CPF Induces GC2spd Cell Injury via ROS/AKT/Efcab6 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
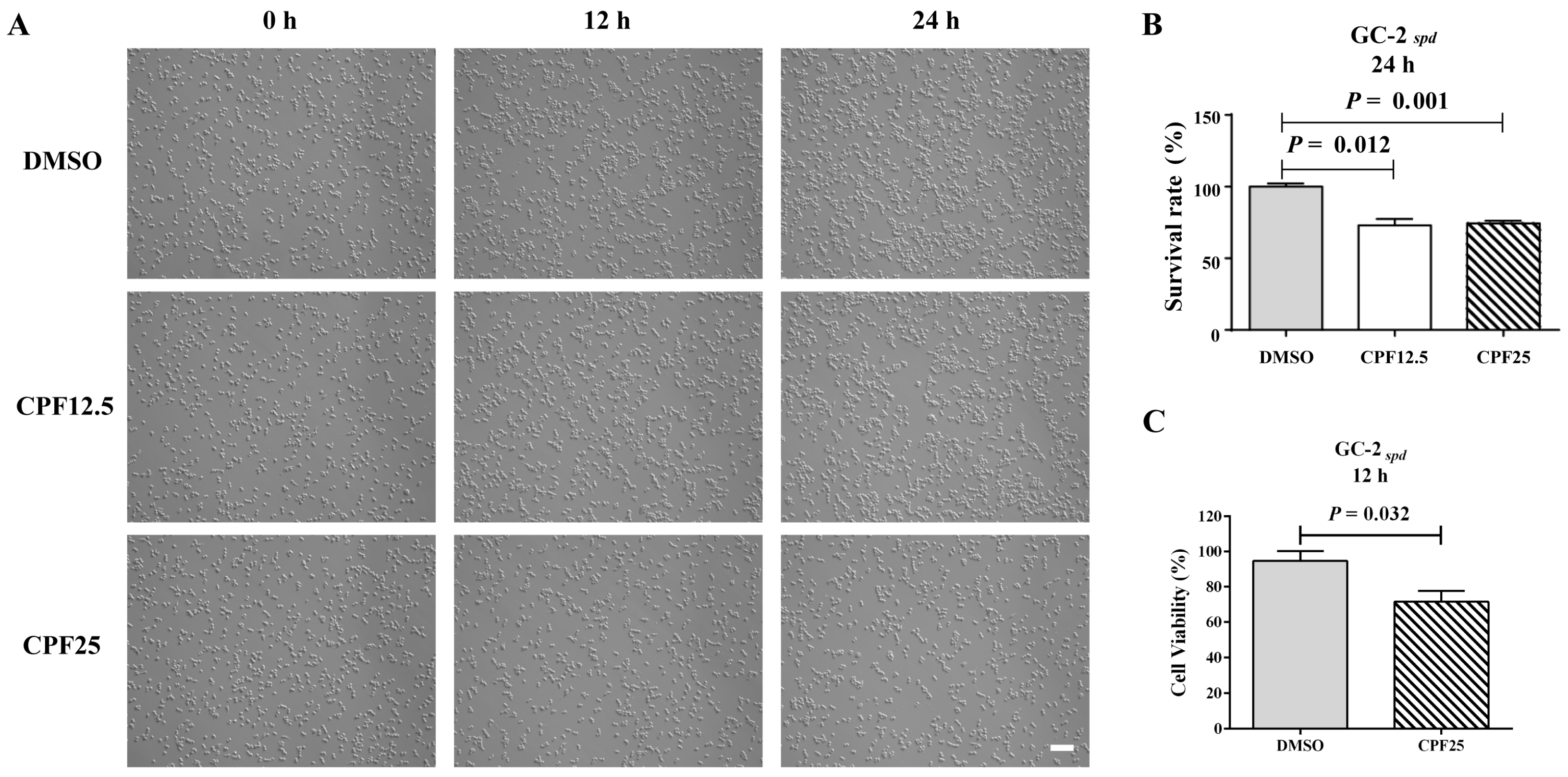
2.2. The Detection of Cell Viability
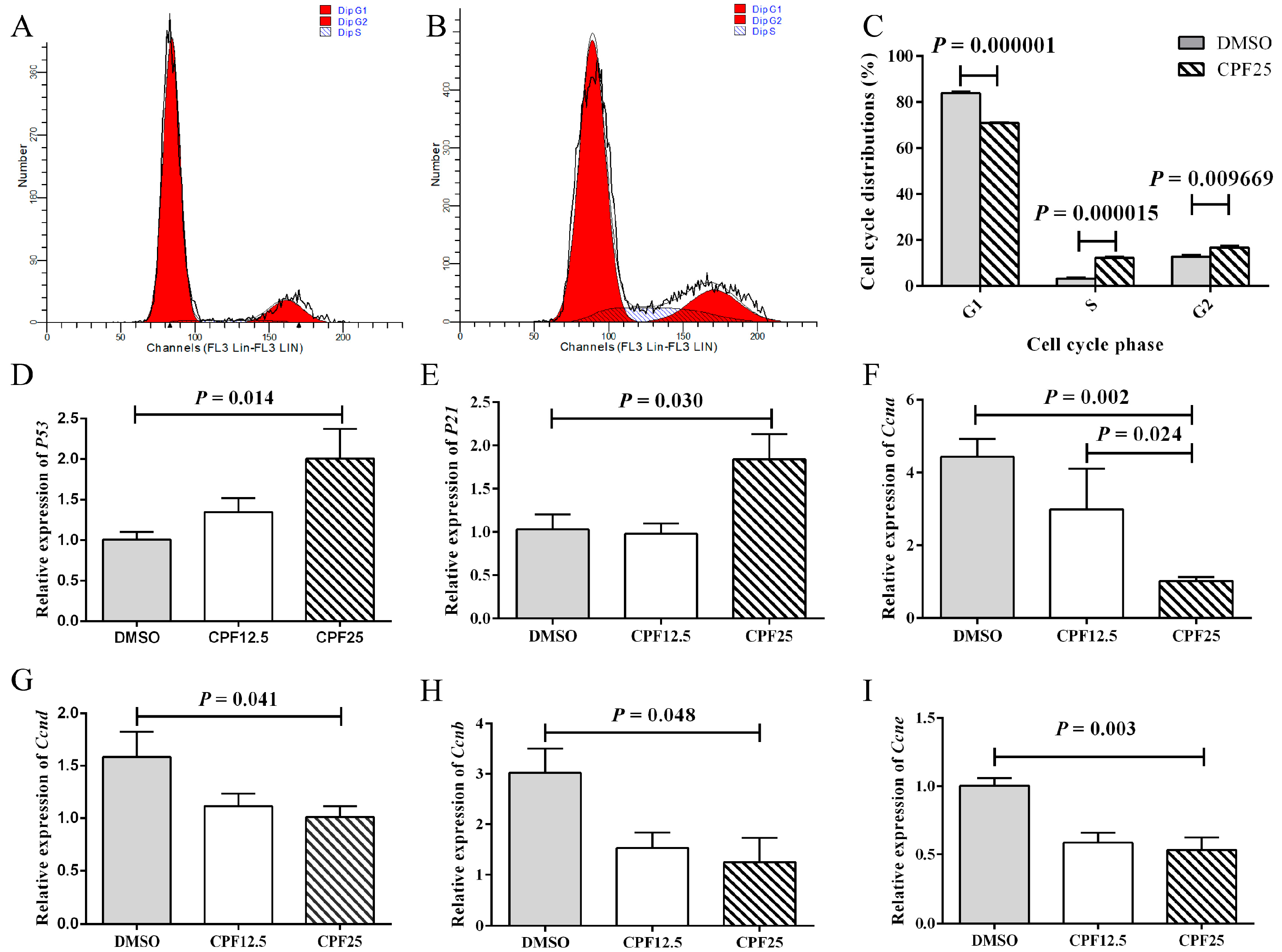
2.3. The Detection of Cell Cycle
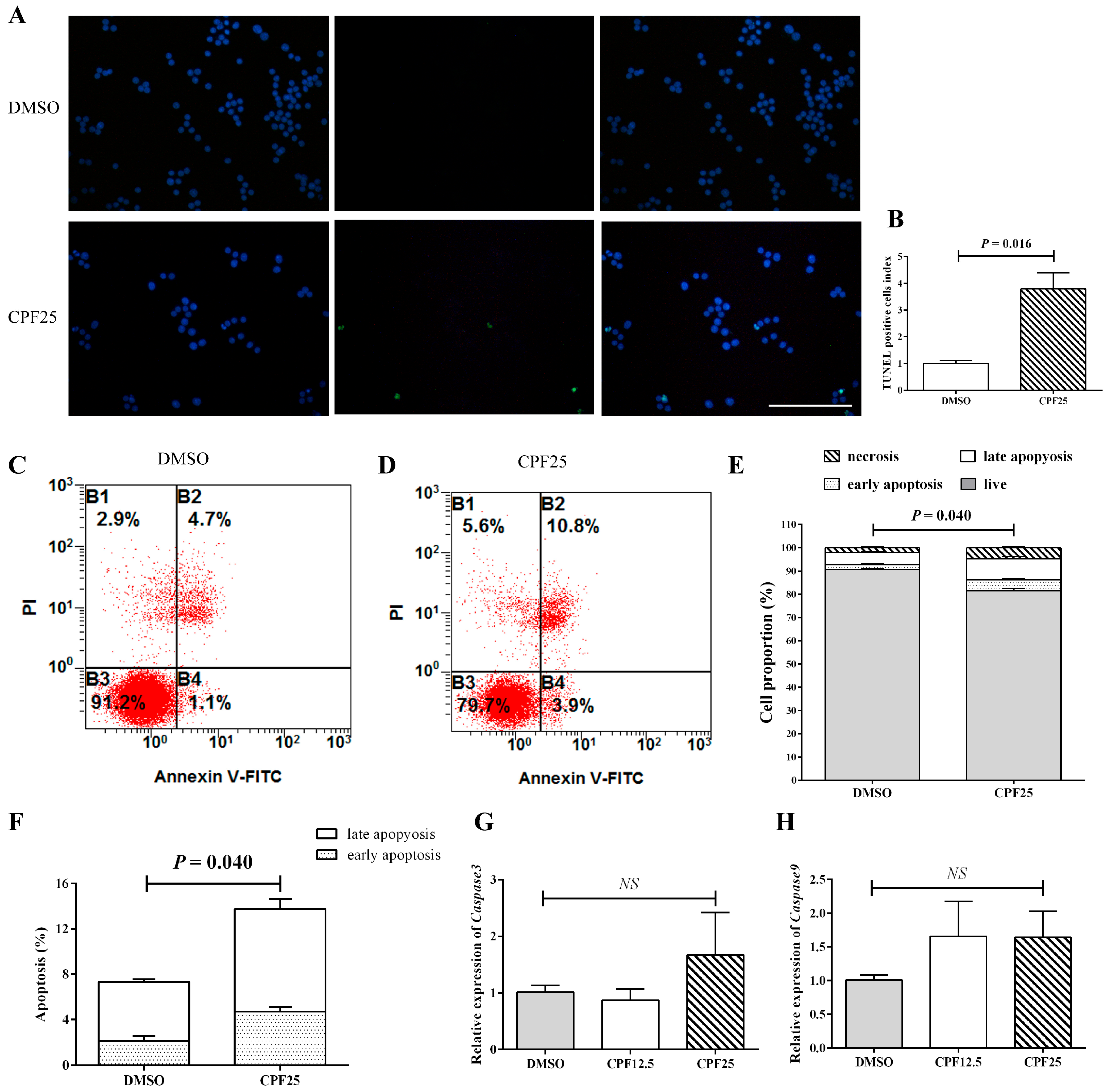
2.4. The Detection of Apoptosis
2.5. RNA Extraction and qRT-PCR
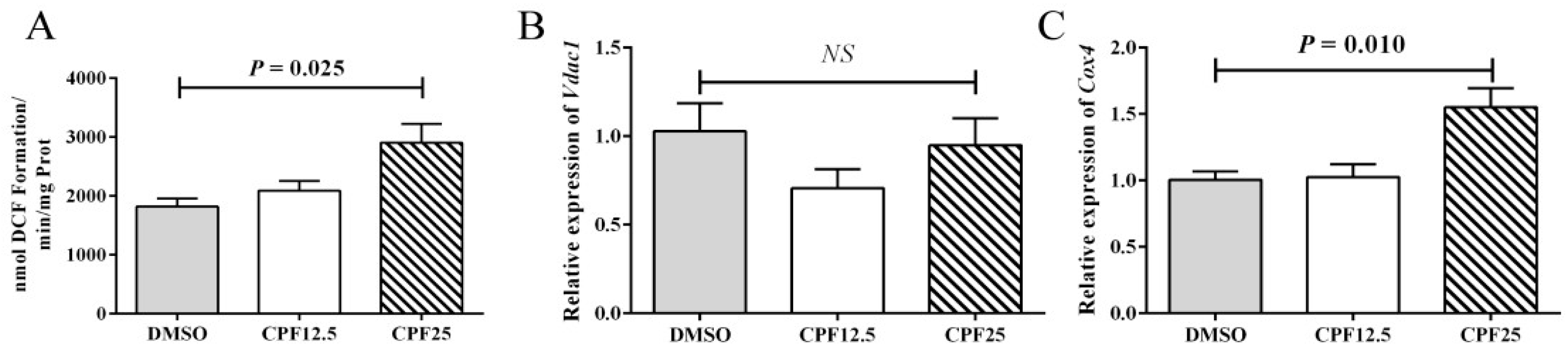
2.6. Detection of Oxidative Stress Level
2.7. RNA-Seq and Data Analyze
2.8. Western Blot (WB)
2.9. The Prediction of Molecular Docking
2.10. SC79 and CPF Co-Treatment
