The Effects of Different Types of Exercise on Pulmonary Inflammation and Fibrosis in Mice with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. T2DM Mouse Model Induction
2.3. Exercise Protocol
2.3.1. Swimming
2.3.2. Resistance Exercise
2.3.3. High-Intensity Interval Training (HIIT)
2.4. Tissue Collection
2.5. Body Composition
2.6. Hematoxylin-Eosin (H&E) Staining
2.7. Masson Staining
2.8. Immunofluorescence Staining
2.9. RNA Extraction and Gene Expression Analysis
2.10. Western Blotting Analysis
2.11. Statistical Analysis
3. Results
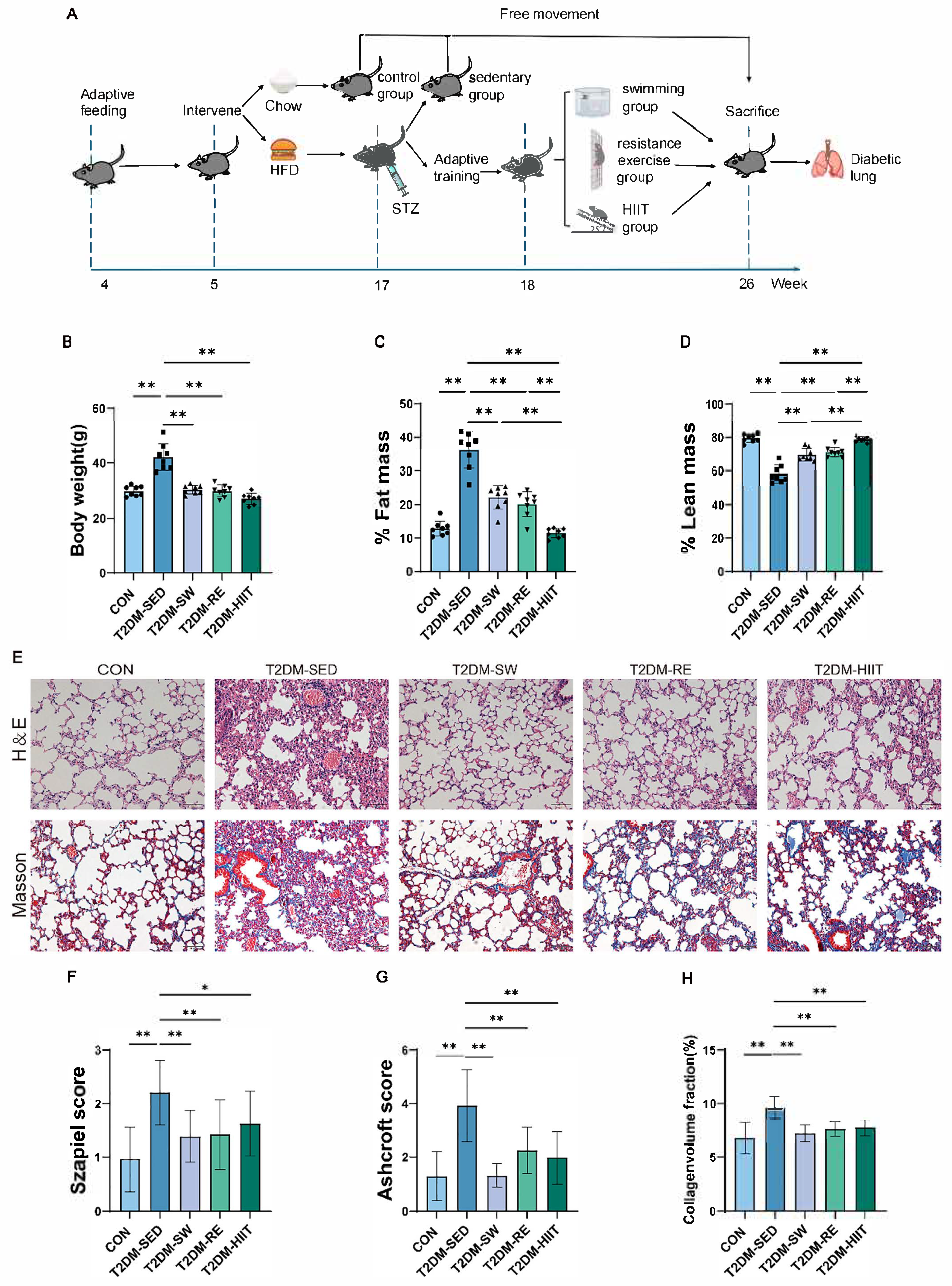
3.1. Exercise Reduced Body Weight and Body Fat Percentage in T2DM Mice
3.2. Exercise Improved Pulmonary Morphological Damage and Fibrosis in T2DM Mice
3.3. Exercise Attenuated Oxidative Stress in the Lungs of T2DM Mice
3.4. Exercise Suppressed the Expression of Inflammatory Cytokines in the Lungs of T2DM Mice
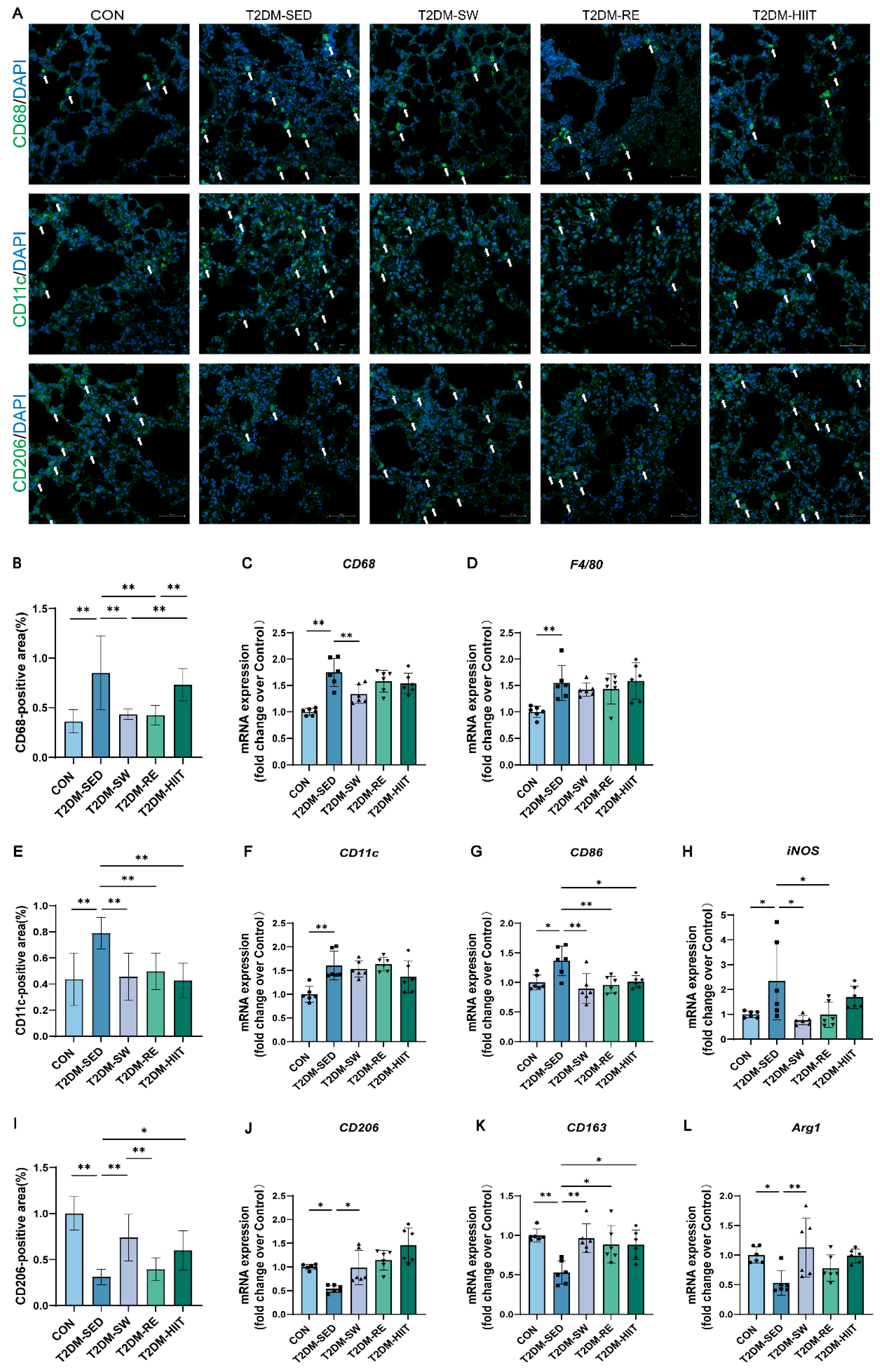
3.5. Exercise Reduced Macrophage Infiltration, Inhibited Pro-Inflammatory Macrophage Polarization, and Promoted Anti-Inflammatory Polarization in the Lungs of T2DM Mice
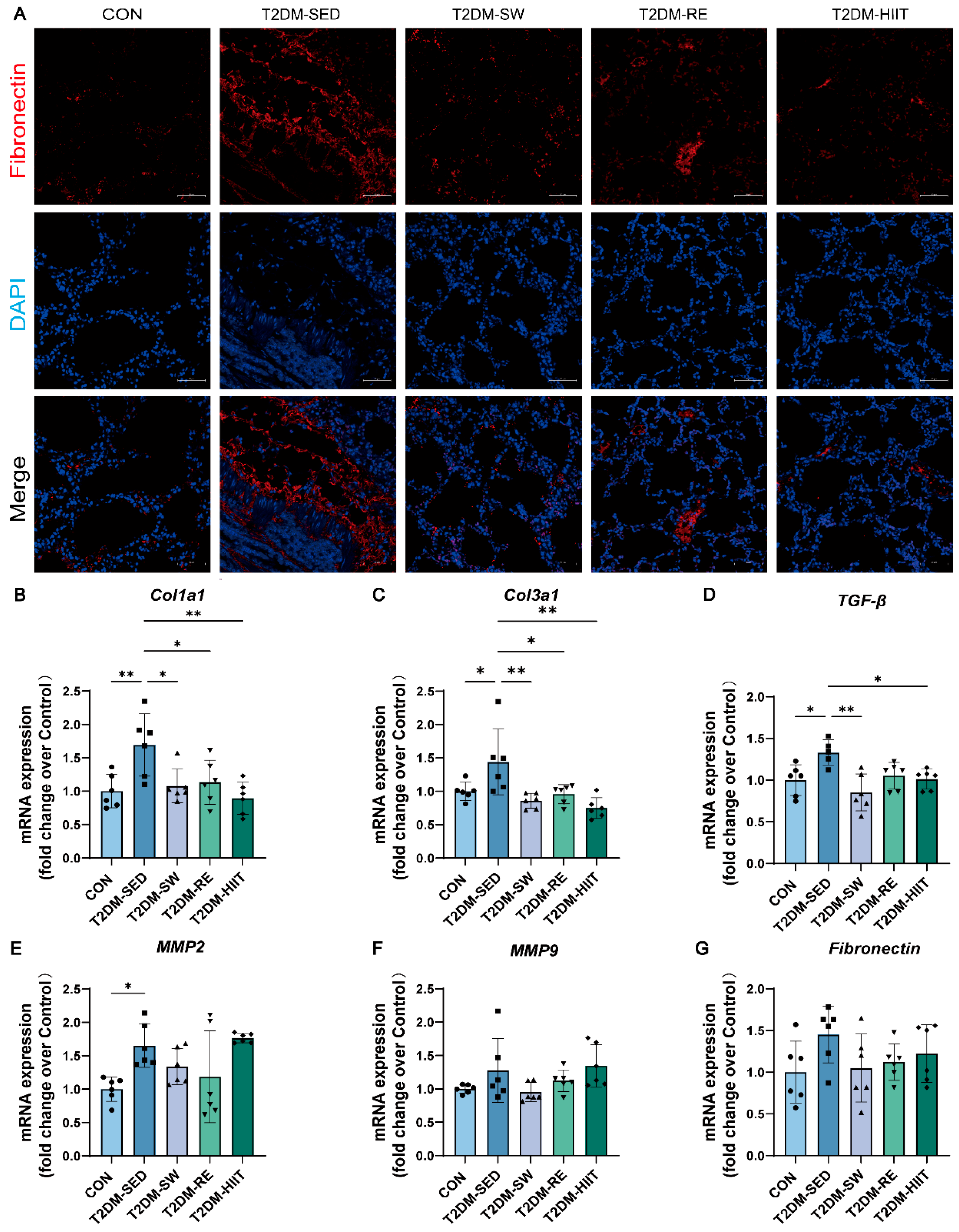
3.6. Exercise Ameliorated Pulmonary Fibrosis in T2DM Mice
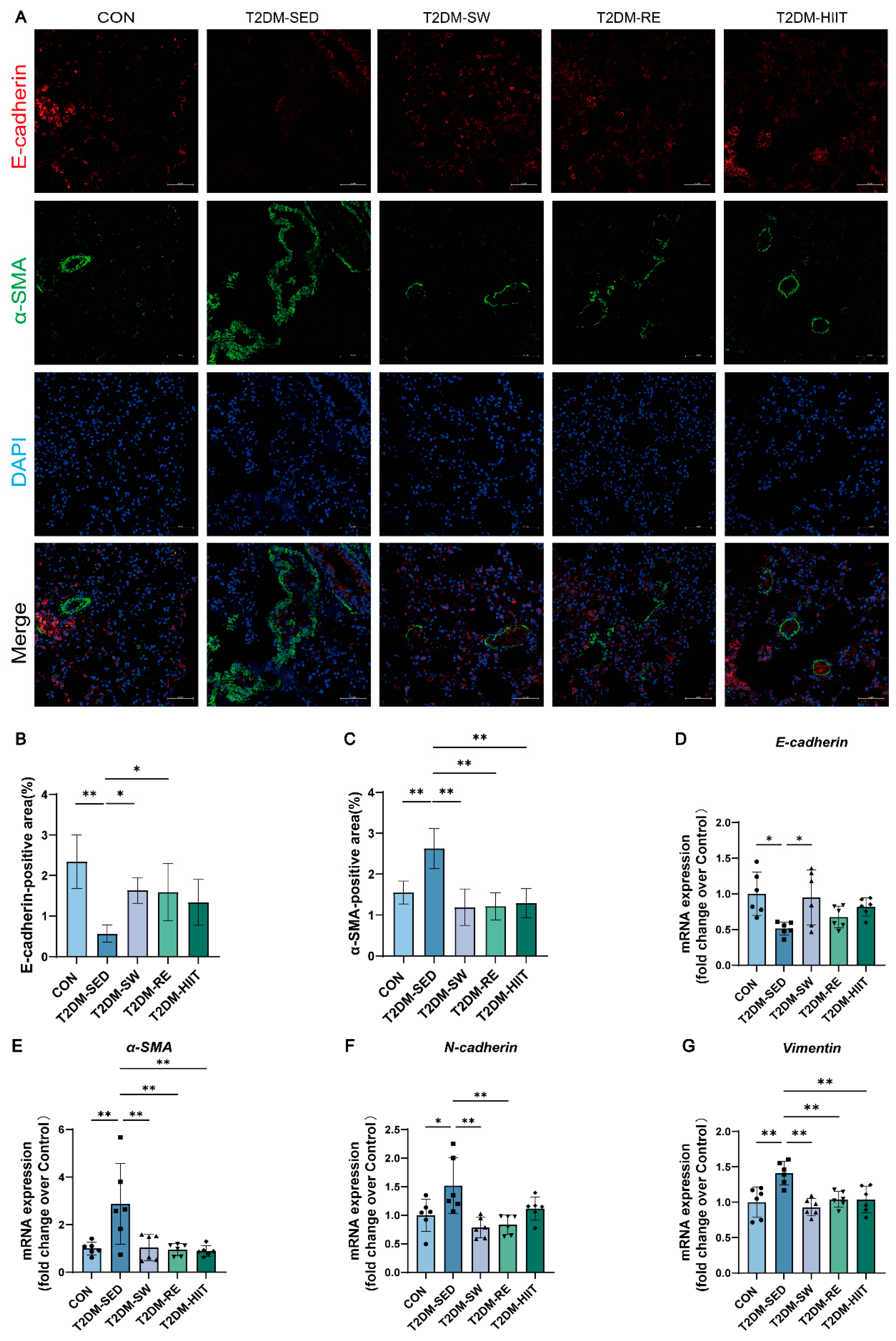
3.7. Exercise Suppressed EMT in the Lungs of T2DM Mice
3.8. Exercise Inhibited TGF-β1/Smad Signaling Pathway Activation in the Lungs of T2DM Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; E Sheffer, K.; Carrillo-Larco, R.M.; E Bennett, J.; E Shaw, J.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Eid, S.A.; Rumora, A.E.; Beirowski, B.; Bennett, D.L.; Hur, J.; Savelieff, M.G.; Feldman, E.L. New perspectives in diabetic neuropathy. Neuron 2023, 111, 2623–2641. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Paulus, W.J.; Rosano, G.; Polovina, M.; Petrie, M.C.; Jhund, P.S.; Tschope, C.; Sattar, N.; Piepoli, M.; Papp, Z.; et al. Diabetic myocardial disorder. A clinical consensus statement of the Heart Failure Association of the ESC and the ESC Working Group on Myocardial & Pericardial Diseases. Eur. J. Heart Fail. 2024, 26, 1893–1903. [Google Scholar] [CrossRef]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Schuyler, M.R.; Niewoehner, D.E.; Inkley, S.R.; Kohn, R. Abnormal lung elasticity in juvenile diabetes mellitus. Am. Rev. Respir. Dis. 1976, 113, 37–41. [Google Scholar] [CrossRef]
- Sandler, M. Is the lung a ‘target organ’ in diabetes mellitus? Arch. Intern. Med. 1990, 150, 1385–1388. [Google Scholar] [CrossRef]
- Hsia, C.C.; Raskin, P. Lung function changes related to diabetes mellitus. Diabetes Technol. Ther. 2007, 9 (Suppl. S1), S73–S82. [Google Scholar] [CrossRef]
- Giovannelli, J.; Trouiller, P.; Hulo, S.; Cherot-Kornobis, N.; Ciuchete, A.; Edme, J.L.; Matran, R.; Amouyel, P.; Meirhaeghe, A.; Dauchet, L. Low-grade systemic inflammation: A partial mediator of the relationship between diabetes and lung function. Ann. Epidemiol. 2018, 28, 26–32. [Google Scholar] [CrossRef]
