathological Microbiota and the Induction of Cellular Senescence and SASP
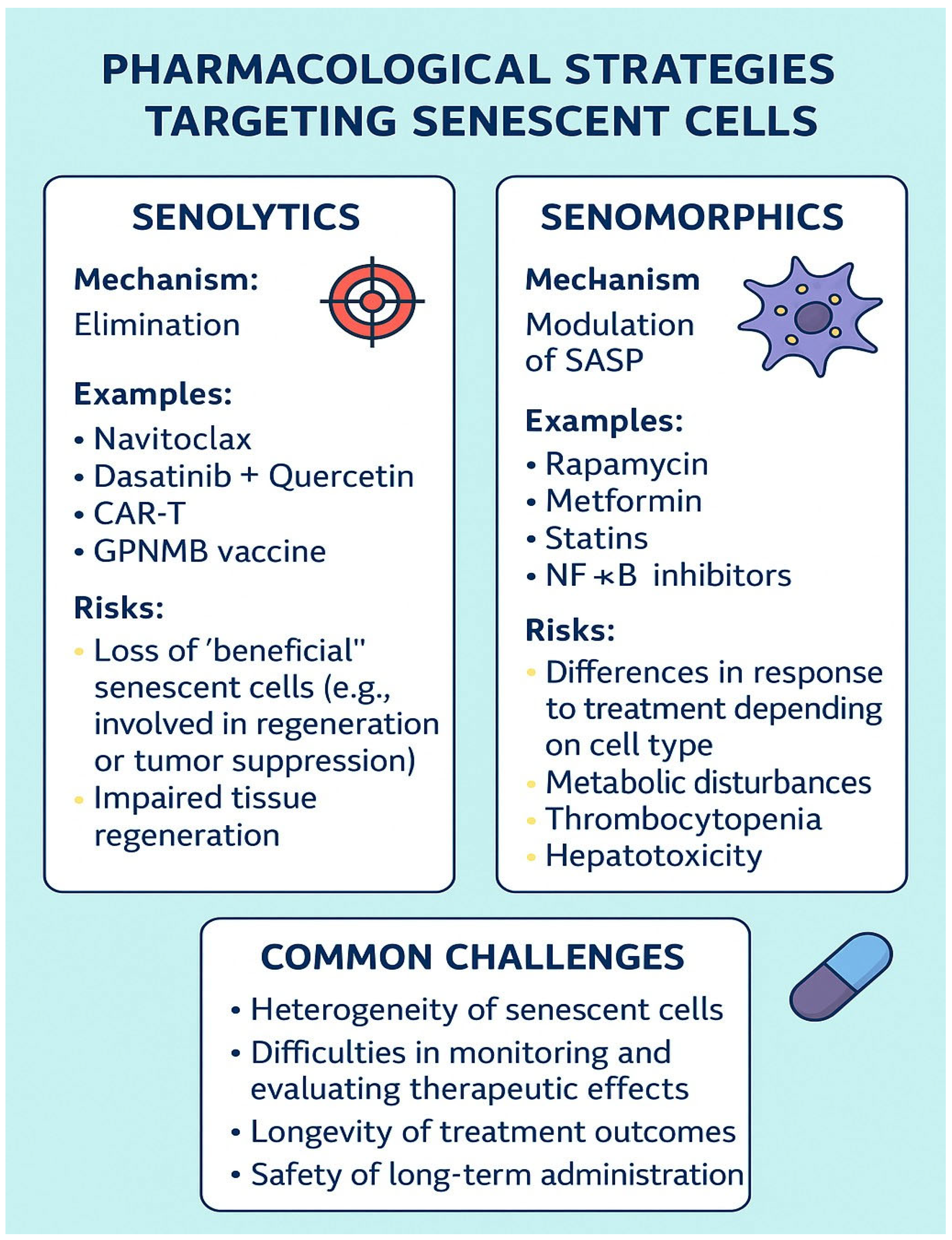
5. Pharmacological Strategies Targeting Senescent Cells: Senolytics, Senomorphics, and Therapeutic Challenges
5.1. Senolytics
5.2. Senomorphics
5.3. Therapeutic Challenges
6. Conclusions
- Cellular senescence acts as both a barrier to cancer and a driver of chronic diseases via the SASP.
- Senolytic and senomorphic therapies represent promising approaches, but their selectivity, clinical efficacy, and safety require further study.
- Most clinical data are still preliminary; preclinical findings should be cautiously interpreted before translation into clinical practice.
- The heterogeneity of senescent cells and lack of specific markers remain key challenges.
- Circulating SASP factors, including IL-6, may serve as valuable clinical biomarkers for predicting health status and complications in the elderly.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Laberge, R.-M.; Demaria, M.; Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- de Mera-Rodríguez, J.A.; Infantes-Rojas, L.; Hernández-Núñez, I.; Herrera-Gómez, I.G.; Martín-Partido, G.; Francisco-Morcillo, J. Senescence-Associated β-Galactosidase Activity in the Retina Is Linked to Müller Glia Proliferation During Zebrafish Development and Regeneration. Front. Cell Dev. Biol. 2021, 9, 693451. [Google Scholar]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Sanders, Y.Y.; Liu, H.; Zhang, X.; Hecker, L.; Bernard, K.; Desai, L.; Liu, G.; Thannickal, V.J. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox. Biol. 2013, 1, 8–16. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; Demaria, M. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 2017, 27, 2652–2660. [Google Scholar] [CrossRef]
- Vizioli, M.G.; Liu, T.; Miller, K.N.; Robertson, N.; Gilroy, K.; Lagnado, A.B.; Perez-Garcia, A.; Kiourtis, C.; Dasgupta, N.; Lei, X.; et al. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 2020, 34, 428–445. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and regulation of cellular senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Lackner, D.H.; Hayashi, M.; Cesare, A.; Karlseder, J. A genomics approach identifies senescence-specific gene expression regulation. Aging Cell 2014, 13, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cheng, J.; Zeng, H.; Shao, L. Senescent cell depletion through targeting BCL-family proteins and mitochondria. Front. Physiol. 2020, 11, 593630. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; An, J.; Zou, M.H. Immune clearance of senescent cells to combat ageing and chronic diseases. Cells 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Chen, X.C.; Song, Y.; Pan, X.D.; Dai, X.M.; Zhang, J.; Cui, X.L.; Wu, X.L.; Zhu, Y.G. Amyloid β protein aggravates neuronal senescence and cognitive deficits in 5XFAD mouse model of Alzheimer’s disease. Chin. Med. J. 2016, 129, 1835–1844. [Google Scholar] [CrossRef]
- Xu, M.; Bradley, E.W.; Weivoda, M.M.; Hwang, S.M.; Pirtskhalava, T.; Decklever, T.; Curran, G.L.; Ogrodnik, M.; Jurk, D.; Johnson, K.O.; et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 780–785. [Google Scholar] [CrossRef]
- Itahana, K.; Campisi, J.; Dimri, G.P. Mechanisms of cellular senescence in human and mouse cells. Biogerontology 2004, 5, 1–10. [Google Scholar] [CrossRef]
- Xiao, S.; Qin, D.; Hou, X.; Tian, L.; Yu, Y.; Zhang, R.; Lyu, H.; Guo, D.; Chen, X.Z.; Zhou, C.; et al. Cellular senescence: A double-edged sword in cancer therapy. Front. Oncol. 2023, 13, 1189015. [Google Scholar] [CrossRef]
- Ewald, J.A.; Desotelle, J.A.; Wilding, G.; Jarrard, D.F. Therapy-induced senescence in cancer. J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef]
- Vergel, M.; Marin, J.J.; Estevez, P.; Carnero, A. Cellular senescence as a target in cancer control. J. Aging Res. 2011, 2011, 725365. [Google Scholar] [CrossRef]
- Hou, J.G.; Jeon, B.M.; Yun, Y.J.; Cui, C.H.; Kim, S.C. Ginsenoside Rh2 ameliorates doxorubicin-induced senescence bystander effect in breast carcinoma cell MDA-MB-231 and normal epithelial cell MCF-10A. Int. J. Mol. Sci. 2019, 20, 1244. [Google Scholar] [CrossRef]
- Roberson, R.S.; Kussick, S.J.; Vallieres, E.; Chen, S.Y.; Wu, D.Y. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005, 65, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Yin, G.; Huang, J.R.; Xi, S.J.; Qian, F.; Lee, R.X.; Peng, X.C.; Tang, F.R. Ionizing radiation-induced brain cell aging and the potential underlying molecular mechanisms. Cells 2021, 10, 3570. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Adv. Hyg. Exp. Med. 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Jeong, S.G.; Cho, G.W. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem. Biophys. Res. Commun. 2015, 460, 971–976. [Google Scholar] [CrossRef]
- Caporali, A.; Meloni, M.; Nailor, A.; Mitić, T.; Shantikumar, S.; Riu, F.; Sala-Newby, G.B.; Rose, L.; Besnier, M.; Katare, R.; et al. p75NTR-dependent activation of NF-κB regulates microRNA-503 transcription and pericyte–endothelial crosstalk in diabetes after limb ischaemia. Nat. Commun. 2015, 6, 8024. [Google Scholar] [CrossRef]
- Liu, X.-L.; Ding, J.; Meng, L.-H. Oncogene-induced senescence: A double-edged sword in cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef]
- Courtois-Cox, S.; Jones, S.L.; Cichowski, K. Many roads lead to oncogene-induced senescence. Oncogene 2008, 27, 2801–2808. [Google Scholar] [CrossRef]
- Sosińska, P.; Mikuła-Pietrasik, J.; Książek, K. Molecular bases of cellular senescence: Hayflick phenomenon 50 years later. Postepy Hig. Med. Dosw. 2016, 70, 231–242. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Y.; Qian, L.; Wang, P. Emerging strategies to target RAS signaling in human cancer therapy. J. Hematol. Oncol. 2021, 14, 116. [Google Scholar] [CrossRef]
