Therapeutic Horizons: Gut Microbiome, Neuroinflammation, and Epigenetics in Neuropsychiatric Disorders
Abstract
1. Introduction
2. Neuroinflammation in the Pathogenesis of Neuropsychiatric Disorders
3. The Gut Microbiome May Orchestrate the Function and Behavior of Immune System
4. Epigenetic Alterations Linked to Immune System and Immune-Relevant Genes in Neuropsychiatric Disorders
4.1. DNA Methylation Patterns
4.2. Histone Modifications
4.3. MicroRNAs (miRNAs)
| Neuropsychiatric Disorders/Study Subjects | Epigenetic Study/Samples | Immune-Relevant Genes | Key Findings | Ref. |
|---|---|---|---|---|
| SCZ/human | Histone acetylation/peripheral blood mononuclear cells (PBMCs) | IL-6 and IFN-γ | Histone H4 hypoacetylation in PBMCs decreases IL-6 and IFN-γ following 90 days exercise in SCZ vs. day 0. | [99] |
| MDD/human | Histone acetylation/dentate gyrus | ISG15, IFI44L, IFI6, NR4A1/Nur-77, GABBR1, CCL2/MCP-1, KANSL1 | Differential expression of 30 genes involved in histone acetylation and inflammation (e.g., CCL2 and KANSL1 in MDD) | [100] |
| MDD/human | Histone methylation/peripheral blood cells (PBCs) | TNFAIP3, TLR4, TNIP2, miR-146a, miR-155 | Lower histone 3 lysine 4 tri-methylation (H3K4me3) levels at the promoters of TNFAIP3, TLR4, TNIP2, miR-146a, and miR-155 in MDD | [101] |
| Alzheimer’s disease (AD)/APP/PS1 mice | Histone acetylation/Entorhinal Cortex | CREB, IL-1β, and TNF-α and NF-kB | Reduced H3K9K14 acetylation, increasing Aβ deposition, microglia and astrocytes activation, and inflammatory factors via the CREB/BDNF/NF-kB pathway | [102] |
| Postpartum psychosis/human | miR-146a and miR-212/monocytes | ADAM17, EGR3, IRAK2, PTGS2, CXCL2, and PTGS2 | Reduced miR-146a expression in monocytes diminishes natural T regulator cells; reduced expression of miR-212 elevated Adrenomedulin, reduced IL-6, and increased Th2 cells | [103] |
| SCZ/human | miR-337-3p, miR-127-5p, miR-206, miR-1185-1-3p/human iPSC-derived astrocytes from SCZ patients | IL-1β, LAMTOR4, IL23R, ERBB3, ERBB2, and IRAK1 | Lower expression of miR-337-3p, miR-127-5p, miR-206, and miR-1185-1-3p in SCZ astrocytes | [104] |
| SCZ/human | hsa-miR-16-5p, hsa-miR-186-5p, hsa-miR-19a-3p, and hsa-miR-19b-3p/blood | IL-1β, IL-6, and TNFα | Higher PANSS scores is linked to down-regulation of four miRNAs that negatively regulate pro-inflammatory cytokines | [105] |
| Bipolar Disorder (BD)/human | hsa-miR-34a-5p, hsa-miR-152-3p hsa-miR-574-3p, hsa-miR-3128 and hsa-miR-3201/Lymphoblastoid cell lines | NF-κB, STAT3, and TNF | 46 up-regulated and 31 down-regulated miRNAs with immune-related functions in responders vs. non-responders to Lithium in BD | [106] |
| MDD/human | let-7e, miR-21-5p miR-145, miR-223, miR-146a, and miR-155/PBMCs and monocytes | TLR4 | Lower levels of let-7e, miR-146a, and miR-155 in PBMCs and miR-146a and miR-155 in monocytes in MDD vs. controls | [107] |
| MDD/human | miRNA-144-5p/plasma | CXCL6, STAMPB, CXCL1, CXCL5, IL-7, MCP-4, MCP-2, MCP_1, MMP-1, IL-8, and IL-18 | An inverse correlation between miR-144-5p and some inflammatory proteins | [108] |
| MDD/human | miR-342, miR-146a, and miR-155/PBMCs and plasma | TNF-α, IL-6, and CCL2 (plasma) | Positive correlation between miR-342 expression and TNF-α level | [109] |
| Parkinson’s disease (PD)/mice | miR-335/serum samples | LRRK2 | Mitigating neuroinflammation by miR-335 via targeting LRRK2 | [110] |
| Autism/mice | miRNA profiling/prefrontal cortex (PFC) | NF-κB, IRAK1, and TLR7 | Neuroinflammation is linked to miR-146a, let- 7b, and miR-592 | [111] |
| AD/mice | Several miRNAs/PFC and the hippocampus | Cst7 and Gfap | Neuroinflammation is linked to miR-124-3p, miR-125b-5p, miR-21-5p, miR-146a-5p, and miR-155-5p | [112] |
5. Therapeutic Approach in Neuroinflammatory Diseases
5.1. Probiotics
5.2. Prebiotics/Postbiotics
5.3. Methyl Rich Diets and Inflammatory Responses
5.4. Modified Mediterranean Diet and Ketogenic Diet
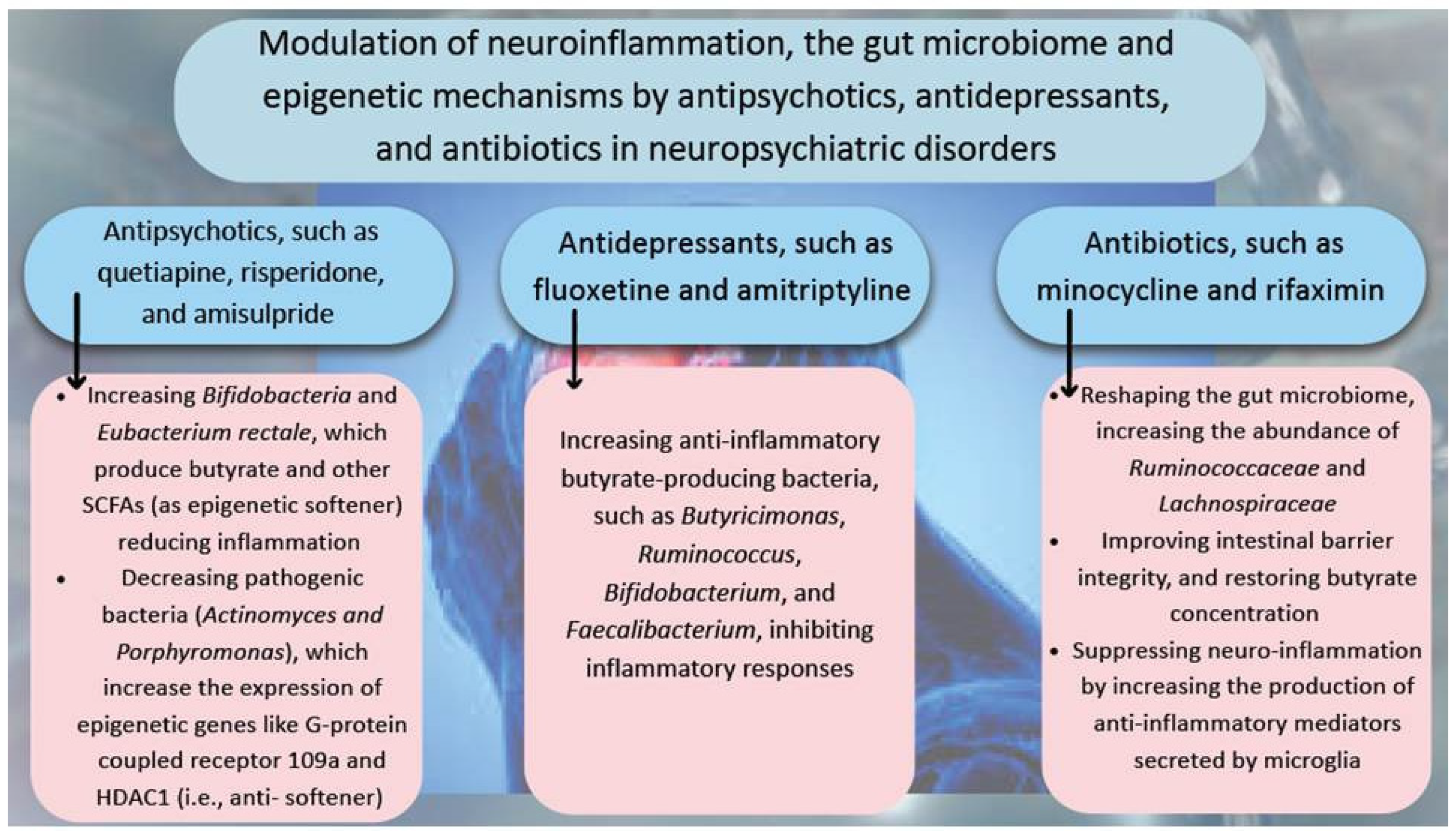
5.5. Immunomodulatory Effects of Different Drugs in the Treatment of Neuropsychiatric Disorders by Targeting GM via Epigenetic Mechanisms
5.5.1. Psychiatric Medications That Influence GM, Inflammation and the Epigenome
5.5.2. Antidepressant and Antibiotic Medications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuo, Y.; Wei, D.; Zhu, C.; Naveed, O.; Hong, W.; Yang, X. Unveiling the pathogenesis of psychiatric disorders using network models. Genes 2021, 12, 1101. [Google Scholar] [CrossRef]
- Ilieva, M.S. Non-coding RNAs in neurological and neuropsychiatric disorders: Unraveling the hidden players in disease pathogenesis. Cells 2024, 13, 1063. [Google Scholar] [CrossRef]
- Morozova, A.; Zorkina, Y.; Abramova, O.; Pavlova, O.; Pavlov, K.; Soloveva, K.; Volkova, M.; Alekseeva, P.; Andryshchenko, A.; Kostyuk, G.; et al. Neurobiological highlights of cognitive impairment in psychiatric disorders. Int. J. Mol. Sci. 2022, 23, 1217. [Google Scholar] [CrossRef]
- McGrath, T.; Baskerville, R.; Rogero, M.; Castell, L. Emerging evidence for the widespread role of glutamatergic dysfunction in neuropsychiatric diseases. Nutrients 2022, 14, 917. [Google Scholar] [CrossRef]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The role of the inflammasome in neurodegenerative diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. A Decade of Dedication: Pioneering Perspectives on Neurological Diseases and Mental Illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]
- Łoś, K.; Waszkiewicz, N. Biological markers in anxiety disorders. J. Clin. Med. 2021, 10, 1744. [Google Scholar] [CrossRef]
- Cătălina, G.R.; Gheorman, V.; Gheorman, V.; Forțofoiu, M.-C. The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases. Healthcare 2025, 13, 837. [Google Scholar] [CrossRef]
- Schmidt-Morgenroth, I.; Michaud, P.; Gasparini, F.; Avrameas, A. Central and peripheral inflammation in mild cognitive impairment in the context of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 10523. [Google Scholar] [CrossRef]
- Upthegrove, R.; Khandaker, G.M. Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Neuroinflamm. Schizophr. 2019, 44, 49–66. [Google Scholar]
- Balõtšev, R.; Koido, K.; Vasar, V.; Janno, S.; Kriisa, K.; Mahlapuu, R.; Ljubajev, U.; Parksepp, M.; Veiksaar, P.; Volke, V.; et al. Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur. Psychiatry 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Lussier, A.A.; Bodnar, T.S.; Weinberg, J. Intersection of epigenetic and immune alterations: Implications for fetal alcohol spectrum disorder and mental health. Front. Neurosci. 2021, 15, 788630. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. An overview of the epigenetic modifications in the brain under normal and pathological conditions. Int. J. Mol. Sci. 2024, 25, 3881. [Google Scholar] [CrossRef]
- Scholz, R.; Brösamle, D.; Yuan, X.; Beyer, M.; Neher, J.J. Epigenetic control of microglial immune responses. Immunol. Rev. 2024, 323, 209–226. [Google Scholar] [CrossRef]
- Cabanel, M.; Brand, C.; Oliveira-Nunes, M.C.; Cabral-Piccin, M.P.; Lopes, M.F.; Brito, J.M.; de Oliveira, F.L.; El-Cheikh, M.C.; Carneiro, K. Epigenetic control of macrophage shape transition towards an atypical elongated phenotype by histone deacetylase activity. PLoS ONE 2015, 10, e0132984. [Google Scholar] [CrossRef]
- Peedicayil, J. Genome–environment interactions and psychiatric disorders. Biomedicines 2023, 11, 1209. [Google Scholar] [CrossRef]
- Bekdash, R.A. Epigenetics, nutrition, and the brain: Improving mental health through diet. Int. J. Mol. Sci. 2024, 25, 4036. [Google Scholar] [CrossRef]