2.11. N-Acetylcysteine (NAC) and CPF Co-Treatment
2.12. Statistical Analysis
3. Results
3.1. The Morphology and Viability of GC2spd Cells After CPF Treatment
3.2. Cell Cycle Arrest of GC2spd Cells After CPF Treatment
3.3. Apoptosis of GC2spd Cells After CPF Treatment
3.4. The Oxidative Stress Level of GC2spd Cells After CPF Treatment
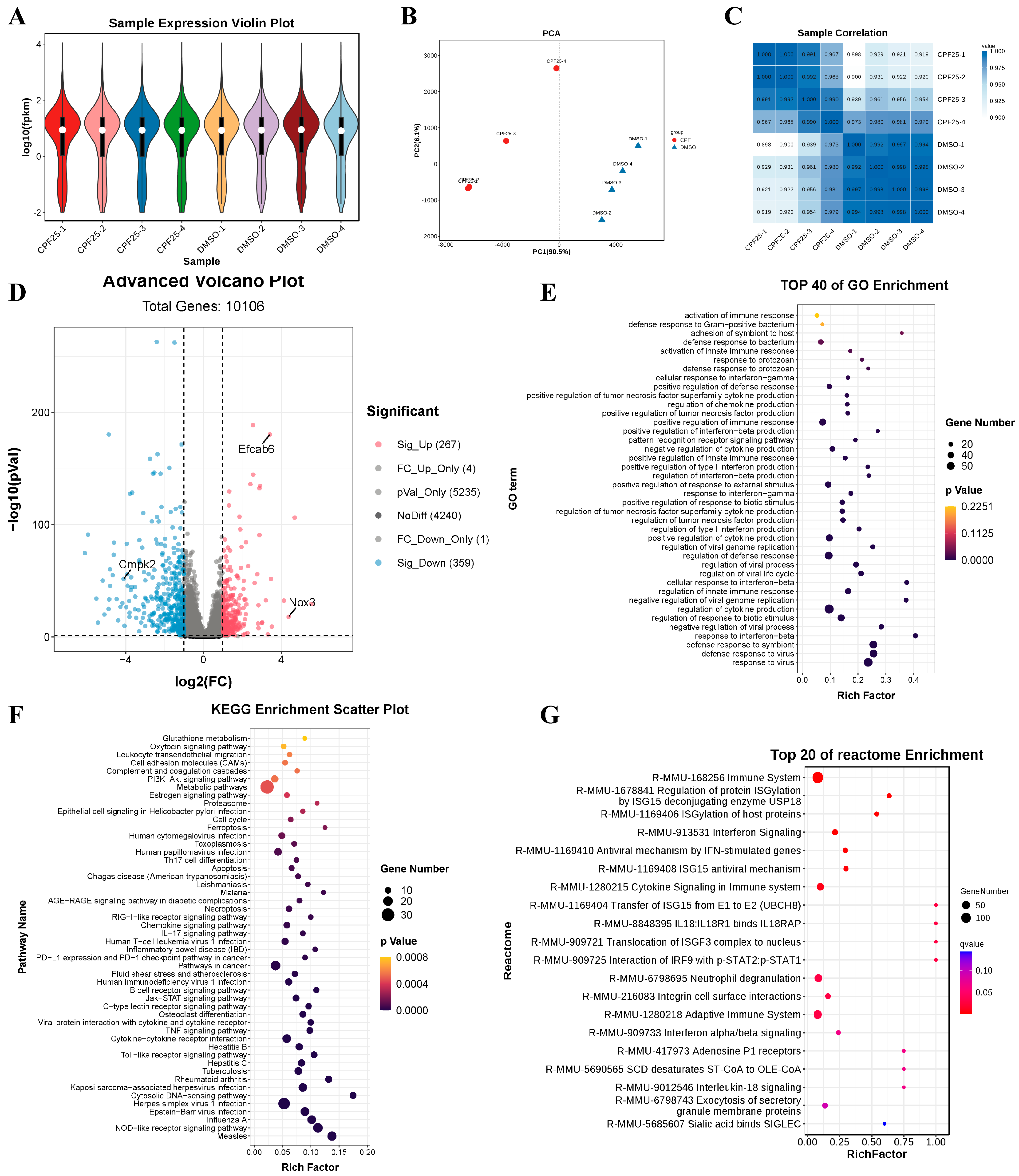
3.5. RNA-Seq of GC2spd Cells After CPF Treatment
3.6. The Gene and Protein Expression of GC2spd Cells After CPF Treatment
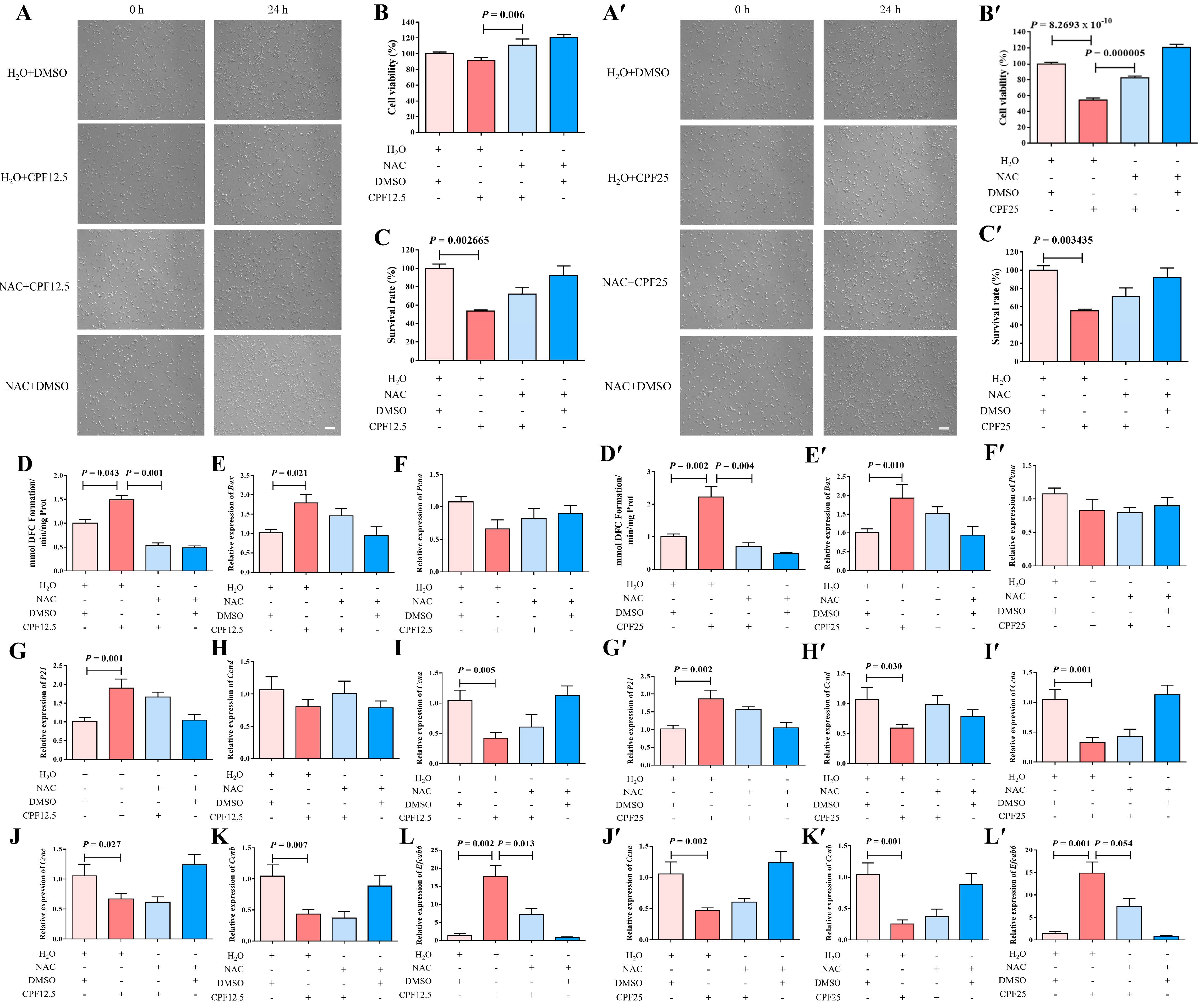
