Insulin Modulates NK Cell Activity in Liver Fibrosis MASH Patients via the STING Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Donors
2.2. Peripheral NK Cell Isolation
2.3. Isolation of Primary Hepatic Stellate Cells (pHSCs) from Liver Biopsies
2.4. NK Cell Cytotoxicity Assay
2.5. NK Cell Treatment
2.6. Flow Cytometry
2.7. RNA Isolation, cDNA Preparation, and Real-Time PCR
2.8. Statistical Analysis
3. Results
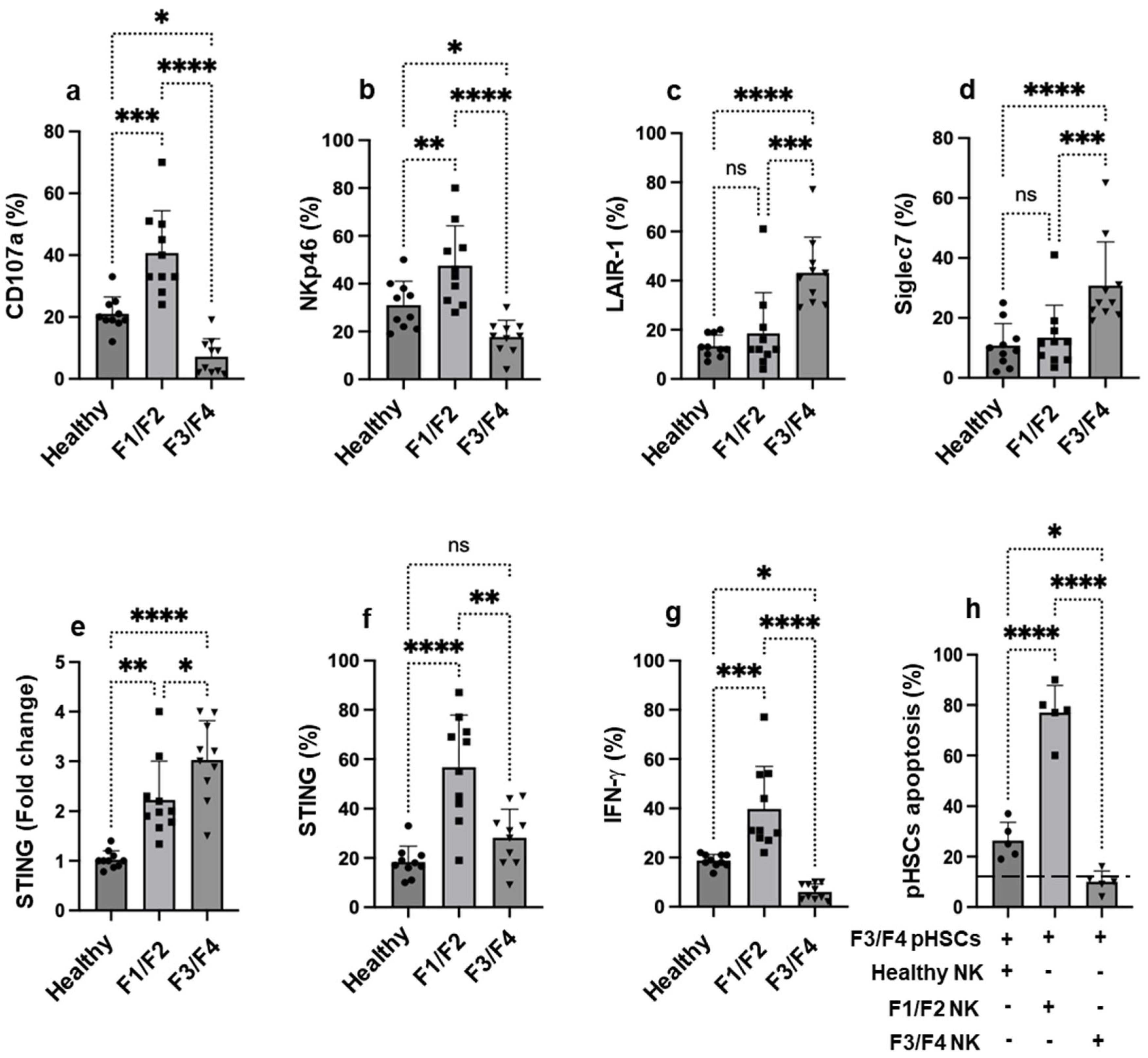
3.1. NK Cells Activity from MASH Patients Is Inversely Correlated with STING Expression in Advanced Liver Fibrosis and Influences Their Ability to Kill pHSCs
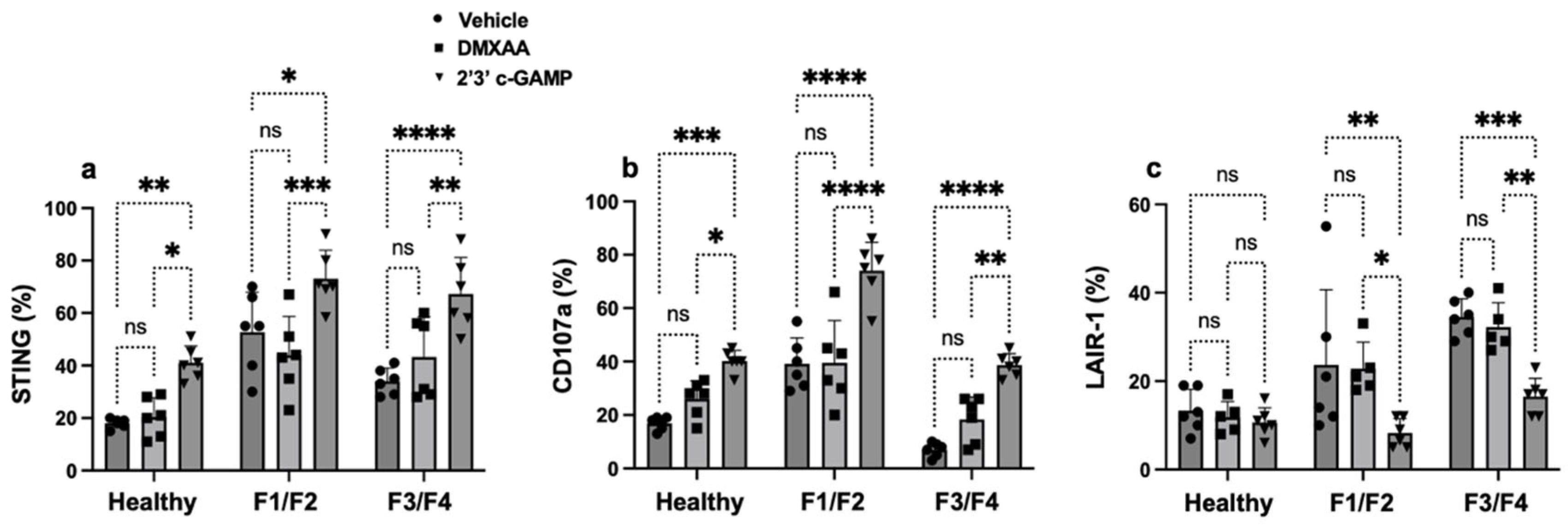
3.2. STING Signaling Regulates NK Cell Dysfunction Across Liver Fibrosis Stages in MASH
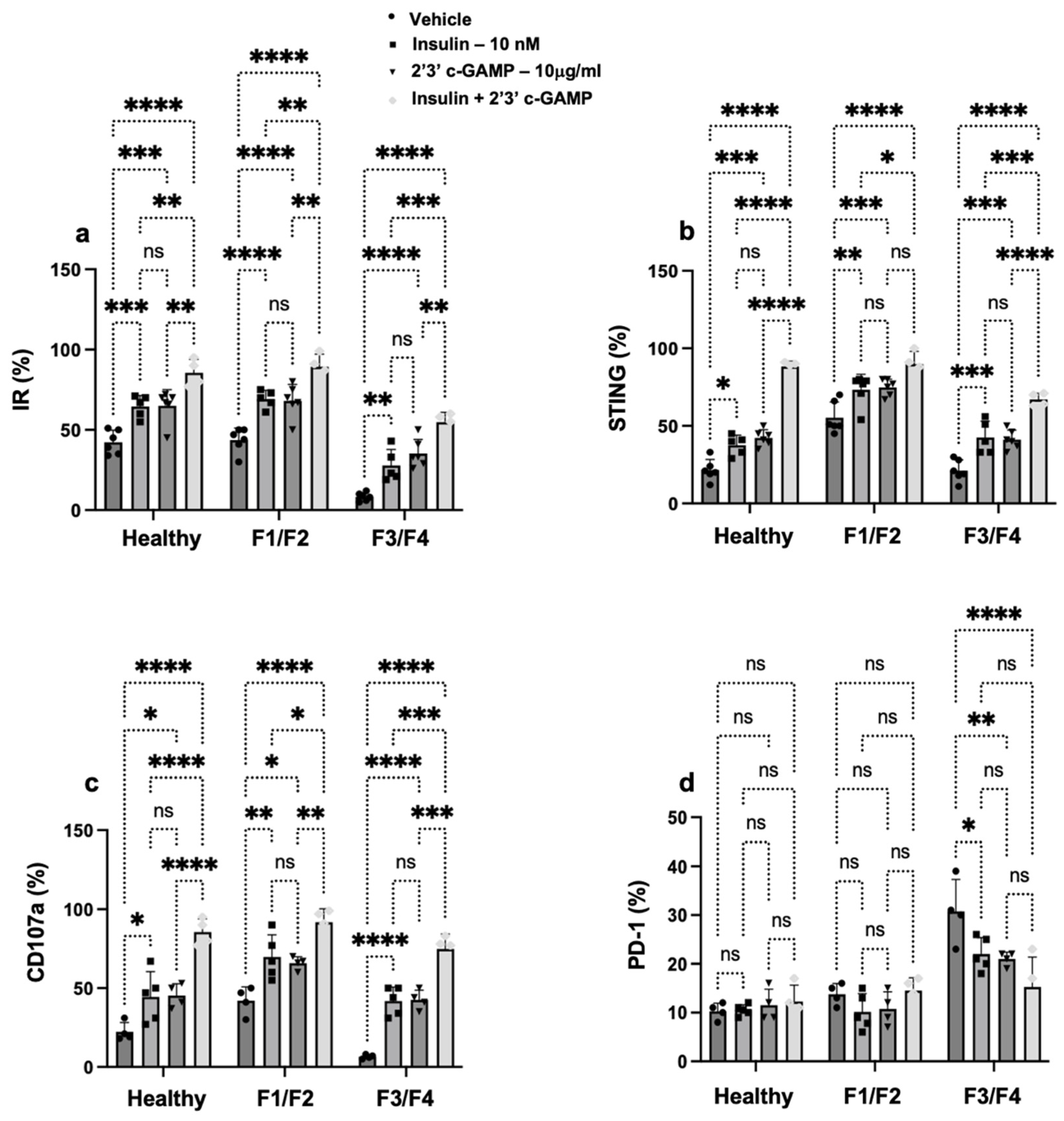
3.3. Insulin Signaling Promotes STING Expression and Restores NK Cell Function in Patients with MASH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Friedman, S.L. Found in translation—Fibrosis in metabolic dysfunction–associated steatohepatitis (MASH). Sci. Transl. Med. 2023, 15, eadi0759. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction–associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2023, 79, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, S.; Dong, B.; Tian, Y. Metabolic reprograming of immune cells in MASH. Hepatology 2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, L.; Zhang, H. Natural Killer Cells in Liver Disease and Hepatocellular Carcinoma and the NK Cell-Based Immunotherapy. J. Immunol. Res. 2018, 2018, 1206737. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yoshio, S.; Doi, H.; Mori, T.; Matsuda, M.; Kawai, H.; Shimagaki, T.; Yoshikawa, S.; Aoki, Y.; Osawa, Y.; et al. Increased Frequency of Dysfunctional Siglec-7−CD57+PD-1+ Natural Killer Cells in Patients With Non-alcoholic Fatty Liver Disease. Front. Immunol. 2021, 12, 603133. [Google Scholar] [CrossRef]
