Human Extravillous Trophoblasts Require SRC-2 for Sustained Viability, Migration, and Invasion
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Placenta Tissue
2.2. Immortalized Human Extravillous Trophoblast Cell Line
2.3. Transfection of Small Interfering RNAs
2.4. Quantitative Real-Time PCR
2.5. Genome-Scale RNA Expression Profiling
2.6. Immunoblotting
2.7. Cell Viability Assay
2.8. Clonogenic Survival Assay
2.9. Transwell Cell Migration Assay
2.10. Transwell Cell Invasion Assay
2.11. Statistical Analysis
3. Results
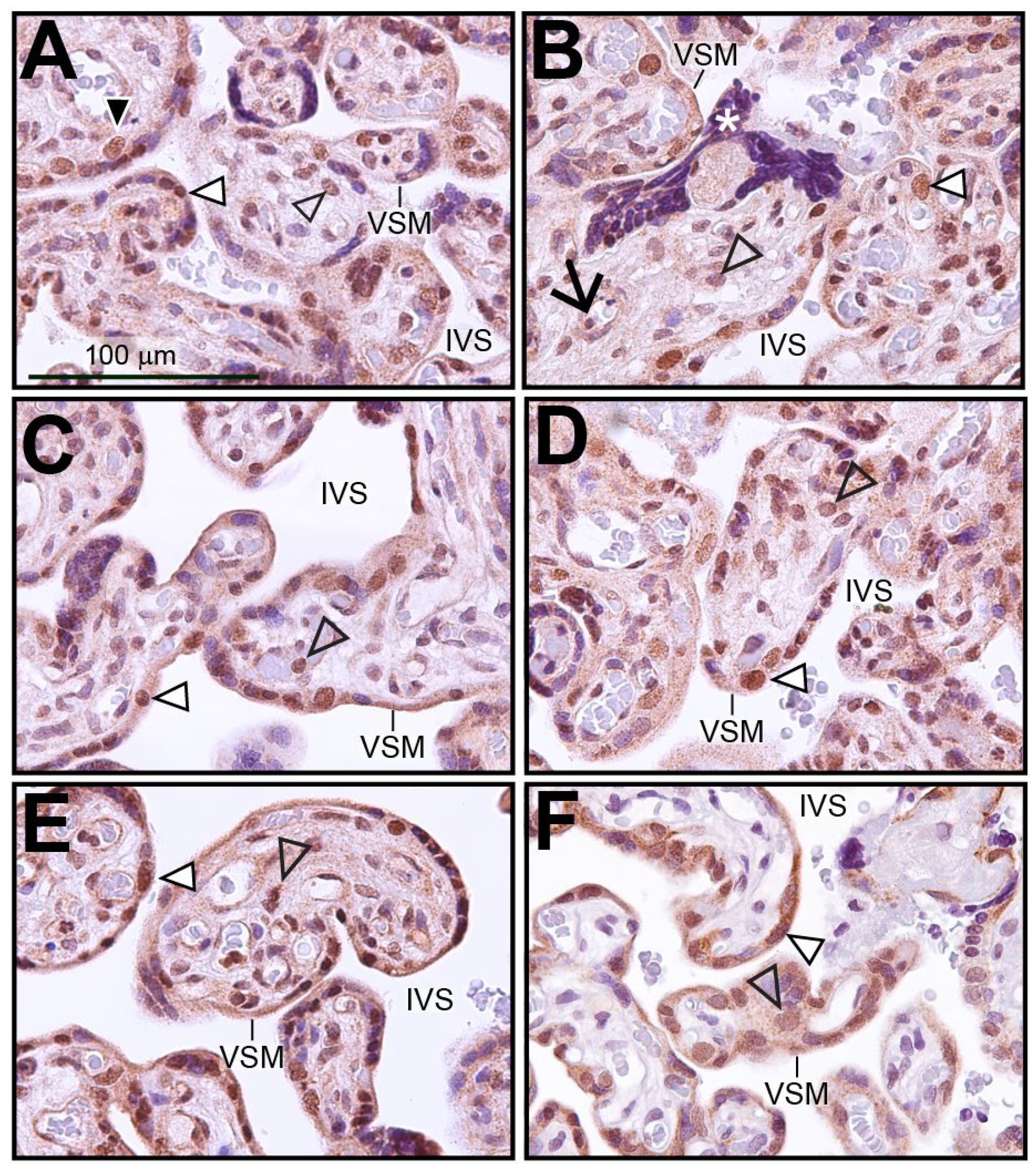
3.1. Human Term-Placenta Expresses SRC-2
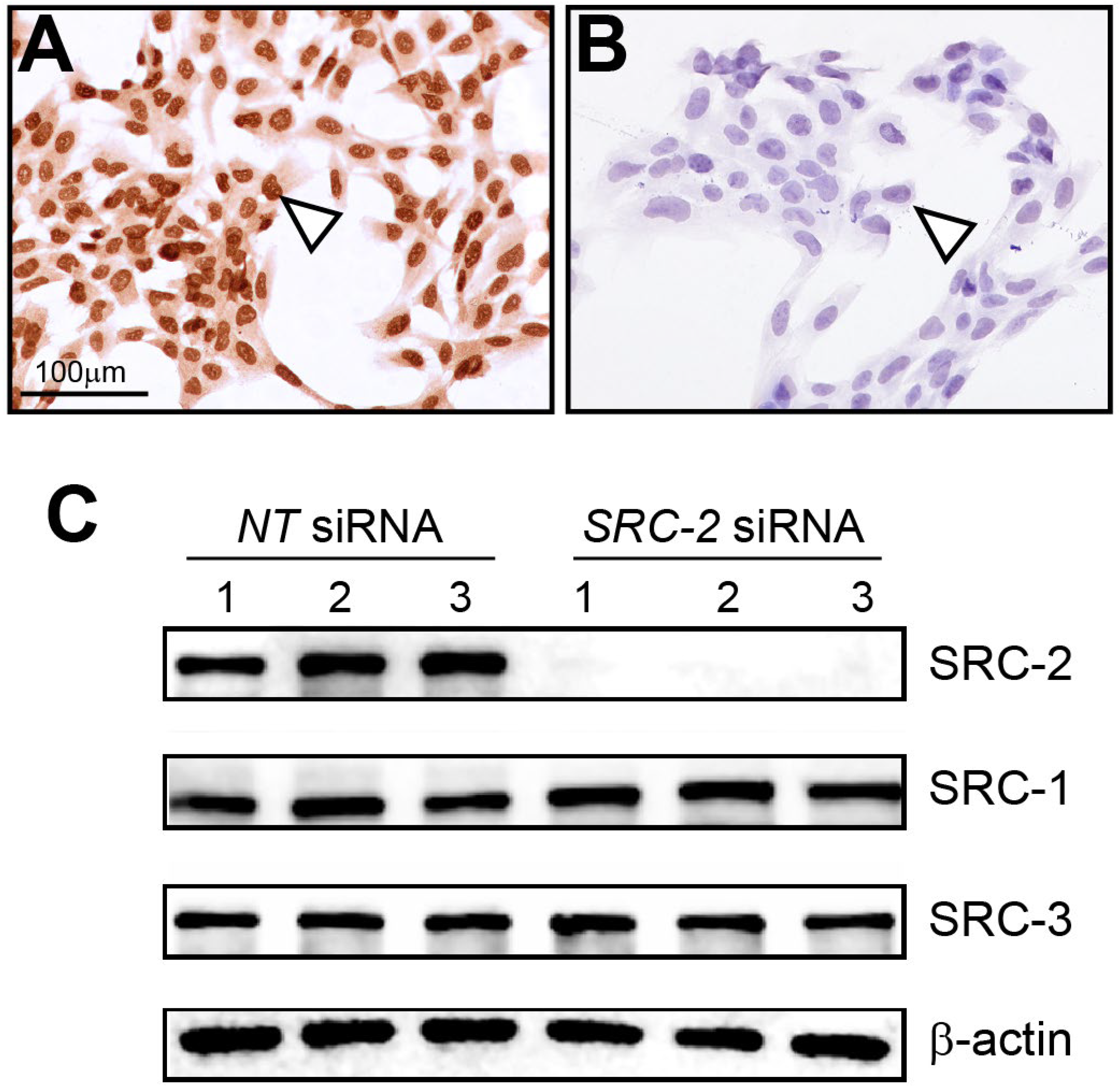
3.2. The HTR-8/SVneo Cell Line Expresses SRC-2
3.3. Sustained Viability, Motility, and Invasiveness of HTR-8/SVneo Cells Require Normal SRC-2 Expression Levels
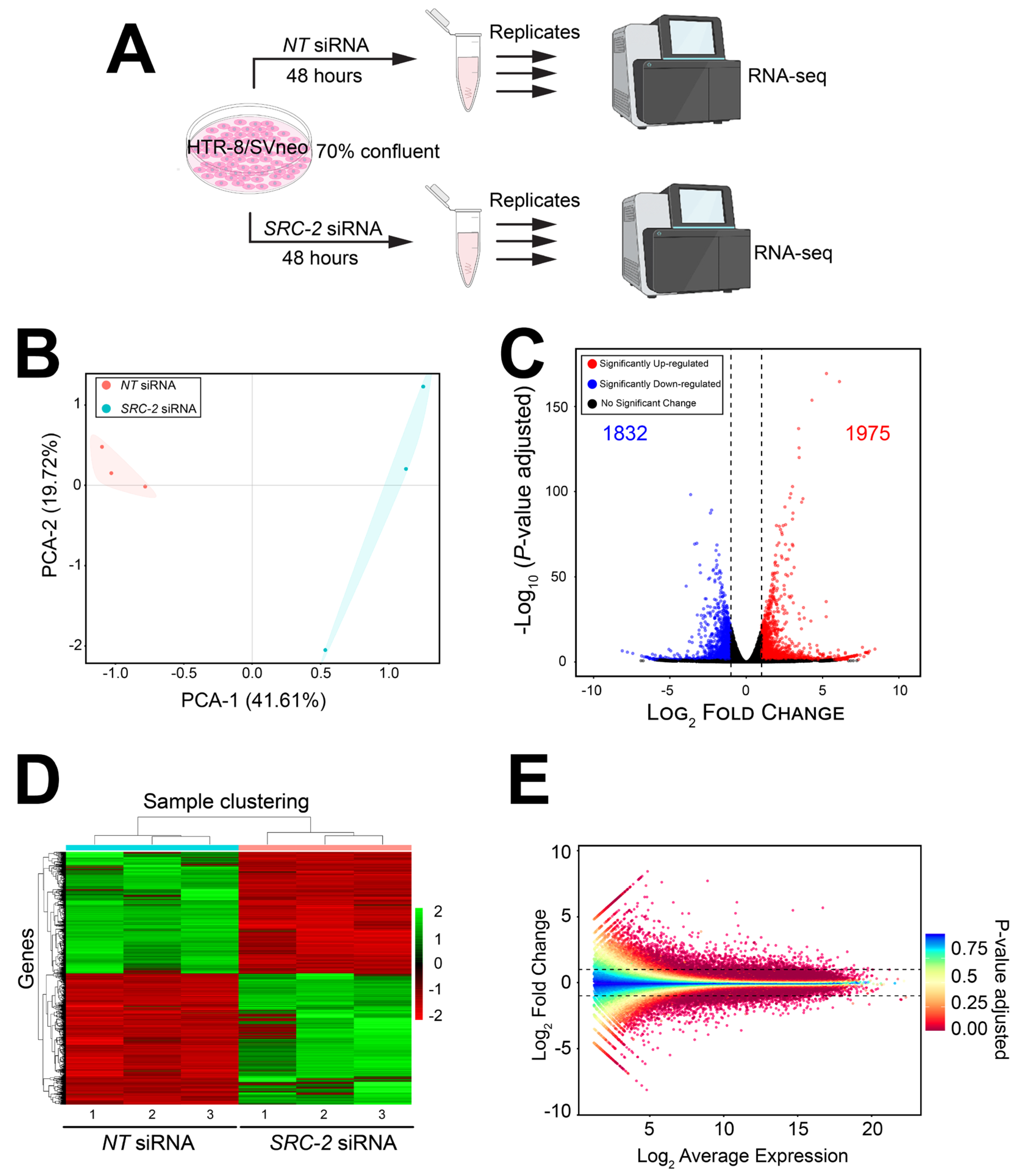
3.4. Genome-Wide Changes in the HTR-8/SVneo Cell Transcriptome Following SRC-2 Depletion
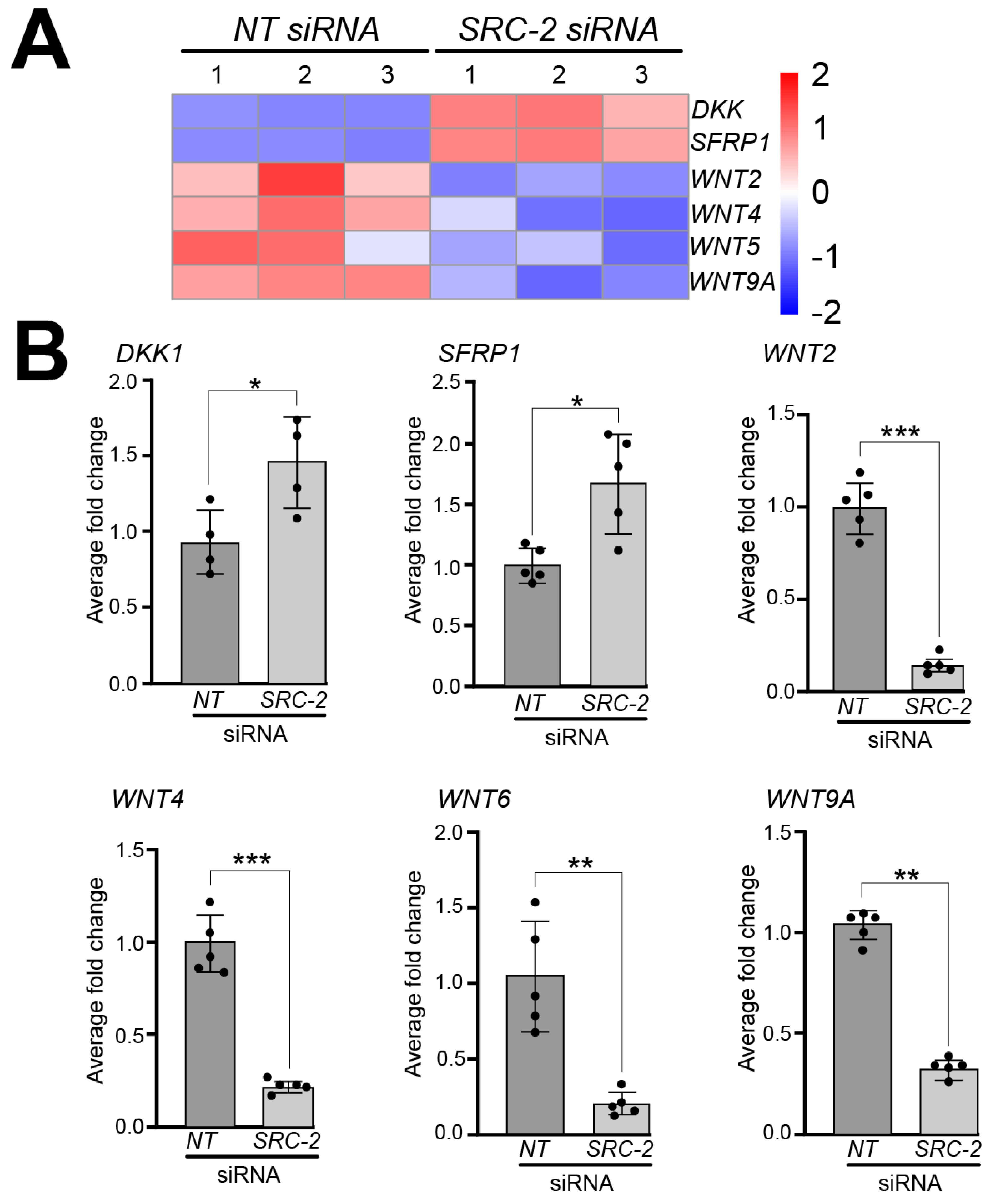
3.5. Gene Expression Associated with Trophoblastic Biology and WNT Signaling Are Significantly Reduced in HTR-8/SVneo Cells Following SRC-2 Depletion
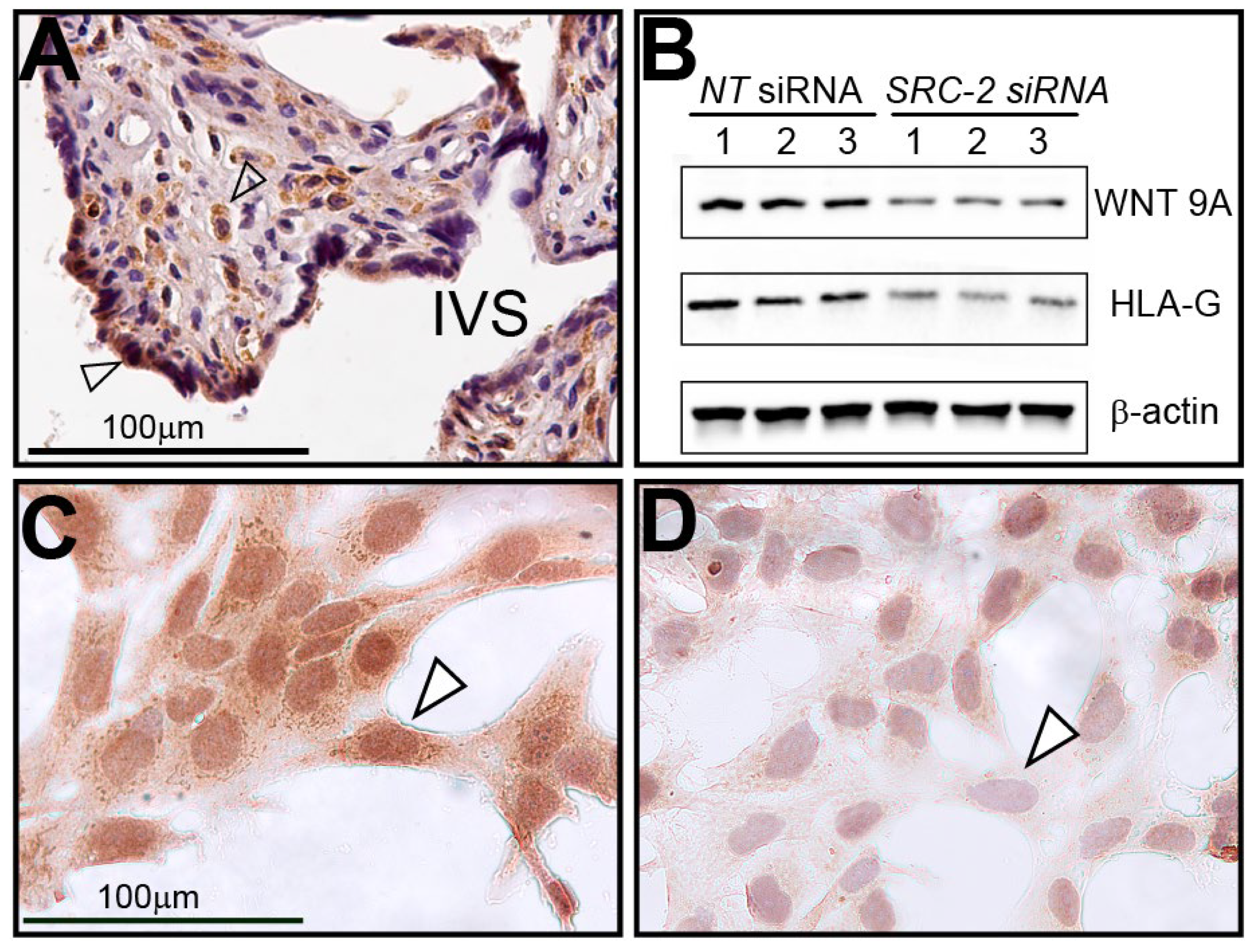
3.6. Human Term-Placental Tissue and HTR-8/SVneo Cells Express WNT9A
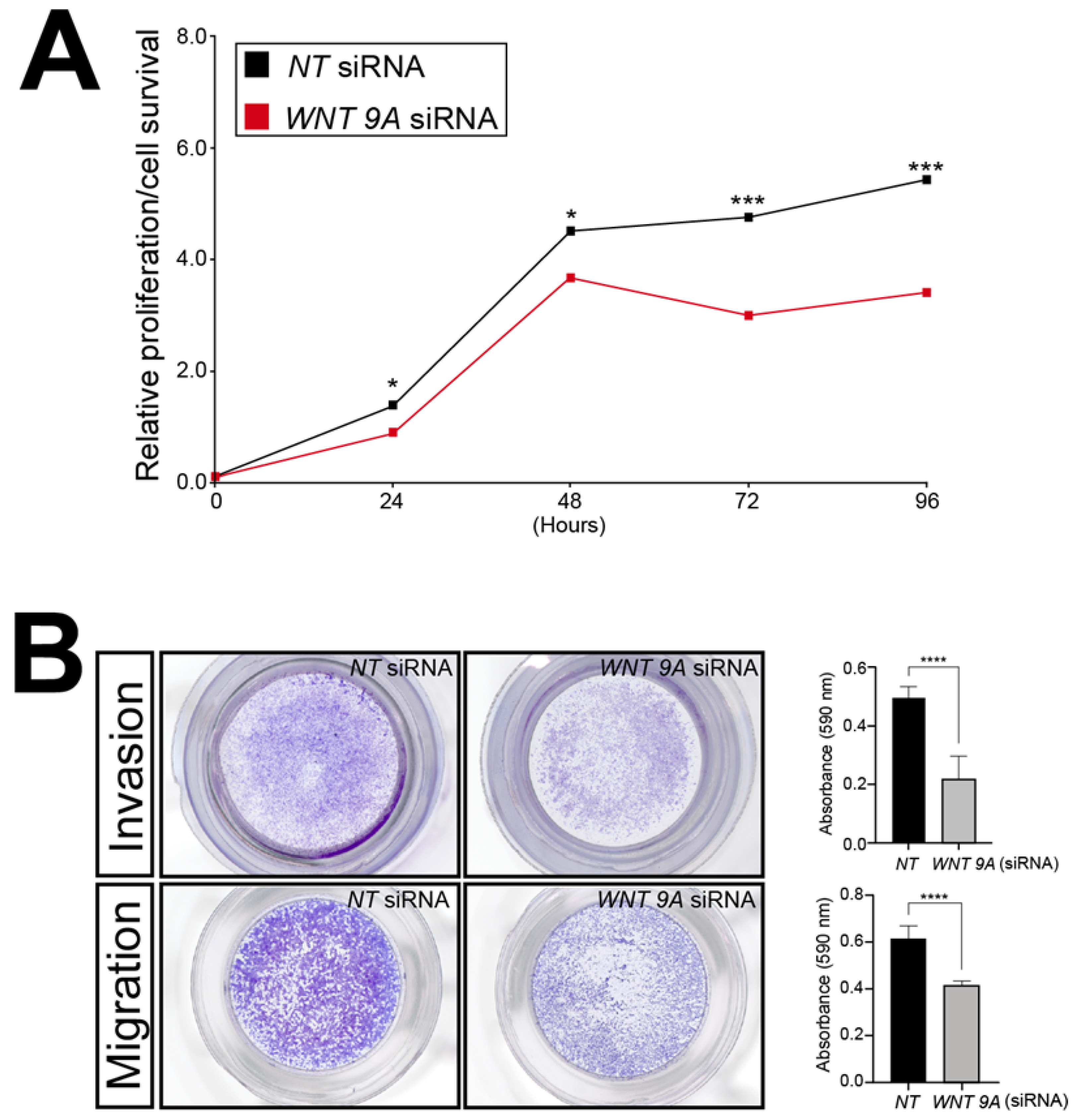
3.7. Attenuation of WNT9A Expression Significantly Reduces the Sustained Viability, Motility, and Invasiveness of HTR-8/SVneo Trophoblast Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Varberg, K.M.; Iqbal, K. Hemochorial placentation: Development, function, and adaptations. Biol. Reprod. 2018, 99, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Watson, A.L.; Hempstock, J.; Skepper, J.N.; Jauniaux, E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 2002, 87, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J. Embryo implantation-coordination of maternal and embryonic adaptations. Int. J. Dev. Biol. 2014, 58, 71–74. [Google Scholar] [CrossRef]
- Kojima, J.; Ono, M.; Kuji, N.; Nishi, H. Human Chorionic Villous Differentiation and Placental Development. Int. J. Mol. Sci. 2022, 23, 8003. [Google Scholar] [CrossRef]
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43. [Google Scholar] [CrossRef]
- Soares, M.J.; Vivian, J.L. Tipping the balance toward trophoblast development. Proc. Natl. Acad. Sci. USA 2016, 113, 5144–5146. [Google Scholar] [CrossRef]
- Ruane, P.T.; Garner, T.; Parsons, L.; Babbington, P.A.; Wangsaputra, I.; Kimber, S.J.; Stevens, A.; Westwood, M.; Brison, D.R.; Aplin, J.D. Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation. Hum. Reprod. 2022, 37, 777–792. [Google Scholar] [CrossRef]
- Knofler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Burton, G.J.; Moffett, A. Trophoblast remodeling of the uterine veins and maternal placental blood flow. Am. J. Obstet. Gynecol. 2023, 229, 704–705. [Google Scholar] [CrossRef]
