Abstract
Chlorpyrifos (CPF) has been extensively utilized in recent decades due to its highly efficient insecticidal properties. However, the widespread use of pesticides has posed new challenges to male reproduction. This study aims to explore the potential molecular mechanisms of male reproductive decline induced by CPF. We employ flow cytometry, qRT-PCR, Western blot, RNA sequencing, and bioinformatics analysis to investigate the potential molecular mechanisms involved in CPF-induced male reproductive damage in GC2spd cells. Our results revealed that after 24 h of CPF treatment, the cell viability, cell cycle, apoptosis, and reactive oxygen species (ROS) accumulation of GC2spd cells were significantly affected in vitro. RNA sequencing analysis data indicated that a total of 626 genes were differentially expressed compared to the DMSO group, especially for Efcab6, Nox3, and Cmpk2. These differential genes were mainly enriched in signaling pathways such as PI3K-AKT and glutamine metabolism. In addition, further validation through qRT-PCR, Western blot, and experiments involving the inhibition of intracellular ROS generation with N-acetylcysteine collectively confirmed that CPF induces male reproductive damage through the ROS/AKT/Efcab6 pathway. These studies elucidate potential targets and molecular mechanisms underlying CPF-induced male infertility, providing a theoretical basis for the prevention of male reproductive damage caused by pesticide residues.
1. Introduction
In recent years, the decline in semen quality has attracted increasing attention globally. A study of semen quality changes in sperm donor candidates in Henan Province, China, showed significant decreases in volume, sperm concentration, total sperm count, progressive motility, and total motility between 2009 and 2019 [1]. The increasing use of pesticides has been one of the major contributing factors to the decline in semen quality [2]. Recently, it has been shown that annual spraying of organophosphorus pesticides resulted in a 6.253 unit decrease in sperm quality index from An Giang Province, Vietnam [3]. Chlorpyrifos (CPF) is one of the most widely used organophosphate pesticides in recent decades [4]. Organisms can be exposed to CPF through oral ingestion, inhalation, and dermal contact [5]. Even after the ban was enacted by the European Union in 2020, it continues to be used in some developing countries due to its highly efficient insecticidal properties [6]. The main insecticidal mechanism of CPF involves the inhibition of acetylcholinesterase (AChE) activity, leading to the induction of neurotoxicity [7]. However, AChE inhibition alone cannot explain all the symptoms of CPF toxicity, as CPF exposure can also affect other systems, such as inducing hematological, immune, and reproductive toxicities [8,9]. However, up to now, the targets and molecular mechanisms of CPF-induced reproductive toxicity are still unelucidated.
In rodents, during embryonic development, the expression of Sry triggers a battery of critical events. Primordial germ cells (PGCs) interact with Sertoli cells (SCs), Leydig cells (LCs), and myoid cells to complete sex determination, form seminiferous tubules, and constitute the embryonic testis [10,11]. Subsequently, PGCs undergo a period of mitotic division to prospermatogonia [12]. Once a threshold of prospermatogonia is reached, they enter a non-proliferative quiescent phase. Later, these prospermatogonia migrate to the spermatogonial stem cell (SSC) niche located on the periphery of the seminiferous tubules, where some prospermatogonia transition into SSCs [13,14]. Most SSCs remain undifferentiated until they begin to differentiate into A paired spermatogonia (Apr) or A aligned spermatogonia (Aal) after adolescence [15]. The majority of Aal spermatogonia transition to A1 differentiated spermatogonia without cell division. Subsequently, A1 differentiated spermatogonia generate A2, A3, A4, In, and B spermatogonia through cell divisions. These spermatogonia undergo a series of transformations and enter meiotic division, which leads to the formation of various types of spermatocytes and, ultimately, the development of round spermatids. These round spermatids then undergo spermatid morphogenesis to become elongated spermatids. This entire process takes approximately 35 days and is referred to as the cycle of the seminiferous epithelium. However, this process occurs every 8.6 days [16]. Previous studies have shown that CPF can impede sperm maturation in the epididymis [17]. To further investigate the potential molecular mechanism of CPF-induced reproductive impairment in male mice, this study selected GC2spd cells for experimentation. GC2spd cells represent a cell line that leans toward spermatocytes and possesses certain characteristics of sperm cells, providing better continuity with previous experiments.
In approximately 40–50% of male infertility cases, oxidative stress (OS) mechanisms are considered a significant cause of male fertility parameter disruptions [18]. Sperm, due to their limited antioxidant defense and DNA repair systems, are highly vulnerable to the deleterious influence of OS [19]. Research has indicated that low levels of reactive oxygen species (ROS) within spermatozoa are necessary for normal male reproduction. However, when OS occurs, an excess of ROS is generated, which can impact cellular components, nucleic acids, lipids, and proteins, leading to cell damage [20]. Notably, prior studies have shown that CPF exposure can increase OS in the mouse brain [21]. Additionally, our previous experiments have demonstrated that CPF can increase OS in the sperm of mouse cauda epididymidis. Therefore, this study aims to further validate these findings using GC2spd cells and explore the potential molecular mechanisms underlying CPF-induced male reproductive disorders.
Based on this, the experiment employed CPF treatment on the GC2spd cell line (a spermatocytes cell line, purchased from ATCC, Cat number CRL-2196) and utilized RNA sequencing to identify potential targets of CPF-induced male reproductive damage. This study aims to reveal the potential mechanisms of CPF-induced male reproductive toxicity and provide reference materials for the sustainable and healthy development of animal husbandry. The overarching goal of this research endeavor is to shed light on the intricate mechanisms underlying CPF-induced male reproductive toxicity, which aspires to furnish valuable reference materials that contribute to the sustainable and healthy development of animal husbandry practices.
2. Materials and Methods
2.1. Cell Culture and Treatments
The GC2spd cell line was purchased from ATCC: The Global Bioresource Center (https://www.atcc.org (accessed on 20 May 2025)) (CRL-2196, ATCC, Manassas, VA, USA). The medium contained DMEM/HIGH GLUCOSE (E600003, Sangon, Shanghai, China), fetal bovine serum (Z7186FBS-500, Zeta Life, New York, NY, USA), Penicillin-Streptomycin (15140122, Gibco, New York, NY, USA), GlutaMAX (35050061, Gibco, New York, NY, USA), AdvanceSTEM ES Qualified Non-Essential Amino Acids (100X) (SH30853.01, HyClone, Logan, UT, USA), and Sodium Pyruvate (2276850, Gibco, New York, NY, USA); their proportions were 86%, 10%, 1%, 1%, 1%, and 1%, respectively. Six-well plates were initially seeded with a cell density of 3.5 × 105, and cells were cultured for 12 h and then treated with CPF. CPF was dissolved in Dimethyl sulfoxide (DMSO) (HY-Y0320, MedChemExpress, Monmouth Junction, NJ, USA). Then, 12.5 μmol/L CPF (CPF12.5) and 25 μmol/L CPF (CPF25) were finally selected to treat the GC2spd cells for 12 or 24 h based on published articles and pre-experiment results on GC2spd cells [17,22]. CPF came from Shanghai Aladdin Bio-Chem Technology Company, Ltd. (C109843, Shanghai, China). The same volume of DMSO was used as the control (DMSO).
2.2. The Detection of Cell Viability
According to the manufacturer’s instructions, the viability of GC2spd cells was assessed after treatment with 0 and 25 μmol/L CPF for 12 h using the Cell Counting Kit 8 (CCK8) (C0037, Beyotime Institute of Biotechnology, Shanghai, China). The specific experimental methods are as follows: 10 μL of CCK8 solution was added per 100 μL of cell culture medium; after incubation at 37 °C in darkness for 2~4 h, the absorbance of the cells was measured at 450 nm using a microplate reader.
2.3. The Detection of Cell Cycle
After treating GC2spd cells with 0 and 25 μmol/L CPF for 24 h, the floating dead cells in the supernatant were removed, and the live cells digested with trypsin were collected by centrifugation at 450× g for 5 min. Flow cytometry was used to detect each cell cycle. For detailed methods, refer to the article by Zhang et al. [23].
2.4. The Detection of Apoptosis
Apoptosis was detected using an apoptosis kit (Annexin V-FITC Apoptosis Detection Kit) (BA1150, Nanjing Biobox Biotech. Co., Ltd., Nanjing, China) on GC2spd cells treated with CPF (0 and 25 μmol/L) for 24 h according to the commercial instructions. More than 1 × 104 cells were required in each tube. Subsequently, the treated cells were stained using Annexin-V-FLUOS and PI, respectively, and apoptosis was detected after incubation. The specific steps are described in Chen et al. [22].
In addition, the One-step TUNEL In Situ Apoptosis Kit (Green, Elab Fluor® 488) (E-CK-A321, Elabscience Biotechnology Co., Ltd., Wuhan, China) was utilized for detecting late apoptosis cells in CPF-treated GC2spd cells. The specific method was described in the kit instructions. Firstly, GC2spd cells were fixed using 4% paraformaldehyde at room temperature for 20–30 min, and the fixed samples were immersed in PBS and rinsed 3 times for 5 min each time; subsequently, the cells were permeabilized using 0.2% Triton-100 at 37 °C for 10 min; the permeabilized samples were immersed in PBS and rinsed 3 times for 5 min each time; 100 μL of TdT Equilibration Buffer was added to each sample, equilibrated at 37 °C for 30 min. Prepare the labeling working solution and add an appropriate amount to each well, react at 37 °C for 60 min in the dark; rinse the labeled samples by immersing them in PBS for 3 times, for 5 min each time; add DAPI working solution and incubate for 5 min at room temperature, avoiding light; rinse the samples by adding PBS for 4 times, for 5 min each time. Finally, the stained cells were photographed and counted under a fluorescence microscope to calculate the late apoptosis rate of GC2spd cells after 25 μmol/L CPF treatment.
2.5. RNA Extraction and qRT-PCR
RNA in GC2spd cells treated with CPF (0, 12.5 and 25 μmol/L) was extracted using RNAiso Plus (9109, TAKARA, Beijing, China), chloroform, isopropanol, anhydrous ethanol, and enzyme-free water (R1600, Solarbio, Beijing, China). The RNA purity of samples was assessed by its OD260/280 value using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). In addition, electrophoretic analysis was performed on 1% agarose gel to assess the RNA quality [24]. Then the cDNA of the GC2spd cells was obtained from RNA using Hifair® II 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) (11123ES10, YEASEN, Shanghai, China) according to the manufacturer’s protocol. Briefly, genomic DNA is first removed, followed by reverse transcription of RNA to cDNA. cDNA obtained was stored in a −80 °C refrigerator. The gene expression levels in GC2spd cells were detected using qRT-PCR and performed using ChamQ SYBR qPCR Master Mix (Q311-02, vazyme, Nanjing, China). The primer sequences were designed by NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 16 April 2023)) and are shown in Table 1, and the specific qRT-PCR reaction system and reaction procedures are shown in Tables S1 and S2.

Table 1.
The primers used for qRT-PCR.