- Sarkisian, C.J.; Keister, B.A.; Stairs, D.B.; Boxer, R.B.; Moody, S.E.; Chodosh, L.A. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007, 9, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Braig, M.; Lee, S.; Loddenkemper, C.; Rudolph, C.; Peters, A.H.; Schlegelberger, B.; Stein, H.; Dörken, B.; Jenuwein, T.; Schmitt, C.A. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005, 436, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Rattanavirotkul, N.; Kirschner, K.; Chandra, T. Induction and transmission of oncogene-induced senescence. Cell. Mol. Life Sci. 2021, 78, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. J. Cell Biol. 2017, 217, 65–75. [Google Scholar] [CrossRef]
- Suda, M.; Shimizu, I.; Katsuumi, G.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Matsumoto, N.; Yoshida, Y.; Mikawa, R.; Katayama, A.; et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 2021, 1, 1117–1126. [Google Scholar] [CrossRef]
- Brauning, A.; Rae, M.; Zhu, G.; Fulton, E.; Admasu, T.D.; Stolzing, A.; Sharma, A. Aging of the immune system: Focus on natural killer cells phenotype and functions. Cells 2022, 11, 1017. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.D.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence study of T cells: A systematic review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef]
- Nga, H.T.; Nguyen, T.L.; Yi, H.-S. T-cell senescence in human metabolic diseases. Diabetes Metab. J. 2024, 48, 864–881. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Cellular senescence: When growth stimulation meets cell cycle arrest. Aging 2023, 15, 905–913. [Google Scholar] [CrossRef]
- Domen, A.; Deben, C.; Verswyvel, J.; Flieswasser, T.; Prenen, H.; Peeters, M.; Lardon, F.; Wouters, A. Cellular senescence in cancer: Clinical detection and prognostic implications. J. Exp. Clin. Cancer Res. 2022, 41, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–115. [Google Scholar] [CrossRef]
- Lee, C.W.; Martinez-Yamout, M.A.; Dyson, H.J.; Wright, P.E. Structure of the p53 transactivation domain in complex with the nuclear coactivator binding domain of CBP. Biochemistry 2010, 49, 9964–9971. [Google Scholar] [CrossRef]
- Crighton, D.; Wilkinson, S.; O’Prey, J.; Syed, N.; Smith, P.; Harrison, P.R.; Gasco, M.; Garrone, O.; Crook, T.; Ryan, K.M. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 2006, 126, 121–134. [Google Scholar] [CrossRef]
- Moll, U.M.; Marchenko, N.; Zhang, X.-K. p53 and Nur77/TR3—Transcription factors that directly target mitochondria for cell death induction. Oncogene 2006, 25, 4725–4743. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Xu, D.; Zhang, T.; Hu, W.; Feng, Z. Gain-of-function mutant p53 in cancer progression and therapy. J. Mol. Cell Biol. 2020, 12, 674–687. [Google Scholar] [CrossRef]
- Chen, Q.M.; Liu, J.; Merrett, J.B. Apoptosis or senescence-like growth arrest: Influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem. J. 2000, 347, 543–551. [Google Scholar] [CrossRef]
- Spiegelberg, D.; Mortensen, A.C.; Lundsten, S.; Brown, C.J.; Lane, D.P.; Nestor, M. The MDM2/MDMX-p53 antagonist PM2 radiosensitizes wild-type p53 tumors. Cancer Res. 2018, 78, 5084–5093. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Voltan, R.; Gonelli, A.; Secchiero, P.; Zauli, G. MDM2/X inhibitors under clinical evaluation: Perspectives for the management of hematological malignancies and pediatric cancer. J. Hematol. Oncol. 2017, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Sheekey, E.; Narita, M. p53 in senescence—It’s a marathon, not a sprint. FEBS J. 2023, 290, 1212–1220. [Google Scholar] [CrossRef]
- Undas, A.; Ariëns, R.A. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef]
- Xu, D.; Neville, R.; Finkel, T. Homocysteine accelerates endothelial cell senescence. FEBS Lett. 2000, 470, 20–24. [Google Scholar] [CrossRef]
- Kodaman, N.; Aldrich, M.C.; Sobota, R.; Asselbergs, F.W.; Brown, N.J.; Moore, J.H.; Williams, S.M. Plasminogen activator inhibitor-1 and diagnosis of the metabolic syndrome in a West African population. J. Am. Heart Assoc. 2016, 5, e003867. [Google Scholar] [CrossRef]
- Brown, N.J.; Nakamura, S.; Ma, L.; Nakamura, I.; Donnert, E.; Freeman, M.; Vaughan, D.E.; Fogo, A.B. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int. 2000, 58, 1219–1227. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Higgins, P.J.; Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 2006, 8, 877–884. [Google Scholar] [CrossRef]
- Sun, T.; Ghosh, A.K.; Eren, M.; Miyata, T.; Vaughan, D.E. PAI-1 contributes to homocysteine-induced cellular senescence. Cell Signal. 2019, 64, 109394. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Rai, R.; Park, K.E.; Eren, M.; Miyata, T.; Wilsbacher, L.D.; Vaughan, D.E. A small molecule inhibitor of PAI-1 protects against doxorubicin-induced cellular senescence. Oncotarget 2016, 7, 72443–72457. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Choi, J.H.; Park, E.M.; Choi, Y.H. Interaction of promyelocytic leukemia/p53 affects signal transducer and activator of transcription-3 activity in response to oncostatin M. Korean J. Physiol. Pharmacol. 2020, 24, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.P.; Moretto, F.; Makkou, M.; Papamatheakis, J.; Kretsovali, A. Promyelocytic leukemia protein (PML) and stem cells: From cancer to pluripotency. Int. J. Dev. Biol. 2022, 66, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Kao, H.Y. The function, regulation and therapeutic implications of the tumor suppressor protein, PML. Cell Biosci. 2015, 5, 60. [Google Scholar] [CrossRef]
- Hsu, K.S.; Kao, H.Y. PML: Regulation and multifaceted function beyond tumor suppression. Cell Biosci. 2018, 8, 5. [Google Scholar] [CrossRef]
- Pearson, M.; Carbone, R.; Sebastiani, C.; Cioce, M.; Fagioli, M.; Saito, S.; Higashimoto, Y.; Appella, E.; Minucci, S.; Pandolfi, P.P.; et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 2000, 406, 207–210. [Google Scholar] [CrossRef]
- Wolyniec, K.; Shortt, J.; de Stanchina, E.; Levav-Cohen, Y.; Alsheich-Bartok, O.; Louria-Hayon, I.; Corneille, V.; Kumar, B.; Woods, S.J.; Opat, S.; et al. E6AP ubiquitin ligase regulates PML-induced senescence in Myc-driven lymphomagenesis. Blood 2012, 120, 822–832. [Google Scholar] [CrossRef]
- Rabellino, A.; Scaglioni, P.P. PML degradation: Multiple ways to eliminate PML. Front. Oncol. 2013, 3, 60. [Google Scholar] [CrossRef]