- Li, W.; Ning, Y.; Ma, Y.; Lin, X.; Man, S.; Wang, B.; Wang, C.; Yang, T. Association of lung function and blood glucose level: A 10-year study in China. BMC Pulm. Med. 2022, 22, 444. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, M.K.; Joung, K.H.; Lee, J.H.; Kim, H.J.; Ku, B.J. Association between glycemic state and pulmonary function and effect of walking as a protective factor in subjects with diabetes mellitus. Ann. Transl. Med. 2019, 7, 530. [Google Scholar] [CrossRef]
- Zhang, R.H.; Zhou, J.B.; Cai, Y.H.; Shu, L.P.; Simo, R.; Lecube, A. Non-linear association between diabetes mellitus and pulmonary function: A population-based study. Respir. Res. 2020, 21, 292. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, F.; Xie, Y.; Mo, Y.; Zhang, X.; Liu, C. Diabetic endothelial microangiopathy and pulmonary dysfunction. Front. Endocrinol. 2023, 14, 1073878. [Google Scholar] [CrossRef]
- Kumar, V.; Nawroth, P.P. Is the association between diabetes mellitus and pulmonary fibrosis real? Nat. Rev. Endocrinol. 2021, 17, 703–704. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wei, D.G.; Li, R.S. Ghrelin attenuates inflammation in diabetic lung disease by TLR4 pathway In Vivo and In Vitro. BMJ Open Diabetes Res. Care 2023, 11, e003027. [Google Scholar] [CrossRef]
- Oztay, F.; Kandil, A.; Gurel, E.; Ustunova, S.; Kapucu, A.; Balci, H.; Akgun-Dar, K.; Demirci, C. The relationship between nitric oxide and leptin in the lung of rat with streptozotocin-induced diabetes. Cell Biochem. Funct. 2008, 26, 162–171. [Google Scholar] [CrossRef]
- Zou, X.Z.; Gong, Z.C.; Liu, T.; He, F.; Zhu, T.T.; Li, D.; Zhang, W.F.; Jiang, J.L.; Hu, C.P. Involvement of epithelial-mesenchymal transition afforded by activation of LOX-1/TGF-beta1/KLF6 signaling pathway in diabetic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2017, 44, 70–77. [Google Scholar] [CrossRef]
- Zheng, F.; Lu, W.; Wu, F.; Li, H.; Hu, X.; Zhang, F. Recombinant decorin ameliorates the pulmonary structure alterations by down-regulating transforming growth factor-beta1/SMADS signaling in the diabetic rats. Endocr. Res. 2010, 35, 35–49. [Google Scholar] [CrossRef]
- Kolahian, S.; Leiss, V.; Nürnberg, B. Diabetic lung disease: Fact or fiction? Rev. Endocr. Metab. Disord. 2019, 20, 303–319. [Google Scholar] [CrossRef]
- Ho, T.W.; Huang, C.T.; Ruan, S.Y.; Tsai, Y.J.; Lai, F.; Yu, C.J. Diabetes mellitus in patients with chronic obstructive pulmonary disease-The impact on mortality. PLoS ONE 2017, 12, e0175794. [Google Scholar] [CrossRef]
- Brunetti, V.C.; Ayele, H.T.; Yu, O.H.Y.; Ernst, P.; Filion, K.B. Type 2 diabetes mellitus and risk of community-acquired pneumonia: A systematic review and meta-analysis of observational studies. CMAJ Open 2021, 9, E62–E70. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Murray, M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008, 5, e152. [Google Scholar] [CrossRef]