2.6. Detection of Oxidative Stress Level
Relative ROS levels in GC2spd cells were measured using a ROS assay kit (S0033, Beyotime Institute of Biotechnology, Shanghai, China). The principle of this kit is that DCFH-DA itself is non-fluorescent, but intracellular ROS can oxidize non-fluorescent DCFH to produce fluorescent DCF, so the fluorescence of DCF can be detected to determine the level of ROS in the cell. Briefly, the GC2spd cells were collected, the fluorescent probe DCFH-DA in the ROS assay kit was added, and the cells were bathed in the dark at 37 °C for 30 min. The concentration of the DCFH-DA working solution is 10 μmol/L. Then, the cells washed three times using serum-free medium, and finally, the fluorescence intensity and absorbance values were measured using a microplate reader at 488 nm and 525 nm to calculate the intracellular ROS levels in GC2spd cells.
2.7. RNA-Seq and Data Analyze
The obtained RNA was sent to the company for sequencing, and the steps of Zhang et al. [23] were followed. Subsequently, the R package (https://www.omicstudio.cn/home (accessed on 16 April 2023)) was utilized for data analysis, including principal component analysis (PCA) and differentially expressed genes (DEGs), including GO, KEGG, Reactome enrichment, etc.
2.8. Western Blot (WB)
Proteins were collected from GC2spd cells after CPF treatment for 24 h using RIPA (PL001, Shaanxi Zhonghui Hecai Biological Medicine Technology Co., Ltd., Xi’an, China), then centrifuged at 12,000× g for 20 min in 4 °C to collect the supernatant, followed by the addition of loading buff (P1040, Solarbio, Beijing, China) and bathing at 100 °C for 20 min. Subsequently, the prepared protein samples were subjected to electrophoresis, transferred to PVDF membranes (ISEQ00010, Millipore, Burlington, MA, USA), sealed with skimmed milk at room temperature for 2 h, and incubated with primary antibodies at 4 °C for 12 h. Then, the secondary antibodies were incubated at room temperature for 2 h after being purged in TBST for 3 times. Protein expression was detected using Ultra-sensitive ECL Reagent (DY30208, DIYIBIO, Shanghai, China) and Bio-Rad Chemidoc. Details of the primary and secondary antibodies are shown in Table S3.
2.9. The Prediction of Molecular Docking
The prediction of molecular docking was performed in the 3D protein structures of candidate genes and CPF using HOME for Researchers (https://www.dockeasy.cn/DockCompound (accessed on 16 April 2023)). When the binding energy between the ligand small molecule and the receptor protein is less than −4.25 kcal/mol, it indicates that there is some binding activity between the two; when the binding energy is less than −5.0 kcal/mol, it can be regarded as good binding activity; when the binding energy is less than −7.0 kcal/mol, it suggests that there is strong binding activity.
2.10. SC79 and CPF Co-Treatment
SC79 was purchased from MedChemExpress (HY-18749, Monmouth Junction, NJ, USA). GC2spd cells were passaged to 6-well (3.5 × 105) plates and cultured for 12 h. After 12 h, 0, 5, and 10 μmol/L SC79 was added into the medium for 24 h, and SC79 was dissolved in DMSO. Then, 5 μmol/L SC79 was selected and added into culture medium for pre-protecting the adhered GC2spd cells. After 1 h of pre-protection, CPF (0, 12.5, and 25 μmol/L) was added into the medium to co-treat for 24 h. The cells were named DMSO + DMSO, DMSO + CPF12.5, DMSO + CPF25, SC79 + DMSO, SC79 + CPF12.5, and SC79 + CPF25, respectively. Subsequently, the co-treated GC2spd cells were assayed for cellular morphology and CCK8.
2.11. N-Acetylcysteine (NAC) and CPF Co-Treatment
NAC scavenges intracellular ROS by enhancing the intracellular cysteine pool, increasing glutathione (GSH) levels and enhancing the activity of antioxidant enzymes (glutathione peroxidase, thioredoxin) [25]. NAC was purchased from MedChemExpress (HY-B0215, Monmouth Junction, NJ, USA). GC2spd cells were passaged to 6-well (3.5 × 105) or 96-well (2 × 104) plates and cultured for 12 h. After 12 h, NAC was dissolved in H2O and 0 and 5 mmol/L NAC were added for pre-protection. The choice of NAC dose was screened according to Zhang and Wei et al. and no toxic effect of 5 mmol/L NAC on GC2spd cells was detected in pre-tests [26,27]. After 1 h of pre-protection, CPF (0, 12.5, and 25 μmol/L) was added into the medium to co-treat for 24 h. The cells were named H2O + DMSO, H2O + CPF12.5, H2O + CPF25, NAC + DMSO, NAC + CPF12.5, and NAC + CPF25, respectively. Subsequently, the co-treated GC2spd cells were assayed for cellular morphology, CCK8, ROS, and gene expression.
2.12. Statistical Analysis
SPSS 23.0 software was used for statistical analysis of the data. Independent samples t-test (t-test) or one-way analysis of variance (ANOVA) was used to analyze the differences between all groups. When p < 0.05 or< 0.01, these differences were considered significant or extremely significant, respectively. All data are expressed as mean ± standard error of the mean (SEM). And all biological replicates in the experiments were more than or equal to 3 (n ≥ 3).
3. Results
3.1. The Morphology and Viability of GC2spd Cells After CPF Treatment
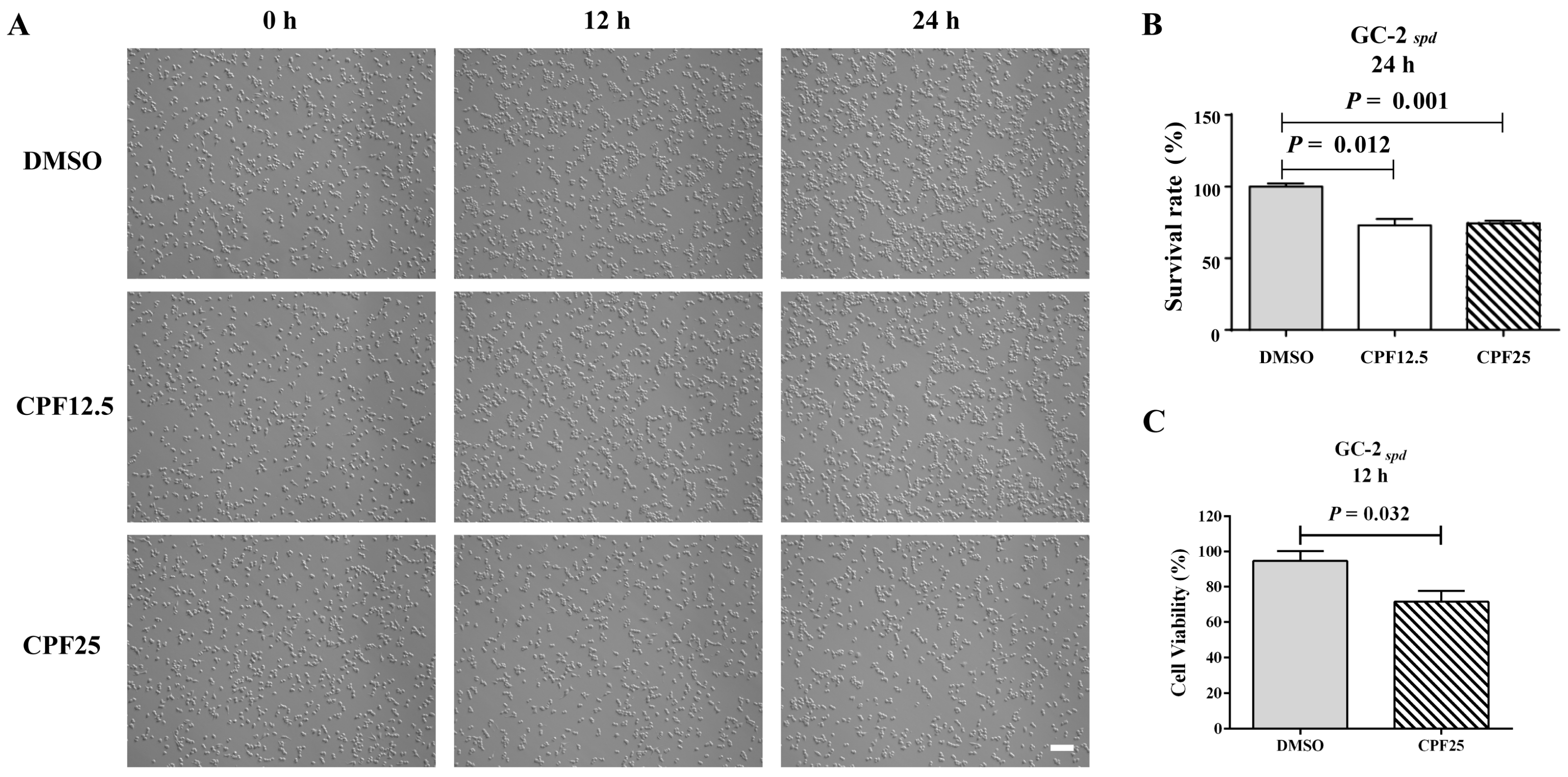
Preliminary research findings have indicated that CPF has male reproductive toxicity in vivo [17]. In order to further investigate the potential mechanisms of male infertility induced by CPF in mice, the GC2spd cell line, which is biased towards spermatocytes, was selected for in vitro validation. Firstly, CPF was added to the culture medium of GC2spd cells for 12 h and 24 h of in vitro experiments. After treatment with CPF at 12.5 and 25 μmol/L for 12 h, there was a significant reduction in adherent GC2spd cells (Figure 1A); counting the GC2spd cells after 24 h of CPF treatment, it was found that the CPF12.5 and CPF25 treatment groups exhibited approximately a 20% decrease in cell number (Figure 1B). Additionally, CCK8 results showed that exposure to 25 μmol/L CPF for 12 h led to a significant decrease in cell viability of GC2spd cells compared to the control group (p = 0.032) (Figure 1C).
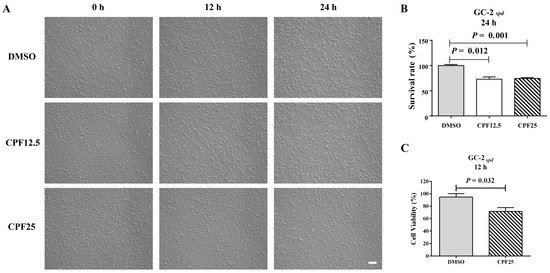
Figure 1.
Status of GC2spd cells after CPF treatment for 24 h. (A) Phenotypes of GC2spd cells; (B) cell number of GC2spd cells; (C) cell viability of GC2spd cells. Scale bar indicates 100 μm.