- Kreis, N.N.; Louwen, F.; Yuan, J. The multifaceted p21 (Cip1/Waf1/CDKN1A) in cell differentiation, migration and cancer therapy. Cancers 2019, 11, 1220. [Google Scholar] [CrossRef]
- Kreis, N.N.; Friemel, A.; Zimmer, B.; Roth, S.; Rieger, M.A.; Rolle, U.; Louwen, F.; Yuan, J. Mitotic p21Cip1/CDKN1A is regulated by cyclin-dependent kinase 1 phosphorylation. Oncotarget 2016, 7, 50215–50228. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- de Renty, C.; DePamphilis, M.L.; Ullah, Z. Cytoplasmic localization of p21 protects trophoblast giant cells from DNA damage induced apoptosis. PLoS ONE 2014, 9, e97434. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.N.; Louwen, F.; Yuan, J. Less understood issues: p21(Cip1) in mitosis and its therapeutic potential. Oncogene 2015, 34, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Shtutman, M.; Chang, B.D.; Schools, G.P.; Broude, E.V. Cellular model of p21-induced senescence. Methods Mol. Biol. 2017, 1534, 31–39. [Google Scholar]
- Mansour, M.A.; Rahman, M.; Ayad, A.A.; Warrington, A.E.; Burns, T.C. p21 overexpression promotes cell death and induces senescence in human glioblastoma. Cancers 2023, 15, 1279. [Google Scholar] [CrossRef]
- Trost, T.M.; Lausch, E.U.; Fees, S.A.; Schmitt, S.; Enklaar, T.; Reutzel, D.; Brixel, L.R.; Schmidtke, P.; Maringer, M.; Schiffer, I.B.; et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 2005, 65, 840–849. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–407. [Google Scholar] [CrossRef]
- Engelanda, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Buj, R.; Chen, C.W.; Dahl, E.S.; Leon, K.E.; Kuskovsky, R.; Maglakelidze, N.; Navaratnarajah, M.; Zhang, G.; Doan, M.T.; Jiang, H.; et al. Suppression of p16 induces mTORC1-mediated nucleotide metabolic reprogramming. Cell Rep. 2019, 28, 1971–1980. [Google Scholar] [CrossRef]
- Coppé, J.P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.Y.; Campisi, J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohtani, N.; Hara, E. Irreversibility of cellular senescence: Dual roles of p16INK4a/Rb-pathway in cell cycle control. Cell Div. 2007, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Capasso, S.; Ferone, A.; Di Bernardo, G.; Cipollaro, M.; Casale, F.; Peluso, G.; Giordano, A.; Galderisi, U. Misidentified human gene functions with mouse models: The case of the retinoblastoma gene family in senescence. Neoplasia 2017, 19, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Pereira-Smith, O.M. Primary and compensatory roles for RB family members at cell cycle gene promoters that are deacetylated and downregulated in doxorubicin-induced senescence of breast cancer cells. Mol. Cell. Biol. 2006, 26, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.; Kataura, T.; Panek, J.; Ma, G.; Salmonowicz, H.; Davis, A.; Kendall, H.; Brookes, C.; Ayine-Tora, D.M.; Banks, P.; et al. Suppressed basal mitophagy drives cellular aging phenotypes that can be reversed by a p62-targeting small molecule. Dev. Cell 2024, 59, 1287–1302.e7. [Google Scholar] [CrossRef]
- Martín-Maestro, P.; Gargini, R.; Perry, G.; Avila, J.; García-Escudero, V. PARK2 enhancement is able to compensate mitophagy alterations found in sporadic Alzheimer’s disease. Hum. Mol. Genet. 2016, 25, 792–806. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef]
- Zhao, S.; Qiao, Z.; Pfeifer, R.; Pape, H.C.; Mao, K.; Tang, H.; Meng, B.; Chen, S.; Liu, H. Modulation of fracture healing by senescence-associated secretory phenotype (SASP): A narrative review of the current literature. Eur. J. Med. Res. 2024, 29, 38. [Google Scholar] [CrossRef]
- Neves, J.; Demaria, M.; Campisi, J.; Jasper, H. Of flies, mice, and men: Evolutionarily conserved tissue damage responses and aging. Dev. Cell 2015, 32, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.D.; Zacarias Fluck, M.F.; Pedersen, K.; Parra-Palau, J.L.; Guiu, M.; Bernadó Morales, C.; Vicario, R.; Luque-García, A.; Navalpotro, N.P.; Giralt, J.; et al. Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res. 2013, 73, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Kauser, K.; Campisi, J.; Beauséjour, C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Flanagan, K.C.; Luo, X.; Ruhland, M.K.; Huang, H.; Pazolli, E.; Donlin, M.J.; Marsh, T.; Piwnica-Worms, D.; Monahan, J.; et al. p38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov. 2014, 4, 716–729. [Google Scholar] [CrossRef]
- Raynard, C.; Ma, X.; Huna, A.; Tessier, N.; Massemin, A.; Zhu, K.; Flaman, J.M.; Moulin, F.; Goehrig, D.; Medard, J.J.; et al. NF-κB-dependent secretome of senescent cells can trigger neuroendocrine transdifferentiation of breast cancer cells. Aging Cell 2022, 21, e13632. [Google Scholar] [CrossRef]
- Weber, C.E.; Li, N.Y.; Wai, P.Y.; Kuo, P.C. Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound healing and tissue remodeling after injury. J. Burn Care Res. 2012, 33, 311–318. [Google Scholar] [CrossRef]
- Flanagan, K.C.; Alspach, E.; Pazolli, E.; Parajuli, S.; Ren, Q.; Arthur, L.L.; Tapia, R.; Stewart, S.A. c-Myb and C/EBPβ regulate OPN and other senescence-associated secretory phenotype factors. Oncotarget 2017, 9, 21–36. [Google Scholar] [CrossRef]
- Song, J.; Bae, Y.S. CK2 down-regulation increases the expression of senescence-associated secretory phenotype factors through NF-κB activation. Int. J. Mol. Sci. 2021, 22, 406. [Google Scholar] [CrossRef]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. mTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Akkari, L.; Simon, J.; Grace, D.; Tschaharganeh, D.F.; Bolden, J.E.; Zhao, Z.; Thapar, V.; Joyce, J.A.; Krizhanovsky, V.; Lowe, S.W. Non-cell-autonomous tumor suppression by p53. Cell 2013, 153, 449–460. [Google Scholar]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. IGF binding protein-5 induces cell senescence. Front. Endocrinol. 2018, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Vadgama, J.V.; Wu, Y.; Datta, G.; Khan, H.; Chillar, R. Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology 1999, 57, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Pirtskhalava, T.; Thomou, T.; Cartwright, M.J.; Wise, B.; Karagiannides, I.; Shpilman, A.; Lash, T.L.; Becherer, J.D.; Kirkland, J.L. Increased TNFα and CCAAT/enhancer binding protein homologous protein (CHOP) with aging predispose preadipocytes to resist adipogenesis. Am. J. Physiol. 2007, 293, E1810–E1819. [Google Scholar]