- Li, Q.; Sharkey, A.; Sheridan, M.; Magistrati, E.; Arutyunyan, A.; Huhn, O.; Sancho-Serra, C.; Anderson, H.; McGovern, N.; Esposito, L.; et al. Human uterine natural killer cells regulate differentiation of extravillous trophoblast early in pregnancy. Cell Stem Cell 2024, 31, 181–195.e189. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Shreeve, N. Local immune recognition of trophoblast in early human pregnancy: Controversies and questions. Nat. Rev. Immunol. 2023, 23, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.P.; Myatt, L.; Colvis, C.; et al. Placental origins of adverse pregnancy outcomes: Potential molecular targets: An Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef]
- Kadyrov, M.; Kingdom, J.C.; Huppertz, B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am. J. Obstet. Gynecol. 2006, 194, 557–563. [Google Scholar] [CrossRef]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef]
- Khong, T.Y.; De Wolf, F.; Robertson, W.B.; Brosens, I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br. J. Obstet. Gynaecol. 1986, 93, 1049–1059. [Google Scholar] [CrossRef]
- Romero, R.; Kusanovic, J.P.; Chaiworapongsa, T.; Hassan, S.S. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 313–327. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, R.C.; O’Malley, B.W. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat. Rev. Cancer 2009, 9, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, M.M.; Kommagani, R.; Lessey, B.A.; Lydon, J.P. The p160/steroid receptor coactivator family: Potent arbiters of uterine physiology and dysfunction. Biol. Reprod. 2014, 91, 122. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, M.M.; Lydon, J.P.; O’Malley, B.W. Steroid receptor coactivators as therapeutic targets in the female reproductive system. J. Steroid Biochem. Mol. Biol. 2015, 154, 32–38. [Google Scholar] [CrossRef]
- Kommagani, R.; Szwarc, M.M.; Kovanci, E.; Creighton, C.J.; O’Malley, B.W.; Demayo, F.J.; Lydon, J.P. A murine uterine transcriptome, responsive to steroid receptor coactivator-2, reveals transcription factor 23 as essential for decidualization of human endometrial stromal cells. Biol. Reprod. 2014, 90, 75. [Google Scholar] [CrossRef]
- Kommagani, R.; Szwarc, M.M.; Kovanci, E.; Gibbons, W.E.; Putluri, N.; Maity, S.; Creighton, C.J.; Sreekumar, A.; DeMayo, F.J.; Lydon, J.P.; et al. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet. 2013, 9, e1003900. [Google Scholar] [CrossRef]
- Szwarc, M.M.; Hai, L.; Gibbons, W.E.; White, L.D.; Mo, Q.; Kommagani, R.; Lanz, R.B.; DeMayo, F.J.; O’Malley, B.W.; Lydon, J.P. Retinoid signaling controlled by SRC-2 in decidualization revealed by transcriptomics. Reproduction 2018, 156, 387–395. [Google Scholar] [CrossRef]