3.2. Cell Cycle Arrest of GC2spd Cells After CPF Treatment
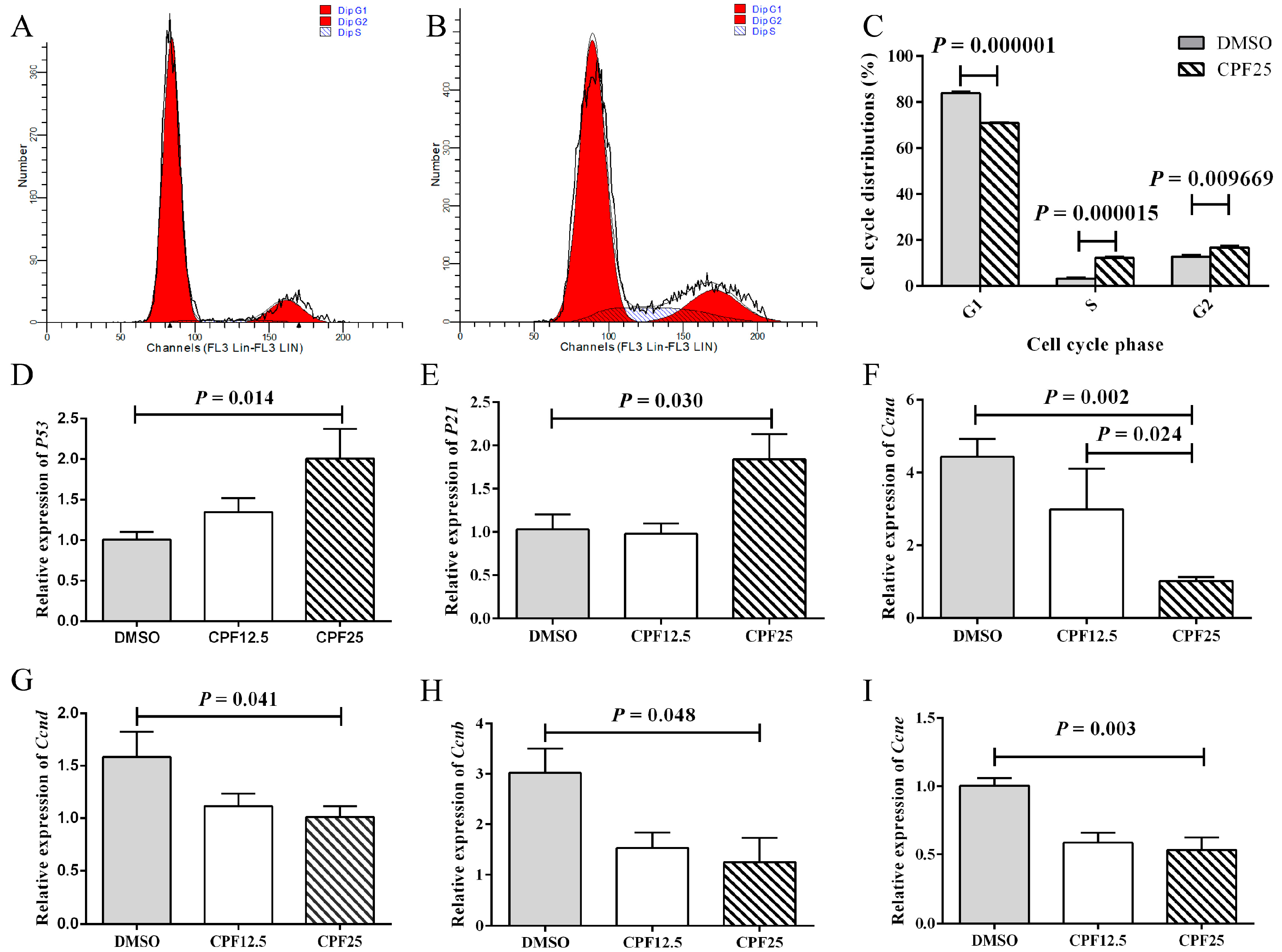
After CPF treatment, the reduction in adherent GC2spd cells may be attributed to factors such as decreased cell proliferation or increased cell apoptosis. Based on this, cell cycle analysis was performed on GC2spd cells after 24 h of CPF treatment. Firstly, flow cytometry showed that in the DMSO group, the proportions of cells in the G1, G2, and S phases were 83.97 ± 0.60%, 12.76 ± 0.85%, and 3.27 ± 0.44%, respectively; after CPF treatment, the proportions of cells in the G1, G2, and S phases were 70.95 ± 0.26%, 16.82 ± 0.68%, and 12.24 ± 0.56%, respectively (Figure 2A,B). After 24 h of CPF treatment, there was a significant decrease in G1 phase cells (p = 0.000001), and a significant increase in S phase (p = 0.000015) and G2 phase (p = 0.009669) cells, indicating that GC2spd cells were arrested in the S and G2 phases (Figure 2C).
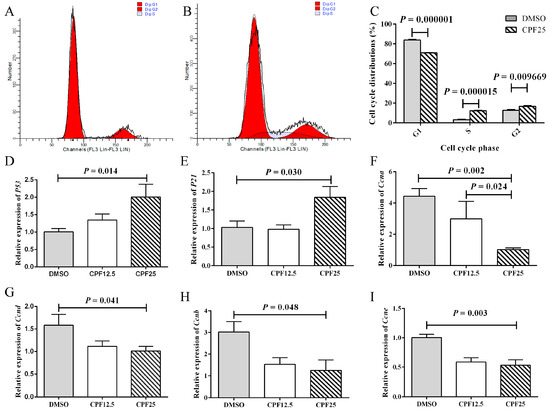
Figure 2.
Cell cycle arrest of GC2spd cells after CPF treatment for 24 h. (A) Cell distributions of vehicle (DMSO) and CPF (25 μmol/L) group; (B) cell distributions of CPF (25 μmol/L) group; (C) statistics on cell cycle distribution; (D) mRNA expression of P53; (E) mRNA expression of P21; (F) mRNA expression of Ccna; (G) mRNA expression of Ccnd; (H) mRNA expression of Ccnb; (I) mRNA expression of Ccne.
Subsequently, the expression of cell cycle-related genes was examined. After 24 h of CPF treatment, the CPF25 group showed significant upregulation of P53 (p = 0.014) and P21 (p = 0.030) (Figure 2D,E), while the expression of cell cycle-related genes Ccna (p = 0.002), Ccnd (p = 0.041), Ccnb (p = 0.048), and Ccne (p = 0.003) was significantly downregulated (Figure 2F–I). These genes collectively regulate GC2spd cells, leading to cell cycle arrest in the S and G2 phases after 24 h of CPF treatment.
3.3. Apoptosis of GC2spd Cells After CPF Treatment
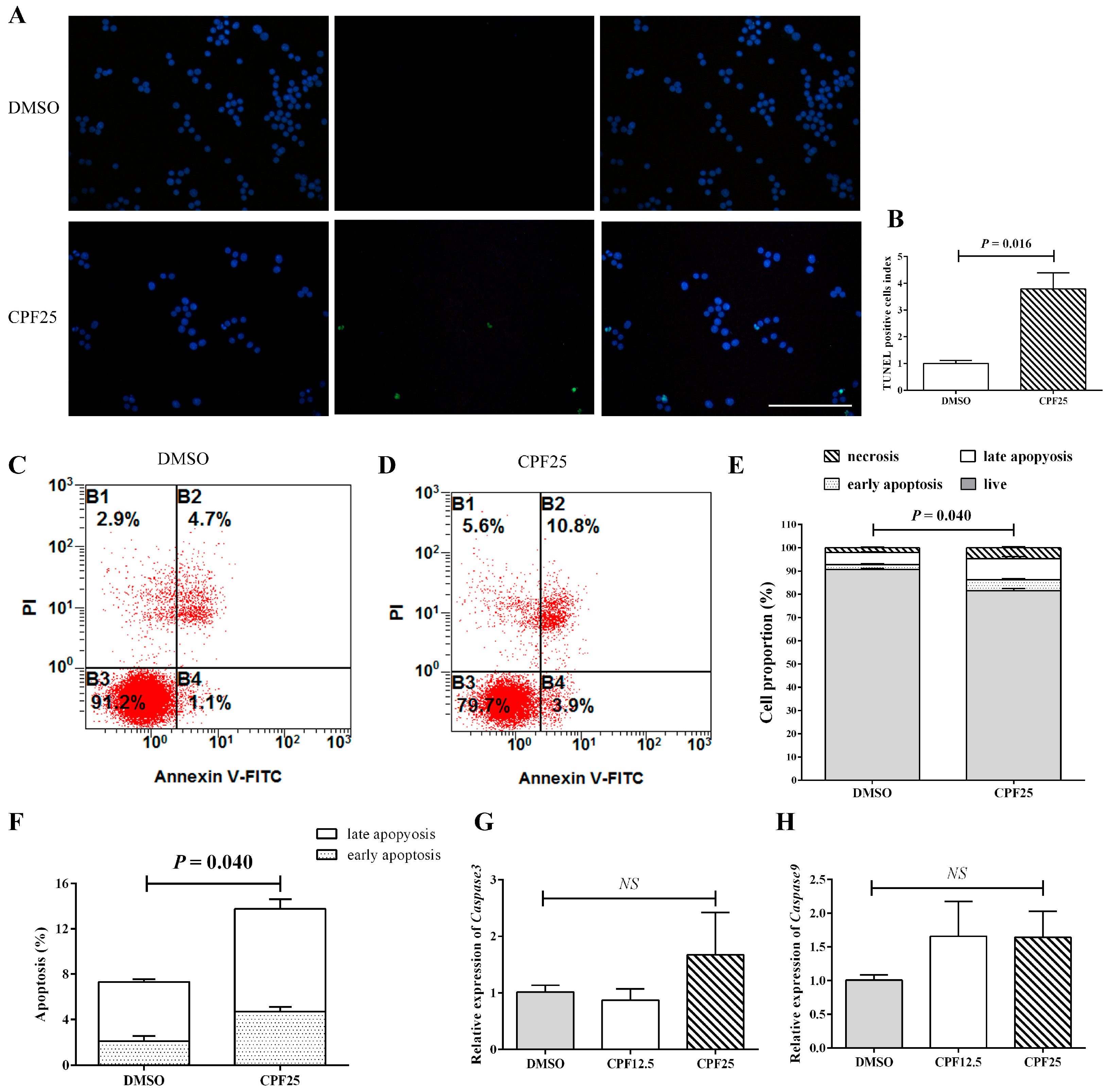
An increase in apoptosis is also one of the important reasons for the decrease in the number of adherent cells. Subsequently, the apoptosis of CPF-treated GC2spd cells was investigated. First, the TUNEL assay was used to detect GC2spd cells treated with 25 μmol/L of CPF for 24 h. The results showed that after 24 h of CPF treatment, the proportion of TUNEL-positive cells in the CPF25-treated group was significantly higher than that in the DMSO group (p = 0.016) (Figure 3A,B). The proportion of TUNEL-positive cells in the DMSO group was 0.8424 ± 0.10372, while in the CPF25-treated group, it was 3.1959 ± 0.50442.