- Ruhland, M.K.; Loza, A.J.; Capietto, A.H.; Luo, X.; Knolhoff, B.L.; Flanagan, K.C.; Belt, B.A.; Alspach, E.; Leahy, K.; Luo, J.; et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 2016, 7, 11762. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Højfeldt, G.; Hojman, P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013, 138, 657–664. [Google Scholar] [CrossRef]
- Culig, Z.; Puhr, M. Interleukin-6: A multifunctional targetable cytokine in human prostate cancer. Mol. Cell Endocrinol. 2012, 360, 52–58. [Google Scholar] [CrossRef]
- Altundag, O.; Altundag, K.; Gunduz, E. Interleukin-6 and C-reactive protein in metastatic renal cell carcinoma. J. Clin. Oncol. 2005, 23, 1044. [Google Scholar] [CrossRef]
- Sanguinete, M.M.M.; Oliveira, P.H.; Martins-Filho, A.; Micheli, D.C.; Tavares-Murta, B.M.; Murta, E.F.C.; Nomelini, R.S. Serum IL-6 and IL-8 correlate with prognostic factors in ovarian cancer. Immunol. Investig. 2017, 46, 677–688. [Google Scholar] [CrossRef]
- Miura, T.; Mitsunaga, S.; Ikeda, M.; Shimizu, S.; Ohno, I.; Takahashi, H.; Furuse, J.; Inagaki, M.; Higashi, S.; Kato, H.; et al. Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas 2015, 44, 756–763. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keffe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signaling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Chang, K.W.; Liu, C.J.; Tseng, Y.H.; Lu, H.H.; Lee, S.Y.; Lin, S.C. Ripe areca nut extract induces G1 phase arrests and senescence-associated phenotypes in normal human oral keratinocytes. Carcinogenesis 2006, 27, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.J.; Sasser, A.K.; Axel, A.E.; Vesuna, F.; Raman, V.; Ramirez, N.; Oberyszyn, T.M.; Hall, B.M. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009, 28, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, J.; Lee, Y.H.; Eom, M.; Choi, J. Interleukin-6/STAT3 signaling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells. Sci. Rep. 2018, 8, 8859. [Google Scholar] [CrossRef]
- Liu, H.; Ren, G.; Wang, T.; Chen, Y.; Gong, C.; Bai, Y.; Wang, B.; Qi, H.; Shen, J.; Zhu, L.; et al. Aberrantly expressed Fra-1 by IL-6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial-mesenchymal transition. Carcinogenesis 2015, 36, 459–468. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, J.; Sun, Y.; Song, J.; Gao, D.; Huang, S.; Pang, A.; Zhang, J.; Wang, J.; Wang, Y.; et al. Interleukin-6 and hypoxia synergistically promote EMT-mediated invasion in epithelial ovarian cancer via the IL-6/STAT3/HIF-1α feedback loop. Anal. Cell Pathol. 2023, 2023, 8334881. [Google Scholar] [CrossRef]
- Diepenbruck, M.; Christofori, G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Herbstein, F.; Sapochnik, M.; Attorresi, A.; Pollak, C.; Senin, S.; Gonilski-Pacin, D.; Ciancio Del Giudice, N.; Fiz, M.; Elguero, B.; Fuertes, M.; et al. The SASP factor IL-6 sustains cell-autonomous senescent cells via a cGAS-STING-NFκB intracrine senescent noncanonical pathway. Aging Cell 2024, 23, e14258. [Google Scholar] [CrossRef]
- Singh, J.K.; Simões, B.M.; Howell, S.J.; Farnie, G.; Clarke, R.B. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013, 15, 210. [Google Scholar] [CrossRef]
- Bieche, I.; Chavey, C.; Andrieu, C.; Busson, M.; Vacher, S.; Le Corre, L.; Guinebretiere, J.-M.; Burlinchon, S.; Lidereau, R.; Lazennec, G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr. Relat. Cancer 2007, 14, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- González-Puertos, V.Y.; Maciel-Barón, L.Á.; Barajas-Gómez, B.A.; López-Diazguerrero, N.E.; Königsberg, M. Involvement of phenotype secretor of senescent cells in the development of cancer, aging and the diseases associated with age. Gac. Med. Mex. 2015, 151, 491–500. [Google Scholar] [PubMed]
- Ben-Baruch, A. The tumor-promoting flow of cells into, within and out of the tumor site: Regulation by the inflammatory axis of TNFα and chemokines. Cancer Microenviron. 2012, 5, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Kawata, M.; Koinuma, D.; Ogami, T.; Umezawa, K.; Iwata, C.; Watabe, T.; Miyazono, K. TGF-β-induced epithelial-mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells. J. Biochem. 2012, 151, 205–216. [Google Scholar] [CrossRef]
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Bar, J.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Kahani, H.; et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res. Ther. 2015, 6, 87. [Google Scholar] [CrossRef]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef]
- Mantovani, A.; Bonecchi, R.; Locati, M. Tuning inflammation and immunity by chemokine sequestration: Decoys and more. Nat. Rev. Immunol. 2006, 6, 907–918. [Google Scholar] [CrossRef]
- Zhou, J.; Down, J.M.; George, C.N.; Murphy, J.; Lefley, D.V.; Tulotta, C.; Alsharif, M.A.; Leach, M.; Ottewell, P.D. Novel methods of targeting IL-1 signaling for the treatment of breast cancer bone metastasis. Cancers 2022, 14, 4816. [Google Scholar] [CrossRef]
- Voronov, E.; Apte, R.N. Targeting the tumor microenvironment by intervention in interleukin-1 biology. Curr. Pharm. Des. 2017, 23, 4893–4905. [Google Scholar] [CrossRef]
- Zhu, W.; London, N.R.; Gibson, C.C.; Davis, C.T.; Tong, Z.; Sorensen, L.K.; Shi, D.S.; Guo, J.; Smith, M.C.; Grossmann, A.H.; et al. Receptor interleukin activates the MYD88-ARNO-ARF6 cascade, disrupting vascular stability. Nature 2012, 492, 252–255. [Google Scholar] [CrossRef]