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the role of the human gut Microbiome in Health and diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. The profound influence of gut microbiome and extracellular vesicles on animal health and disease. Int. J. Mol. Sci. 2024, 25, 4024. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Lopizzo, N.; Marizzoni, M.; Begni, V.; Mazzelli, M.; Provasi, S.; Borruso, L.; Riva, M.A.; Cattaneo, A. Social isolation in adolescence and long-term changes in the gut microbiota composition and in the hippocampal inflammation: Implications for psychiatric disorders–Dirk Hellhammer Award Paper 2021. Psychoneuroendocrinology 2021, 133, 105416. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Kowalski, K.; Szponar, B.; Bochen, P.; Żebrowska-Różańska, P.; Łaczmański, Ł.; Samochowiec, J.; Misiak, B. Altered levels of fecal short-chain fatty acids are associated with subclinical inflammation and worse cognitive performance in patients with schizophrenia. J. Psychiatr. Res. 2023, 165, 298–304. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, C.-C.; Liao, H.-Y.; Lin, Y.-T.; Wu, Y.-W.; Liou, J.-M.; Wu, M.-S.; Kuo, C.-H.; Lin, C.-H. Association of fecal and plasma levels of short-chain fatty acids with gut microbiota and clinical severity in patients with Parkinson disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and functional dysbiosis of fecal microbiota in Chinese patients with Alzheimer’s disease. Front. Cell Dev. Biol. 2021, 8, 634069. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, Y. Peripheral inflammation is a potential etiological factor in Alzheimer’s disease. Rev. Neurosci. 2024, 35, 99–120. [Google Scholar] [CrossRef]
- Zhao, Y.; Sharfman, N.M.; Jaber, V.R.; Lukiw, W.J. Down-regulation of essential synaptic components by GI-tract microbiome-derived lipopolysaccharide (LPS) in LPS-treated human neuronal-glial (HNG) cells in primary culture: Relevance to Alzheimer’s disease (AD). Front. Cell Neurosci. 2019, 13, 314. [Google Scholar] [CrossRef]
- Kouli, A.; Spindler, L.R.; Fryer, T.D.; Hong, Y.T.; Malpetti, M.; Aigbirhio, F.I.; White, S.R.; Camacho, M.; O’Brien, J.T.; Williams-Gray, C.H. Neuroinflammation is linked to dementia risk in Parkinson’s disease. Brain 2024, 147, 923–935. [Google Scholar] [CrossRef]
- Nascimento, C.; Nunes, P.V.; Leite, R.E.P.; Grinberg, L.T.; Suemoto, C.K.; Lafer, B. The relationship of neuropsychiatric symptoms with inflammatory markers in the hippocampus and cingulate cortex of bipolar disorder subjects: A post-mortem study. J. Psychiatr. Res. 2024, 173, 25–33. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Piehl, N.; Simonton, B.; Parikh, M.; Zhang, Z.; Teregulova, V.; van Olst, L.; Gate, D. Epigenetic dysregulation in Alzheimer’s disease peripheral immunity. Neuron 2024, 112, 1235–1248.e1235. [Google Scholar] [CrossRef]
- Murphy, C.E.; Walker, A.K.; O’Donnell, M.; Galletly, C.; Lloyd, A.R.; Liu, D.; Weickert, C.S.; Weickert, T.W. Peripheral NF-κB dysregulation in people with schizophrenia drives inflammation: Putative anti-inflammatory functions of NF-κB kinases. Transl. Psychiatry 2022, 12, 21. [Google Scholar] [CrossRef]
- Gong, H.; Lu, Y.; Deng, S.-L.; Lv, K.-Y.; Luo, J.; Luo, Y.; Du, Z.-L.; Wu, L.-F.; Liu, T.-Y.; Wang, X.-Q.; et al. Targeting S100A9 attenuates social dysfunction by modulating neuroinflammation and myelination in a mouse model of autism. Pharmacol. Res. 2025, 211, 107568. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Alam, R.; Nohesara, S.; Deth, R.C.; Zhou, J.-R. iPSC-Derived Astrocytes and Neurons Replicate Brain Gene Expression, Epigenetic, Cell Morphology and Connectivity Alterations Found in Autism. Cells 2024, 13, 1095. [Google Scholar] [CrossRef]
- Megagiannis, P.; Mei, Y.; Yan, R.E.; Yuan, L.; Wilde, J.J.; Eckersberg, H.; Suresh, R.; Tan, X.; Chen, H.; Farmer, W.T.; et al. Autism-associated CHD8 controls reactive gliosis and neuroinflammation via remodeling chromatin in astrocytes. Cell Rep. 2024, 43, 114637. [Google Scholar] [CrossRef]
- Zhao, T.; Piao, L.-H.; Li, D.-P.; Xu, S.-H.; Wang, S.-Y.; Yuan, H.-B.; Zhang, C.-X. BDNF gene hydroxymethylation in hippocampus related to neuroinflammation-induced depression-like behaviors in mice. J. Affect. Disord. 2023, 323, 723–730. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Guan, X.; Li, Z. The neutrophil-to-Lymphocyte ratio is associated with clinical symptoms in first-episode medication-naïve patients with schizophrenia. Schizophrenia 2024, 10, 13. [Google Scholar] [CrossRef]
- Mendez-Victoriano, G.; Zhu, Y.; Middleton, F.; Massa, P.T.; Ajulu, K.; Webster, M.J.; Weickert, C.S. Increased Parenchymal Macrophages are associated with decreased Tyrosine Hydroxylase mRNA levels in the Substantia Nigra of people with Schizophrenia and Bipolar Disorder. Psychiatry Res. 2024, 340, 116141. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Sarawi, W.; Attia, S.M.; Bakheet, S.A.; Alqarni, S.A.; Ali, N.; AsSobeai, H.M. Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Mol. Immunol. 2022, 141, 297–304. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, W.; Wei, H.; Dean, M.N.; Standaert, D.G.; Cutter, G.R.; Benveniste, E.N.; Qin, H. Dysregulation of the adaptive immune system in patients with early-stage Parkinson disease. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1036. [Google Scholar] [CrossRef]
- Jiang, S.-S.; Wang, Y.-L.; Xu, Q.-H.; Gu, L.-Y.; Kang, R.-Q.; Yang, W.-Y.; Zhang, B.-R.; Tian, J.; Pu, J.-L. Cytokine and chemokine map of peripheral specific immune cell subsets in Parkinson’s disease. npj Park. Dis. 2023, 9, 117. [Google Scholar] [CrossRef]
- Deecke, L.; Goldeck, D.; Ohlei, O.; Homann, J.; Demuth, I.; Bertram, L.; Pawelec, G.; Lill, C.M. Immune cell distributions in the blood of healthy individuals at high genetic risk of Parkinson’s disease. Int. J. Mol. Sci. 2024, 25, 13655. [Google Scholar] [CrossRef]
- Lu, Y.; Li, K.; Hu, Y.; Wang, X. Expression of immune related genes and possible regulatory mechanisms in Alzheimer’s disease. Front. Immunol. 2021, 12, 768966. [Google Scholar] [CrossRef]
- Xu, H.; Jia, J. Single-cell RNA sequencing of peripheral blood reveals immune cell signatures in Alzheimer’s disease. Front. Immunol. 2021, 12, 645666. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, F.; Zhou, Y.; Wu, B.; Li, C. Peripheral immune cells contribute to the pathogenesis of Alzheimer’s disease. Mol. Neurobiol. 2025, 62, 264–270. [Google Scholar] [CrossRef]
- Rachayon, M.; Jirakran, K.; Sodsai, P.; Sughondhabirom, A.; Maes, M. T cell activation and deficits in T regulatory cells are associated with major depressive disorder and severity of depression. Sci. Rep. 2024, 14, 11177. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Sanmarco, L.M.; Wheeler, M.A.; Gutiérrez-Vázquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-licensed IFNγ+ NK cells drive LAMP1+ TRAIL+ anti-inflammatory astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef]
- Noe, J.T.; Rendon, B.E.; Geller, A.E.; Conroy, L.R.; Morrissey, S.M.; Young, L.E.; Bruntz, R.C.; Kim, E.J.; Wise-Mitchell, A.; Rizzo, M.B.d.S.; et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci. Adv. 2021, 7, eabi8602. [Google Scholar] [CrossRef]
- Ling, Z.; Jin, G.; Yan, X.; Cheng, Y.; Shao, L.; Song, Q.; Liu, X.; Zhao, L. Fecal dysbiosis and immune dysfunction in Chinese elderly patients with schizophrenia: An observational study. Front. Cell Infect. Microbiol. 2022, 12, 886872. [Google Scholar] [CrossRef]
- Huang, T.; Shang, Y.; Dai, C.; Zhang, Q.; Hu, S.; Xie, J. Gut microbiota and its relation to inflammation in patients with bipolar depression: A cross-sectional study. Ann. Gen. Psychiatry 2023, 22, 21. [Google Scholar] [CrossRef]
- Hu, S.; Li, A.; Huang, T.; Lai, J.; Li, J.; Sublette, M.E.; Lu, H.; Lu, Q.; Du, Y.; Hu, Z. Gut microbiota changes in patients with bipolar depression. Adv. Sci. 2019, 6, 1900752. [Google Scholar] [CrossRef]
- Li, Z.; Tao, X.; Wang, D.; Pu, J.; Liu, Y.; Gui, S.; Zhong, X.; Yang, D.; Zhou, H.; Tao, W.; et al. Alterations of the gut microbiota in patients with schizophrenia. Front. Psychiatry 2024, 15, 1366311. [Google Scholar] [CrossRef]
- Nie, S.; Wang, J.; Deng, Y.; Ye, Z.; Ge, Y. Inflammatory microbes and genes as potential biomarkers of Parkinson’s disease. npj Biofilm. Microbiomes 2022, 8, 101. [Google Scholar] [CrossRef]
- Kozhakhmetov, S.; Kaiyrlykyzy, A.; Jarmukhanov, Z.; Vinogradova, E.; Zholdasbekova, G.; Alzhanova, D.; Kunz, J.; Kushugulova, A.; Askarova, S. Inflammatory Manifestations Associated with Gut Dysbiosis in Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2024, 2024, 9741811. [Google Scholar] [CrossRef]