- Machado, L.M.Q.; Serra, D.S.; Neves, T.G.; Cavalcante, F.S.A.; Ceccatto, V.M.; Leal-Cardoso, J.H.; Zin, W.A.; Moreira-Gomes, M.D. Pulmonary impairment in type 2 diabetic rats and its improvement by exercise. Acta Physiol. 2022, 234, e13708. [Google Scholar] [CrossRef]
- Rajizadeh, M.A.; Khoramipour, K.; Joukar, S.; Darvishzadeh-Mahani, F.; Iranpour, M.; Bejeshk, M.A.; Zaboli, M.D. Lung molecular and histological changes in type 2 diabetic rats and its improvement by high-intensity interval training. BMC Pulm. Med. 2024, 24, 37. [Google Scholar] [CrossRef]
- Athari, S.Z.; Mirzaei Bavil, F.; Keyhanmanesh, R.; Lotfi, H.; Sajed, Y.; Delkhosh, A.; Ghiasi, F. Voluntary exercise improves pulmonary inflammation through NF-kappaB and Nrf2 in type 2 diabetic male rats. Iran. J. Basic Med. Sci. 2024, 27, 74–80. [Google Scholar] [CrossRef]
- Azizi, N.; Rahbarghazi, A.; Bavil, F.M.; Rahbarghazi, R.; Ghaffari-Nasab, A.; Rezaie, J.; Delkhosh, A.; Ahmadi, M. Swimming training reduced inflammation and apoptotic changes in pulmonary tissue in type 1 diabetic mice. J. Diabetes Metab. Disord. 2023, 22, 793–800. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Xu, Y.; Wang, W.; Zhuang, S.; Wang, R.; Xiao, W. HIIT Ameliorates Inflammation and Lipid Metabolism by Regulating Macrophage Polarization and Mitochondrial Dynamics in the Liver of Type 2 Diabetes Mellitus Mice. Metabolites 2022, 13, 14. [Google Scholar] [CrossRef]
- Zheng, L.; Qin, R.; Rao, Z.; Xiao, W. High-intensity interval training induces renal injury and fibrosis in type 2 diabetic mice. Life Sci. 2023, 324, 121740. [Google Scholar] [CrossRef]
- Fu, T.L.; Li, G.R.; Li, D.H.; He, R.Y.; Liu, B.H.; Xiong, R.; Xu, C.Z.; Lu, Z.L.; Song, C.K.; Qiu, H.L.; et al. Mangiferin alleviates diabetic pulmonary fibrosis in mice via inhibiting endothelial-mesenchymal transition through AMPK/FoxO3/SIRT3 axis. Acta Pharmacol. Sin. 2024, 45, 1002–1018. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Q.; Zheng, L.; Shou, J.; Zhuang, S.; Xiao, W.; Chen, P. Depot-specific adaption of adipose tissue for different exercise approaches in high-fat diet/streptozocin-induced diabetic mice. Front. Physiol. 2023, 14, 1189528. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, D.; Zhang, T.; Sun, Y.; Ding, S. Swimming Differentially Affects T2DM-Induced Skeletal Muscle ER Stress and Mitochondrial Dysfunction Related to MAM. Diabetes Metab. Syndr. Obes. 2020, 13, 1417–1428. [Google Scholar] [CrossRef]
