Muscle Spatial Transcriptomic Reveals Heterogeneous Profiles in Juvenile Dermatomyositis and Persistence of Abnormal Signature After Remission
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Muscle Biopsies
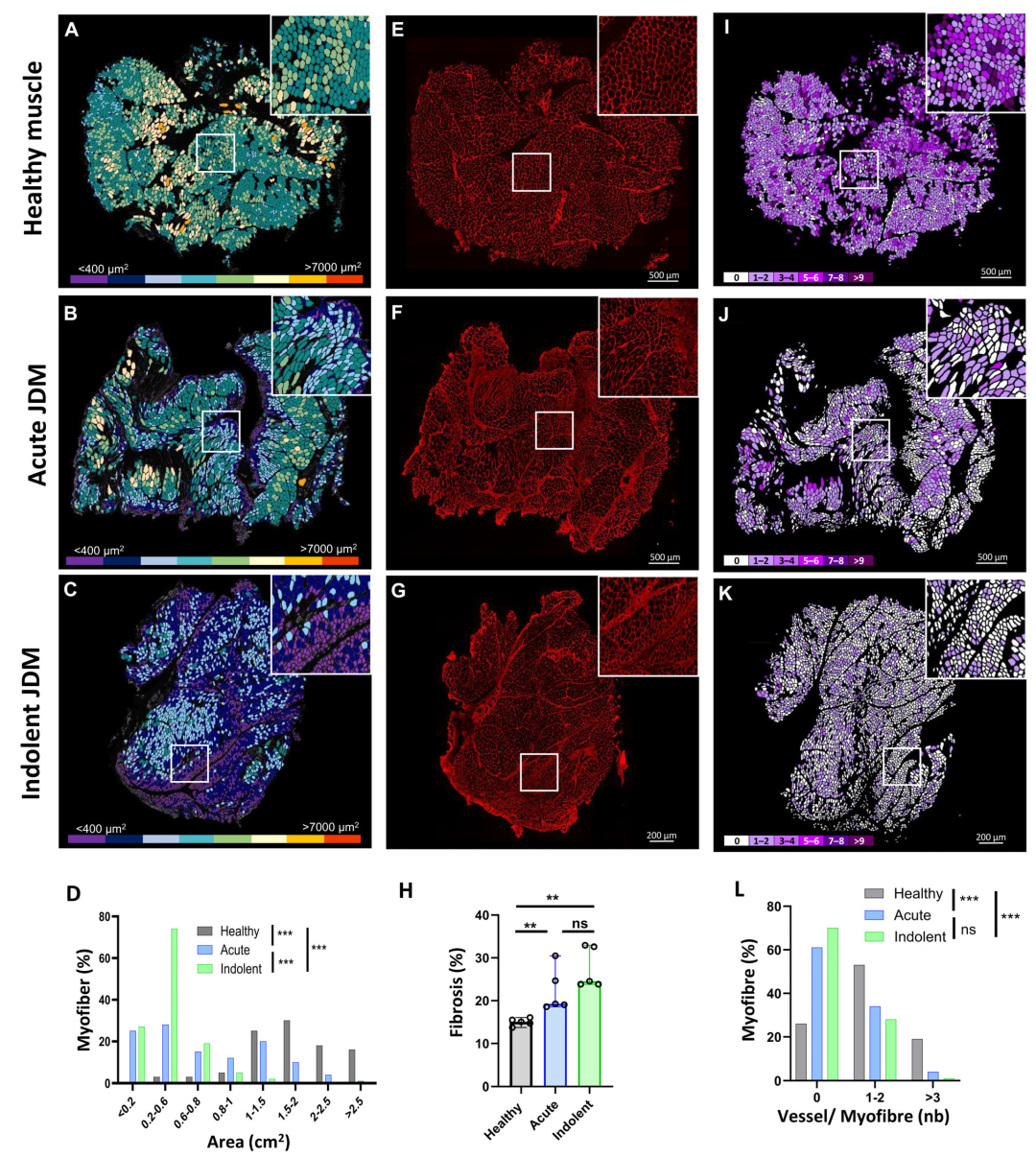
2.2. Methods for Histology, Immunohistochemistry and Morphometric Analysis
2.3. Visium Spatial Gene Expression Library Construction