- Donaldson, A.I.; Fyfe, C.L.; Martin, J.C.; Smith, E.E.; Horgan, G.W.; Myint, P.K.; Johnstone, A.M.; Scott, K.P. Aging Gut-Brain Interactions: Pro-Inflammatory Gut Bacteria Are Elevated in Fecal Samples from Individuals Living with Alzheimer’s Dementia. Geriatrics 2025, 10, 37. [Google Scholar] [CrossRef]
- Krueger, M.E.; Boles, J.S.; Simon, Z.D.; Alvarez, S.D.; McFarland, N.R.; Okun, M.S.; Zimmermann, E.M.; Forsmark, C.E.; Tansey, M.G. Comparative analysis of Parkinson’s and inflammatory bowel disease gut microbiomes reveals shared butyrate-producing bacteria depletion. npj Park. Dis. 2025, 11, 50. [Google Scholar] [CrossRef]
- Cao, X.; Liu, K.; Liu, J.; Liu, Y.; Xu, L.; Wang, H.; Zhu, Y.; Wang, P.; Li, Z.; Wen, J.; et al. Dysbiotic gut microbiota and dysregulation of cytokine profile in children and teens with autism spectrum disorder. Front Neurosci. 2021, 15, 635925. [Google Scholar] [CrossRef]
- Ghorbani, M.; Joseph, G.B.S.; Mei, T.M.; Ramly, S.S.; Rasat, M.A.M.; Croft, L.; Parimannan, S.; Rajandas, H.; Lee, S.Y. Functional associations of the gut microbiome with dopamine, serotonin, and BDNF in schizophrenia: A pilot study. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 123. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Han, Y.; Liu, B.; Chen, S.; Ye, Z.; Li, J.; Xie, L.; Wu, X. Integrated Analysis of Gut Microbiome, Inflammation, and Neuroimaging Features Supports the Role of Microbiome–Gut–Brain Crosstalk in Schizophrenia. Schizophr. Bull. Open 2024, 5, sgae026. [Google Scholar] [CrossRef]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef]
- Coello, K.; Hansen, T.H.; Sørensen, N.; Munkholm, K.; Kessing, L.V.; Pedersen, O.; Vinberg, M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav. Immun. 2019, 75, 112–118. [Google Scholar] [CrossRef]
- Guo, Z.; Xiao, S.; Chen, G.; Zhong, S.; Zhong, H.; Sun, S.; Chen, P.; Tang, X.; Yang, H.; Jia, Y. Disruption of the gut microbiota-inflammation-brain axis in unmedicated bipolar disorder II depression. Transl. Psychiatry 2024, 14, 495. [Google Scholar] [CrossRef]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, Y.; Chen, F.; Yan, X.; Liu, X.; Shao, L.; Jin, G.; Zhou, D.; Jiang, G.; Li, H.; et al. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: A case-control study. Front. Immunol. 2022, 13, 964910. [Google Scholar] [CrossRef]
- Liu, P.; Gao, M.; Liu, Z.; Zhang, Y.; Tu, H.; Lei, L.; Wu, P.; Zhang, A.; Yang, C.; Li, G.; et al. Gut microbiome composition linked to inflammatory factors and cognitive functions in first-episode, drug-naive major depressive disorder patients. Front. Neurosci. 2022, 15, 800764. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Z.; Wang, J.; Wang, J.; Gao, M.; Zhang, Y.; Yang, C.; Zhang, A.; Li, G.; Li, X.; et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat. Commun. 2024, 15, 3003. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Z.; Yang, Z.; Liu, X.; Qian, X.; Zhu, J.; Hu, X.; Jiang, P.; Cui, T.; Wang, Y.; et al. Alterations in fecal microbiota composition and cytokine expression profiles in adolescents with depression: A case-control study. Sci. Rep. 2025, 15, 12177. [Google Scholar] [CrossRef]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.-C.; Ardura-Fabregat, A.; De Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Zhang, X.; Wang, J.; Tian, W.; Ren, Y.; Liu, Y.; Wang, T.; Li, Y.; Liu, Y. Butyrate alleviates alcoholic liver disease-associated inflammation through macrophage regulation and polarization via the HDAC1/miR-155 axis. Int. Immunopharmacol. 2024, 131, 111852. [Google Scholar] [CrossRef]
- Yu, H.; Li, R.; Liang, X.-j.; Yang, W.-M.; Guo, L.; Liu, L.; Tan, Q.-r.R.; Peng, Z.-w. A cross-section study of the comparison of plasma inflammatory cytokines and short-chain fatty acid in patients with depression and schizophrenia. BMC Psychiatry 2024, 24, 834. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Ehrlich, S.; Walton, E.; White, T.; Perrone-Bizzozero, N.; Bustillo, J.; Turner, J.A.; Calhoun, V.D. Methylation patterns in whole blood correlate with symptoms in schizophrenia patients. Schizophr. Bull. 2014, 40, 769–776. [Google Scholar] [CrossRef]
- Shindo, R.; Tanifuji, T.; Okazaki, S.; Otsuka, I.; Shirai, T.; Mouri, K.; Horai, T.; Hishimoto, A. Accelerated epigenetic aging and decreased natural killer cells based on DNA methylation in patients with untreated major depressive disorder. npj Aging 2023, 9, 19. [Google Scholar] [CrossRef]
- Luo, C.; Pi, X.; Hu, N.; Wang, X.; Xiao, Y.; Li, S.; Sweeney, J.A.; Bishop, J.R.; Gong, Q.; Xie, D.; et al. Subtypes of schizophrenia identified by multi-omic measures associated with dysregulated immune function. Mol. Psychiatry 2021, 26, 6926–6936. [Google Scholar] [CrossRef]
- Luo, C.; Pi, X.; Zhang, Q.; Hu, N.; Xiao, Y.; Sweeney, J.A.; Bishop, J.R.; Gong, Q.; Xie, D.; Lui, S. A subtype of schizophrenia patients with altered methylation level of genes related to immune cell activity. Psychol. Med. 2024, 54, 2538–2546. [Google Scholar] [CrossRef]
- Sabunciyan, S.; Maher, B.; Bahn, S.; Dickerson, F.; Yolken, R.H. Association of DNA methylation with acute mania and inflammatory markers. PLoS ONE 2015, 10, e0132001. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, J.; Tang, X.; Feng, X.; Yu, M.; Sha, W.; Wang, X.; Zhang, X.; Yi, H.; Zhang, X. DNA methylation and gene expression of the chemokine (CXC motif) ligand 1 in patients with deficit and non-deficit schizophrenia. Psychiatry Res. 2018, 268, 82–86. [Google Scholar] [CrossRef]
- Garcia-Ruiz, B.; Moreno, L.; Muntané, G.; Sánchez-Gistau, V.; Gutiérrez-Zotes, A.; Martorell, L.; Labad, J.; Vilella, E. Leukocyte and brain DDR1 hypermethylation is altered in psychosis and is correlated with stress and inflammatory markers. Epigenomics 2020, 12, 251–265. [Google Scholar] [CrossRef]
- Ni, C.; Jiang, W.; Wang, Z.; Wang, Z.; Zhang, J.; Zheng, X.; Liu, Z.; Ou, H.; Jiang, T.; Liang, W. LncRNA-AC006129. 1 reactivates a SOCS3-mediated anti-inflammatory response through DNA methylation-mediated CIC downregulation in schizophrenia. Mol. Psychiatry 2021, 26, 4511–4528. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, Y.; Palaniyappan, L.; Yao, Y.; Luo, Q.; Li, Y. Epigenetic profile of the immune system associated with symptom severity and treatment response in schizophrenia. J. Psychiatry Neurosci. 2024, 49, E45–E58. [Google Scholar] [CrossRef]
- Mirza, S.; Lima, C.N.C.; Del Favero-Campbell, A.; Rubinstein, A.; Topolski, N.; Cabrera-Mendoza, B.; Kovács, E.H.C.; Blumberg, H.P.; Richards, J.G.; Williams, A.J.; et al. Blood epigenome-wide association studies of suicide attempt in adults with bipolar disorder. Transl. Psychiatry 2024, 14, 70. [Google Scholar] [CrossRef]
- Garcia-Ruiz, B.; Jiménez, E.; Aranda, S.; Verdolini, N.; Gutiérrez-Zotes, A.; Sáez, C.; Losantos, E.; Alonso-Lana, S.; Fatjó-Vilas, M.; Sarró, S. Associations of altered leukocyte DDR1 promoter methylation and childhood trauma with bipolar disorder and suicidal behavior in euthymic patients. Mol. Psychiatry 2024, 29, 2478–2486. [Google Scholar] [CrossRef]
- Crawford, B.; Craig, Z.; Mansell, G.; White, I.; Smith, A.; Spaull, S.; Imm, J.; Hannon, E.; Wood, A.; Yaghootkar, H.; et al. DNA methylation and inflammation marker profiles associated with a history of depression. Hum. Mol. Genet. 2018, 27, 2840–2850. [Google Scholar] [CrossRef]
- Draganov, M.; Arranz, M.J.; Salazar, J.; de Diego-Adeliño, J.; Gallego-Fabrega, C.; Jubero, M.; Carceller-Sindreu, M.; Portella, M.J. Association study of polymorphisms within inflammatory genes and methylation status in treatment response in major depression. Eur. Psychiatry 2019, 60, 7–13. [Google Scholar] [CrossRef]
- Rasmusson, A.J.; Gallwitz, M.; Soltanabadi, B.; Ciuculete, D.M.; Mengel-From, J.; Christensen, K.; Nygaard, M.; Soerensen, M.; Boström, A.E.; Fredriksson, R.; et al. Toll-like receptor 4 methylation grade is linked to depressive symptom severity. Transl. Psychiatry 2021, 11, 371. [Google Scholar] [CrossRef]
- Han, K.-M.; Choi, K.W.; Kim, A.; Kang, W.; Kang, Y.; Tae, W.-S.; Han, M.-R.; Ham, B.-J. Association of DNA methylation of the NLRP3 gene with changes in cortical thickness in major depressive disorder. Int. J. Mol. Sci. 2022, 23, 5768. [Google Scholar] [CrossRef]