- Zheng, L.; Rao, Z.; Wu, J.; Ma, X.; Jiang, Z.; Xiao, W. Resistance Exercise Improves Glycolipid Metabolism and Mitochondrial Biogenesis in Skeletal Muscle of T2DM Mice via miR-30d-5p/SIRT1/PGC-1alpha Axis. Int. J. Mol. Sci. 2024, 25, 12416. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Q.; Yang, D.; Chen, P.; Xiao, W. HIIT Promotes M2 Macrophage Polarization and Sympathetic Nerve Density to Induce Adipose Tissue Browning in T2DM Mice. Biomolecules 2024, 14, 246. [Google Scholar] [CrossRef]
- Song, D.; Li, Z.; Sun, F.; Wu, K.; Zhang, K.; Liu, W.; Liu, K.; An, B.; Wang, Z.; Zhao, T.; et al. Optimized administration of human embryonic stem cell-derived immunity-and-matrix regulatory cells for mouse lung injury and fibrosis. Stem Cell Res. Ther. 2024, 15, 344. [Google Scholar] [CrossRef]
- Shariati, S.; Kalantar, H.; Pashmforoosh, M.; Mansouri, E.; Khodayar, M.J. Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed. Pharmacother. 2019, 114, 108776. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Xiang, Z.; Ren, Y.; Zheng, X.; Zhao, Q.; Zhou, Q.; Zhou, Y.; Xu, L.; Wang, Y. Microcystin-LR ameliorates pulmonary fibrosis via modulating CD206(+) M2-like macrophage polarization. Cell Death Dis. 2020, 11, 136. [Google Scholar] [CrossRef]
- Su, H.; Tian, C.J.; Wang, Y.; Shi, J.; Chen, X.; Zhen, Z.; Bai, Y.; Deng, L.; Feng, C.; Ma, Z.; et al. Ginsenoside Rb1 reduces oxidative/carbonyl stress damage and ameliorates inflammation in the lung of streptozotocin-induced diabetic rats. Pharm. Biol. 2022, 60, 2229–2236. [Google Scholar] [CrossRef]
- Aisanjiang, M.; Dai, W.; Wu, L.; Yuan, Y.; Liu, S.; Liao, G.; Li, L.; Tong, X.; Zhang, H.; Chen, Y.; et al. Ameliorating lung fibrosis and pulmonary function in diabetic mice: Therapeutic potential of mesenchymal stem cell. Biochem. Biophys. Res. Commun. 2024, 737, 150495. [Google Scholar] [CrossRef]
- Klein, O.L.; Krishnan, J.A.; Glick, S.; Smith, L.J. Systematic review of the association between lung function and Type 2 diabetes mellitus. Diabet. Med. 2010, 27, 977–987. [Google Scholar] [CrossRef]
- De Santi, F.; Zoppini, G.; Locatelli, F.; Finocchio, E.; Cappa, V.; Dauriz, M.; Verlato, G. Type 2 diabetes is associated with an increased prevalence of respiratory symptoms as compared to the general population. BMC Pulm. Med. 2017, 17, 101. [Google Scholar] [CrossRef]
- Pan, B.; Ge, L.; Xun, Y.Q.; Chen, Y.J.; Gao, C.Y.; Han, X.; Zuo, L.Q.; Shan, H.Q.; Yang, K.H.; Ding, G.W.; et al. Exercise training modalities in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 72. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, H.; Yang, X.; Shi, J.; Sheng, X.; Wang, L.; Li, T.; Quan, H.; Zhai, X.; Li, W. Effects of dapagliflozin monotherapy and combined aerobic exercise on skeletal muscle mitochondrial quality control and insulin resistance in type 2 diabetes mellitus rats. Biomed. Pharmacother. 2023, 169, 115852. [Google Scholar] [CrossRef]
- Cheng, F.; Dun, Y.; Cheng, J.; Ripley-Gonzalez, J.W.; Jiang, W.; You, B.; Liu, S. Exercise activates autophagy and regulates endoplasmic reticulum stress in muscle of high-fat diet mice to alleviate insulin resistance. Biochem. Biophys. Res. Commun. 2022, 601, 45–51. [Google Scholar] [CrossRef]