- Szwarc, M.M.; Kommagani, R.; Jeong, J.W.; Wu, S.P.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W.; DeMayo, F.J.; Lydon, J.P. Perturbing the cellular levels of steroid receptor coactivator-2 impairs murine endometrial function. PLoS ONE 2014, 9, e98664. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Xu, J. The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Mol. Endocrinol. 2010, 24, 1917–1934. [Google Scholar] [CrossRef]
- He, C.; Shan, N.; Xu, P.; Ge, H.; Yuan, Y.; Liu, Y.; Zhang, P.; Wen, L.; Zhang, F.; Xiong, L.; et al. Hypoxia-induced Downregulation of SRC-3 Suppresses Trophoblastic Invasion and Migration Through Inhibition of the AKT/mTOR Pathway: Implications for the Pathogenesis of Preeclampsia. Sci. Rep. 2019, 9, 10349. [Google Scholar] [CrossRef]
- Yuan, Y.; Shan, N.; Tan, B.; Deng, Q.; Liu, Y.; Wang, H.; Luo, X.; He, C.; Luo, X.; Zhang, H.; et al. SRC-3 Plays a Critical Role in Human Umbilical Vein Endothelial Cells by Regulating the PI3K/Akt/mTOR Pathway in Preeclampsia. Reprod. Sci. 2018, 25, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Fang, L.; Li, Y.; Thakur, A.; Hoodless, P.A.; Guo, Y.; Wang, Z.; Wu, Z.; Yan, Y.; Jia, Q.; et al. G protein-coupled estrogen receptor stimulates human trophoblast cell invasion via YAP-mediated ANGPTL4 expression. Commun. Biol. 2021, 4, 1285. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, D.; Zhu, J.; Wang, A.; Zhou, X.; Han, T.L.; Zhang, H. The metabolic role of the CD73/adenosine signaling pathway in HTR-8/SVneo cells: A Double-Edged Sword? Heliyon 2024, 10, e25252. [Google Scholar] [CrossRef]
- Liu, Y.; Du, L.; Gu, S.; Liang, J.; Huang, M.; Huang, L.; Lai, S.; Zhang, S.; Tu, Z.; Sun, W.; et al. Identification of the role of DAB2 and CXCL8 in uterine spiral artery remodeling in early-onset preeclampsia. Cell Mol. Life Sci. 2024, 81, 180. [Google Scholar] [CrossRef]
- Van Buren, E.; Azzara, D.; Rangel-Moreno, J.; Garcia-Hernandez, M.L.; Murphy, S.P.; Cohen, E.D.; Lewis, E.; Lin, X.; Park, H.R. Single-cell RNA sequencing reveals placental response under environmental stress. Nat. Commun. 2024, 15, 6549. [Google Scholar] [CrossRef]
- Maurya, V.K.; Szwarc, M.M.; Fernandez-Valdivia, R.; Lonard, D.M.; Yong, S.; Joshi, N.; Fazleabas, A.T.; Lydon, J.P. Early growth response 1 transcription factor is essential for the pathogenic properties of human endometriotic epithelial cells. Reproduction 2022, 164, 41–54. [Google Scholar] [CrossRef]
- Maurya, V.K.; Szwarc, M.M.; Lonard, D.M.; Kommagani, R.; Wu, S.P.; O’Malley, B.W.; DeMayo, F.J.; Lydon, J.P. Steroid receptor coactivator-2 drives epithelial reprogramming that enables murine embryo implantation. FASEB J. 2023, 37, e23313. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Loukeris, K.; Sela, R.; Baergen, R.N. Syncytial knots as a reflection of placental maturity: Reference values for 20 to 40 weeks’ gestational age. Pediatr. Dev. Pathol. 2010, 13, 305–309. [Google Scholar] [CrossRef]