- Daily, K.P.; Badr, A.; Eltobgy, M.; Estfanous, S.; Whitham, O.; Tan, M.H.; Carafice, C.; Krause, K.; McNamara, A.; Hamilton, K.; et al. DNA hypomethylation promotes the expression of CASPASE-4 which exacerbates inflammation and amyloid-β deposition in Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 29. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Alshehri, S.; Alqarni, S.S.; Ahmad, S.F.; Alghibiwi, H.; Al-Harbi, N.O.; Alqarni, S.A.; Al-Ayadhi, L.Y.; Attia, S.M.; Alfardan, A.S.; et al. DNA hypomethylation is associated with increased inflammation in peripheral blood neutrophils of children with autism spectrum disorder: Understanding the role of ubiquitous pollutant Di (2-ethylhexyl) phthalate. Metabolites 2023, 13, 458. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Zheng, T.; Yang, X.; Liu, L.; Gao, S.-S.; Xu, H.-B.; Song, Y.-T.; Tong, K.; Yang, L.; Gao, Y.; et al. HDAC2 hyperexpression alters hippocampal neuronal transcription and microglial activity in neuroinflammation-induced cognitive dysfunction. J. Neuroinflamm. 2019, 16, 249. [Google Scholar] [CrossRef]
- Correa, F.; Mallard, C.; Nilsson, M.; Sandberg, M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: Restoring effects of inhibitors of HDACs, p38 MAPK and GSK3β. Neurobiol. Dis. 2011, 44, 142–151. [Google Scholar] [CrossRef]
- Rigillo, G.; Vilella, A.; Benatti, C.; Schaeffer, L.; Brunello, N.; Blom, J.M.; Zoli, M.; Tascedda, F. LPS-induced histone H3 phospho (Ser10)-acetylation (Lys14) regulates neuronal and microglial neuroinflammatory response. Brain Behav. Immun. 2018, 74, 277–290. [Google Scholar] [CrossRef]
- Rodriguez-Zas, S.L.; Wu, C.; Southey, B.R.; O’Connor, J.C.; Nixon, S.E.; Garcia, R.; Zavala, C.; Lawson, M.; McCusker, R.H.; Romanova, E.V.; et al. Disruption of microglia histone acetylation and protein pathways in mice exhibiting inflammation-associated depression-like symptoms. Psychoneuroendocrinology 2018, 97, 47–58. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Ni, J.; Cheng, J.; Jia, J.; Zhen, X. miRNA-3473b contributes to neuroinflammation following cerebral ischemia. Cell Death Dis. 2018, 9, 11. [Google Scholar] [CrossRef]
- Slota, J.A.; Booth, S.A. MicroRNAs in neuroinflammation: Implications in disease pathogenesis, biomarker discovery and therapeutic applications. Non-Coding RNA 2019, 5, 35. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An important regulator of neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Thomas, K.T.; Zakharenko, S.S. MicroRNAs in the Onset of Schizophrenia. Cells 2021, 10, 2679. [Google Scholar] [CrossRef]
- Ye, Y.; Hao, J.; Hong, Z.; Wu, T.; Ge, X.; Qian, B.; Chen, X.; Zhang, F. Downregulation of microRNA-145-5p in activated microglial exosomes promotes astrocyte proliferation by removal of Smad3 inhibition. Neurochem. Res. 2022, 47, 382–393. [Google Scholar] [CrossRef]
- Amoah, S.K.; Rodriguez, B.A.; Logothetis, C.N.; Chander, P.; Sellgren, C.M.; Weick, J.P.; Sheridan, S.D.; Jantzie, L.L.; Webster, M.J.; Mellios, N. Exosomal secretion of a psychosis-altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacology 2020, 45, 656–665. [Google Scholar] [CrossRef]
- Kaurani, L.; Islam, M.R.; Heilbronner, U.; Krüger, D.M.; Zhou, J.; Methi, A.; Strauss, J.; Pradhan, R.; Schröder, S.; Burkhardt, S.; et al. Regulation of Zbp1 by miR-99b-5p in microglia controls the development of schizophrenia-like symptoms in mice. EMBO J. 2024, 43, 1420–1444. [Google Scholar] [CrossRef]
- Lavratti, C.; Dorneles, G.; Pochmann, D.; Peres, A.; Bard, A.; Schipper, L.d.L.; Lago, P.D.; Wagner, L.C.; Elsner, V.R. Exercise-induced modulation of histone H4 acetylation status and cytokines levels in patients with schizophrenia. Physiol. Behav. 2017, 168, 84–90. [Google Scholar] [CrossRef]
- Mahajan, G.J.; Vallender, E.J.; Garrett, M.R.; Challagundla, L.; Overholser, J.C.; Jurjus, G.; Dieter, L.; Syed, M.; Romero, D.G.; Benghuzzi, H.; et al. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 177–186. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Wang, S.-C.; Yang, Y.-C.; Fu, H.-C.; Chou, C.-K.; Kang, H.-Y.; Hung, Y.-Y. Aberrant histone modification of TNFAIP3, TLR4, TNIP2, miR-146a, and miR-155 in major depressive disorder. Mol. Neurobiol. 2023, 60, 4753–4760. [Google Scholar] [CrossRef]
- Wang, C.; Shen, D.; Hu, Y.; Chen, J.; Liu, J.; Huang, Y.; Yu, X.; Chu, H.; Zhang, C.; Yin, L.; et al. Selective targeting of class I HDAC reduces microglial inflammation in the entorhinal cortex of young APP/PS1 mice. Int. J. Mol. Sci. 2023, 24, 4805. [Google Scholar] [CrossRef]
- Weigelt, K.; Bergink, V.; Burgerhout, K.M.; Pescatori, M.; Wijkhuijs, A.; Drexhage, H.A. Down-regulation of inflammation-protective microRNAs 146a and 212 in monocytes of patients with postpartum psychosis. Brain Behav. Immun. 2013, 29, 147–155. [Google Scholar] [CrossRef]
- Akkouh, I.A.; Hughes, T.; Steen, V.M.; Glover, J.C.; Andreassen, O.A.; Djurovic, S.; Szabo, A. Transcriptome analysis reveals disparate expression of inflammation-related miRNAs and their gene targets in iPSC-astrocytes from people with schizophrenia. Brain Behav. Immun. 2021, 94, 235–244. [Google Scholar] [CrossRef]
- Miyano, T.; Mikkaichi, T.; Nakamura, K.; Yoshigae, Y.; Abernathy, K.; Ogura, Y.; Kiyosawa, N. Circulating microRNA profiles identify a patient subgroup with high inflammation and severe symptoms in Schizophrenia experiencing Acute psychosis. Int. J. Mol. Sci. 2024, 25, 4291. [Google Scholar] [CrossRef]
- Cattane, N.; Courtin, C.; Mombelli, E.; Maj, C.; Mora, C.; Etain, B.; Bellivier, F.; Marie-Claire, C.; Cattaneo, A. Transcriptomics and miRNomics data integration in lymphoblastoid cells highlights the key role of immune-related functions in lithium treatment response in Bipolar disorder. BMC Psychiatry 2022, 22, 665. [Google Scholar] [CrossRef]
- Hung, Y.-Y.; Wu, M.-K.; Tsai, M.-C.; Huang, Y.-L.; Kang, H.-Y. Aberrant expression of intracellular let-7e, miR-146a, and miR-155 correlates with severity of depression in patients with major depressive disorder and is ameliorated after antidepressant treatment. Cells 2019, 8, 647. [Google Scholar] [CrossRef]
- Sundquist, K.; Memon, A.A.; Palmér, K.; Sundquist, J.; Wang, X. Inflammatory proteins and miRNA-144-5p in patients with depression, anxiety, or stress-and adjustment disorders after psychological treatment. Cytokine 2021, 146, 155646. [Google Scholar] [CrossRef]
- Brás, J.P.; Pinto, S.; von Doellinger, O.; Prata, J.; Coelho, R.; Barbosa, M.A.; Almeida, M.I.; Santos, S.G. Combining inflammatory miRNA molecules as diagnostic biomarkers for depression: A clinical study. Front. Psychiatry 2023, 14, 1227618. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Dionísio, P.A.; Gaspar, M.M.; Guedes, L.C.; Coelho, M.; Rosa, M.M.; Ferreira, J.J.; Amaral, J.D.; Rodrigues, C.M. miR-335 targets LRRK2 and mitigates inflammation in Parkinson’s disease. Front. Cell Dev. Biol. 2021, 9, 661461. [Google Scholar] [CrossRef]
- Mooney, C.; Parlante, A.; Canarutto, G.; Grigoli, A.; Scattoni, M.L.; Ricceri, L.; Jimenez-Mateos, E.M.; Sanz-Rodriguez, A.; Clementi, E.; Piazza, S.; et al. Deregulated mRNA and microRNA Expression Patterns in the Prefrontal Cortex of the BTBR Mouse Model of Autism. Mol. Neurobiol. 2025, 1–21. [Google Scholar] [CrossRef]
- Ianni, M.; Corraliza-Gomez, M.; Costa-Coelho, T.; Ferreira-Manso, M.; Inteiro-Oliveira, S.; Alemãn-Serrano, N.; Sebastião, A.M.; Garcia, G.; Diógenes, M.J.; Brites, D. Spatiotemporal Dysregulation of Neuron–Glia Related Genes and Pro-/Anti-Inflammatory miRNAs in the 5xFAD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 9475. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Borthakur, A.; Anbazhagan, A.N.; Kumar, A.; Raheja, G.; Singh, V.; Ramaswamy, K.; Dudeja, P.K. The probiotic Lactobacillus plantarum counteracts TNF-α-induced downregulation of SMCT1 expression and function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G928–G934. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Chen, Z.; Wang, Y.; Xu, F.; Kuang, Q.; Zhang, Y.; Zheng, H. Bacillus coagulans and Clostridium butyricum synergistically alleviate depression in a chronic unpredictable mild stress mouse model through altering gut microbiota and prefrontal cortex gene expression. Front. Pharmacol. 2024, 15, 1393874. [Google Scholar] [CrossRef]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.-A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Singh, D.; Singh, S.; Pinnaka, A.K.; Boparai, R.; Bishnoi, M.; Kondepudi, K.; Chopra, K. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef]