- Guo, X.; Sunil, C.; Qian, G. Obesity and the Development of Lung Fibrosis. Front. Pharmacol. 2021, 12, 812166. [Google Scholar] [CrossRef]
- Wang, X.; Yi, X.; Tang, D. Aerobic Exercise Improves Pulmonary Fibrosis by Improving Insulin Resistance and Inflammation in Obese Mice. Front. Physiol. 2021, 12, 785117. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Q.; Shi, W.; Peng, L.; Jiang, Y.; Zhu, M.; Guo, J.; Peng, D.; Wang, M.; Men, L.; et al. ATF3/SPI1/SLC31A1 Signaling Promotes Cuproptosis Induced by Advanced Glycosylation End Products in Diabetic Myocardial Injury. Int. J. Mol. Sci. 2023, 24, 1667. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Forgiarini, L.A., Jr.; Kretzmann, N.A.; Porawski, M.; Dias, A.S.; Marroni, N.A. Experimental diabetes mellitus: Oxidative stress and changes in lung structure. J. Bras. Pneumol. 2009, 35, 788–791. [Google Scholar] [CrossRef]
- Sacan, O.; Turkyilmaz, I.B.; Bayrak, B.B.; Mutlu, O.; Akev, N.; Yanardag, R. Zinc supplementation ameliorates glycoprotein components and oxidative stress changes in the lung of streptozotocin diabetic rats. Biometals 2016, 29, 239–248. [Google Scholar] [CrossRef]
- Liao, Y.F.; Yin, S.; Chen, Z.Q.; Li, F.; Zhao, B. High glucose promotes tumor cell proliferation and migration in lung adenocarcinoma via the RAGE-NOXs pathway. Mol. Med. Rep. 2018, 17, 8536–8541. [Google Scholar] [CrossRef]
- Luan, R.; Ding, D.; Yang, J. The protective effect of natural medicines against excessive inflammation and oxidative stress in acute lung injury by regulating the Nrf2 signaling pathway. Front. Pharmacol. 2022, 13, 1039022. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.H. Recent Advances in Understanding Nrf2 Agonism and Its Potential Clinical Application to Metabolic and Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef]
- Eren, G.; Cukurova, Z.; Hergunsel, O.; Demir, G.; Kucur, M.; Uslu, E.; Dalo, E.; Uhri, M.; Tugcu, V. Protective effect of the nuclear factor kappa B inhibitor pyrrolidine dithiocarbamate in lung injury in rats with streptozotocin-induced diabetes. Respiration 2010, 79, 402–410. [Google Scholar] [CrossRef]
- Baldissera, G.; Sperotto, N.D.; Rosa, H.T.; Henn, J.G.; Peres, V.F.; Moura, D.J.; Roehrs, R.; Denardin, E.L.; Dal Lago, P.; Nunes, R.B.; et al. Effects of crude hydroalcoholic extract of Syzygium cumini (L.) Skeels leaves and continuous aerobic training in rats with diabetes induced by a high-fat diet and low doses of streptozotocin. J. Ethnopharmacol. 2016, 194, 1012–1021. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, S.; Qiao, L.; Peng, Z.; Zhao, J.; Xu, D.; Wu, C.; Li, M.; Zeng, X.; Wang, Q. Non-apoptotic programmed cell deaths in diabetic pulmonary dysfunction: The new side of advanced glycation end products. Front. Endocrinol. 