- Bozickovic, O.; Skartveit, L.; Engelsen, A.S.T.; Helland, T.; Jonsdottir, K.; Flageng, M.H.; Fenne, I.S.; Janssen, E.; Lorens, J.B.; Bjorkhaug, L.; et al. A novel SRC-2-dependent regulation of epithelial-mesenchymal transition in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 185, 57–70. [Google Scholar] [CrossRef]
- Cai, M.; Liang, X.; Sun, X.; Chen, H.; Dong, Y.; Wu, L.; Gu, S.; Han, S. Nuclear Receptor Coactivator 2 Promotes Human Breast Cancer Cell Growth by Positively Regulating the MAPK/ERK Pathway. Front. Oncol. 2019, 9, 164. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, F.; Lu, D.; Sun, W.; Zhu, G.; Lan, B. Knockdown of NCOA2 Inhibits the Growth and Progression of Gastric Cancer by Affecting the Wnt Signaling Pathway-Related Protein Expression. Technol. Cancer Res. Treat. 2020, 19, 1533033820928072. [Google Scholar] [CrossRef]
- Szwarc, M.M.; Kommagani, R.; Putluri, V.; Dubrulle, J.; Stossi, F.; Mancini, M.A.; Coarfa, C.; Lanz, R.B.; Putluri, N.; DeMayo, F.J.; et al. Steroid Receptor Coactivator-2 Controls the Pentose Phosphate Pathway through RPIA in Human Endometrial Cancer Cells. Sci. Rep. 2018, 8, 13134. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Liu, H.; Chen, D.; Liu, Z.; Lu, Q.; Lai, X.; Hou, H.; Zhang, M.; Zhang, J.Y.; Duan, Y.; et al. Interleukin-1 beta signals through the ERK signalling pathway to modulate human placental trophoblast migration and invasion in the first trimester of pregnancy. Placenta 2024, 151, 67–78. [Google Scholar] [CrossRef]
- Kamei, T.; Jones, S.R.; Chapman, B.M.; McGonigle, K.L.; Dai, G.; Soares, M.J. The phosphatidylinositol 3-kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol. Endocrinol. 2002, 16, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, J.; Knofler, M. Signalling pathways regulating the invasive differentiation of human trophoblasts: A review. Placenta 2005, 26 (Suppl. A), S21–S30. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Klaman, L.D.; Chen, B.; Araki, T.; Harada, H.; Thomas, S.M.; George, E.L.; Neel, B.G. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev. Cell 2006, 10, 317–327. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, D.; Tang, F.; Gao, H.; Li, X. CAPN6 in disease: An emerging therapeutic target (Review). Int. J. Mol. Med. 2020, 46, 1644–1652. [Google Scholar] [CrossRef]
- Saunders, A.C.; McGonnigal, B.; Uzun, A.; Padbury, J. The developmental expression of the CDK inhibitor p57(kip2) (Cdkn1c) in the early mouse placenta. Mol. Reprod. Dev. 2016, 83, 405–412. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Lamptey, J.; Chen, X.M.; Li, F.F.; Li, C.; Ruan, L.L.; Yang, X.N.; Liu, T.H.; Wang, Y.X.; Ding, Y.B. Iodothyronine deiodinase 2 (DiO(2)) regulates trophoblast cell line cycle, invasion and apoptosis; and its downregulation is associated with early recurrent miscarriage. Placenta 2021, 111, 54–68. [Google Scholar] [CrossRef]