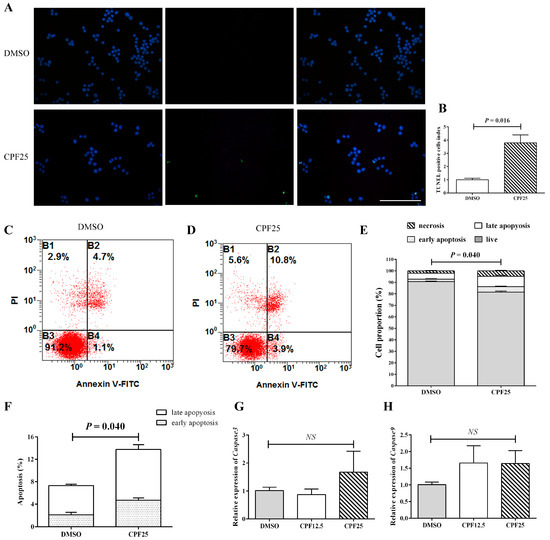
Figure 3.
Detection of apoptosis in GC2spd cells after CPF treatment for 24 h. (A) TUNEL staining in GC2spd cells; (B) TUNEL cell positivity rate in GC2spd cells; (C,D) cell distributions of vehicle (DMSO) and CPF (25 μmol/L) group; (E,F) statistics of apoptotic cells in GC2spd cells; (G) mRNA expression of Caspase3; (H) mRNA expression of Caspase9. Blue fluorescence represents cells stained with DAPI, and green fluorescence indicates apoptotic cells. B1: membrane rupture cells; B2: represents late apoptotic cells and necrotic cells; B3: live cells; B4: early apoptotic cells. Scale bar indicates 100 μm. “NS” represents no significant difference between each group.
Flow cytometry analysis showed that after 24 h of CPF treatment, the proportion of early apoptosis, late apoptosis, and necrotic cells in the CPF25-treated group was significantly higher than that in the DMSO group (p = 0.009, p = 0.040, and p = 0.005), and the proportion of live cells was significantly lower than the control group (p = 0.000241) (Figure 3C–F). The proportions of live cells, early apoptotic cells, late apoptotic cells, and necrotic cells in the DMSO group were 90.6500 ± 0.43684, 2.10000 ± 0.43970, 5.2250 ± 0.22867, and 2.0250 ± 0.29262, respectively; while in the CPF25 group, they were 81.6000 ± 0.98489, 4.70000 ± 0.40415, 9.0667 ± 0.86859, and 4.6333 ± 0.50442, respectively.
Apoptosis-related gene detection showed that after 24 h of CPF treatment, there was an upward trend in the expression of apoptosis-related genes (Caspase3 and Caspase9), but it was not statistically significant (Figure 3G,H), suggesting the possible presence of post-transcriptional regulation.
3.4. The Oxidative Stress Level of GC2spd Cells After CPF Treatment
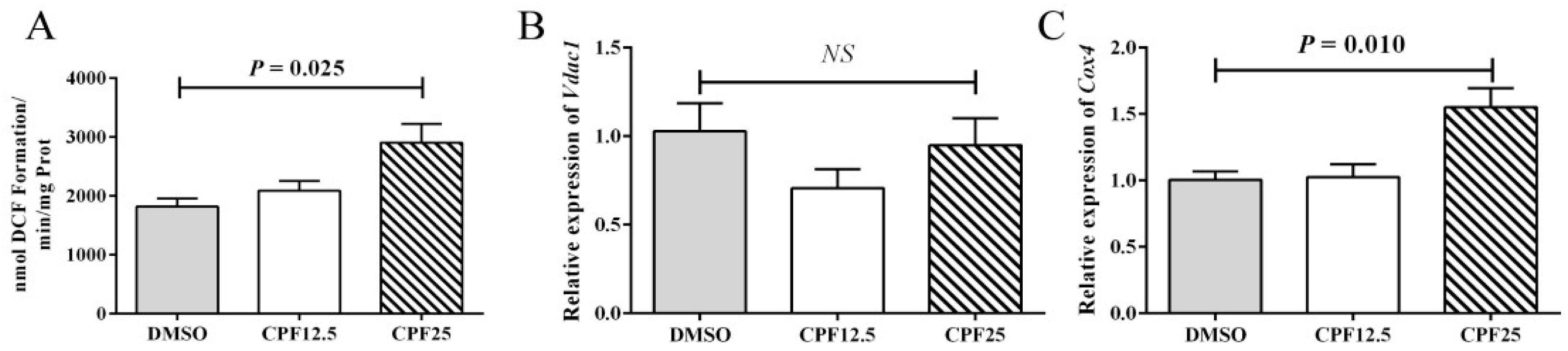
It is known that CPF can cause increased oxidative stress levels in the mouse cauda epididymidis. Therefore, further investigation was carried out to measure the oxidative stress levels in GC2spd cells after 24 h of CPF treatment. The results showed that after 24 h of CPF treatment, the ROS levels significantly increased in the 25 μmol/L treatment group (p = 0.025) (Figure 4A), which is consistent with the in vivo results. The elevation of ROS levels is associated with mitochondrial dysfunction. Subsequently, the expression of mitochondrial marker genes was examined, and it was found that there was no significant change in Vdac1 expression, but the expression level of Cox4 significantly increased (p = 0.010) (Figure 4B,C). This indicates that mitochondrial dysfunction occurs after CPF treatment, leading to an increase in oxidative stress levels.
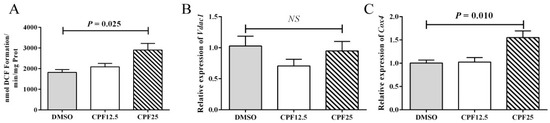
Figure 4.
Mitochondrial homeostasis assay in GC2spd cells after 24 h of CPF treatment. (A) ROS levels in GC2spd cells; (B) expression of Vdac1; (C) expression of Cox4. “NS” represents no significant difference between each group.
3.5. RNA-Seq of GC2spd Cells After CPF Treatment
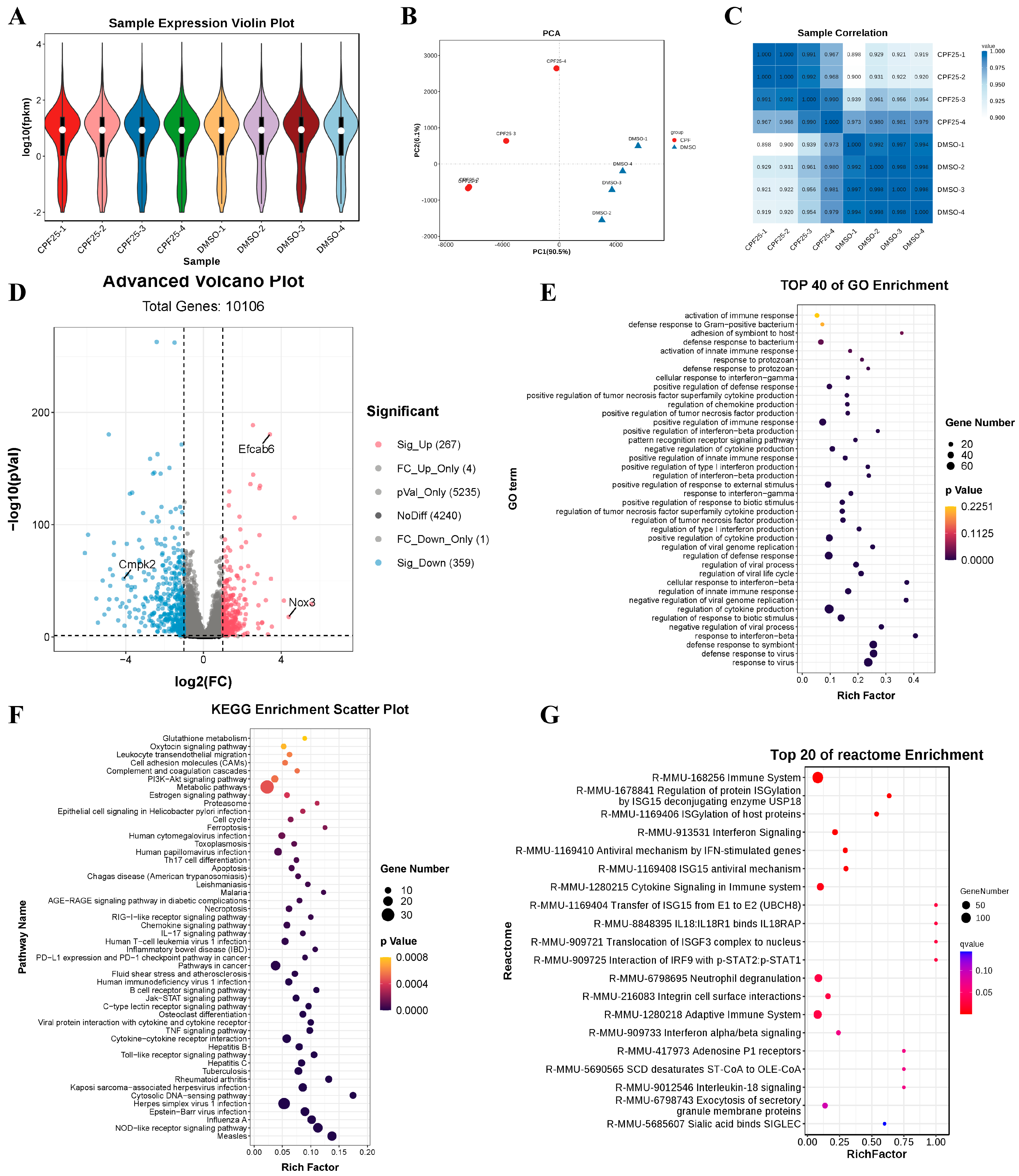
Based on the above results, to investigate the potential mechanisms underlying CPF-induced male reproductive damage, RNA sequencing was performed on GC2spd cells treated with CPF for 24 h, and the sequencing data was analyzed. The violin plots show that the quality of the sequencing data met the criteria for further analysis (Figure 5A). Results from PCA and heatmaps indicated good consistency within the group, confirming the suitability of the samples for subsequent analysis (Figure 5B,C). Subsequently, statistical analysis was conducted on the differentially expressed genes. The results showed that after 24 h of CPF treatment at 25 μmol/L, a total of 626 genes were differentially expressed compared to the DMSO group, with 267 upregulated and 359 downregulated (Figure 5D). Further analysis of the differentially expressed genes revealed significant upregulation of the male reproductive-related gene EF-hand calcium binding domain 6 (Efcab6), significant upregulation of the oxidative stress-related gene NADPH oxidase 3 (Nox3), and significant downregulation of the mitochondrial function-related gene cytidine/uridine monophosphate kinase 2 (Cmpk2). These genes will be the focus of further study. Additionally, GO enrichment analysis of the differentially expressed genes showed that compared to the DMSO group, the CPF25 group exhibited significant enrichment in pathways such as cellular immunity, inflammation response, and protein synthesis (Figure 5E). The KEGG enrichment analysis of the differentially expressed genes identified the top 20 pathways including the TNF and Jak-STAT pathways that were significantly enriched in the CPF25 group compared to the DMSO group (Figure 5F). In addition, the top 50 pathways involved TNF, Jak-STAT, PI3K-AKT, and glutamine metabolism (Figure 5F). Lastly, analysis using Reactome revealed that differentially expressed genes were mainly enriched in cellular immunity, inflammation, and the Jak-STAT pathway (Figure 5G).