3.7. The Morphology of GC2spd Cells After NAC and CPF Co-Treatment
3.8. The ROS Levels of GC2spd Cells After NAC and CPF Co-Treatment
3.9. Apoptosis of GC2spd Cells After NAC and CPF Co-Treatment
3.10. Cell Cycle Arrest of GC2spd Cells After NAC and CPF Co-Treatment
3.11. The Expression of Efcab6 in GC2spd Cells After NAC and CPF Co-Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Dai, Y.; Li, Y.; Yuan, E.; Wang, Q.; Wang, X.; Guan, Y. A longitudinal study of semen quality among Chinese sperm donor candidates during the past 11 years. Sci. Rep. 2020, 10, 10771. [Google Scholar] [CrossRef] [PubMed]
- Knapke, E.T.; Magalhaes, D.P.; Dalvie, M.A.; Mandrioli, D.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology 2022, 465, 153017. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Ngo, Q.D.; Nguyen, V.C.; Ngo, K.D.; Lam, V.N.; Dang, T.N.; Tran, Q.H.; Phung, T.D.; Nguyen, K.T.; Nguyen, T.V.; et al. Organophosphate Pesticide Exposure: Effect on Farmers’ Sperm Quality in the Mekong Delta, Vietnam. J. Agromed. 2024, 29, 404–414. [Google Scholar] [CrossRef]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L.; et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008, 38 (Suppl. 2), 1–125. [Google Scholar] [CrossRef]
- Andersen, H.R.; Rambaud, L.; Riou, M.; Buekers, J.; Remy, S.; Berman, T.; Govarts, E. Exposure Levels of Pyrethroids, Chlorpyrifos and Glyphosate in EU-An Overview of Human Biomonitoring Studies Published since 2000. Toxics 2022, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. 2022. Available online: https://echa.europa.eu/documents/10162/8a51d7d9-e9a4-2513-e975-492fb70f825c (accessed on 16 April 2023).
- Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A.; Pietruszyńska, M.; Wydro, U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12209. [Google Scholar] [CrossRef]
- Mehta, A.; Verma, R.S.; Srivastava, N. Chlorpyrifos-induced DNA damage in rat liver and brain. Environ. Mol. Mutagen. 2008, 49, 426–433. [Google Scholar] [CrossRef]
- Shenouda, J.; Green, P.; Sultatos, L. An evaluation of the inhibition of human butyrylcholinesterase and acetylcholinesterase by the organophosphate chlorpyrifos oxon. Toxicol. Appl. Pharmacol. 2009, 241, 135–142. [Google Scholar] [CrossRef]
- Cool, J.; Capel, B. Mixed signals: Development of the testis. Semin. Reprod. Med. 2009, 27, 5–13. [Google Scholar] [CrossRef]
- Cool, J.; DeFalco, T.; Capel, B. Testis formation in the fetal mouse: Dynamic and complex de novo tubulogenesis. Wiley interdisciplinary reviews. Dev. Biol. 2012, 1, 847–859. [Google Scholar]
- McCarrey, J.R. Toward a more precise and informative nomenclature describing fetal and neonatal male germ cells in rodents. Biol. Reprod. 2013, 89, 47. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Huckins, C. The spermatogonial stem cell population in adult rats. 3. Evidence for a long-cycling population. Cell Tissue Kinet. 1971, 4, 335–349. [Google Scholar]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Wang, K.; Chen, R.; Chen, M.; Lan, K.; Wei, Y.; Pan, C.; Lan, X. Chlorpyrifos inhibits sperm maturation and induces a decrease in mouse male fertility. Environ. Res. 2020, 188, 109785. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Sengupta, P.; Dutta, S. Coenzyme Q10 Improves Sperm Parameters, Oxidative Stress Markers and Sperm DNA Fragmentation in Infertile Patients with Idiopathic Oligoasthenozoospermia. World J. Men’s Health 2021, 39, 346–351. [Google Scholar] [CrossRef]
- De Felice, A.; Greco, A.; Calamandrei, G.; Minghetti, L. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J. Neuroinflamm. 2016, 13, 149. [Google Scholar] [CrossRef]
- Chen, R.; Cui, Y.; Zhang, X.; Zhang, Y.; Chen, M.; Zhou, T.; Lan, X.; Dong, W.; Pan, C. Chlorpyrifos Induction of Testicular-Cell Apoptosis through Generation of Reactive Oxygen Species and Phosphorylation of AMPK. J. Agric. Food Chem. 2018, 66, 12455–12470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Li, W.; Yue, L.; Zhang, T.; Tang, Q.; Zhang, N.; Lan, X.; Pan, C. Chlorpyrifos induces male infertility in pigs through ROS and PI3K-AKT pathway. iScience 2023, 26, 106558. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, C.; Lettieri, G.; Chianese, T.; Bianchi, A.R.; Zarrelli, A.; Palatucci, D.; Scudiero, R.; Rosati, L.; De Maio, A.; Piscopo, M. Exploring the molecular and toxicological mechanism associated with interactions between heavy metals and the reproductive system of Mytilus galloprovincialis. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2024, 275, 109778. [Google Scholar]
- KaKalyanaraman, B. NAC, NAC, Knockin’ on Heaven’s door: Interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022, 57, 102497. [Google Scholar]
- Zhang, X.; Hao, H.; Ma, K.; Pang, H.; Li, X.; Tian, T.; Hou, S.; Ning, X.; Wu, H.; Hou, Q.; et al. The role and mechanism of unfolded protein response signaling pathway in methylmercury-induced apoptosis of mouse spermatocytes germ cell-2 cells. Environ. Toxicol. 2023, 38, 472–482. [Google Scholar] [CrossRef]