- Parra, I.; Martínez, I.; Vásquez-Celaya, L.; Gongora-Alfaro, J.L.; Tizabi, Y.; Mendieta, L. Neuroprotective and immunomodulatory effects of probiotics in a rat model of Parkinson’s disease. Neurotox. Res. 2023, 41, 187–200. [Google Scholar] [CrossRef]
- Valvaikar, S.; Vaidya, B.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Supplementation of probiotic Bifidobacterium breve Bif11 reverses neurobehavioural deficits, inflammatory changes and oxidative stress in Parkinson’s disease model. Neurochem. Int. 2024, 174, 105691. [Google Scholar] [CrossRef]
- Adıgüzel, E.; Çiçek, B.; Ünal, G.; Aydın, M.F.; Barlak-Keti, D. Probiotics and prebiotics alleviate behavioral deficits, inflammatory response, and gut dysbiosis in prenatal VPA-induced rodent model of autism. Physiol. Behav. 2022, 256, 113961. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary probiotic treatment and vitamin B7 in depression—A randomized controlled trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Huang, Y.-Y.; Tsai, S.-Y.; Kuo, Y.-W.; Lin, J.-H.; Ho, H.-H.; Chen, J.-F.; Hsia, K.-C.; Sun, Y. Efficacy of probiotic supplements on brain-derived neurotrophic factor, inflammatory biomarkers, oxidative stress and cognitive function in patients with Alzheimer’s dementia: A 12-week randomized, double-blind active-controlled study. Nutrients 2023, 16, 16. [Google Scholar] [CrossRef]
- Akhgarjand, C.; Vahabi, Z.; Shab-Bidar, S.; Anoushirvani, A.; Djafarian, K. The effects of probiotic supplements on oxidative stress and inflammation in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled study. Inflammopharmacology 2024, 32, 1413–1420. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. An update on prebiotics and on their health effects. Foods 2024, 13, 446. [Google Scholar] [CrossRef]
- Akram, W.; Garud, N.; Joshi, R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov. Ther. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef]
- Chen, P.; Li, X.; Yu, Y.; Zhang, J.; Zhang, Y.; Li, C.; Li, J.; Li, K. Administration Time and Dietary Patterns Modified the Effect of Inulin on CUMS-Induced Anxiety and Depression. Mol. Nutr. Food Res. 2023, 67, 2200566. [Google Scholar] [CrossRef]
- Lin, S.; Li, Q.; Xu, Z.; Chen, Z.; Tao, Y.; Tong, Y.; Wang, T.; Chen, S.; Wang, P. Detection of the role of intestinal flora and tryptophan metabolism involved in antidepressant-like actions of crocetin based on a multi-omics approach. Psychopharmacology 2022, 239, 3657–3677. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, P.; Zhang, X.; Yang, Y.; Yang, M.; Wang, T.; Lu, H.; Tian, H.; Wang, H.; Liu, J. Inulin ameliorates schizophrenia via modulation of the gut microbiota and anti-inflammation in mice. Food Funct. 2021, 12, 1156–1175. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Lan, Y.; Tuo, Y.; Ma, S.; Liu, X. Inulin attenuates blood–brain barrier permeability and alleviates behavioral disorders by modulating the TLR4/MyD88/NF-κB pathway in mice with chronic stress. J. Agric. Food Chem. 2023, 71, 13325–13337. [Google Scholar] [CrossRef]
- Szala-Rycaj, J.; Szewczyk, A.; Zagaja, M.; Kaczmarczyk-Ziemba, A.; Maj, M.; Andres-Mach, M. The Influence of topinambur and inulin preventive supplementation on microbiota, anxious behavior, cognitive functions and neurogenesis in mice exposed to the chronic unpredictable mild stress. Nutrients 2023, 15, 2041. [Google Scholar] [CrossRef]
- Zou, H.; Gao, H.; Liu, Y.; Zhang, Z.; Zhao, J.; Wang, W.; Ren, B.; Tan, X. Dietary inulin alleviated constipation induced depression and anxiety-like behaviors: Involvement of gut microbiota and microbial metabolite short-chain fatty acid. Int. J. Biol. Macromol. 2024, 259, 129420. [Google Scholar] [CrossRef]
- Buchanan, R.W.; Werkheiser, A.E.M.; Michel, H.B.; Zaranski, J.M.; Glassman, M.; Adams, H.A.P.; Vyas, G.D.; Blatt, F.; Pilli, N.R.; Pan, Y.; et al. Prebiotic Treatment in People With Schizophrenia. J. Clin. Psychopharmacol. 2024, 44, 457–461. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Y.; Cui, L.; Wang, Z.; Luo, Y.; Gao, C.; Ge, W.; Huang, T.; Wen, J.; Zhou, T. Butyrate emerges as a crucial effector of Zhi-Zi-Chi decoctions to ameliorate depression via multiple pathways of brain-gut axis. Biomed. Pharmacother. 2022, 149, 112861. [Google Scholar] [CrossRef]
- de Paiva, I.H.R.; Maciel, L.M.; da Silva, R.S.; Mendonça, I.P.; de Souza, J.R.B.; Peixoto, C.A. Prebiotics modulate the microbiota–gut–brain axis and ameliorate anxiety and depression-like behavior in HFD-fed mice. Food Res. Int. 2024, 182, 114153. [Google Scholar] [CrossRef]
- Xiong, L.; Mao, M.; Shu, Q. A preliminary study on the diversity of butyrate-producing bacteria in response to the treatment of depression with Xiaoyaosan. Lett. Appl. Microbiol. 2022, 75, 844–856. [Google Scholar] [CrossRef]
- Xiong, L.; Wu, Y.; Shu, Q.; Xiong, W. The pharmacological mechanism of Xiaoyaosan polysaccharide reveals improvement of CUMS-induced depression-like behavior by carbon source-triggered butyrate-producing bacteria. J. Appl. Microbiol. 2023, 134, lxad052. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Wang, C.; Wang, J.; Yan, W.; Luo, M.; Dong, J.; Li, X.; Liu, X.; Lan, Y. Raspberry Ketone Prevents LPS-Induced Depression-Like Behaviors in Mice by Inhibiting TLR-4/NF-κB Signaling Pathway via the Gut-Brain Axis. Mol. Nutr. Food Res. 2024, 68, 2400090. [Google Scholar] [CrossRef]
- Prince, N.; Marzal, L.N.P.; Markidi, A.; Ahmed, S.; Adolfs, Y.; Pasterkamp, R.J.; Kumar, H.; Roeselers, G.; Garssen, J.; Kraneveld, A.D.; et al. Prebiotic diet normalizes aberrant immune and behavioral phenotypes in a mouse model of autism spectrum disorder. Acta Pharmacol. Sin. 2024, 45, 1591–1603. [Google Scholar] [CrossRef]
- Sarti, G.; Traini, C.; Magni, G.; Attorre, S.; Tognozzi, G.; Calussi, E.; Giovannini, M.G.; Vannucchi, M.G.; Lana, D. Chronic administration of prebiotics and probiotics prevent pathophysiological hallmarks of Alzheimer’s disease in the cortex of APP/PS1 mice. Front. Pharmacol. 2025, 16, 1596469. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Romano, S.; Heinzmann, S.S.; Duncan, A.; Traka, M.H.; Ng, D.; Segovia-Lizano, D.; Simon, M.-C.; Narbad, A.; Wüllner, U.; et al. A prebiotic dietary pilot intervention restores faecal metabolites and may be neuroprotective in Parkinson’s Disease. npj Park. Dis. 2025, 11, 66. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bulotta, R.M.; Ruga, S.; Nucera, S.; Macrì, R.; Scarano, F.; Oppedisano, F.; Carresi, C.; Gliozzi, M.; Musolino, V.; et al. The postbiotic properties of butyrate in the modulation of the gut microbiota: The potential of its combination with polyphenols and dietary fibers. Int. J. Mol. Sci. 2024, 25, 6971. [Google Scholar] [CrossRef]
- Głowacka, P.; Oszajca, K.; Pudlarz, A.; Szemraj, J.; Witusik-Perkowska, M. Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases. Nutrients 2024, 16, 2244. [Google Scholar] [CrossRef]
- Guo, C.; Huo, Y.-J.; Li, Y.; Han, Y.; Zhou, D. Gut-brain axis: Focus on gut metabolites short-chain fatty acids. World J. Clin. Cases 2022, 10, 1754. [Google Scholar] [CrossRef]
- Stilling, R.M.; Van De Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Huuskonen, J.; Suuronen, T.; Nuutinen, T.; Kyrylenko, S.; Salminen, A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br. J. Pharmacol. 2004, 141, 874–880. [Google Scholar] [CrossRef]