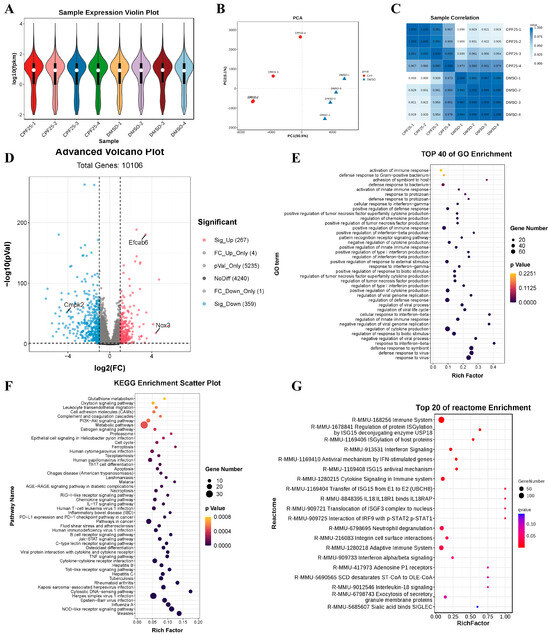
Figure 5.
Analysis of RNA sequencing data for GC2spd cells after CPF treatment for 24 h. (A) Sample expression violin plot; (B) principal component analysis of sample; (C) sample correlation heat map; (D) statistical chart of differential genes; pink dots represent significantly up-regulated genes, blue dots represent significantly down-regulated genes, and the rest are non-significant genes. (E) GO enrichment classification histogram; (F) KEGG enrichment bubble plot; (G) Reactome enrichment bubble chart. Data presented are from four independent experiments (n = 4).
3.6. The Gene and Protein Expression of GC2spd Cells After CPF Treatment
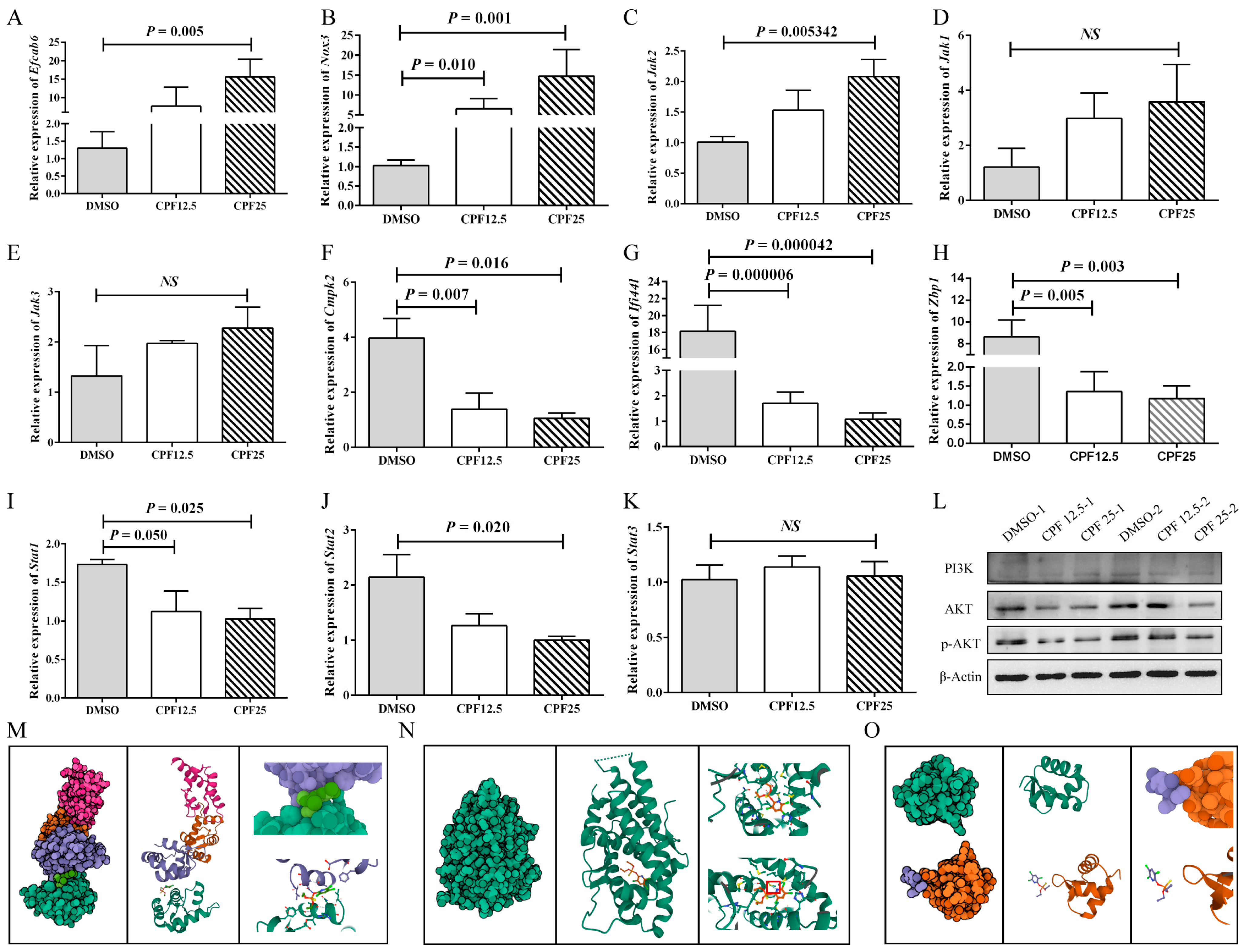
Based on the aforementioned sequencing and bioinformatics analysis results, male reproductive-related genes (Efcab6), oxidative stress-related genes (Nox3 and Cmpk2), immune-related genes (Ifi44l, Zbp1), and Jak-STAT pathway-related genes (Jak2, Jak3, Jak1, Stat1, and Stat2) were selected and validated. After 24 h of CPF treatment in GC2spd cells, the expression of the male reproductive-related gene (Efcab6) was significantly upregulated (p = 0.005) (Figure 6A), which can inhibit the expression of the androgen receptor (AR). The expression of the oxidative stress-related gene (Nox3) significantly increased (p = 0.001) (Figure 6B), while Cmpk2 expression significantly decreased (p = 0.016) (Figure 6F). The expression of Ifi44l and Zbp1 was significantly downregulated (p = 0.000042, p = 0.003) (Figure 6G,H). The expression of Jak-STAT pathway-related genes also demonstrated significant changes. Jak2 expression was significantly upregulated (p = 0.005), while Stat1 and Stat2 expression were significantly downregulated (p = 0.025, p = 0.020). Jak1 and Jak3 showed an upward trend in expression but were not statistically significant (p > 0.05) (Figure 6C–E,I–K). These results are consistent with the RNA sequencing analysis.
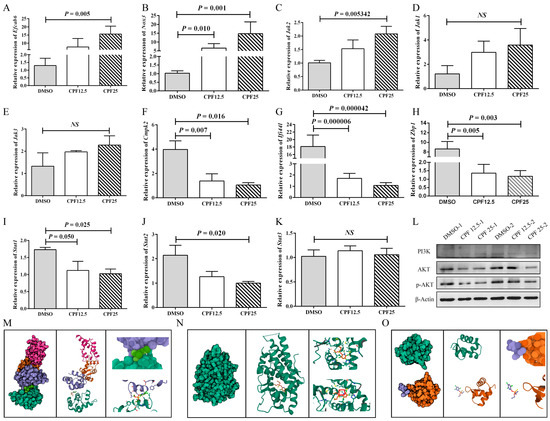
Figure 6.
Validation of RNA sequencing results. (A) Expression of Efcab6; (B) expression of Nox3; (C) expression of Jak2; (D) expression of Jak1; (E) expression of Jak3; (F) expression of Cmpk2; (G) expression of Ifi44l; (H) expression of Zbp1; (I) expression of Stat1; (J) expression of Stat2; (K) expression of Stat3; (L) protein expression of PI3K-AKT pathway; (M) prediction of molecular docking of CPF and EFCAB6; (N) prediction of molecular docking of CPF and AR; (O) prediction of molecular docking of CPF and ZBP1. “NS” represents no significant difference between each group.
Subsequently, based on the sequencing results, related signaling pathways were also screened. In Figure 4A, the results showed a significant increase in ROS levels in GC2spd cells after CPF treatment, and in Figure 5F, the results indicated that differentially expressed genes were enriched in the glutamine metabolism pathway. Therefore, it was speculated that CPF may induce ferroptosis in GC2spd cells. Then, ferroptosis-related proteins were detected in GC2spd cells treated with CPF for 24 h. The results showed that the expression of ferroptosis-related protein ferritin heavy polypeptide 1 (FTH1) did not change significantly (Figure S1A), while transferrin receptor 71 (CD71) expression was significantly reduced (Figure S1B). These results suggest that CPF may not affect the survival of GC2spd cells through inducing iron death.
In addition, existing studies have reported that the accumulation of a large amount of intracellular ROS can inhibit the activity of the PI3K-AKT signaling pathway. Combining the results of mouse GC2spd cell sequencing analysis and sequencing analysis in pig ST cells [23], it is speculated that CPF may induce male reproductive damage through the ROS/PI3K/AKT pathway. Therefore, the protein expression of the PI3K-AKT signaling pathway was further verified. The results showed that after 24 h of CPF treatment, the expression of AKT in GC2spd cells was significantly decreased, and the downstream effector protein p-AKT expression was significantly lower than in the control group, indicating inhibition of the PI3K-AKT signaling pathway (Figure 6L). After that, SC79 was utilized to activate the phosphorylation of AKT in GC2spd cells. The optimal concentration of SC79 was first examined in GC2spd cells, and the results showed that 5 μm/L SC79 did not have a damaging effect on GC2spd cells; 5 μm/L SC79 was used for subsequent experiments (Figure S2A). However, the addition of SC79 did not rescue the decrease in the number and viability of GC2spd cells caused by CPF (Figure S2B,C). The above experiments indicated that CPF reduced p-AKT expression by decreasing the total AKT level in GC2spd cells and thus did not affect the phosphorylation process of AKT.