- Tucci, M.; Stucci, S.; Passarelli, A.; Giudice, G.; Dammacco, F.; Silvestris, F. The immune escape in melanoma: Role of the impaired dendritic cell function. Expert Rev. Clin. Immunol. 2014, 10, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Kandhaya-Pillai, R.; Miro-Mur, F.; Alijotas-Reig, J.; Tchkonia, T.; Kirkland, J.L.; Schwartz, S. TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging 2017, 9, 2411–2435. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, E.; Braeuer, R.R.; Kamiya, T.; Mobley, A.K.; Huang, L.; Vasquez, M.E.; Velazquez-Torres, G.; Chakravarti, N.; Ivan, C.; Prieto, V.; et al. NFAT1 directly regulates IL8 and MMP3 to promote melanoma tumor growth and metastasis. Cancer Res. 2016, 76, 3145–3155. [Google Scholar] [CrossRef]
- Ortiz-Montero, P.; Londono-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.; Zheng, Y.; Wang, S.; Yang, B.; Li, M.; Song, J.; Zhang, F.; Zhang, X.; Wang, Q.; et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018, 9, 880. [Google Scholar] [CrossRef]
- Oliveira-Ferrer, L.; Milde-Langosch, K.; Eylmann, K.; Rossberg, M.; Müller, V.; Schmalfeldt, B.; Witzel, I.; Wellbrock, J.; Fiedler, W. Mechanisms of tumor-lymphatic interactions in invasive breast and prostate carcinoma. Int. J. Mol. Sci. 2020, 21, 602. [Google Scholar] [CrossRef]
- Nie, F.; Zhang, J.; Tian, H.; Zhao, J.; Gong, P.; Wang, H.; Wang, S.; Yang, P.; Yang, C. The role of CXCL2-mediated crosstalk between tumor cells and macrophages in Fusobacterium nucleatum-promoted oral squamous cell carcinoma progression. Cell Death Dis. 2024, 15, 277. [Google Scholar] [CrossRef]
- Guan, J.; Weng, J.; Ren, Q.; Zhang, C.; Hu, L.; Deng, W.; Lu, S.; Dong, X.; Li, W.; Li, Y.; et al. Clinical significance and biological functions of chemokine CXCL3 in head and neck squamous cell carcinoma. Biosci. Rep. 2021, 41, BSR20212403. [Google Scholar] [CrossRef]
- Xin, H.; Cao, Y.; Shao, M.L.; Zhang, W.; Zhang, C.B.; Wang, J.T.; Liang, L.C.; Shao, W.W.; Qi, Y.L.; Li, Y.; et al. Chemokine CXCL3 mediates prostate cancer cells proliferation, migration and gene expression changes in an autocrine/paracrine fashion. Int. Urol. Nephrol. 2018, 50, 861–868. [Google Scholar] [CrossRef]
- Liu, J.F.; Chen, P.C.; Chang, T.M.; Hou, C.H. Monocyte Chemoattractant Protein-1 promotes cancer cell migration via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J. Exp. Clin. Cancer Res. 2020, 39, 254. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, B.S.; Forwood, M.R.; Morrison, N.A. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) drives activation of bone remodeling and skeletal metastasis. Curr. Osteoporos. Rep. 2019, 17, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Ahmadi, Z.; Hassanshahi, G.; Abbasifard, M.; Taghipour, Z.; Falahati-Pour, S.K.; Khorramdelazad, H. Effective treatments for bladder cancer affecting CXCL9/CXCL10/CXCL11/CXCR3 axis: A review. Oman Med. J. 2020, 35, e103. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, K.; Li, L.; Tian, J.; Ruan, W.; Hu, Z.; Peng, D.; Chen, Z. Epigenetic activation of RBM15 promotes clear cell renal cell carcinoma growth, metastasis and macrophage infiltration by regulating the m6A modification of CXCL11. Free Radic. Biol. Med. 2022, 184, 135–147. [Google Scholar] [CrossRef]
- Vassilieva, I.; Kosheverova, V.; Vitte, M.; Kamentseva, R.; Shatrova, A.; Tsupkina, N.; Skvortsova, E.; Borodkina, A.; Tolkunova, E.; Nikolsky, N.; et al. Paracrine senescence of human endometrial mesenchymal stem cells: A role for the insulin-like growth factor binding protein 3. Aging 2020, 12, 1987–2004. [Google Scholar] [CrossRef]
- Severino, V.; Alessio, N.; Farina, A.; Sandomenico, A.; Cipollaro, M.; Peluso, G.; Galderisi, U.; Chambery, A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013, 4, e911. [Google Scholar] [CrossRef]
- David, C.J.; Huang, Y.H.; Chen, M.; Su, J.; Zou, Y.; Bardeesy, N.; Iacobuzio-Donahue, C.A.; Massagué, J. TGF-β suppression in tumors using lethal EMT. Cell 2016, 164, 1015–1030. [Google Scholar] [CrossRef]
- Taylor, M.A.; Sossey-Alaoui, K.; Thompson, C.L.; Danielpour, D.; Schiemann, W.P. TGF-β increases the expression of miR-181a, promoting breast cancer metastasis. J. Clin. Investig. 2013, 123, 150–163. [Google Scholar] [CrossRef]
- Yang, C.Z.; Ma, J.; Zhu, D.W.; Liu, Y.; Montgomery, B.; Wang, L.Z.; Li, J.; Zhang, Z.Y.; Zhang, C.P.; Zhong, L.P. GDF15 is a potential predictive biomarker for TPF induction chemotherapy and promotes tumorigenesis and progression in oral squamous cell carcinoma. Ann. Oncol. 2014, 25, 1215–1222. [Google Scholar] [CrossRef]
- Sugimoto, M.; Suzuki, R.; Nozawa, Y.; Takagi, T.; Konno, N.; Asama, H.; Sato, Y.; Irie, H.; Nakamura, J.; Takasumi, M.; et al. Clinical usefulness and acceleratory effect of macrophage inhibitory cytokine-1 on biliary tract cancer: An experimental biomarker analysis. Cancer Cell Int. 2022, 22, 250. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Jiang, C.; Liu, G.; Miyata, T.; Antony, V.; Thannickal, V.J.; Liu, R.M. PAI-1 regulation of TGF-β1-induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2020, 62, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lademann, U.A.; Rømer, M.U. Regulation of programmed cell death by plasminogen activator inhibitor type 1 (PAI-1). Thromb. Haemost. 2008, 100, 1041–1046. [Google Scholar] [PubMed]
- Kubala, M.H.; Punj, V.; Placencio-Hickok, V.R.; Fang, H.; Fernandez, G.E.; Sposto, R.; DeClerck, Y.A. Plasminogen activator inhibitor-1 promotes the recruitment and polarization of macrophages in cancer. Cell Rep. 2018, 25, 2177–2191. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Zhang, J.; Zhang, F.; Fan, J.; Liu, Y. MMP1 regulated by the NEAT1/miR-361-5p axis promotes proliferation and migration of squamous cell carcinoma. Cancer Biol. Ther. 2021, 22, 381–391. [Google Scholar] [CrossRef]
- Gopal, S.K.; Greening, D.W.; Zhu, H.J.; Simpson, R.J.; Mathias, R.A. Transformed MDCK cells secrete elevated MMP1 that generates LAMA5 fragments promoting endothelial cell angiogenesis. Sci. Rep. 2016, 6, 28321. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Gan, R.H.; Yuan, S.; Lan, T.; Zheng, D.; Lu, Y.G. IL-8 activates fibroblasts to promote invasion of HNSCC. Cell Death Discov. 2024, 10, 65. [Google Scholar] [CrossRef]
- Colombo, G.; Travelli, C.; Porta, C.; Genazzani, A.A. Extracellular nicotinamide phosphoribosyltransferase boosts IFNgamma-induced macrophage polarization independently of TLR4. iScience 2022, 25, 104147. [Google Scholar] [CrossRef]