2.4. Visium Data Processing and Analysis
3. Results
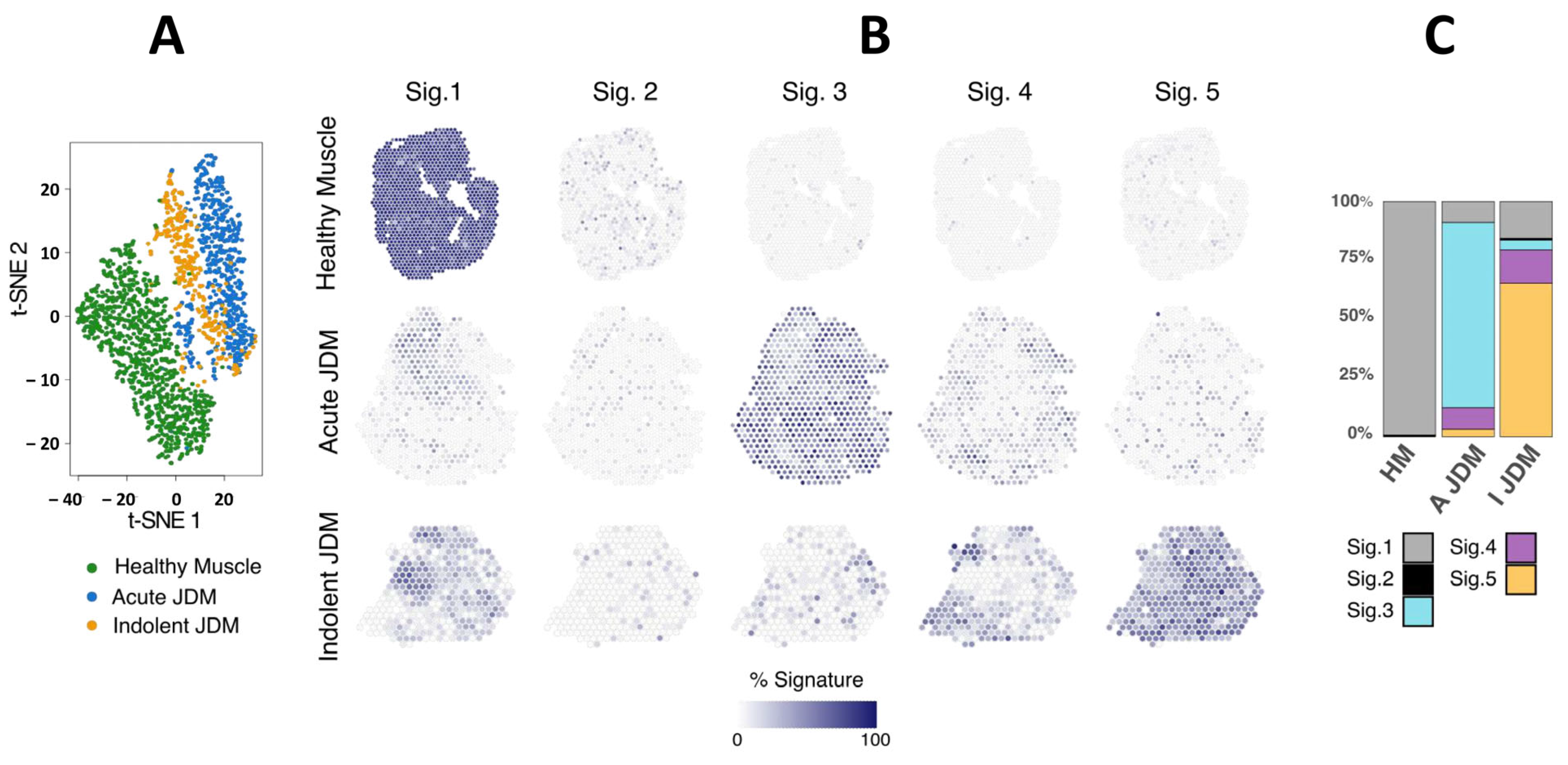
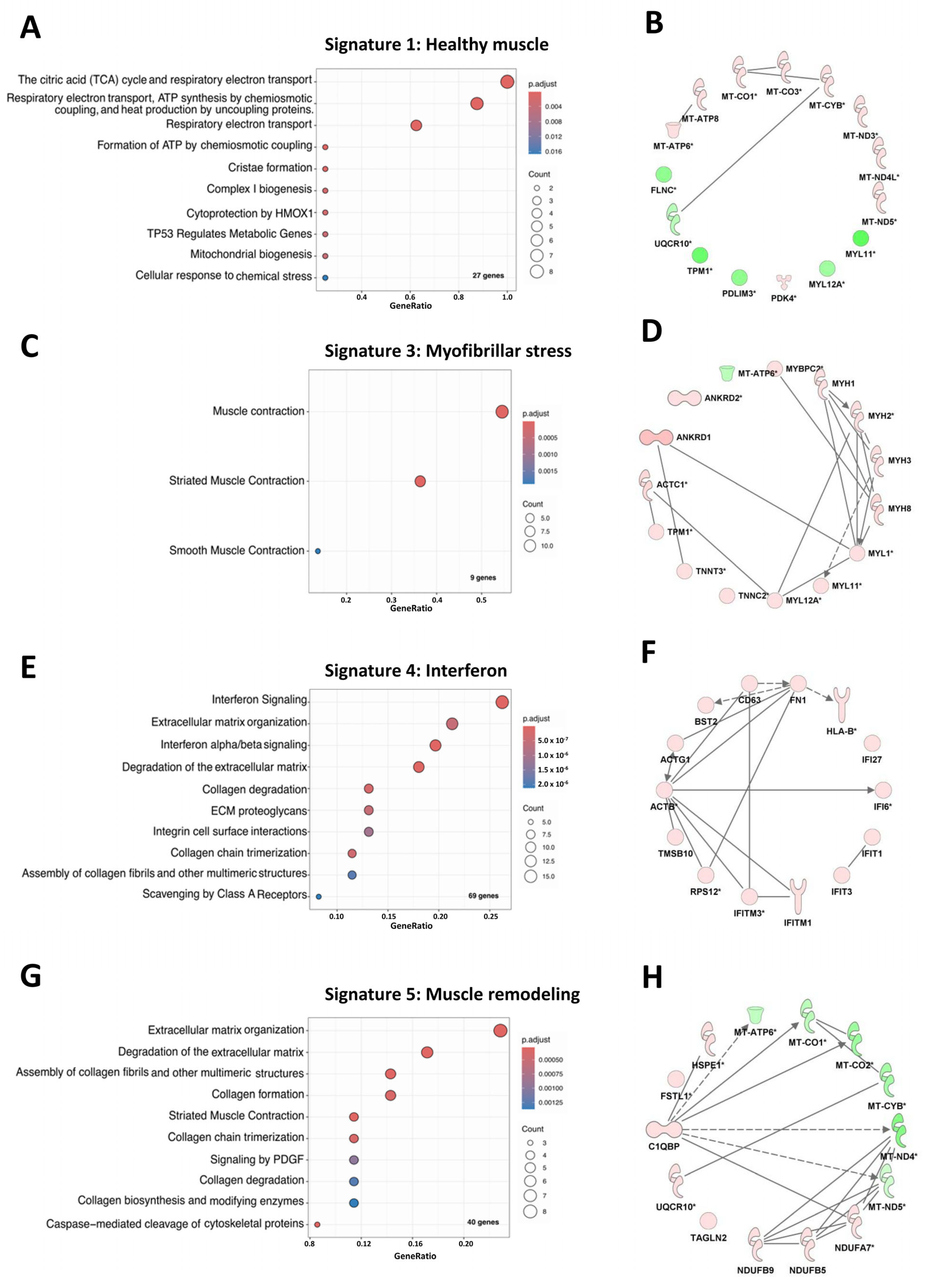
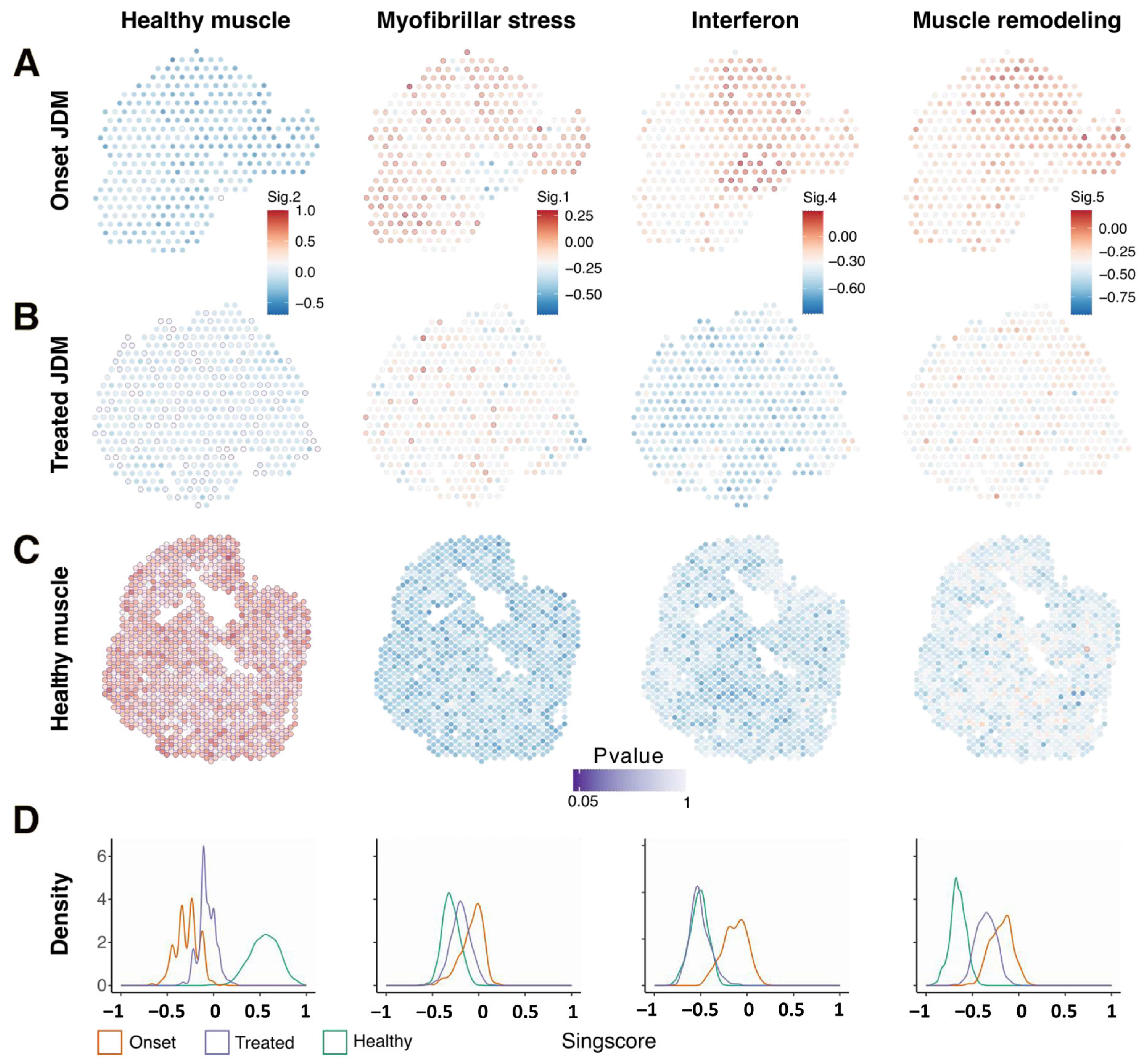
3.1. Spatial Transcriptomic Analysis Reveals Specific and Spatialized Muscle Signatures in JDM as Compared to Healthy Muscle
3.2. Muscles from Acute and Indolent JDM Could Be Distinguished by the Expression