- Tran, S.; Baba, I.; Poupel, L.; Dussaud, S.; Moreau, M.; Gélineau, A.; Marcelin, G.; Magréau-Davy, E.; Ouhachi, M.; Lesnik, P.; et al. Impaired Kupffer Cell Self-Renewal Alters the Liver Response to Lipid Overload during Non-alcoholic Steatohepatitis. Immunity. 2020, 53, 627–640.e5. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Ouyang, X.; Dong, Y.; Sarapultsev, A.; Luo, S.; Hu, D. Multifaceted functions of STING in human health and disease: From molecular mechanism to targeted strategy. Signal Transduct. Target Ther. 2022, 7, 394. [Google Scholar] [CrossRef]
- Hong, Z.; Ma, T.; Liu, X.; Wang, C. cGAS-STING pathway: Post-translational modifications and functions in sterile inflammatory diseases. FEBS J. 2022, 289, 6187–6208. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Jiang, Z. Recent progress on the activation of the cGAS–STING pathway and its regulation by biomolecular condensation. J. Mol. Cell Biol. 2022, 14, mjac042. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zheng, Z.; Zhang, H.; Wu, Y.; Shen, Y.; Chen, Q. cGAS-STING pathway mediates activation of dendritic cell sensing of immunogenic tumors. Cell. Mol. Life Sci. 2024, 81, 149. [Google Scholar] [CrossRef]
- Kanoda, R.; Nakajima, S.; Fukai, S.; Saito, M.; Saito, K.; Suzuki, H.; Kikuchi, T.; Nirei, A.; Okayama, H.; Mimura, K.; et al. High levels of tumor cell-intrinsic STING signaling are associated with increased infiltration of CD8+ T cells in dMMR/MSI-H gastric cancer. Sci. Rep. 2024, 14, 20859. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, X.; Peng, Q.; Oyang, L.; Ren, Z.; Wang, J.; Peng, M.; Zhou, Y.; Deng, X.; Liao, Q. The cGAS–STING pathway in cancer immunity: Mechanisms, challenges, and therapeutic implications. J Hematol Oncol. 2025, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Gall, A.; Treuting, P.; Elkon, K.B.; Loo, Y.-M.; Gale, M.; Barber, G.N.; Stetson, D.B. Autoimmunity Initiates in Nonhematopoietic Cells and Progresses via Lymphocytes in an Interferon-Dependent Autoimmune Disease. Immunity 2012, 36, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, T.; Li, X.D.; Chen, X.; Li, Q.Z.; Wight-Carter, M.; Chen, Z.J. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. USA 2015, 112, E5699–E5705. [Google Scholar] [CrossRef]
- Flood, B.A.; Higgs, E.F.; Li, S.; Luke, J.J.; Gajewski, T.F. STING pathway agonism as a cancer therapeutic. Immunol. Rev. 2019, 290, 24–38. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, H.; Liu, Z.; Xu, J.; Gao, Y.; Zhan, X.; Zhou, S.; Zhong, W.; Wu, D.; Wang, P.; et al. Macrophage STING signaling promotes NK cell to suppress colorectal cancer liver metastasis via 4-1BBL/4-1BB co-stimulation. J. Immunother. Cancer. 2023, 11, e006481. [Google Scholar] [CrossRef]
- Lu, L.; Yang, C.; Zhou, X.; Wu, L.; Hong, X.; Li, W.; Wang, X.; Yang, Y.; Cao, D.; Zhang, A.; et al. STING signaling promotes NK cell antitumor immunity and maintains a reservoir of TCF-1+ NK cells. Cell Rep. 2023, 42, 113108. [Google Scholar] [CrossRef]
- Da, Y.; Liu, Y.; Hu, Y.; Liu, W.; Ma, J.; Lu, N.; Zhang, C.; Zhang, C. STING agonist cGAMP enhances anti-tumor activity of CAR-NK cells against pancreatic cancer. OncoImmunology 2022, 11, 2054105. [Google Scholar] [CrossRef]