- Wei, H.; Yu, C.; Zhang, C.; Ren, Y.; Guo, L.; Wang, T.; Chen, F.; Li, Y.; Zhang, X.; Wang, H.; et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed. Pharmacother. 2023, 160, 114308. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Dal-Pont, G.C.; Steckert, A.V.; Varela, R.B.; Lopes-Borges, J.; Mariot, E.; Resende, W.R.; Arent, C.O.; Carvalho, A.F.; Quevedo, J. Sodium butyrate has an antimanic effect and protects the brain against oxidative stress in an animal model of mania induced by ouabain. Psychiatry Res. 2016, 235, 154–159. [Google Scholar] [CrossRef]
- Kalkan, A.E.; BinMowyna, M.N.; Raposo, A.; Ahmad, M.F.; Ahmed, F.; Otayf, A.Y.; Carrascosa, C.; Saraiva, A.; Karav, S. Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review. Nutrients 2025, 17, 1305. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Fu, S.-P.; Wang, J.-F.; Xue, W.-J.; Liu, H.-M.; Liu, B.-R.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef]
- Guo, T.-T.; Zhang, Z.; Sun, Y.; Zhu, R.-Y.; Wang, F.-X.; Ma, L.-J.; Jiang, L.; Liu, H.-D. Neuroprotective effects of sodium butyrate by restoring gut microbiota and inhibiting TLR4 signaling in mice with MPTP-induced Parkinson’s disease. Nutrients 2023, 15, 930. [Google Scholar] [CrossRef]
- Moretti, M.; Valvassori, S.S.; Varela, R.B.; Ferreira, C.L.; Rochi, N.; Benedet, J.; Scaini, G.; Kapczinski, F.; Streck, E.L.; Zugno, A.I.; et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav. Pharmacol. 2011, 22, 766–772. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, R.; Ma, Y.; Li, Y.; Chen, Z.; He, H.; Chen, J.; Tong, L.; Huang, C.; You, Q. Lipopolysaccharide-induced depression-like behaviors is ameliorated by sodium butyrate via inhibiting neuroinflammation and oxido-nitrosative stress. Pharmacology 2020, 105, 550–560. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, X.; Shi, W.; Zhu, W.; Feng, X.; Xin, H.; Zhang, Y.; Cong, B.; Li, Y. The Gut Microbiota Metabolite Butyrate Modulates Acute Stress-Induced Ferroptosis in the Prefrontal Cortex via the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 1698. [Google Scholar] [CrossRef]
- Firoozi, D.; Masoumi, S.J.; Mohammad-Kazem Hosseini Asl, S.; Fararouei, M.; Jamshidi, S. Effects of Short Chain Fatty Acid-Butyrate Supplementation on the Disease Severity, Inflammation, and Psychological Factors in Patients With Active Ulcerative Colitis: A Double-Blind Randomized Controlled Trial. J. Nutr. Metab. 2025, 2025, 3165876. [Google Scholar] [CrossRef]
- Tong, L.-c.; Wang, Y.; Wang, Z.-b.; Liu, W.-y.; Sun, S.; Li, L.; Su, D.-f.; Zhang, L.-c. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. The anti-inflammatory and antioxidant effects of sodium propionate. Int. J. Mol. Sci. 2020, 21, 3026. [Google Scholar] [CrossRef]
- Dai, H.-y.; Zhang, Z.-x.; Tan, C.; Xian, X.; Ji, D.; Yang, J.; Sun, J.; Yao, H. Propionic acid ameliorates cognitive function through immunomodulatory effects on Th17 cells in perioperative neurocognitive disorders. Heliyon 2024, 10, e28817. [Google Scholar] [CrossRef]
- Hao, C.; Gao, Z.; Liu, X.; Rong, Z.; Jia, J.; Kang, K.; Guo, W.; Li, J. Intravenous administration of sodium propionate induces antidepressant or prodepressant effect in a dose dependent manner. Sci. Rep. 2020, 10, 19917. [Google Scholar] [CrossRef]
- Li, J.; Hou, L.; Wang, C.; Jia, X.; Qin, X.; Wu, C. Short term intrarectal administration of sodium propionate induces antidepressant-like effects in rats exposed to chronic unpredictable mild stress. Front. Psychiatry 2018, 9, 454. [Google Scholar] [CrossRef]
- Behrens, L.M.P.; Gasparotto, J.; Rampelotto, P.H.; Escalona, M.A.R.; Silva, L.d.S.d.; Carazza-Kessler, F.G.; Barbosa, C.P.; Campos, M.S.; Dorn, M.; Gelain, D.P. Sodium propionate oral supplementation ameliorates depressive-like behavior through gut microbiome and histone 3 epigenetic regulation. J. Nutr. Biochem. 2024, 130, 109660. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of folic acid and vitamin B12 supplementation on cognitive impairment and inflammation in patients with Alzheimer’s disease: A randomized, single-blinded, placebo-controlled trial. J. Prev. Alzheimer’s Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef]
- Shaikh, A.; Roy, H. Folate deprivation induced neuroinflammation impairs cognition. Neurosci. Lett. 2023, 807, 137264. [Google Scholar] [CrossRef]
- Śliwiński, W.; Gawlik-Kotelnicka, O. Circulating B vitamins metabolites in depressive disorders-connections with the microbiota-gut-brain axis. Behav. Brain Res. 2024, 472, 115145. [Google Scholar] [CrossRef]
- Menegas, S.; Dal-Pont, G.C.; Cararo, J.H.; Varela, R.B.; Aguiar-Geraldo, J.M.; Possamai-Della, T.; Andersen, M.L.; Quevedo, J.; Valvassori, S.S. Efficacy of folic acid as an adjunct to lithium therapy on manic-like behaviors, oxidative stress and inflammatory parameters in an animal model of mania. Metab. Brain Dis. 2020, 35, 413–425. [Google Scholar] [CrossRef]
- Khosravi, M.; Sotoudeh, G.; Amini, M.; Raisi, F.; Mansoori, A.; Hosseinzadeh, M. The relationship between dietary patterns and depression mediated by serum levels of Folate and vitamin B12. BMC Psychiatry 2020, 20, 63. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.; Wang, Y.; Ma, Y.; Chen, L.; Zhang, H.; Xue, M.; Liang, H. Folic acid rescues dopaminergic neurons in MPTP-induced mice by inhibiting the NLRP3 inflammasome and ameliorating mitochondrial impairment. J. Agric. Food Chem. 2024, 72, 5734–5745. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic acid supplementation mitigates Alzheimer’s disease by reducing inflammation: A randomized controlled trial. Mediat. Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef]
- Baker, J.A.; Bodnar, T.S.; Breit, K.R.; Weinberg, J.; Thomas, J.D. Choline supplementation alters hippocampal cytokine levels in adolescence and adulthood in an animal model of fetal alcohol spectrum disorders. Cells 2023, 12, 546. [Google Scholar] [CrossRef]
- Dave, N.; Judd, J.M.; Decker, A.; Winslow, W.; Sarette, P.; Espinosa, O.V.; Tallino, S.; Bartholomew, S.K.; Bilal, A.; Sandler, J.; et al. Dietary choline intake is necessary to prevent systems-wide organ pathology and reduce Alzheimer’s disease hallmarks. Aging Cell 2023, 22, e13775. [Google Scholar] [CrossRef]
- Egilmez, C.B.; Pazarlar, B.A.; Erdogan, M.A.; Uyanikgil, Y.; Erbas, O. Choline chloride shows gender-dependent positive effects on social deficits, learning/memory impairments, neuronal loss and neuroinflammation in the lipopolysaccharide-induced rat model of autism. Int. J. Dev. Neurosci. 2024, 84, 392–405. [Google Scholar] [CrossRef]
- Li, J.; Kang, X.; Zhang, L.; Luo, J.; Zhang, D. Dietary choline is inversely associated with depressive symptoms: A cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 2011 to 2018. J. Affect. Disord. 2022, 301, 23–29. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in inflammation: Mechanistic aspects and applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]
- Tolmunen, T.; Hintikka, J.; Voutilainen, S.; Ruusunen, A.; Alfthan, G.; Nyyssönen, K.; Viinamäki, H.; Kaplan, G.A.; Salonen, J.T. Association between depressive symptoms and serum concentrations of homocysteine in men: A population study. Am. J. Clin. Nutr. 2004, 80, 1574–1578. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, Y.; Chen, C.; Jing, T.; Hu, Y.; Xu, H.; Wang, S.; He, Y.; Liu, E.; Cui, J. Betaine supplementation alleviates dextran sulfate sodium-induced colitis via regulating the inflammatory response, enhancing the intestinal barrier, and altering gut microbiota. Food Funct. 2022, 13, 12814–12826. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, X.; Yi, Y.; Xie, L.; Zhou, W.; Cao, W.; Chen, L. Prophylactic Effects of Betaine on Depression and Anxiety Behaviors in Mice with Dextran Sulfate Sodium-Induced Colitis. J. Agric. Food Chem. 2024, 72, 21041–21051. [Google Scholar] [CrossRef]
- Hui, R.; Xu, J.; Zhou, M.; Xie, B.; Zhou, M.; Zhang, L.; Cong, B.; Ma, C.; Wen, D. Betaine improves METH-induced depressive-like behavior and cognitive impairment by alleviating neuroinflammation via NLRP3 inflammasome inhibition. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 135, 111093. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J. Betaine mitigates amyloid-β-associated neuroinflammation by suppressing the NLRP3 and NF-κB signaling pathways in microglial cells. J. Alzheimer’s Dis. 2023, 94, S9–S19. [Google Scholar] [CrossRef]