- Biron-Shental, T.; Schaiff, W.T.; Rimon, E.; Shim, T.L.; Nelson, D.M.; Sadovsky, Y. Hypoxia enhances the expression of follistatin-like 3 in term human trophoblasts. Placenta 2008, 29, 51–57. [Google Scholar] [CrossRef]
- Guo, J.; Tian, T.; Lu, D.; Xia, G.; Wang, H.; Dong, M. Alterations of maternal serum and placental follistatin-like 3 and myostatin in pre-eclampsia. J. Obstet. Gynaecol. Res. 2012, 38, 988–996. [Google Scholar] [CrossRef]
- Peiris, H.N.; Ponnampalam, A.P.; Osepchook, C.C.; Mitchell, M.D.; Green, M.P. Placental expression of myostatin and follistatin-like-3 protein in a model of developmental programming. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E854–E861. [Google Scholar] [CrossRef]
- Kozai, K.; Moreno-Irusta, A.; Iqbal, K.; Winchester, M.L.; Scott, R.L.; Simon, M.E.; Muto, M.; Parrish, M.R.; Soares, M.J. The AKT1-FOXO4 axis reciprocally regulates hemochorial placentation. Development 2023, 150, dev201095. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Lim, R.; Riley, C.; Menon, R.; Permezel, M. Expression and localisation of FoxO3 and FoxO4 in human placenta and fetal membranes. Placenta 2010, 31, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Bora, M.; Singha, S.; Madan, T.; Deka, G.; Hazarika, S.G.; Baruah, S. HLA-G isoforms, HLA-C allotype and their expressions differ between early abortus and placenta in relation to spontaneous abortions. Placenta 2024, 149, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Dunk, C.E.; Bucher, M.; Zhang, J.; Hayder, H.; Geraghty, D.E.; Lye, S.J.; Myatt, L.; Hackmon, R. Human leukocyte antigen HLA-C, HLA-G, HLA-F, and HLA-E placental profiles are altered in early severe preeclampsia and preterm birth with chorioamnionitis. Am. J. Obstet. Gynecol. 2022, 227, 641.e641–641.e613. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Meissner, T.B.; Mikkelsen, T.S.; Mallard, W.; O’Donnell, C.W.; Tilburgs, T.; Gomes, H.A.; Camahort, R.; Sherwood, R.I.; Gifford, D.K.; et al. A distant trophoblast-specific enhancer controls HLA-G expression at the maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2016, 113, 5364–5369. [Google Scholar] [CrossRef]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; DeMars, R. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Chu, A.; Najafzadeh, P.; Sullivan, P.; Cone, B.; Elshimali, R.; Shakeri, H.; Janzen, C.; Mah, V.; Wadehra, M. Aldehyde dehydrogenase isoforms and inflammatory cell populations are differentially expressed in term human placentas affected by intrauterine growth restriction. Placenta 2019, 81, 9–17. [Google Scholar] [CrossRef]