- Wang, B.; Yu, W.; Jiang, H.; Meng, X.; Tang, D.; Liu, D. Clinical applications of STING agonists in cancer immunotherapy: Current progress and future prospects. Front. Immunol. 2024, 15, 1485546. [Google Scholar] [CrossRef]
- Muhanna, N.; Tair, L.A.; Doron, S.; Amer, J.; Azzeh, M.; Mahamid, M.; Friedman, S.; Safadi, R. Amelioration of hepatic fibrosis by NK cell activation. Gut 2010, 60, 90–98. [Google Scholar] [CrossRef]
- Melhem, A.; Muhanna, N.; Bishara, A.; Alvarez, C.E.; Ilan, Y.; Bishara, T.; Horani, A.; Nassar, M.; Friedman, S.L.; Safadi, R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J. Hepatol. 2006, 45, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Amer, J.; Salhab, A.; Noureddin, M.; Doron, S.; Abu-Tair, L.; Ghantous, R.; Mahamid, M.; Safadi, R. Insulin signaling as a potential natural killer cell checkpoint in fatty liver disease. Hepatol. Commun. 2018, 2, 285–298. [Google Scholar] [CrossRef] [PubMed]
- De Martin, E.; Fulgenzi, C.A.M.; Celsa, C.; Laurent-Bellue, A.; Torkpour, A.; Lombardi, P.; D’alessio, A.; Pinato, D.J. Immune checkpoint inhibitors and the liver: Balancing therapeutic benefit and adverse events. Gut 2024, 74, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wu, Z.; Xu, Y.; Liu, Y.; Liu, W.; Wang, T.; Li, C.; Zhang, C.; Yi, F.; Gao, L.; et al. Increased Tim-3 expression alleviates liver injury by regulating macrophage activation in MCD-induced NASH mice. Cell. Mol. Immunol. 2018, 16, 878–886. [Google Scholar] [CrossRef]
- Fadriquela, A.; Kim, C.-S.; Lee, K.-J.; Kang, S.H.; Lee, J.-H. Characteristics of immune checkpoint regulators and potential role of soluble TIM-3 and LAG-3 in male patients with alcohol-associated liver disease. Alcohol 2022, 98, 9–17. [Google Scholar] [CrossRef]