2023, 14, 1126661. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Javed Shaikh, M.A.; Thangavelu, L.; Singh, S.K.; Rama Raju Allam, V.S.; Jha, N.K.; et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, F.; Zhao, H.; An, Y. Curcumin alleviates lung injury in diabetic rats by inhibiting nuclear factor-kappaB pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 956–963. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Safarian, B.; Esparham, A.; Mirzaei, M.; Esmaeilzadeh, M.; Boskabady, M.H. The modulatory effects of exercise on lipopolysaccharide-induced lung inflammation and injury: A systemic review. Life Sci. 2022, 293, 120306. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Wang, A.J.; Ren, J.; Wang, A.; Hascall, V.C. Monocyte adhesive hyaluronan matrix induced by hyperglycemia in diabetic lung injuries. J. Biol. Chem. 2023, 299, 104995. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis--a common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Yang, J.; Xue, Q.; Miao, L.; Cai, L. Pulmonary fibrosis: A possible diabetic complication. Diabetes Metab. Res. Rev. 2011, 27, 311–317. [Google Scholar] [CrossRef]
- Ofulue, A.F.; Kida, K.; Thurlbeck, W.M. Experimental diabetes and the lung. I. Changes in growth, morphometry, and biochemistry. Am. Rev. Respir. Dis. 1988, 137, 162–166. [Google Scholar] [CrossRef]
- Daghigh, F.; Karimi, P.; Alihemmati, A.; Majidi Zolbin, M.; Ahmadiasl, N. Swimming training modulates lung injury induced by ovariectomy in diabetic rats: Involvement of inflammatory and fibrotic biomarkers. Arch. Physiol. Biochem. 2022, 128, 514–520. [Google Scholar] [CrossRef]
- Chen, C.M.; Juan, S.H.; Pai, M.H.; Chou, H.C. Hyperglycemia induces epithelial-mesenchymal transition in the lungs of experimental diabetes mellitus. Acta Histochem. 2018, 120, 525–533. [Google Scholar] [CrossRef]
- Talakatta, G.; Sarikhani, M.; Muhamed, J.; Dhanya, K.; Somashekar, B.S.; Mahesh, P.A.; Sundaresan, N.; Ravindra, P.V. Diabetes induces fibrotic changes in the lung through the activation of TGF-beta signaling pathways. Sci. Rep. 2018, 8, 11920. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Guo, Y.; Qian, H.; Xin, X.; Liu, Q. Effects of different exercise modalities on inflammatory markers in the obese and overweight populations: Unraveling the mystery of exercise and inflammation. Front. Physiol. 2024, 15, 1405094. [Google Scholar] [CrossRef]
- Yilmaz, O.F.; Ozdal, M. Acute, chronic, and combined pulmonary responses to swimming in competitive swimmers. Respir. Physiol. Neurobiol. 2019, 259, 129–135. [Google Scholar] [CrossRef]
- Rodrigues, J.; Jesus, B.; Caseiro, P.; Ferreira, A.J.; Rama, L. Lung Function Changes with Swim Training in Healthy and Allergic Endurance Athletes. J. Funct. Morphol. Kinesiol. 2025, 10, 231. [Google Scholar] [CrossRef]