Next, the prediction of molecular docking was performed for genes with large fold changes after CPF treatment, such as Efcab6, Nox3, and Zbp1. Firstly, the 3D structures of the corresponding proteins for these genes were searched in the RCSB PDB database (https://www.rcsb.org (accessed on 16 April 2023)), where NOX3 had no available structure resolution, the PDB ID for EFCAB6 was 1WLZ, and the PDB ID for ZBP1 was 2HEO. In addition, the prediction of molecular docking was also performed for AR (1GS4), the downstream target protein of EFCAB6. According to the simulation results, CPF showed good binding affinity with AR with a binding energy of −6.369 and the formation of hydrogen bonding (Figure 6M); CPF also exhibited good binding affinity with EFCAB6 with a binding energy of −5.364, although no hydrogen bonding was formed (Figure 6N); whereas CPF had poor binding affinity with ZBP1 with a binding energy of −1.262 and no binding occurred (Figure 6O). These results suggest that CPF may directly target the structural domain of EFCAB6 or AR to regulate their expressions, thus reducing male reproductive fertility.
3.7. The Morphology of GC2spd Cells After NAC and CPF Co-Treatment
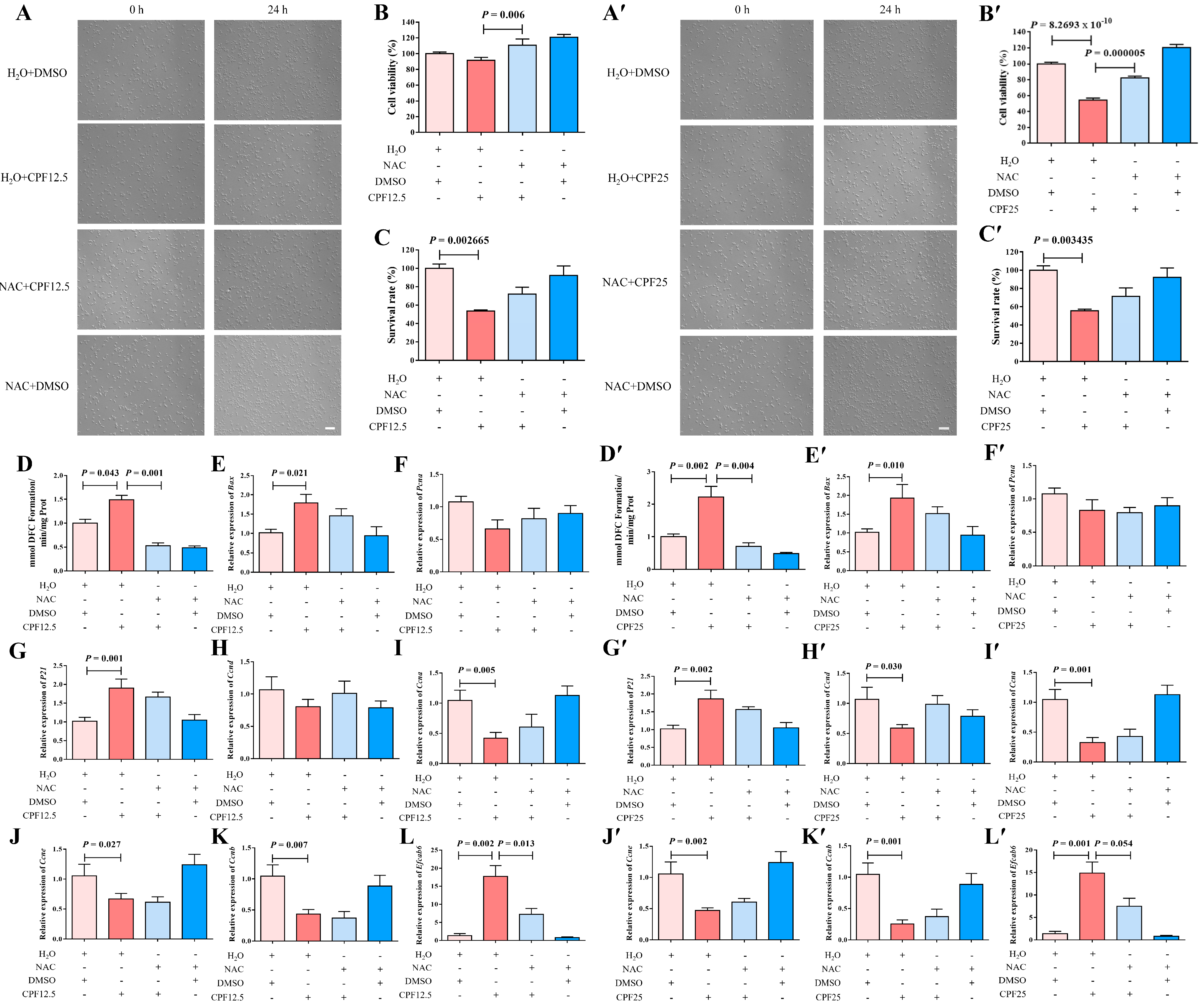
This experiment utilized NAC to clear ROS in GC2spd cells, thereby reverse validating that CPF can damage GC2spd cells through ROS. After the co-treatment of NAC and CPF, live cells were significantly reduced after treatment with H2O + CPF12.5 and H2O + CPF25 for 24 h. However, the addition of NAC could restore this reduction (NAC + CPF12.5 and NAC + CPF25) (Figure 7A,A′). The above cells were counted, and it was found that 5 mmol/L of NAC could partially restore the reduction in cell number induced by CPF12.5 (Figure 7C); at the same time, the same dose of NAC could also partially restore the reduction in cell number induced by CPF25 (Figure 7C′). The above results indicated that NAC could restore CPF-induced damage in GC2spd cells after inhibiting intracellular ROS generation.
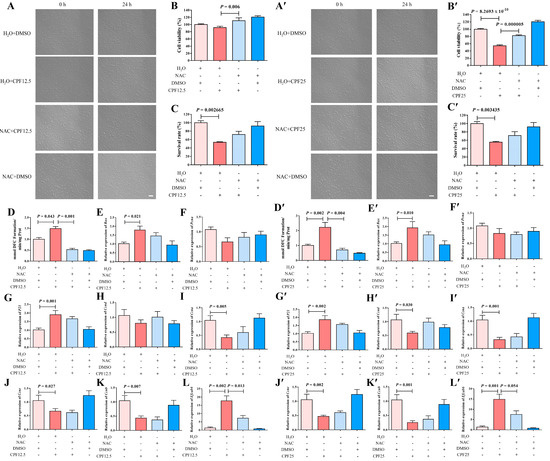
Figure 7.
Restorative effect of NAC on GC2spg cells after CPF treatment for 24 h. (A–L) Restorative effect of NAC on GC2spg cells after 12.5 μmol/L CPF exposure; (A′–L′) restorative effect of NAC on GC2spg cells after 25 μmol/L CPF exposure; (A,A′) phenotypes of GC2spd cells; scale bar indicates 100 μm. (B,B′) cell viability of GC2spd cells; (C,C′) cell number of GC2spd cells; (D,D′) level of ROS in GC2spd cells; (E,E′) expression of Bax; (F,F′) expression of Pcna; (G,G′) expression of P21; (H,H′) expression of Ccnd; (I,I′) expression of Ccna; (J,J′) expression of Ccne; (K,K′) expression of Ccnb; (L,L′) expression of Efcab6. Scale bar indicates 100 μm.
To further investigate the restorative effect of NAC on CPF damage, CCK8 assay was subsequently performed on GC2spd cells after co-treatment with NAC and CPF. The results showed that cell viability was significantly reduced (p = 8.2693 × 10−10) after H2O + CPF25 treatment of GC2spd cells for 24 h (Figure 7B′). After adding NAC for 1 h of pre-protection followed by co-treatment with CPF (NAC + CPF12.5 and NAC + CPF25), cell viability was significantly higher in the NAC + CPF12.5 group than that in the H2O + CPF12.5 (p = 0.006) (Figure 7B); cell viability in the NAC + CPF25 group was lower than that in the H2O + DMSO group, but significantly higher than that in the H2O + CPF25 group (p = 0.000005) (Figure 7B′). The above results indicated that the CPF-induced reduction in GC2spd cell viability could be restored after NAC inhibited intracellular ROS generation.
3.8. The ROS Levels of GC2spd Cells After NAC and CPF Co-Treatment
Subsequently, ROS assay was performed on GC2spd cells after co-treatment with NAC and CPF, and the results showed that ROS levels in GC2spd cells were significantly increased after 24 h of treatment with H2O + CPF12.5 (p = 0.043) (Figure 7D); ROS levels in GC2spd cells were further increased in the H2O + CPF25-treated group, which was significantly higher than that in the H2O + DMSO group (p = 0.002) (Figure 7D′). However, after adding NAC for 1 h of pre-protection followed by CPF for treatment (NAC + CPF12.5 and NAC + CPF25), the intracellular ROS levels in GC2spd cells in the NAC + CPF12.5-treated group were lower than those in the H2O + DMSO group and lower than those in the H2O + CPF12.5 group, and the accumulation of ROS in the GC2spd cells was reduced by about 2-fold (p = 0.001) (Figure 7D); the intracellular ROS levels in GC2spd cells in the NAC + CPF25-treated group were also significantly lower than those in the H2O + DMSO and H2O + CPF25 groups, and the ROS accumulation was reduced by about 3-fold (p = 0.004) (Figure 7D’). The above results indicated that NAC inhibition of intracellular ROS generation restored CPF-induced ROS accumulation in GC2spd.
3.9. Apoptosis of GC2spd Cells After NAC and CPF Co-Treatment
The expression of the above apoptosis-related genes was immediately explored. A 12.5 μmol/L CPF (H2O + CPF12.5) treatment of GC2spd cells for 24 h resulted in a significant increase in Bax expression in the GC2spd cells (p = 0.021); Bax expression in NAC + CPF12.5 was higher than that in the H2O + DMSO group, but lower than that in the H2O + CPF12.5 group (Figure 7E). In addition, the qRT-PCR results showed that Bax expression was significantly upregulated in the H2O + CPF25 group (p = 0.010), and in the NAC + CPF25, the expression of Bax also tended to be lower than that in H2O + CPF25 group (Figure 7E′). All these results indicated that NAC could partially restore the upregulation of apoptosis-related gene expression in GC2spd cells induced by 12.5 μmol/L and 25 μmol/L CPF.