- Wei, Y.; Geng, W.; Zhang, T.; He, H.; Zhai, J. N-acetylcysteine rescues meiotic arrest during spermatogenesis in mice exposed to BDE-209. Environ. Sci. Pollut. Res. 2023, 30, 50952–50968. [Google Scholar] [CrossRef]
- Hombría, J.C.; Brown, S. The fertile field of Drosophila Jak/STAT signalling. Curr. Biol. 2002, 12, R569–R575. [Google Scholar] [CrossRef]
- Singh, S.R.; Chen, X.; Hou, S.X. JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophila. Cell Res. 2005, 15, 1–5. [Google Scholar] [CrossRef]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef]
- Wu, S.F.; Ga, Y.; Ma, D.Y.; Hou, S.L.; Hui, Q.Y.; Hao, Z.H. The role of ferroptosis in environmental pollution-induced male reproductive system toxicity. Environ. Pollut. 2024, 363 Pt 1, 125118. [Google Scholar] [CrossRef]
- Sun, T.C.; Li, D.M.; Yu, H.; Song, L.L.; Jia, Y.J.; Lin, L.; Zhou, S.J. Bilateral varicocele leads to ferroptosis, pyroptosis and necroptosis of human spermatozoa and affects semen quality in infertile men. Front. Cell Dev. Biol. 2023, 11, 1091438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Zhang, M.; OuYang, H.; Li, M.; Jia, D.; Wang, R.; Zhou, W.; Liu, H.; Hu, Y.; et al. Cadmium exposure during puberty damages testicular development and spermatogenesis via ferroptosis caused by intracellular iron overload and oxidative stress in mice. Environ. Pollut. 2023, 325, 121434. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, J.; Wang, X.; Zhang, Y.; Wang, M.; Su, P. Cadmium attenuates testosterone synthesis by promoting ferroptosis and blocking autophagosome-lysosome fusion. Free Radic. Biol. Med. 2021, 176, 176–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Cui, J.G.; Wang, J.X.; Chen, M.S.; Wang, H.R.; Li, X.N.; Li, J.L. Ferroptosis is critical for phthalates driving the blood-testis barrier dysfunction via targeting transferrin receptor. Redox Biol. 2023, 59, 102584. [Google Scholar] [CrossRef]
- Barata, J.T.; Silva, A.; Brandao, J.G.; Nadler, L.M.; Cardoso, A.A.; Boussiotis, V.A. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004, 200, 659–669. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, L.; Chen, M.; Gui, M.; Lu, L.; Deng, F.; Ren, Z. IgA1 isolated from Henoch-Schönlein purpura children promotes proliferation of human mesangial cells in vitro. Cell Biol. Int. 2019, 43, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Huang, W.; Quan, C.; Duan, P.; Tang, S.; Chen, W.; Yang, K. Nonylphenol induced apoptosis and autophagy involving the Akt/mTOR pathway in prepubertal Sprague-Dawley male rats in vivo and in vitro. Toxicology 2016, 373, 41–53. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Zhang, X.; Shi, X.; Gong, D. The antagonistic effect of melatonin on TBBPA-induced apoptosis and necroptosis via PTEN/PI3K/AKT signaling pathway in swine testis cells. Environ. Toxicol. 2022, 37, 2281–2290. [Google Scholar] [CrossRef]
- Yao, W.; Lin, Z.; Shi, P.; Chen, B.; Wang, G.; Huang, J.; Sui, Y.; Liu, Q.; Li, S.; Lin, X.; et al. Delicaflavone induces ROS-mediated apoptosis and inhibits PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in colorectal cancer cells. Biochem. Pharmacol. 2020, 171, 113680. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J.; Sun, X.; Zhou, Z.; Zhu, Q. ROS accumulation contributes to abamectin-induced apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway in TM3 Leydig cells. J. Biochem. Mol. Toxicol. 2020, 34, e22505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Shi, X.; Xu, S. Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J. Hazard. Mater. 