- Tidman, M.M.; White, D.R.; White, T.A. Impact of a keto diet on symptoms of Parkinson’s disease, biomarkers, depression, anxiety and quality of life: A longitudinal study. Neurodegener. Dis. Manag. 2024, 14, 97–110. [Google Scholar] [CrossRef]
- Ozan, E.; Chouinard, V.-A.; Palmer, C.M. The ketogenic diet as a treatment for mood disorders. Curr. Treat. Options Psychiatry 2024, 11, 163–176. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Chen, Y.; Gong, X.; Chen, Z.; Zhang, X. Ketogenic Diet and Gut Microbiota: Exploring New Perspectives on Cognition and Mood. Foods 2025, 14, 1215. [Google Scholar] [CrossRef]
- Joseph, J.; Depp, C.; Shih, P.-a.B.; Cadenhead, K.S.; Schmid-Schönbein, G. Modified mediterranean diet for enrichment of short chain fatty acids: Potential adjunctive therapeutic to target immune and metabolic dysfunction in schizophrenia? Front. Neurosci. 2017, 11, 155. [Google Scholar] [CrossRef]
- Mentzelou, M.; Dakanalis, A.; Vasios, G.K.; Gialeli, M.; Papadopoulou, S.K.; Giaginis, C. The relationship of ketogenic diet with neurodegenerative and psychiatric diseases: A scoping review from basic research to clinical practice. Nutrients 2023, 15, 2270. [Google Scholar] [CrossRef]
- Dąbek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, J.; Sun, J.; Liang, Q.; Zhan, Y.; Yang, Z.; Zhang, Y.; Jin, L.; Hu, C.; Zhao, Y.-T. Ketogenic diet attenuates neuroinflammation and restores hippocampal neurogenesis to improve CUMS induced depression-like behavior in mice. Food Funct. 2025, 16, 3408–3422. [Google Scholar] [CrossRef]
- Di Lucente, J.; Persico, G.; Zhou, Z.; Jin, L.-W.; Ramsey, J.J.; Rutkowsky, J.M.; Montgomery, C.M.; Tomilov, A.; Kim, K.; Giorgio, M.; et al. Ketogenic diet and BHB rescue the fall of long-term potentiation in an Alzheimer’s mouse model and stimulates synaptic plasticity pathway enzymes. Commun. Biol. 2024, 7, 195. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, X.; Cheng, Z.; Dong, Q.; Ruan, G. The Anti-Inflammatory Effect of Preventive Intervention with Ketogenic Diet Mediated by the Histone Acetylation of mGluR5 Promotor Region in Rat Parkinson’s Disease Model: A Dual-Tracer PET Study. Park. Dis. 2022, 2022, 3506213. [Google Scholar] [CrossRef]
- Allan, N.P.; Yamamoto, B.Y.; Kunihiro, B.P.; Nunokawa, C.K.L.; Rubas, N.C.; Wells, R.K.; Umeda, L.; Phankitnirundorn, K.; Torres, A.; Peres, R.; et al. Ketogenic diet induced shifts in the gut microbiome associate with changes to inflammatory cytokines and brain-related miRNAs in children with autism Spectrum disorder. Nutrients 2024, 16, 1401. [Google Scholar] [CrossRef]
- Schweickart, A.; Batra, R.; Neth, B.J.; Martino, C.; Shenhav, L.; Zhang, A.R.; Shi, P.; Karu, N.; Huynh, K.; Meikle, P.J.; et al. Serum and CSF metabolomics analysis shows Mediterranean Ketogenic Diet mitigates risk factors of Alzheimer’s disease. NPJ Metab. Health Dis. 2024, 2, 15. [Google Scholar] [CrossRef]
- Lu, Q.; Lai, J.; Lu, H.; Ng, C.; Huang, T.; Zhang, H.; Ding, K.; Wang, Z.; Jiang, J.; Hu, J.; et al. Gut microbiota in bipolar depression and its relationship to brain function: An advanced exploration. Front. Psychiatry 2019, 10, 784. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Yuan, X.; Pang, L.; Hu, S.; Wang, Y.; Huang, X.; Song, X. The role of butyric acid in treatment response in drug-naive first episode schizophrenia. Front. Psychiatry 2021, 12, 724664. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, Z.; Ko, C.-Y.; Xu, J.-H.; Lin, Y.; Wang, J. Analysis of gut microbiota in patients with exacerbated symptoms of schizophrenia following therapy with amisulpride: A pilot study. Behav. Neurol. 2022, 2022, 4262094. [Google Scholar] [CrossRef]
- Weng, S.; Zheng, J.; Lin, Y.; Fang, H.; Ko, C.-Y. Therapeutic effects of amisulpride in male schizophrenics: Role of short-chain fatty acids and gene expression changes. Physiol. Behav. 2025, 294, 114864. [Google Scholar] [CrossRef]
- Huang, S.; Hu, S.; Liu, S.; Tang, B.; Liu, Y.; Tang, L.; Lei, Y.; Zhong, L.; Yang, S.; He, S. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol. Res. 2022, 175, 105992. [Google Scholar] [CrossRef]
- Lopes, L.d.S.; da Silva, J.S.; da Luz, J.M.R.; Silva, M.d.C.S.d.; Lima, H.S.; Rocha, G.C.; Mantovani, H.C.; Kasuya, M.C.M. Intestinal microbial diversity of swines fed with different sources of lithium. 3 Biotech 2024, 14, 102. [Google Scholar]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Cao, Y.; Wang, C.; Zhao, C.; Wang, H.; Cui, G.; Wang, M.; Pan, Y.; Shi, Y. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int. J. Med. Sci. 2019, 16, 1260. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- Jiang, Y.; Qu, Y.; Shi, L.; Ou, M.; Du, Z.; Zhou, Z.; Zhou, H.; Zhu, H. The role of gut microbiota and metabolomic pathways in modulating the efficacy of SSRIs for major depressive disorder. Transl. Psychiatry 2024, 14, 493. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, L.; Sun, T.; Yang, L.; Cheng, L.-F.; Wang, Y.; Liu, Q.-Q.; Liu, A.; Liu, H.-Y.; Zhao, M.-G.; et al. Chronic minocycline treatment exerts antidepressant effect, inhibits neuroinflammation, and modulates gut microbiota in mice. Psychopharmacology 2020, 237, 3201–3213. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflamm. 2021, 18, 254. [Google Scholar] [CrossRef]
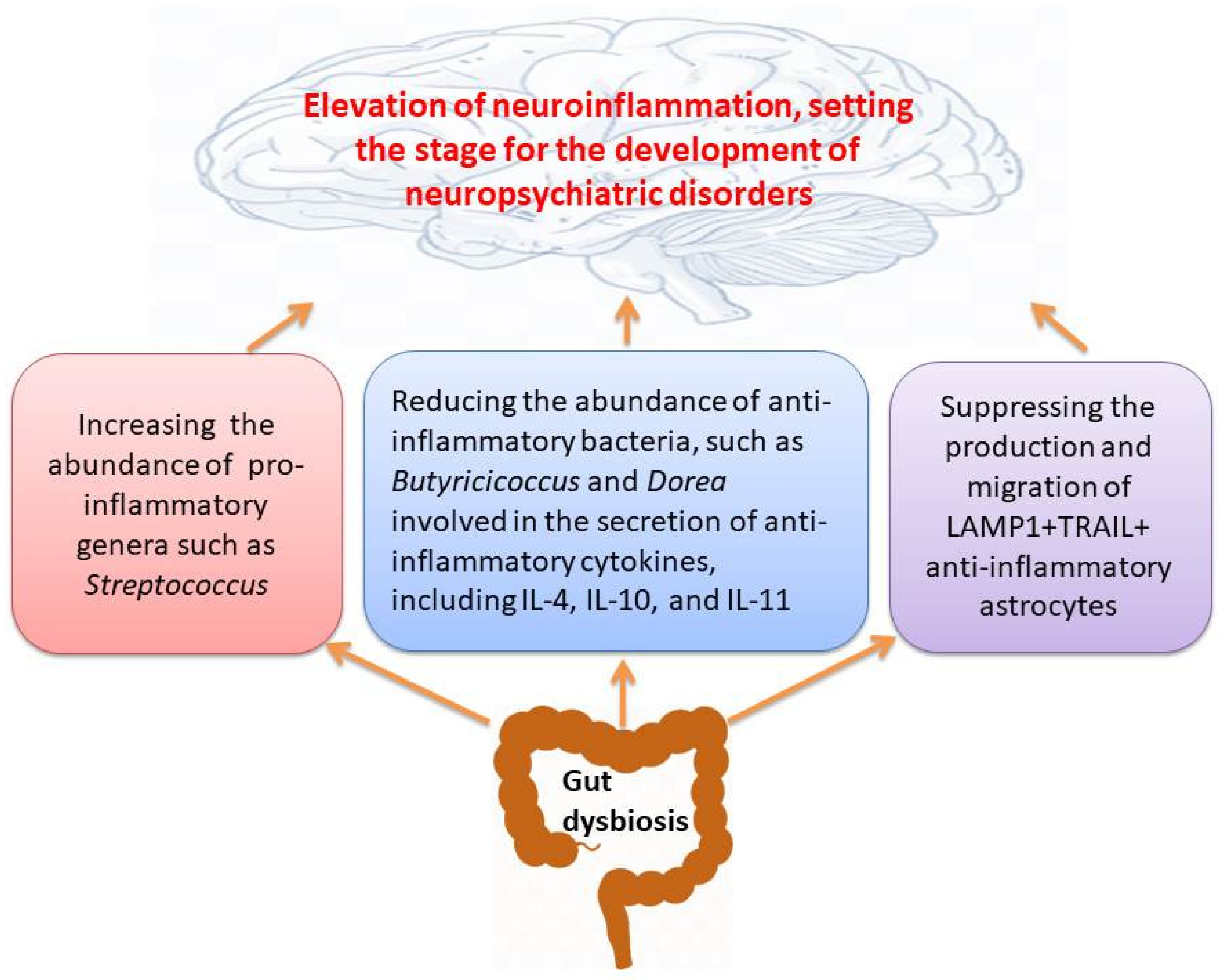
| Neuropsychiatric Disorders | Study Subjects/Sample | Immune Cells | Main Outcomes | Ref. |
|---|---|---|---|---|
| Schizophrenia (SCZ) | Human/blood | Neutrophils and lymphocyte | Association between greater proportions of neutrophils and neutrophil-to-lymphocyte ratio and higher PANSS-total scores in SCZ | [36] |
| SCZ and Bipolar Disorder (BD) | Human/ postmortem midbrain tissue | Parenchymal CD163+ cells | Association between higher proportions of parenchymal CD163+ cells and CD163 protein level, and a reduction in Tyrosine Hydroxylase (TR) in the substantia nigra | [37] |
| Autism spectrum disorder (ASD) | Human/blood | B cells | Reduced expression of anti-inflammatory cytokine IL-10 and elevated expression of TNF-α and IL-6 due to TLR4 activation in B cells in ASD | [38] |
| Parkinson disease (PD) | Human/blood | Central memory CD4+ T cells, naive CD4+ and naive CD8+ T cells | Reductions in naive B cells, naive CD4+ and naive CD8+ T cells; elevation of TNF-α–producing CD19+ B cells, central memory CD4+ T cells, IL-17–producing CD4+ Th17 cells, IL-4–producing CD4+ Th2 cells, and IFN-γ–producing CD8+ T cells in PD | [39] |
| PD | Human/blood | CD8+ T cell and natural killer (NK) cells | Negative association between Montreal Cognitive Assessment scores and Intracellular TNF-α in naïve CD8+ T-cell cluster C16 (CD57− naïve CD8+ T) and NK cell cluster C32 (CD57− CD28− NK) | [40] |
| PD | Human/blood | Myeloid dendritic cells and CD27+ CD4+ memory T cells | Relation between elevated genetic risk for PD and greater levels of myeloid dendritic cells and CD27+ CD4+ memory T cells | [41] |
| Alzheimer’s disease (AD) | Human/blood and brain | NK cells and CD4 T cells | Reduced NK cells and increased CD4 T cells in AD | [42] |
| AD | Human/blood | CD4+ T, CD8+ T, B and, NK cells, monocytes–macrophages | High-frequency amplification clonotypes in T and B cells and reduced T cells diversity in AD | [43] |
| AD | Human/blood | Monocytes, regulatory T cells (Treg), and B cells | AD risk in linked to CD33 on CD14+ monocyte; AD risk is inversely linked to secreting CD4 regulatory T cells, %CD4 regulatory T cells and CD25 on switched memory B cells | [44] |
| Major depressive disorder (MDD) | Human/blood | T regulatory cells | Acute phase of severe MDD is linked to breakdown of immune tolerance, and CD40L activation; elevated levels of CD3+ CD71+, CD3+ CD40L+, CD4+ CD71+, CD4+ CD40L+, CD4+ HLADR+, and CD8+ HLADR+ T cells in MDD | [45] |
| Neuropsychiatric Disorders | Study and Sample Type | Alerted Pro- or Anti-Inflammatory Bacteria and Their Epigenetic Metabolites | Main Finding | Ref. |
|---|---|---|---|---|