- Bernhardsen, G.P.; Stang, J.; Halvorsen, T.; Stensrud, T. Differences in lung function, bronchial hyperresponsiveness and respiratory health between elite athletes competing in different sports. Eur. J. Sport Sci. 2023, 23, 1480–1489. [Google Scholar] [CrossRef]
- Rochat, I.; Cote, A.; Boulet, L.P. Determinants of lung function changes in athletic swimmers. A review. Acta Paediatr. 2022, 111, 259–264. [Google Scholar] [CrossRef]
- Leite, A.B.; Lima, H.N.; Flores, C.O.; Oliveira, C.A.; Cunha, L.E.C.; Neves, J.L.; Correia, T.M.L.; de Melo, F.F.; Oliveira, M.V.; de Magalhaes, A.C.M.; et al. High-intensity interval training is more effective than continuous training to reduce inflammation markers in female rats with cisplatin nephrotoxicity. Life Sci. 2021, 266, 118880. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, F.; Shen, W.; Xia, J.; Wang, J.; Zhao, Y.; Zhang, Z.; Sun, Y.; Qian, M.; Ding, S. High-Intensity Interval Training Attenuates Ketogenic Diet-Induced Liver Fibrosis in Type 2 Diabetic Mice by Ameliorating TGF-beta1/Smad Signaling. Diabetes Metab. Syndr. Obes. 2020, 13, 4209–4219. [Google Scholar] [CrossRef]
- Brown, M.B.; Neves, E.; Long, G.; Graber, J.; Gladish, B.; Wiseman, A.; Owens, M.; Fisher, A.J.; Presson, R.G.; Petrache, I.; et al. High-intensity interval training, but not continuous training, reverses right ventricular hypertrophy and dysfunction in a rat model of pulmonary hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R197–R210. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, F.; Sun, Y.; Li, L. Mechanisms of endurance and resistance exercise in type 2 diabetes mellitus: A Narrative review. Biochem. Biophys. Res. Commun. 2025, 761, 151731. [Google Scholar] [CrossRef]
- Verbrugge, S.A.J.; Alhusen, J.A.; Kempin, S.; Pillon, N.J.; Rozman, J.; Wackerhage, H.; Kleinert, M. Genes controlling skeletal muscle glucose uptake and their regulation by endurance and resistance exercise. J. Cell. Biochem. 2022, 123, 202–214. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Roberts, B.M.; Nuckols, G.; Krieger, J.W. Sex Differences in Resistance Training: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2020, 34, 1448–1460. [Google Scholar] [CrossRef]



| Gene | Forward Primer Sequences | Reverse Primer Sequences |
|---|---|---|
| Nrf2 | 5′-TCTTGGAGTAAGTCGAGAAGTGT-3′ | 5′-GTTGAAACTGAGCGAAAAAGGC-3′ |
| Hmox1 | 5′-AAGCCGAGAATGCTGAGTTCA-3′ | 5′-GCCGTAGATATGGTACAAGGA-3′ |
| Keap1 | 5′-TCGAAGGCATCCACCCTAAG-3′ | 5′-CTCGAACCACGCTGTCAATCT-3′ |
| Gpx3 | 5′-CCTTTTAAGCAGTATGCAGGCA-3′ | 5′-CAAGCCAAATGGCCCAAGTT-3′ |
| Sod1 | 5′-TATGGGGACAATACACAAGGCT-3′ | 5′-TATGGGGACAATACACAAGGCT-3′ |
| Nox2 | 5′-TGATCCTGCTGCCAGTGTGTC-3′ | 5′-TGATCCTGCTGCCAGTGTGTC-3′ |
| TNFα | 5′-CCTGTAGCCCACGTCGTAG-3′ | 5′-GGGAGTAGACAAGGTACAACCC-3′ |