- Luan, X.; Li, S.; Zhao, J.; Zhai, J.; Liu, X.; Chen, Z.J.; Li, W.; Du, Y. Down-regulation of CCR7 via AKT pathway and GATA2 inactivation suppressed trophoblast migration and invasion in recurrent spontaneous abortiondagger. Biol. Reprod. 2020, 102, 424–433. [Google Scholar] [CrossRef]
- Karakis, V.; Jabeen, M.; Britt, J.W.; Cordiner, A.; Mischler, A.; Li, F.; San Miguel, A.; Rao, B.M. Laminin switches terminal differentiation fate of human trophoblast stem cells under chemically defined culture conditions. J. Biol. Chem. 2023, 299, 104650. [Google Scholar] [CrossRef]
- Nayeem, S.B.; Arfuso, F.; Dharmarajan, A.; Keelan, J.A. Role of Wnt signalling in early pregnancy. Reprod. Fertil. Dev. 2016, 28, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, S.; Husslein, H.; Leisser, C.; Knofler, M. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 2007, 28 (Suppl. A), S97–S102. [Google Scholar] [CrossRef] [PubMed]
- Khong, Y.; Brosens, I. Defective deep placentation. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 301–311. [Google Scholar] [CrossRef]
- Jauniaux, E.; Moffett, A.; Burton, G.J. Placental Implantation Disorders. Obstet. Gynecol. Clin. North. Am. 2020, 47, 117–132. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knofler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Knofler, M.; Pollheimer, J. Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 2013, 4, 190. [Google Scholar] [CrossRef]
- Matsumoto, S.; Tanaka, S. Wnt signaling activation confers a syncytiotrophoblast progenitor state on trophoblast stem cells of cynomolgus monkeydagger. Biol. Reprod. 2024, 111, 1262–1281. [Google Scholar] [CrossRef]
- Shukla, V.; Moreno-Irusta, A.; Varberg, K.M.; Kuna, M.; Iqbal, K.; Galligos, A.M.; Aplin, J.D.; Choudhury, R.H.; Okae, H.; Arima, T.; et al. NOTUM-mediated WNT silencing drives extravillous trophoblast cell lineage development. Proc. Natl. Acad. Sci. USA 2024, 121, e2403003121. [Google Scholar] [CrossRef]
- Jiang, L.L.; Yang, D.L.; Han, Q.; Zhang, H.L.; Pan, M.; Yan, J.Y. LncRNA-NEAT1 blocks the Wnt/beta-catenin signaling pathway by targeting miR-217 to inhibit trophoblast cell migration and invasion. J. Assist. Reprod. Genet. 2024, 41, 2107–2115. [Google Scholar] [CrossRef]
- Lan, R.; Yu, Y.; Song, J.; Xue, M.; Gong, H. SFRP2 suppresses trophoblast cell migration by inhibiting the Wnt/beta-catenin pathway. Mol. Med. Rep. 2024, 29, 66. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, S.; Haslinger, P.; Sabri, A.; Leisser, C.; Otten, J.V.; Fiala, C.; Knofler, M. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology 2010, 151, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ran, Y.; Ma, Y.; Huang, H.; Chen, Y.; Qi, H. Elevated Serum SFRP5 Levels During Preeclampsia and Its Potential Association with Trophoblast Dysfunction via Wnt/beta-Catenin Suppression. Reprod. Sci. 2022, 29, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Gao, Y.; Wu, S.; Chen, J.; Ding, J.; Wang, Y.; Yang, J. Ubiquitin-specific protease 7 prevents recurrent spontaneous abortion by targeting enhancer of zeste homolog 2 to regulate trophoblast proliferation, apoptosis, migration, and invasion through the Wnt/beta-catenin pathwaydagger. Biol. Reprod. 2023, 109, 204–214. [Google Scholar] [CrossRef]