| Alzheimer’s disease (AD) | Human/fecal and blood samples | inflammatory bacteria such as Synergistetes and the Christensenellaceae family | Increased abundance of inflammatory bacteria and elevated levels of inflammatory cytokines in AD | [54] |
| AD | Human/fecal samples | Pro-inflammatory (e.g., Escherichia/Shigella, Clostridium_sensu_stricto_1 and anti-inflammatory genera (Faecalibacterium, Blautia, Bacteroides, and Roseburia | Higher levels of pro-inflammatory bacteria and lower levels of anti-inflammatory genera and total SCFAs in AD | [55] |
| Parkinson’s disease (PD) | Human/ fecal samples | Anti-inflammatory butyrate-producing bacteria, including Roseburia intestinalis, Faecalibacterium prausnitzii, Anaerostipes hadrus, and Eubacterium rectale | Depletion of anti-inflammatory butyrate-producing bacteria, derangements in SCFA-synthesis, and increased neuro-inflammation due to intestinal inflammation | [56] |
| Autism spectrum disorder (ASD) | Human/Stool and blood samples | Anti-inflammatory bacteria like Lachnospiraceae family | Negative correlation between pro-inflammatory cytokines IFN-γ and IL-6 and Lachnospiraceae family | [57] |
| SCZ | Human/fecal samples | Butyrate-producing and succinate-producing bacteria (Phascolarctobacterium succinatutens and Paraprevotella clara) | Association between increased levels of succinate-producing bacteria and inflammation | [58] |
| SCZ | Human/fecal and blood samples | Pro-inflammatory genera such as Proteus and Succinivibrio and anti-inflammatory butyrate-producing bacteria | Reduced levels of butyrate-producing bacteria (e.g., Faecalibacterium, Blautia, Alistipes, Gemmiger, and Butyricicoccus) and elevated levels of genera such as Proteus and Succinivibrio; positive correlations between pro-inflammatory cytokines (IL-1β, IL-2, IL-6, and TNF-α) and Succinivibrio | [59] |
| BD | Human/stool samples | Pro-inflammatory genera, like Streptococcus | Association between higher IL-6 levels and greater abundance of pro-inflammatory bacteria, like Streptococcus | [60] |
| BD | Human/Stool samples | Pro-inflammatory genera, like Flavonifractor | Positive correlation between Flavonifractor and oxidative stress and inflammation | [61] |
| Depressive BD II | Human/fecal samples | Pro -inflammatory bacteria (e.g., Proteobacteria, Enterobacteriaceae, Porphyromonadaceae, and Pseudescherichia) | Higher levels of Proteobacteria, Enterobacteriaceae, Porphyromonadaceae, and Pseudescherichia, along with inflammatory cytokines in unmedicated depressive BD II vs. controls | [62] |
| Depression | Human/fecal samples | Gut anti-inflammatory (Faecalibacterium and Subdoligranulum) and pro-inflammatory (Flavonifractor and Gammaproteobacteria) bacteria | Decreased abundance of Faecalibacterium and Subdoligranulum and increased abundance of Flavonifractor and Gammaproteobacteria in depressed vs. control subjects | [63] |
| Depression | Human/fecal sample | Pro-inflammatory genera such as Streptococcus and anti-inflammatory genera, like Faecalibacterium | Elevated abundance of Streptococcus and Escherichia/Shigella, and reduced abundance of Faecalibacterium; higher levels of pro-inflammatory cytokines like IL-17, and lower levels of anti-inflammatory cytokines, like IFN-γ | [64] |
| Depression | Human/fecal and blood sample | Anti-inflammatory butyrate-producing bacteria such as Turicibacter, Roseburia, and Clostridium | Reduced levels of anti-inflammatory bacteria; negative correlation between Turicibacter and Turicibacteraceae and IL-1β and IL-6 levels | [65] |
| Inflammatory depression | Human and mouse/fecal, blood, and colon biopsy samples | Anti-inflammatory bacteria such as Clostridium and Faecalibacterium | Elevated levels of Bacteroides and reduced levels of Clostridium and Faecalibacterium in inflammatory vs. non-inflammatory depression and HCs; lower levels of propionic and butyric acids in depressed patients vs. HCs | [66] |
| Depression | Human/fecal samples | Pro-inflammatory genera such as Streptococcus and anti-inflammatory genera, like Faecalibacterium | Lower α-diversity and richness, changes in β-diversity, elevated abundance of Streptococcus and reduced abundance of Faecalibacterium | [67] |
| Neuropsychiatric Disorders | Type of Sample/Study Population | Immune-Relevant Genes | Key Findings | Ref. |
|---|---|---|---|---|
| Acute mania | Serum samples/20 mania and 20 unaffected controls | CYP11A1 | Relationship between methylation of CYP11A1 and three inflammatory markers in patients | [75] |
| Schizophrenia (SCZ) | Blood/deficit SCZ (n = 53), non-deficit SCZ (n = 55), and 63 healthy controls (HCs) | CXCL1 | Hypomethylation of most CpG sites within CXCL1 gene in SCZ vs. HCs | [76] |
| Psychosis | Leukocyte/60 non-affective psychosis and 40 HCs | DDR1 | Association between DDR1 hypermethylation and inflammatory markers | [77] |
| SCZ | Peripheral blood cells (PBCs)/monozygotic twins discordant for SCZ | SOCS3 and CASP1 | Reactivating a SOCS3-mediated anti-inflammatory response by LncRNA-AC006129.1 via DNA methylation-mediated down-regulation of Capicua gene | [78] |
| SCZ | PBCs/469 Han Chinese patients with SCZ | The Th1 regulatory-related genes (SLC11A1, TNFSF4, IL27, and IL1R1); L12B, IL27, S100A12, and ZAP70 | Symptom severity is linked to DNA methylation of immune-relevant genes; hypermethylation of L12B, IL27, S100A12, and ZAP70 correlate to better response to antipsychotics | [79] |
| Bipolar disorder (BD) | PBCs/84 BD subjects with a history of suicide attempt (SA) (BD + SA), 79 BD subjects without history of SA (BD–SA) | CXCL8, CD300LG, LFNG, TRIM40, RNF14, and HIVEP3 | Six differentially methylated positions (DMPs) and seven differentially methylated regions (DMRs) in BD + SA vs. BD–SA in immune-related genes | [80] |
| BD | Leukocyte/128 BD patients in remission and 141 HCs | DDR1 | DDR1 hypermethylation at cg19215110 and cg23953820, and hypomethylation at cg14279856 and cg03270204 sites are linked to immune and inflammatory mechanisms in BD | [81] |
| Major depressive disorder (MDD) | Whole blood and serum samples/self-reported history of depression (n = 100) vs. no depression (n = 100) | LTB4R2 and IL-6 | Six DMRs in exon 1 of LTB4R2 gene; one depression-associated co-methylation module relevant to telomere length and IL-6 levels | [82] |
| MDD | Blood/153 subjects with MDD | IL1-β and IL6R | Higher methylation percentage of treatment responders in an IL6R CpG island | [83] |
| MDD | Blood/52 young patients with MDD in Scandinavian adults | TLR4 | Reduced methylation of TLR4 in blood is linked to greater depression scores | [84] |
| MDD | 220 MDD and 82 HCs | NLRP3 | DMPs in NLRP3 are linked to brain structural alterations (NLRP3 DNA methylation may elevate NLRP3 inflammasome-related neuroinflammation) | [85] |
| Alzheimer’s disease (AD) | Human brain/5 from AD and age-matched non-dementia controls | CASPASE-4 (CASP4) | Hypomethylation of CASP4 in AD, and elevated expression of CASP4, and IL-1β | [86] |
| Autism spectrum disorder (ASD) | Peripheral blood neutrophils/52 ASD children and 24 controls | CCR2 and MCP-1 | DNA hypo-methylation and increased levels of inflammatory mediators (CCR2 and MCP-1) | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohesara, S.; Mostafavi Abdolmaleky, H.; Pirani, A.; Thiagalingam, S. Therapeutic Horizons: Gut Microbiome, Neuroinflammation, and Epigenetics in Neuropsychiatric Disorders. Cells 2025, 14, 1027. https://doi.org/10.3390/cells14131027
Nohesara S, Mostafavi Abdolmaleky H, Pirani A, Thiagalingam S. Therapeutic Horizons: Gut Microbiome, Neuroinflammation, and Epigenetics in Neuropsychiatric Disorders. Cells. 2025; 14(13):1027. https://doi.org/10.3390/cells14131027
Chicago/Turabian StyleNohesara, Shabnam, Hamid Mostafavi Abdolmaleky, Ahmad Pirani, and Sam Thiagalingam. 2025. "Therapeutic Horizons: Gut Microbiome, Neuroinflammation, and Epigenetics in Neuropsychiatric Disorders" Cells 14, no. 13: 1027. https://doi.org/10.3390/cells14131027
APA StyleNohesara, S., Mostafavi Abdolmaleky, H., Pirani, A., & Thiagalingam, S. (2025). Therapeutic Horizons: Gut Microbiome, Neuroinflammation, and Epigenetics in Neuropsychiatric Disorders. Cells, 14(13), 1027. https://doi.org/10.3390/cells14131027