- Camp, S.M.; Ceco, E.; Evenoski, C.L.; Danilov, S.M.; Zhou, T.; Chiang, E.T.; Moreno-Vinasco, L.; Mapes, B.; Zhao, J.; Gursoy, G.; et al. Unique TLR4 activation by NAMPT/PBEF induces NFκB signaling and inflammatory lung injury. Sci. Rep. 2015, 5, 13135. [Google Scholar] [CrossRef]
- Guo, F.; Li, Y.; Wang, J.; Li, Y.; Li, Y.; Li, G. Stanniocalcin1 (STC1) inhibits cell proliferation and invasion of cervical cancer cells. PLoS ONE 2013, 8, e53989. [Google Scholar] [CrossRef]
- Hou, J.; Cheng, J.; Dai, Z.; Wei, N.; Chen, H.; Wang, S.; Dai, M.; Li, L.; Wang, H.; Ni, Q. Molecular and clinical significance of stanniocalcin-1 expression in breast cancer through promotion of homologous recombination-mediated DNA damage repair. Front. Cell Dev. Biol. 2021, 9, 731086. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Saravi, S.S.; Pugin, B.; Constancias, F.; Shabanian, K.; Spalinger, M.; Thomas, A.; Le Gludic, S.; Shabanian, T.; Karsai, G.; Colucci, M.; et al. Gut microbiota-dependent increase in phenylacetic acid induces endothelial cell senescence during aging. Nat. Aging 2025, 5, 1025–1045. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y.; et al. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Prata, L.G.P.L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Chen, L.S.; Balakrishnan, K.; Gandhi, V. Inflammation and survival pathways: Chronic lymphocytic leukemia as a model system. Biochem. Pharmacol. 2010, 80, 1936–1945. [Google Scholar] [CrossRef]
- Basu, A. The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy. Pharmacol. Ther. 2022, 230, 107943. [Google Scholar] [CrossRef]
- Gupta, V.; Ackley, J.; Kaufman, J.L.; Boise, L.H. BCL2 family inhibitors in the biology and treatment of multiple myeloma. Blood Lymphat. Cancer 2021, 11, 11–24. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Garcia, J.S.; Somervaille, T.C.P.; Foran, J.M.; Verstovsek, S.; Jamieson, C.; Mesa, R.; Ritchie, E.K.; Tantravahi, S.K.; Vachhani, P.; et al. Addition of navitoclax to ongoing ruxolitinib treatment in patients with myelofibrosis (REFINE): A post-hoc analysis of molecular biomarkers in a phase 2 study. Lancet Haematol. 2022, 9, e434–e444. [Google Scholar]
- Aldoss, I.; Wang, X. Phase 1 trial of navitoclax/venetoclax/decitabine combination in relapsed/refractory (R/R) acute myeloid leukemia (AML). Blood 2024, 144, 4264. [Google Scholar]
- Lafontaine, J.; Cardin, G.B.; Malaquin, N.; Boisvert, J.-S.; Rodier, F.; Wong, P. Senolytic targeting of Bcl-2 anti-apoptotic family increases cell death in irradiated sarcoma cells. Cancers 2021, 13, 386. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, S.J.; Choi, Y.J.; Kim, M.J.; Kim, T.Y.; Ko, S.G. Quercetin induces apoptosis in glioblastoma cells by suppressing Axl/IL-6/STAT3 signaling pathway. Am. J. Chin. Med. 2021, 49, 767–784. [Google Scholar] [CrossRef]
- Primikyri, A.; Chatziathanasiadou, M.V.; Karali, E.; Kostaras, E.; Mantzaris, M.D.; Hatzimichael, E.; Shin, J.S.; Chi, S.W.; Briasoulis, E.; Kolettas, E.; et al. Direct binding of Bcl-2 family proteins by quercetin triggers its pro-apoptotic activity. ACS Chem. Biol. 2014, 9, 2737–2741. [Google Scholar] [CrossRef]
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. OncoTargets Ther. 2017, 10, 4719–4729. [Google Scholar] [CrossRef]
- Farr, J.N.; Atkinson, E.J.; Achenbach, S.J.; Volkman, T.L.; Tweed, A.J.; Vos, S.J.; Ruan, M.; Sfeir, J.; Drake, M.T.; Saul, D.; et al. Effects of intermittent senolytic therapy on bone metabolism in postmenopausal women: A phase 2 randomized controlled trial. Nat. Med. 2024, 30, 2605–2612. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Tharmapalan, V.; Du Marchie Sarvaas, M.; Bleichert, M.; Wessiepe, M.; Wagner, W. Senolytic compounds reduce epigenetic age of blood samples in vitro. NPJ Aging Mech. Dis. 2025, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Bisson, D.; Jarzem, P.; Weber, M.; Ouellet, J.; Haglund, L. Curcumin and o-vanillin exhibit evidence of senolytic activity in human IVD cells in vitro. J. Clin. Med. 2019, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lian, N.; Zhang, F.; Chen, L.; Chen, Q.; Lu, C.; Bian, M.; Shao, J.; Wu, L.; Zheng, S. Activation of PPARγ/P53 signaling is required for curcumin to induce hepatic stellate cell senescence. Cell Death Dis. 2016, 7, e2189. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Herranz, N.; Sun, B.; Wagner, V.; Gallage, S.; Guiho, R.; Wolter, K.; Pombo, J.; Irvine, E.E.; Innes, A.J.; et al. Cardiac glycosides are broad-spectrum senolytics. Nat. Metab. 2019, 1, 1074–1088. [Google Scholar] [CrossRef]
- Ijima, S.; Saito, Y.; Nagaoka, K.; Yamamoto, S.; Sato, T.; Miura, N.; Iwamoto, T.; Miyajima, M.; Chikenji, T.S. Fisetin reduces the senescent tubular epithelial cell burden and also inhibits proliferative fibroblasts in murine lupus nephritis. Front. Immunol. 2022, 13, 960601. [Google Scholar] [CrossRef]
- Ji, J.; Bae, M.; Wong, F.L.; Crespi, C.M.; Yee, L.D.; Sedrak, M.S. A phase II randomized double-blind placebo-controlled study of fisetin to improve physical function in frail older breast cancer survivors (TROFFi) [abstract]. J. Clin. Oncol. 2024, 42, TPS1645. [Google Scholar] [CrossRef]
- Magkouta, S.; Veroutis, D.; Papaspyropoulos, A.; Georgiou, M.; Lougiakis, N.; Pippa, N.; Havaki, S.; Palaiologou, A.; Thanos, D.F.; Kambas, K.; et al. Generation of a selective senolytic platform using a micelle-encapsulated Sudan Black B conjugated analog. Nat. Aging 2025, 5, 162–175. [Google Scholar] [CrossRef]
- Matsubayashi, S.; Ito, S.; Araya, J.; Kuwano, K. Drugs against metabolic diseases as potential senotherapeutics for aging-related respiratory diseases. Front. Endocrinol. 2023, 14, 1079626. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2022, 289, 1209–1236. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- University of Washington School of Dentistry. Rapamycin for Periodontal Disease (RAPID). Available online: https://www.rapamycintrial.com/ (accessed on 5 June 2025).