- Bao, H.; Liu, D.; Xu, Y.; Sun, Y.; Mu, C.; Yu, Y.; Wang, C.; Han, Q.; Liu, S.; Cai, H.; et al. Hyperactivated Wnt-beta-catenin signaling in the absence of sFRP1 and sFRP5 disrupts trophoblast differentiation through repression of Ascl2. BMC Biol. 2020, 18, 151. [Google Scholar] [CrossRef]
- Chronopoulou, E.; Koika, V.; Tsiveriotis, K.; Stefanidis, K.; Kalogeropoulos, S.; Georgopoulos, N.; Adonakis, G.; Kaponis, A. Wnt4, Wnt6 and beta-catenin expression in human placental tissue—Is there a link with first trimester miscarriage? Results from a pilot study. Reprod. Biol. Endocrinol. 2022, 20, 51. [Google Scholar] [CrossRef]
- Huang, L.; Ying, H.; Chen, Z.; Zhu, Y.L.; Gu, Y.; Hu, L.; Chen, D.; Zhong, N. Down-regulation of DKK1 and Wnt1/beta-catenin pathway by increased homeobox B7 resulted in cell differentiation suppression of intrauterine fetal growth retardation in human placenta. Placenta 2019, 80, 27–35. [Google Scholar] [CrossRef]
- Li, N.; Li, S.; Wang, Y.; Wang, J.; Wang, K.; Liu, X.; Li, Y.; Liu, J. Decreased expression of WNT2 in villi of unexplained recurrent spontaneous abortion patients may cause trophoblast cell dysfunction via downregulated Wnt/beta-catenin signaling pathway. Cell Biol. Int. 2017, 41, 898–907. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Yu, N.; Wei, S.; Liu, Z. Involvement of WNT2 in trophoblast cell behavior in preeclampsia development. Cell Cycle 2020, 19, 2207–2215. [Google Scholar] [CrossRef]
- Monkley, S.J.; Delaney, S.J.; Pennisi, D.J.; Christiansen, J.H.; Wainwright, B.J. Targeted disruption of the Wnt2 gene results in placentation defects. Development 1996, 122, 3343–3353. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhang, L.; Jia, L.; Wang, P.; Gao, Y. Association of Wnt2 and sFRP4 expression in the third trimester placenta in women with severe preeclampsia. Reprod. Sci. 2013, 20, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Ide, A.D.; Grainger, S. WNT9A and WNT9B in Development and Disease. Differentiation 2024, 142, 100820. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.; Willems, E.; Abdo, S.; Lambiv, L.; Leyns, L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev. Dyn. 2005, 233, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Gaccioli, F.; Dopierala, J.; Sovio, U.; Cook, E.; Volders, P.J.; Martens, L.; Kirk, P.D.W.; Richardson, S.; Smith, G.C.S.; et al. The RNA landscape of the human placenta in health and disease. Nat. Commun. 2021, 12, 2639. [Google Scholar] [CrossRef]