2020, 398, 122905. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pei, H.; Chen, W.; Zhu, X.; Wang, Y.; Li, J.; He, Z.; Du, R. Evaluating the effect of ginsenoside Rg1 on CPF-induced brain injury in mice via PI3k/AKT pathway. J. Biochem. Mol. Toxicol. 2023, 37, e23319. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Ubaid Ur Rahman, H.; Asghar, W.; Nazir, W.; Sandhu, M.A.; Ahmed, A.; Khalid, N. A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: Evidence of mechanisms, exposures and mitigation strategies. Sci. Total Environ. 2021, 755, 142649. [Google Scholar] [CrossRef]
- Hazarika, J.; Ganguly, M.; Borgohain, G.; Sarma, S.; Bhuyan, P.; Mahanta, R. Disruption of androgen receptor signaling by chlorpyrifos (CPF) and its environmental degradation products: A structural insight. J. Biomol. Struct. Dyn. 2022, 40, 6027–6038. [Google Scholar] [CrossRef]
- Niki, T.; Takahashi-Niki, K.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. DJBP: A novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol. Cancer Res. MCR 2003, 1, 247–261. [Google Scholar]
- Zhang, S.; Mo, J.; Wang, Y.; Ni, C.; Li, X.; Zhu, Q.; Ge, R.S. Endocrine disruptors of inhibiting testicular 3β-hydroxysteroid dehydrogenase. Chem.-Biol. Interact. 2019, 303, 90–97. [Google Scholar] [CrossRef]

| Gene Names | Forward Primers (5′ to 3′) | Reverse Primers (5′ to 3′) | Production Sizes (bp) |
|---|---|---|---|
| P21 | CCTGGTGATGTCCGACCTG | CCATGAGCGCATCGCAATC | 103 |
| Ccnd | TGCTGCAAATGGAACTGCTT | CCACAAAGGTCTGTGCATGCT | 150 |
| Ccne | GTGGCTCCGACCTTTCAGTC | CACAGTCTTGTCAATCTTGGCA | 101 |
| Ccna | GCCTTCACCATTCATGTGGAT | TTGCTGCGGGTAAAGAGACAG | 118 |
| Ccnb | AAGGTGCCTGTGTGTGAACC | GTCAGCCCCATCATCTGCG | 228 |
| Efcab6 | CACCCTGAAAAGCAACACGG | TTAGCCTCCCCTTGGCATTG | 131 |
| Cmpk2 | TTCTGAGGAGAGAGTGCGGA | AGATGGCAGCTTGGGTTCTC | 138 |
| Zbp1 | AAGAGTCCCCTGCGATTATTTG | TCTGGATGGCGTTTGAATTGG | 102 |
| Ifi44l | GCTGTGTGATTCAATGGGGC | GCTCACAGGGGTTGAACTGA | 116 |
| Igf2bp3 | GCTACGCGTTCGTGGACT | GTGGGGCGGGATATTTCGT | 161 |
| Jak1 | GCCAGTGCCCTGAGTTACTT | TGTCTGGATCTTGCCTGGTC | 166 |
| Jak2 | GGAATGGCCTGCCTTACAATG | TGGCTCTATCTGCTTCACAGAAT | 108 |
| Jak3 | GATCTGCAAGGGCATGGAGT | ACTCCGGGGCATACCAAAAG | 197 |
| Stat1 | TCACAGTGGTTCGAGCTTCAG | CGAGACATCATAGGCAGCGTG | 150 |
| Stat2 | AGAAGTCCTGCATTGGAGCC | TTCAGTAGCTGCCGAAGGTG | 106 |
| Vdac1 | GAGTATGGGCTGACGTTTACAG | GAGCTTCAGTCCACGAGCAAG | 96 |
| Cox4i1 | ATTGGCAAGAGAGCCATTTCTAC | TGGGGAAAGCATAGTCTTCACT | 82 |
| β-Actin | CCTAAGGCCAACCGTGAAA | TGGTACGACCAGAGGCATA | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, M.; Wang, C.; Song, Q.; Yang, H.; Tang, Q.; Zhao, Q.; Wang, J.; Pan, C. CPF Induces GC2spd Cell Injury via ROS/AKT/Efcab6 Pathway. Cells 2025, 14, 940. https://doi.org/10.3390/cells14130940
Zhang X, Zhang M, Wang C, Song Q, Yang H, Tang Q, Zhao Q, Wang J, Pan C. CPF Induces GC2spd Cell Injury via ROS/AKT/Efcab6 Pathway. Cells. 2025; 14(13):940. https://doi.org/10.3390/cells14130940
Chicago/Turabian StyleZhang, Xuelian, Mengyang Zhang, Chunzhi Wang, Qingchuan Song, Haiyan Yang, Qi Tang, Qiaoling Zhao, Jing Wang, and Chuanying Pan. 2025. "CPF Induces GC2spd Cell Injury via ROS/AKT/Efcab6 Pathway" Cells 14, no. 13: 940. https://doi.org/10.3390/cells14130940
APA StyleZhang, X., Zhang, M., Wang, C., Song, Q., Yang, H., Tang, Q., Zhao, Q., Wang, J., & Pan, C. (2025). CPF Induces GC2spd Cell Injury via ROS/AKT/Efcab6 Pathway. Cells, 14(13), 940. https://doi.org/10.3390/cells14130940