- Fadriquela, A.; Kim, C.-S.; Lee, K.-J.; Kang, S.H.; Kim, M.Y.; Lee, J.-H. Soluble type immune checkpoint regulators using multiplex luminex immunoassay in chronic hepatitis B patients. J. Clin. Pathol. 2021, 74, 780–786. [Google Scholar] [CrossRef]
| Variable | Healthy (n = 10) | F1/F2 (n = 10) | F3/F4 (n = 10) | p-Value |
|---|---|---|---|---|
| Age, Years | 36.5 ± 8.2 | 39.3 ± 9.1 | 38.10 ± 9.6 | ns |
| Male, n (%) | 6 (60%) | 5 (50%) | 4 (40%) | ns |
| Male, n (%) | 4 (40%) | 5 (50%) | 6 (60%) | ns |
| Fibroscan CAP (dB/m) | 210 ± 12 | 258 ± 13 | 359.5 ± 16 | <0.01 |
| ALT, U/L | 22 ± 7 | 29 ± 7 | 59 ± 18 | <0.001 |
| AST, U/L | 29 ± 5 | 39 ± 10 | 49 ± 17 | <0.001 |
| NK Donor | PD-1 (%) | TIGIT (%) | LAG-3 (%) |
|---|---|---|---|
| Healthy | 8 ± 2.5 | 13 ± 1.4 | 9 ± 1.5 |
| F1/F2 | 11 ± 3.3 | 15 ± 3.2 | 8 ± 1.5 |
| F3/F4 | 32 ± 4.7 * | 28 ± 5.3 * | 21 ± 4.9 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, J.; Salhab, A.; Ariel, A.; Safadi, R. Insulin Modulates NK Cell Activity in Liver Fibrosis MASH Patients via the STING Pathway. Cells 2025, 14, 941. https://doi.org/10.3390/cells14130941
Amer J, Salhab A, Ariel A, Safadi R. Insulin Modulates NK Cell Activity in Liver Fibrosis MASH Patients via the STING Pathway. Cells. 2025; 14(13):941. https://doi.org/10.3390/cells14130941
Chicago/Turabian StyleAmer, Johnny, Ahmad Salhab, Amiram Ariel, and Rifaat Safadi. 2025. "Insulin Modulates NK Cell Activity in Liver Fibrosis MASH Patients via the STING Pathway" Cells 14, no. 13: 941. https://doi.org/10.3390/cells14130941
APA StyleAmer, J., Salhab, A., Ariel, A., & Safadi, R. (2025). Insulin Modulates NK Cell Activity in Liver Fibrosis MASH Patients via the STING Pathway. Cells, 14(13), 941. https://doi.org/10.3390/cells14130941