| IL6 | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| IL-1β | 5′-GAAATGCCACCTTTTGACAGTG-3′ | 5′-TGGATGCTCTCATCAGGACAG-3′ |
| IL-15 | 5′-CATCCATCTCGTGCTACTTGTG-3′ | 5′-GCCTCTGTTTTAGGGAGACCT-3′ |
| NF-κB | 5′-GACACGACAGAATCCTCAGCATCC-3′ | 5′-GACACGACAGAATCCTCAGCATCC-3′ |
| CCL2 | 5′-TAAAAACCTGGATCGGAACCAAA-3′ | 5′-GCATTAGCTTCAGATTTACGGGT-3′ |
| CCL5 | 5′-GCTGCTTTGCCTACCTCTCC-3′ | 5′-TCGAGTGACAAACACGACTGC-3′ |
| IL-10 | 5′-CTTACTGACTGGCATGAGGATCA-3′ | 5′-GCAGCTCTAGGAGCATGTGG-3′ |
| CD68 | 5′-TGATCCTGCTGCCAGTGTGTC-3′ | 5′-TGATCCTGCTGCCAGTGTGTC-3′ |
| F4/80 | 5-GCAAAAACTGGCAGTGGG-3′ | 5-GAATCTTGGCCAAGAAGAGAC-3′ |
| CD11c | 5′-GGTGCAAAGACCACCTTCAT-3′ | 5′-GACGTTTGAAGAAGCCAAGC-3′ |
| CD86 | 5-GCAAAAACTGGCAGTGGG-3′ | 5-GCAAAAACTGGCAGTGGG-3′ |
| iNOS | 5′-GTTCTCAGCCCAACAATACAAGA-3′ | 5′-GTGGACGGGTCGATGTCAC-3′ |
| CD163 | 5-GTTCCTGTCAAAGCTCGTGC-3′ | 5-GCAAAAACTGGCAGTGGG-3′ |
| CD206 | 5-CTCTGTTCAGCTATTGGACGC-3 | 5-CGGAATTTCTGGGATTCAGCTTC-3′ |
| Arg1 | 5′-CTCCAAGCCAAAGTCCTTAGAG-3′ | 5′-GGAGCTGTCATTAGGGACATCA-3′ |
| Col1a1 | 5′-CTGGCGGTTCAGGTCCAAT-3′ | 5′-TTCCAGGCAATCCACGAGC-3′ |
| Col3a1 | 5′-CTGTAACATGGAAACTGGGGAAA-3′ | 5′-CCATAGCTGAACTGAAAACCACC-3′ |
| MMP2 | 5′-ACAAGTGGTCCGCGTAAAGT-3 | 5′-GTAAACAAGGCTTCATGGGGG-3′ |
| MMP9 | 5′-GGACCCGAAGCGGACATTG-3′ | 5′-CGTCGTCGAAATGGGCATCT-3′ |
| Fibronectin | 5′-ATGTGGACCCCTCCTGATAGT-3′ | 5′-GCCCAGTGATTTCAGCAAAGG-3′ |
| TGF-β | 5′-CTTCAATACGTCAGACATTCGGG-3′ | 5′-GTAACGCCAGGAATTGTTGCTA-3′ |
| α-SMA | 5′-GTCCCAGACATCAGGGAGTAA-3′ | 5′-TCGGATACTTCAGCGTCAGGA-3′ |
| E-cadherin | 5′-CAGTTCCGAGGTCTACACCTT-3′ | 5′-TGAATCGGGAGTCTTCCGAAAA-3′ |
| N-cadherin | 5′-AGGCTTCTGGTGAAATTGCAT-3′ | 5′-GTCCACCTTGAAATCTGCTGG-3′ |
| Vimentin | 5′-TCCACACGCACCTACAGTCT-3′ | 5′-CCGAGGACCGGGTCACATA-3′ |
| β-actin | 5′-CGTGCGTGACATCAAAGAGAA-3′ | 5′-GCTCGTTGCCAATAGTGATGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Ma, X.; Wang, Z.; Zhu, D.; Guo, Y.; Zhao, L.; Xiao, W. The Effects of Different Types of Exercise on Pulmonary Inflammation and Fibrosis in Mice with Type 2 Diabetes Mellitus. Cells 2025, 14, 1026. https://doi.org/10.3390/cells14131026
Gao H, Ma X, Wang Z, Zhu D, Guo Y, Zhao L, Xiao W. The Effects of Different Types of Exercise on Pulmonary Inflammation and Fibrosis in Mice with Type 2 Diabetes Mellitus. Cells. 2025; 14(13):1026. https://doi.org/10.3390/cells14131026
Chicago/Turabian StyleGao, Haoyang, Xiaotong Ma, Ze Wang, Danlin Zhu, Yifan Guo, Linlin Zhao, and Weihua Xiao. 2025. "The Effects of Different Types of Exercise on Pulmonary Inflammation and Fibrosis in Mice with Type 2 Diabetes Mellitus" Cells 14, no. 13: 1026. https://doi.org/10.3390/cells14131026
APA StyleGao, H., Ma, X., Wang, Z., Zhu, D., Guo, Y., Zhao, L., & Xiao, W. (2025). The Effects of Different Types of Exercise on Pulmonary Inflammation and Fibrosis in Mice with Type 2 Diabetes Mellitus. Cells, 14(13), 1026. https://doi.org/10.3390/cells14131026