- ClinicalTrials.Gov. Validating Benefits of Rapamycin for Reproductive Aging Treatment (VIBRANT). Available online: https://clinicaltrials.gov/study/NCT05836025 (accessed on 30 July 2024).
- Fang, J.; Yang, J.; Wu, X.; Zhang, G.; Li, T.; Wang, X.; Zhang, H.; Wang, C.C.; Liu, G.H.; Wang, L. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 2018, 17, e12765. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Perez, T.; Chang, H.; Mehta, P.; Steffener, J.; Pradabhan, G.; Ichise, M.; Manly, J.; Devanand, D.P.; Bagiella, E. Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. J. Alzheimer’s Dis. 2016, 51, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of endothelial nitric oxide synthase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Bell, K.L.; Galasko, D.; Galvin, J.E.; Thomas, R.G.; van Dyck, C.H.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2011, 77, 556–563. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef]
- Yoon, S.; Han, Y.M. Cellular senescence and senolytic therapies: Current status and future directions. BMB Rep. 2023, 56, 395–405. [Google Scholar]
- Oguma, Y.; Alessio, N.; Aprile, D.; Dezawa, M.; Peluso, G.; Di Bernardo, G.; Galderisi, U. Meta-analysis of senescent cell secretomes to identify common and specific features of the different senescent phenotypes: A tool for developing new senotherapeutics. Cell Commun. Signal. 2023, 21, 262. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]

| Components of SASP | Factor | Effects | Reference |
|---|---|---|---|
| Cytokines | IL-1α | Formation and maintenance of SASP | [126] |
| Promote cancer progression | [127] | ||
| IL-1β | Induce angiogenesis | [114] | |
| Promote tumor invasiveness | [128] | ||
| Induce immunosuppression | [129] | ||
| Increase vascular permeability | [130] | ||
| IL-6 | Induce EMT | [106] | |
| Inhibition of dendritic cells (DCs) | [131] | ||
| Induce cell proliferation | [132] | ||
| TNFα | Remodeling of the extracellular matrix | [114] | |
| Reinforce cellular senescence | [133] | ||
| Chemokines | IL-8 | Promote tumor growth and migration | [134] |
| Induce cellular senescence | [135] | ||
| Groα | Induce EMT | [136] | |
| Promote metastasis | [137] | ||
| Groβ | Promote cell proliferation | [138] | |
| Promote recruitment and polarization of M2 macrophages | |||
| Groγ | Inhibition of tumor cell apoptosis | [139] | |
| Mediates cancer cells migration and proliferation | [140] | ||
| MCP-1 | Promote tumor growth and migration | [141,142] | |
| Induction of angiogenesis | [143] | ||
| Activation of tumor-associated macrophages | |||
| CXCL-11 | Induce tumor-suppressive cells (NKs, CTLs) | [144] | |
| Promote cancer cells migration and proliferation | [145] | ||
| Growth factors | IGFBP3 | Induce premature cellular senescence | [146] |
| IGFBP7 | Induce cellular senescence | [147] | |
| TGF-β | Promote tumor cells apoptosis | [148] | |
| Induce EMT and metastasis | [149] | ||
| MIC-1 | Promote cancer cells invasiveness | [150] | |
| Acceleration tumor cells proliferation and invasion | [151] | ||
| Others | PAI-1 | Synergistic TGF-β induced cellular senescence | [152] |
| Inhibition of tumor cells apoptosis | [153] | ||
| Promote polarization of M2 macrophages | [154] | ||
| MMP-1 | Promote cancer cells migration and proliferation | [155] | |
| Induce angiogenesis | [156] | ||
| Promote cancer cells invasiveness | [157] | ||
| NAMPT | Promote polarization of M1 macrophages | [158] | |
| Induce inflammation | [159] | ||
| STC1 | Inhibition of tumor migration and invasion | [160] | |
| Promote tumorigenesis | [161] |
| Compound/Class | Mechanism of Action | Cell Type/Model | Clinical Status | Gen. | Main Findings/Conclusions | Adverse Effects/Risks (Reported) | Ref. |
|---|---|---|---|---|---|---|---|
| Navitoclax (ABT-263/737) | BCL-2 family inhibition | IMR-90 (in vitro) MEFs (in vitro) HUVECs (in vitro) mouse lung tissue (in vivo) mouse skin (in vivo) irradiated mice (in vivo) | No clinical trials | I | Effectively clears senescent cells, particularly after irradiation or stress-induced senescence; promotes apoptosis by inhibiting BCL-2 | thrombocytopenia | [169,171,172] |
| Navitoclax + Ruxolitinib | BCL-2 inhibition (navitoclax), selective JAK1/JAK2 inhibition (ruxolitinib) | Human myelofibrosis patients (in vivo) | Yes, Phase II NCT03222609 | I | Improved spleen volume, reduced symptoms, and bone marrow fibrosis in myelofibrosis patients | Thrombocytopenia (88%), anemia, fatigue | [173] |
| Navitoclax + Venetoclax + Decitabine | BCL-2 family inhibition (navitoclax), selective BCL-2 inhibition (venetoclax), DNA hypomethylation (decitabine) | Human refractory acute myeloid leukemia (AML) patients (in vivo) | Yes, Phase Ib NCT05222984 | I | 20% achieved complete remission with incomplete/partial hematologic recovery (CRi/CRh), 60% had reduction in bone marrow blasts, 20% proceeded to allogeneic stem cell transplantation | Thrombocytopenia, neutropenia, anemia, febrile neutropenia, gastrointestinal symptoms (nausea, diarrhea) | [174] |
| Venetoclax (ABT-199) | Selective BCL-2 inhibition | Human sarcoma cell lines STS93, STS109, STS117 (in vitro) | No clinical trials | I | Induces apoptosis in irradiated, senescent sarcoma cells. | Not applicable | [175] |
| Quercetin | BCL-2/Bcl-xL inhibition Axl/STAT3/IL-6 pathway suppression, EMT blockade | Jurkat T cells (in vitro) U87MG, U373MG, glioblastoma cells (in vitro) glioblastoma PANC-1 (in vitro) PATU-8988 pancreatic cancer cells (in vitro) | No clinical trials | I | Induces apoptosis in cancer cells, suppresses EMT and invasiveness, reduces STAT3/IL-6 signaling | Not applicable | [176,177,178] |
| Dasatinib + Quercetin (D + Q) | Multi-kinase inhibition (dasatinib) BCL-2/Bcl-xL inhibition (quercetin) | Postmenopausal women aged 55–80 with osteopenia or generally healthy (in vivo) | Yes, Phase II NCT04313634 | I | In exploratory analyses, women with higher baseline levels of T cell senescence markers showed improvements in bone formation, reduced bone resorption, and increased radial bone mineral density after treatment | No significant adverse effects reported | [179] |
| Adults with diabetic kidney disease (in vivo) | Yes, Phase II NCT02848131 | I | Short-term D + Q treatment reduced the burden of senescent cells (p16INK4A, p21CIP1, and SA-β-gal positive cells) in adipose tissue and skin, decreased SASP factors and inflammatory markers | Mild to moderate gastrointestinal symptoms, transient chills, or headache observed. | [180] | ||
| Piperlongumine | OXR1 protein degradation | Human peripheral blood mononuclear cells (PBMCs) (in vitro) | No clinical trials | I | Senolytic compounds reduced the epigenetic age of blood samples in vitro; supports rejuvenation potential of senolytic treatment in human cells | Not applicable | [181] |
| Curcumin | Nrf2 and NF-κB inhibition | Human intervertebral disc cells (in vitro) | No clinical trials | I | Induces apoptosis in senescent cells | Not applicable | [182] |