3.10. Cell Cycle Arrest of GC2spd Cells After NAC and CPF Co-Treatment
Subsequently, NAC was utilized to restore the alterations in proliferation and cell cycle-related gene expression in GC2spd cells induced by CPF. The results showed that the expression of P21 was significantly elevated in GC2spd cells after 24 h of treatment with H2O + CPF12.5 (p = 0.001); the expression of Pcna, Ccnd, Ccne (p = 0.027), Ccna (p = 0.005), and Ccnb (p = 0.007) were lower than that in the control group (H2O + DMSO) (Figure 7F–K). However, the addition of NAC for 1 h of pre-protection followed by the addition of 12.5 μmol/L CPF for treatment (NAC + CPF12.5) showed that the expression of P21 in the cells, although higher than that in the H2O + DMSO group, was lower than that in the H2O + CPF12.5; moreover, the expression of Pcna, Ccnd, and Ccna in GC2spd cells in the NAC + CPF12.5-treated group was lower than that in the control group (H2O + DMSO), but higher than that in the H2O + CPF12.5 group (Figure 7G–I). In the 25 μmol/L CPF (H2O + CPF25) treatment group, the results demonstrated that P21 expression was significantly elevated in GC2spd cells after 24 h (p = 0.002); the expression of Pcna, Ccnd (p = 0.030), Ccne (p = 0.002), Ccna (p = 0.001), and Ccnb (p = 0.001) was lower than that in the control group (H2O + DMSO) (Figure 7F′–K′), which is consistent with the damage induced by 12.5 μmol/L CPF. After adding NAC for 1 h of pre-protection and then adding 25 μmol/L CPF for treatment (NAC + CPF25), the expression of P21 in the cells was lower than that of H2O + CPF25 although higher than that of the H2O + DMSO group (Figure 7G′); moreover, the expression of Ccnd, Ccne, Ccna, and Ccnb of the GC2spd cells in the NAC + CPF25-treated group were lower than those in the control group (H2O + DMSO) but higher than those in the 25 μmol/L CPF-treated group (H2O + CPF25) (Figure 7H′–K′). All of the above results indicated that NAC inhibition of intracellular ROS generation partially restored the CPF-induced alteration of cell cycle-related gene expression in GC2spd.
3.11. The Expression of Efcab6 in GC2spd Cells After NAC and CPF Co-Treatment
Previous results showed that CPF might have a direct targeting effect with Efcab6, so the expression of the potential target gene Efcab6 in GC2spd cells after co-treatment with NAC and CPF was examined. The qRT-PCR results indicated that the expression of Efcab6 in GC2spd cells was significantly elevated after treatment with 12.5 μmol/L CPF (H2O + CPF12.5) and 25 μmol/L CPF (H2O + CPF25) for 24 h (p = 0.002; p = 0.001) (Figure 7L,L′). But concerning the addition of NAC to pre-protect the cells for 1 h followed by the addition of CPF for co-treatment, the expression of Efcab6 was higher than that in the H2O + DMSO group, but notably lower than that in the H2O + CPF12.5 and H2O + CPF25 groups (p = 0.013; p = 0.054) (Figure 7L,L′). These findings indicated that NAC partially restored the upregulation of potential target gene Efcab6 expression in GC2spd cells induced by CPF.
4. Discussion
The advent of industrialization has undeniably positioned pesticides as vital components of agriculture and everyday life. However, the widespread use of pesticides also has brought about numerous adverse consequences. Pesticide residues can accumulate in animals through biomagnification and have an impact on the reproductive system, especially causing significant damage to the male reproductive system. Numerous studies have demonstrated that pesticide residues can affect male reproduction. Preliminary experiments have shown that CPF causes reproductive damage in male mice by reducing the expression of genes related to steroid synthesis, damaging mitochondrial function, increasing sperm oxidative stress, and reducing sperm motility and density, ultimately leading to male infertility [17]. However, the potential targets of CPF-induced male reproductive damage have not yet been identified. In light of this consideration, the primary objective of this experiment is to unveil the intricate mechanisms underpinning CPF-induced male reproductive toxicity, providing reference materials for the prevention and treatment of male reproductive disorders.
After CPF treatment of GC2spd cells, there was a decrease in adherent cells, cell cycle arrest, a significant increase in apoptotic cells, and a significant elevation of ROS levels, aligning with the previous in vivo experiments in mice. In-depth RNA sequencing analysis showed that after 24 h of treatment with 25 μmol/L CPF, several key pathways were enriched, including glutamine metabolism, Jak-STAT signaling, and PI3K-AKT pathways. Previous studies have shown that the Jak-STAT pathway is essential for self-renewal of male SSCs [28]. And in embryonic stem cells (ESs), Jak-STAT signaling is necessary for maintaining ESs [29]. Singh et al. showed that Jak-STAT signaling was also shown to be counteracted by Ras-Raf-MAPK, and that the two signals can subsequently converge on a number of downstream targets to co-regulate cell fate [29]. The RNA sequencing results suggest that in addition to its classic regulatory roles, the Jak-STAT pathway may be involved in CPF-induced damage in GC2spd cells. However, it is crucial to note that further research is warranted to delve deeper into the specific mechanisms.
Ferroptosis is a relatively novel form of regulated cell death that occurs when there is a significant increase in intracellular iron levels. This leads to the buildup of lipid peroxides within the cell membrane, which subsequently breaks down into aldehydes, reactive oxygen species, and other active derivatives, causing damage to cellular proteins, lipids, and nucleic acids, ultimately resulting in cell death [30]. Previous studies have shown that ferroptosis has negative effects on male reproduction [31], and recent research has shown that ferroptosis is involved in the death of sperm caused by bilateral varicocele [32]. In recent years, there has been a growing interest in exploring the adverse effects of ferroptosis in studies focused on male reproductive toxicity. Wang and Zeng et al. have shown that in cadmium-treated models, ferroptosis can inhibit testicular development, impair spermatogenesis, and affect testosterone synthesis, consequently reducing male fertility [33,34]. Additionally, Phthalic acid esters (PAEs) can induce ferroptosis in the testes, damage the blood–testis barrier (BTB), and inhibit male fertility [35]. Based on this, our experiment employed KEGG analysis, which revealed alterations in the glutamine metabolism pathway. When combined with the significant increase in oxidative stress levels in mouse sperm and GC2spd cells after CPF treatment, we speculated that CPF may induce male reproductive damage through the ferroptosis pathway. However, a closer examination of ferroptosis marker proteins showed that after 24 h of CPF treatment in GC2spd cells, there was no significant change in FTH1 expression, but CD71 expression was significantly reduced, indicating that CPF does not affect the viability of GC2spd cells through the induction of ferroptosis. It is important to note that CD71 expression is dependent on p-AKT, so the reduction in CD71 expression may be attributed to a decline in p-AKT levels [36,37].
The PI3K/AKT pathway is crucial for regulating various cellular processes and serves as an important signal transduction pathway in cell growth, energy conversion, cell cycle, apoptosis, and autophagy [38]. It also holds importance in male reproduction [39,40]. KEGG analysis and Western blot results showed that after CPF treatment, the levels of ROS were significantly elevated in GC2spd cells. Simultaneously, the activity of the PI3K/AKT pathway was inhibited. Previous studies have shown that in colorectal cancer research, Delicaflavone can induce the accumulation of ROS, suppress the PI3K/AKT/mTOR and Ras/MEK/Erk signaling cascades, inhibit the proliferation of colorectal cancer cells, and promote apoptosis [41]. Zhu et al. also demonstrated that avibactam can induce apoptosis and autophagy in TM3 cells by accumulating ROS and inhibiting the PI3K/AKT signaling pathway [42]. Additionally, related studies have shown that CPF can inhibit the PI3K/AKT signaling pathway, leading to apoptosis in brain and liver tissues [43,44]. Moreover, high levels of ROS can inhibit the activation of AKT through PI3K [45]. Therefore, CPF may induce apoptosis in GC2spd cells and reduce male fertility through the ROS/AKT pathway.
Numerous studies have established the endocrine-disrupting function of CPF in males, resulting in a decrease in testosterone synthesis and an increase in follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels [46]. The action of androgens in target cells is mediated by the androgen receptor (AR). Hazarika et al. harnessed techniques such as the prediction of molecular docking and molecular dynamics to demonstrate the molecular interactions between CPF and its degradation products with AR, identifying them as potent disruptors of androgen activity [47]. As early as 2003, researchers determined that Efcab6 (also called DJBP) can bind to the COOH-terminal region of DJ-1. It also can bind to the DNA-binding domain of AR, both in vitro and in vivo. This interaction leads to the recruitment of histone deacetylase (HDAC) complexes, including HDAC1 and mSin1, ultimately culminating in the inhibition of AR activation [48]. Based on sequencing data, the Efcab6 gene exhibited differential expression in GC2spd cells before and after CPF treatment, with particularly high expression after CPF treatment. We postulated that CPF may regulate the expression of the Efcab6 gene to inhibit AR activity, in turn, diminishing male fertility. In line with this, the prediction of molecular docking results also supports our speculation. Furthermore, inhibiting intracellular ROS with NAC also resulted in decreased expression of the Efcab6 gene, suggesting that CPF can induce apoptosis and reduce male fertility in GC2spd cells through the ROS/AKT/Efcab6 pathway.
Comparative analysis of mouse and pig transcriptome sequencing data revealed that differentially expressed genes were enriched in pathways such as PI3K-AKT, Jak-STAT, and inflammation [23]. Subsequent experiments also confirmed the toxic effects of CPF on mouse GC2spd and ST cells through the ROS/PI3K/AKT pathway. This implies a certain degree of conservation in the potential molecular mechanisms underlying CPF-induced male reproductive damage across different species. However, due to differences in species and cell types, the action mode of CPF and downstream effect genes may vary. For example, in GC2spd cells, CPF reduces AKT expression and subsequently decreases p-AKT. In contrast, in ST cells, CPF directly affects the phosphorylation process of AKT, reducing p-AKT expression. Additionally, in GC2spd cells, the expression of the male reproductive-related gene Efcab6 is significantly upregulated, whereas in ST cells, the expression of the male reproductive-related gene Hsd3b1 is significantly downregulated. Moreover, these genes share similar effects. Previous studies have shown that high expression of Efcab6 in cells can inhibit transactivation of AR, thereby suppressing AR expression and ultimately causing male infertility [48]. Conversely, decreased expression of Hsd3b1 can lead to a reduction in androgen synthesis, such as testosterone, ultimately causing male reproductive damage [49].
In this study, we examined in detail the potential mechanisms of male injury induced by CPF. However, unfortunately, we have not yet performed recovery experiments in vivo using NAC. In subsequent studies, we will aim to further investigate the restorative effects of NAC on CPF-induced male reproductive injury in vivo and to find natural compounds to replace the function of NAC from a food-borne perspective.
5. Conclusions
In summary, CPF can affect the cell viability, cell cycle, apoptosis, and ROS levels in GC2spd cells. And RNA sequencing illuminated that CPF can regulate the survival of GC2spd cells through the PI3K-AKT pathway. Rescue experiments using NAC confirm that CPF can activate ROS accumulation in GC2spd cells, thereby inhibiting the PI3K-AKT signaling pathway and activating Efcab6 expression, leading to a decrease in cell survival.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells14130940/s1, Table S1: qRT-PCR reaction system; Table S2: qRT-PCR reaction program; Table S3: Antibody information; Figure S1: The protein expression of ferroptosis in GC2spd cells after CPF treatment for 24 h; Figure S2: The detection of GC2spd cells after SC79 and CPF co-treatment for 24 h.