- Bei, Y.; Hogan, J.; Berkowitz, L.A.; Soto, M.; Rocheleau, C.E.; Pang, K.M.; Collins, J.; Mello, C.C. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev. Cell 2002, 3, 113–125. [Google Scholar] [CrossRef]
- Tong, Z.; Zhang, Y.; Guo, P.; Wang, W.; Chen, Q.; Jin, J.; Liu, S.; Yu, C.; Mo, P.; Zhang, L.; et al. Steroid receptor coactivator 1 promotes human hepatocellular carcinoma invasiveness through enhancing MMP-9. J. Cell Mol. Med. 2024, 28, e18171. [Google Scholar] [CrossRef]
- Triki, M.; Lapierre, M.; Cavailles, V.; Mokdad-Gargouri, R. Expression and role of nuclear receptor coregulators in colorectal cancer. World J. Gastroenterol. 2017, 23, 4480–4490. [Google Scholar] [CrossRef]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U.; Ellinger, A.; Burkard, T.R.; Fiala, C.; Pollheimer, J.; et al. Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta. Stem Cell Rep. 2018, 11, 537–551. [Google Scholar] [CrossRef]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 2018, 22, 50–63.e56. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Catalog Number |
|---|---|---|
| ALDH1A1 | 216 | Hs00946916_m1 |
| CAPN6 | 827 | Hs00560073_m1 |
| CCR7 | 1236 | Hs01013469_m1 |
| CDKN1C | 1028 | Hs00175938_m1 |
| DIO2 | 1734 | Hs05050546_s1 |
| DKK1 | 22943 | Hs00183740_m1 |
| FSTL3 | 10272 | Hs00610505_m1 |
| FOXO4 | 4303 | Hs00172973_m1 |
| HLA-G | 3135 | Hs00365950_g1 |
| LAMB3 | 3914 | Hs00165078_m1 |
| NCOA2 | 10499 | Hs00896109_m1 |
| SFRP1 | 6422 | Hs00610060_m1 |
| WNT2 | 7472 | Hs00608224_m1 |
| WNT4 | 54361 | Hs01573505_m1 |
| WNT6 | 7475 | Hs00362452_m1 |
| WNT 9A | 7483 | Hs01573829_m1 |
| HPRT | ThermoFisher Scientific Inc. | 4333768T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurya, V.K.; Popli, P.; Nikolai, B.C.; Lonard, D.M.; Kommagani, R.; O’Malley, B.W.; Lydon, J.P. Human Extravillous Trophoblasts Require SRC-2 for Sustained Viability, Migration, and Invasion. Cells 2025, 14, 1024. https://doi.org/10.3390/cells14131024
Maurya VK, Popli P, Nikolai BC, Lonard DM, Kommagani R, O’Malley BW, Lydon JP. Human Extravillous Trophoblasts Require SRC-2 for Sustained Viability, Migration, and Invasion. Cells. 2025; 14(13):1024. https://doi.org/10.3390/cells14131024
Chicago/Turabian StyleMaurya, Vineet K., Pooja Popli, Bryan C. Nikolai, David M. Lonard, Ramakrishna Kommagani, Bert W. O’Malley, and John P. Lydon. 2025. "Human Extravillous Trophoblasts Require SRC-2 for Sustained Viability, Migration, and Invasion" Cells 14, no. 13: 1024. https://doi.org/10.3390/cells14131024
APA StyleMaurya, V. K., Popli, P., Nikolai, B. C., Lonard, D. M., Kommagani, R., O’Malley, B. W., & Lydon, J. P. (2025). Human Extravillous Trophoblasts Require SRC-2 for Sustained Viability, Migration, and Invasion. Cells, 14(13), 1024. https://doi.org/10.3390/cells14131024