| PPARγ/p53 activation | Rat hepatic stellate cells (in vivo) | No clinical trials | I | Induction of senescence in rat HSCs; increased expression of p16 and p21 | No significant adverse effects reported | [183] | |
| Ouabain | Na+/K+-ATPase inhibition | Senescent IMR-90 human fibroblasts (in vitro) | No clinical trials | I | Selectively induces apoptosis in senescent cells by upregulating NOXA; a broad-spectrum senolytic effect has been demonstrated both in vitro and in vivo | Potential cardiac toxicity | [184] |
| Fisetin | PI3K/Akt and mTOR pathway inhibition | MRL/lpr mice with lupus nephritis (in vivo) | No clinical trials | I | Reduced senescent tubular epithelial cells, inhibited fibroblast proliferation, reduced fibrosis, and improved kidney function | No significant adverse effects reported | [185] |
| Postmenopausal women, survivors of stage I–III breast cancer after chemotherapy (in vivo) | Yes, Phase II NCT05595499 | I | Study ongoing; results not yet available | Study ongoing; adverse effects not yet reported | [186] | ||
| mGL392 (micelle-encapsulated GL392) | Selective delivery to senescent cells via lipofuscin-binding domain dasatinib-induced apoptosis | Senescent IMR-90 human fibroblasts (in vitro) | No clinical trials | II | Effectively eliminates senescent cells, demonstrated improved tissue function and reduced SASP in animal models. | No significant adverse effects reported | [187] |
| uPAR-targeting CAR T cells | uPAR-targeted recognition and elimination of senescent cells (CAR T cells) | Senescent IMR-90 (in vitro) mouse models of liver fibrosis (in vivo) mouse models of lung adenocarcinoma (in vivo) | No clinical trials | II | Selectively eliminate senescent cells, restore tissue homeostasis, reduce fibrosis, and improve physical function in vivo | CRS, transient weight loss, hypothermia, increased serum cytokines at high doses; mild, transient macrophage infiltration in lungs; no significant toxicity at therapeutic doses | [170] |
| GPNMB-targeted senolytic vaccine | Induction of immune responses against GPNMB-expressing senescent cells | Senescent mouse fibroblasts (in vitro) aged mouse models (in vivo) | No clinical trials | II | Selectively eliminates senescent cells, improves physical function, delays age-related pathologies, and extends lifespan in mice | No significant adverse effects reported | [36] |
| Compound/Class | Mechanism of Action | Cell Type/Model | Clinical Status | Main Findings/Conclusions | Adverse Effects/Risks (Reported) | Ref. |
|---|---|---|---|---|---|---|
| Rapamycin (Sirolimus) | mTOR inhibition suppression of SASP factors | Genetically heterogeneous mice (in vivo) | Approved for other indications (immunosuppression), not approved for anti-aging | Feeding rapamycin late in life (600 days of age) significantly extended median and maximal lifespan in both male and female mice. Effect observed despite late intervention. | No significant adverse effects reported | [190] |
| Older adults with periodontal disease | Yes, Phase II—RAPID | Results pending | No significant adverse effects reported | [191] | ||
| Women aged 35–45 (in vivo) | Yes, Phase I NCT05836025 | Ovarian aging deceleration (~20%) potential menopause delay (~5 years) Improvement of memory, energy, skin, hair | No significant adverse effects reported | [192] | ||
| Metformin | Upregulation of endoplasmic reticulum (GPX7), reduction of cellular oxidative stress | IMR-90 human lung fibroblasts (in vitro) | Approved for other indications (type 2 diabetes), not approved for anti-aging | Alleviation of cellular aging, reduction of senescence-associated markers, improvement of redox homeostasis | Not applicable | [193] |
| AMPK activation, improved insulin sensitivity, possible reduction of neuroinflammation | Older adults with amnestic Mild Cognitive Impairment (in vivo) | Yes, Phase II NCT00620191 | Improvement in memory and cognitive function in metformin group versus placebo | No significant adverse effects reported | [194] | |
| Simvastatin | Induction of endothelial nitric oxide synthase (eNOS) via Akt pathway, inhibition of endothelial senescence | HUVEC—human endothelial cells (in vitro) Mice (in vivo) | Approved for hypercholesterolemia, no clinical trials for anti-aging in this model | Suppression of endothelial cell senescence, improved endothelial function, reduction of senescence markers both in vitro and in vivo | No significant adverse effects reported | [195] |
| Reduction of cholesterol levels, potential modulation of neuroinflammation | Older adults with mild to moderate Alzheimer’s disease (in vivo) | Yes, Phase III NCT00053599 | No slowing of cognitive decline or disease progression despite significant lipid lowering | No significant difference in serious adverse effects compared to placebo | [196] | |
| Resveratrol | Inhibits TLR4 oligomerization and pro-inflammatory signaling (NF-κB, STAT1/3, Akt), suppresses cytokine (IL-6, TNF-α, IL-1β) production; inhibits Aβ-induced microglial/macrophage activation | Murine microglial and macrophage cell line (in vitro) | No clinical trials | Significantly decreased cytokine production and inflammatory signaling in LPS- and Aβ-stimulated cells. | Not applicable | [197] |
| Reduces number of activated microglia around amyloid plaques in the brain (partly independent of amyloid burden) | APP/PS1 transgenic mice (in vivo) | No clinical trials | Reduces activation of microglia surrounding amyloid plaques in the cortex | No significant adverse effects reported | ||
| Reduction of neuroinflammation (↓ MMP9 in CSF), induction of adaptive immunity (↑ IL-4, MDC, FGF-2 in CSF | Patients with mild to moderate Alzheimer’s disease (in vivo) | Yes, Phase II NCT01504854 | 52 weeks of treatment significantly reduced MMP9 in CSF, increased anti-inflammatory and neuroprotective cytokines (IL-4, MDC, FGF-2) in CSF, attenuated the decline in cognitive (MMSE) and functional (ADCS-ADL) scores compared to placebo | Well-tolerated overall; most common: nausea, diarrhea, weight loss | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klepacki, H.; Kowalczuk, K.; Łepkowska, N.; Hermanowicz, J.M. Molecular Regulation of SASP in Cellular Senescence: Therapeutic Implications and Translational Challenges. Cells 2025, 14, 942. https://doi.org/10.3390/cells14130942
Klepacki H, Kowalczuk K, Łepkowska N, Hermanowicz JM. Molecular Regulation of SASP in Cellular Senescence: Therapeutic Implications and Translational Challenges. Cells. 2025; 14(13):942. https://doi.org/10.3390/cells14130942
Chicago/Turabian StyleKlepacki, Hubert, Krystyna Kowalczuk, Natalia Łepkowska, and Justyna Magdalena Hermanowicz. 2025. "Molecular Regulation of SASP in Cellular Senescence: Therapeutic Implications and Translational Challenges" Cells 14, no. 13: 942. https://doi.org/10.3390/cells14130942
APA StyleKlepacki, H., Kowalczuk, K., Łepkowska, N., & Hermanowicz, J. M. (2025). Molecular Regulation of SASP in Cellular Senescence: Therapeutic Implications and Translational Challenges. Cells, 14(13), 942. https://doi.org/10.3390/cells14130942