Author Contributions
Conceptualization, X.Z. and M.Z.; methodology, X.Z., M.Z. and C.W.; validation, X.Z., M.Z. and Q.S.; formal analysis, X.Z., M.Z., C.W., Q.S., H.Y. and Q.T.; investigation, X.Z., H.Y. and Q.T.; resources, J.W. and C.P.; data curation, X.Z., M.Z., C.W. and Q.S.; writing—original draft preparation, X.Z.; writing—review and editing, X.Z., M.Z., Q.Z., J.W. and C.P.; visualization, X.Z., M.Z. and H.Y.; supervision, J.W. and C.P.; project administration, H.Y., J.W. and C.P.; funding acquisition, J.W. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Key Research and Development Program of Henan Province (251111110200) and the Natural Science Foundation of Shaanxi Province of China (2022JM-125).
Data Availability Statement
The original data of the paper are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, J.; Dai, Y.; Li, Y.; Yuan, E.; Wang, Q.; Wang, X.; Guan, Y. A longitudinal study of semen quality among Chinese sperm donor candidates during the past 11 years. Sci. Rep. 2020, 10, 10771. [Google Scholar] [CrossRef] [PubMed]
- Knapke, E.T.; Magalhaes, D.P.; Dalvie, M.A.; Mandrioli, D.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology 2022, 465, 153017. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Ngo, Q.D.; Nguyen, V.C.; Ngo, K.D.; Lam, V.N.; Dang, T.N.; Tran, Q.H.; Phung, T.D.; Nguyen, K.T.; Nguyen, T.V.; et al. Organophosphate Pesticide Exposure: Effect on Farmers’ Sperm Quality in the Mekong Delta, Vietnam. J. Agromed. 2024, 29, 404–414. [Google Scholar] [CrossRef]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L.; et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008, 38 (Suppl. 2), 1–125. [Google Scholar] [CrossRef]
- Andersen, H.R.; Rambaud, L.; Riou, M.; Buekers, J.; Remy, S.; Berman, T.; Govarts, E. Exposure Levels of Pyrethroids, Chlorpyrifos and Glyphosate in EU-An Overview of Human Biomonitoring Studies Published since 2000. Toxics 2022, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. 2022. Available online: https://echa.europa.eu/documents/10162/8a51d7d9-e9a4-2513-e975-492fb70f825c (accessed on 16 April 2023).
- Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A.; Pietruszyńska, M.; Wydro, U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12209. [Google Scholar] [CrossRef]
- Mehta, A.; Verma, R.S.; Srivastava, N. Chlorpyrifos-induced DNA damage in rat liver and brain. Environ. Mol. Mutagen. 2008, 49, 426–433. [Google Scholar] [CrossRef]
- Shenouda, J.; Green, P.; Sultatos, L. An evaluation of the inhibition of human butyrylcholinesterase and acetylcholinesterase by the organophosphate chlorpyrifos oxon. Toxicol. Appl. Pharmacol. 2009, 241, 135–142. [Google Scholar] [CrossRef]
- Cool, J.; Capel, B. Mixed signals: Development of the testis. Semin. Reprod. Med. 2009, 27, 5–13. [Google Scholar] [CrossRef]
- Cool, J.; DeFalco, T.; Capel, B. Testis formation in the fetal mouse: Dynamic and complex de novo tubulogenesis. Wiley interdisciplinary reviews. Dev. Biol. 2012, 1, 847–859. [Google Scholar]
- McCarrey, J.R. Toward a more precise and informative nomenclature describing fetal and neonatal male germ cells in rodents. Biol. Reprod. 2013, 89, 47. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Huckins, C. The spermatogonial stem cell population in adult rats. 3. Evidence for a long-cycling population. Cell Tissue Kinet. 1971, 4, 335–349. [Google Scholar]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Wang, K.; Chen, R.; Chen, M.; Lan, K.; Wei, Y.; Pan, C.; Lan, X. Chlorpyrifos inhibits sperm maturation and induces a decrease in mouse male fertility. Environ. Res. 2020, 188, 109785. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Sengupta, P.; Dutta, S. Coenzyme Q10 Improves Sperm Parameters, Oxidative Stress Markers and Sperm DNA Fragmentation in Infertile Patients with Idiopathic Oligoasthenozoospermia. World J. Men’s Health 2021, 39, 346–351. [Google Scholar] [CrossRef]
- De Felice, A.; Greco, A.; Calamandrei, G.; Minghetti, L. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J. Neuroinflamm. 2016, 13, 149. [Google Scholar] [CrossRef]
- Chen, R.; Cui, Y.; Zhang, X.; Zhang, Y.; Chen, M.; Zhou, T.; Lan, X.; Dong, W.; Pan, C. Chlorpyrifos Induction of Testicular-Cell Apoptosis through Generation of Reactive Oxygen Species and Phosphorylation of AMPK. J. Agric. Food Chem. 2018, 66, 12455–12470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Li, W.; Yue, L.; Zhang, T.; Tang, Q.; Zhang, N.; Lan, X.; Pan, C. Chlorpyrifos induces male infertility in pigs through ROS and PI3K-AKT pathway. iScience 2023, 26, 106558. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, C.; Lettieri, G.; Chianese, T.; Bianchi, A.R.; Zarrelli, A.; Palatucci, D.; Scudiero, R.; Rosati, L.; De Maio, A.; Piscopo, M. Exploring the molecular and toxicological mechanism associated with interactions between heavy metals and the reproductive system of Mytilus galloprovincialis. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2024, 275, 109778. [Google Scholar]
- KaKalyanaraman, B. NAC, NAC, Knockin’ on Heaven’s door: Interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022, 57, 102497. [Google Scholar]
- Zhang, X.; Hao, H.; Ma, K.; Pang, H.; Li, X.; Tian, T.; Hou, S.; Ning, X.; Wu, H.; Hou, Q.; et al. The role and mechanism of unfolded protein response signaling pathway in methylmercury-induced apoptosis of mouse spermatocytes germ cell-2 cells. Environ. Toxicol. 2023, 38, 472–482. [Google Scholar] [CrossRef]
- Wei, Y.; Geng, W.; Zhang, T.; He, H.; Zhai, J. N-acetylcysteine rescues meiotic arrest during spermatogenesis in mice exposed to BDE-209. Environ. Sci. Pollut. Res. 2023, 30, 50952–50968. [Google Scholar] [CrossRef]
- Hombría, J.C.; Brown, S. The fertile field of Drosophila Jak/STAT signalling. Curr. Biol. 2002, 12, R569–R575. [Google Scholar] [CrossRef]
- Singh, S.R.; Chen, X.; Hou, S.X. JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophila. Cell Res. 2005, 15, 1–5. [Google Scholar] [CrossRef]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef]
- Wu, S.F.; Ga, Y.; Ma, D.Y.; Hou, S.L.; Hui, Q.Y.; Hao, Z.H. The role of ferroptosis in environmental pollution-induced male reproductive system toxicity. Environ. Pollut. 2024, 363 Pt 1, 125118. [Google Scholar] [CrossRef]
- Sun, T.C.; Li, D.M.; Yu, H.; Song, L.L.; Jia, Y.J.; Lin, L.; Zhou, S.J. Bilateral varicocele leads to ferroptosis, pyroptosis and necroptosis of human spermatozoa and affects semen quality in infertile men. Front. Cell Dev. Biol. 2023, 11, 1091438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Zhang, M.; OuYang, H.; Li, M.; Jia, D.; Wang, R.; Zhou, W.; Liu, H.; Hu, Y.; et al. Cadmium exposure during puberty damages testicular development and spermatogenesis via ferroptosis caused by intracellular iron overload and oxidative stress in mice. Environ. Pollut. 2023, 325, 121434. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, J.; Wang, X.; Zhang, Y.; Wang, M.; Su, P. Cadmium attenuates testosterone synthesis by promoting ferroptosis and blocking autophagosome-lysosome fusion. Free Radic. Biol. Med. 2021, 176, 176–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Cui, J.G.; Wang, J.X.; Chen, M.S.; Wang, H.R.; Li, X.N.; Li, J.L. Ferroptosis is critical for phthalates driving the blood-testis barrier dysfunction via targeting transferrin receptor. Redox Biol. 2023, 59, 102584. [Google Scholar] [CrossRef]
- Barata, J.T.; Silva, A.; Brandao, J.G.; Nadler, L.M.; Cardoso, A.A.; Boussiotis, V.A. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004, 200, 659–669. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, L.; Chen, M.; Gui, M.; Lu, L.; Deng, F.; Ren, Z. IgA1 isolated from Henoch-Schönlein purpura children promotes proliferation of human mesangial cells in vitro. Cell Biol. Int. 2019, 43, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Huang, W.; Quan, C.; Duan, P.; Tang, S.; Chen, W.; Yang, K. Nonylphenol induced apoptosis and autophagy involving the Akt/mTOR pathway in prepubertal Sprague-Dawley male rats in vivo and in vitro. Toxicology 2016, 373, 41–53. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Zhang, X.; Shi, X.; Gong, D. The antagonistic effect of melatonin on TBBPA-induced apoptosis and necroptosis via PTEN/PI3K/AKT signaling pathway in swine testis cells. Environ. Toxicol. 2022, 37, 2281–2290. [Google Scholar] [CrossRef]
- Yao, W.; Lin, Z.; Shi, P.; Chen, B.; Wang, G.; Huang, J.; Sui, Y.; Liu, Q.; Li, S.; Lin, X.; et al. Delicaflavone induces ROS-mediated apoptosis and inhibits PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in colorectal cancer cells. Biochem. Pharmacol. 2020, 171, 113680. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J.; Sun, X.; Zhou, Z.; Zhu, Q. ROS accumulation contributes to abamectin-induced apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway in TM3 Leydig cells. J. Biochem. Mol. Toxicol. 2020, 34, e22505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Shi, X.; Xu, S. Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J. Hazard. Mater. 2020, 398, 122905. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pei, H.; Chen, W.; Zhu, X.; Wang, Y.; Li, J.; He, Z.; Du, R. Evaluating the effect of ginsenoside Rg1 on CPF-induced brain injury in mice via PI3k/AKT pathway. J. Biochem. Mol. Toxicol. 2023, 37, e23319. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Ubaid Ur Rahman, H.; Asghar, W.; Nazir, W.; Sandhu, M.A.; Ahmed, A.; Khalid, N. A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: Evidence of mechanisms, exposures and mitigation strategies. Sci. Total Environ. 2021, 755, 142649. [Google Scholar] [CrossRef]
- Hazarika, J.; Ganguly, M.; Borgohain, G.; Sarma, S.; Bhuyan, P.; Mahanta, R. Disruption of androgen receptor signaling by chlorpyrifos (CPF) and its environmental degradation products: A structural insight. J. Biomol. Struct. Dyn. 2022, 40, 6027–6038. [Google Scholar] [CrossRef]
- Niki, T.; Takahashi-Niki, K.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. DJBP: A novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol. Cancer Res. MCR 2003, 1, 247–261. [Google Scholar]
- Zhang, S.; Mo, J.; Wang, Y.; Ni, C.; Li, X.; Zhu, Q.; Ge, R.S. Endocrine disruptors of inhibiting testicular 3β-hydroxysteroid dehydrogenase. Chem.-Biol. Interact. 2019, 303, 90–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).