of a Predominant “Myofibrillar Stress” Signature or an “Muscle Remodeling” Signature Respectively but Shared “IFN Signaling” Signature
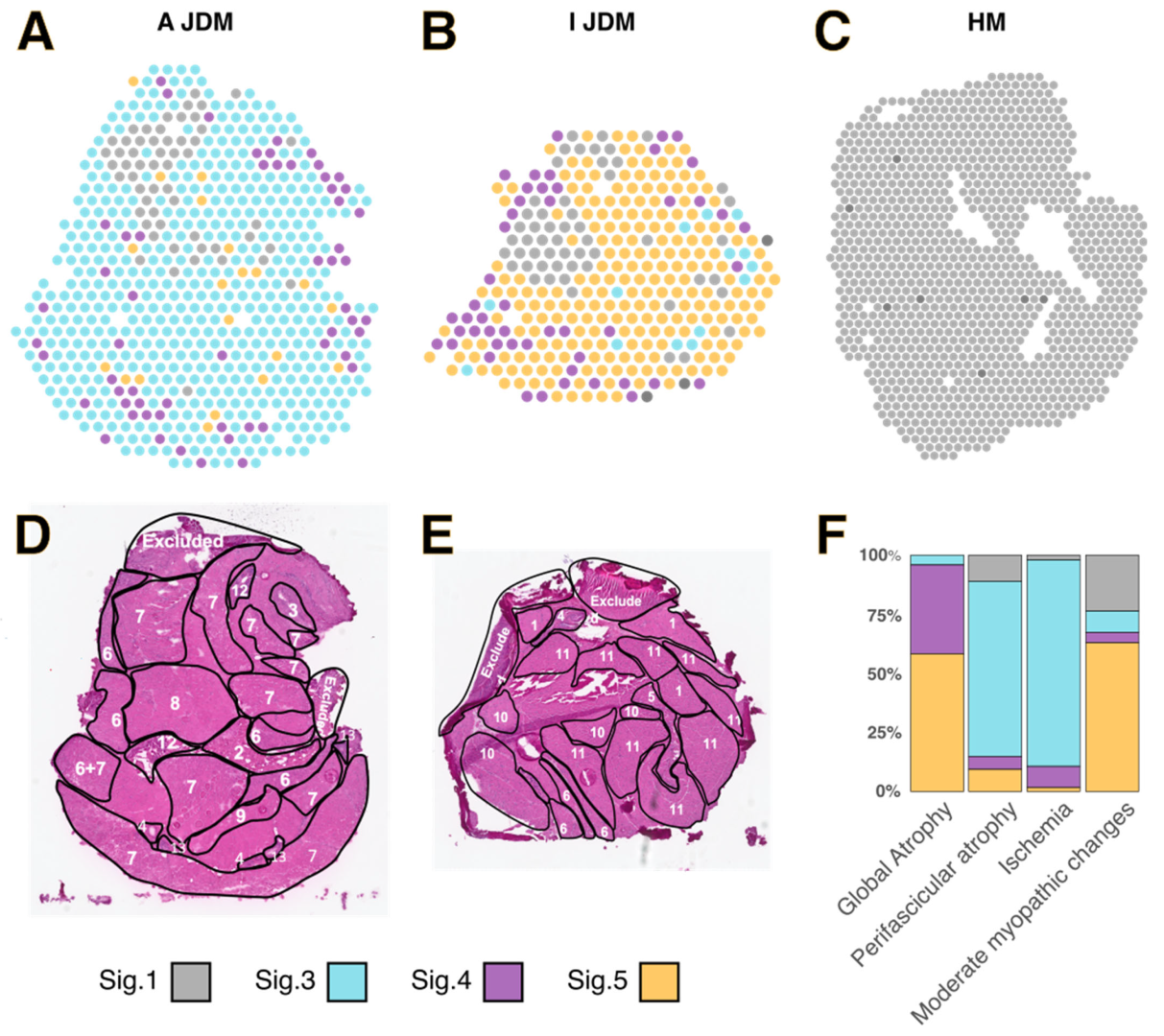
3.3. Spatialized Transcriptomic Pathological Signatures Are Associated with Myopathological Changes
3.4. Persistence of Myofibrillar Stress Signature and Muscle Remodeling Signature in the Muscle of a Patient in Two-Years Remission After JAK Inhibitor Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dabbak, I.; Rodero, M.P.; Aeschlimann, F.A.; Authier, F.-J.; Bodemer, C.; Quartier, P.; Bondet, V.; Charuel, J.-L.; Duffy, D.; Gitiaux, C.; et al. Efficacy and Tolerance of Corticosteroids and Methotrexate in Patients with Juvenile Dermatomyositis: A Retrospective Cohort Study. Rheumatology 2022, 61, 4514–4520. [Google Scholar] [CrossRef]
- Maccora, I.; Brunner, H.I.; Cassedy, A.; Altaye, M.; Quinlan-Waters, M.; Lovell, D.J.; Grom, A.; Angeles-Han, S.T. Achieving Medication-Free Remission in Patients With Juvenile Dermatomyositis. ACR Open Rheumatol. 2025, 7, e11751. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, W.; Goebel, H.-H.; Bader-Meunier, B.; Gitiaux, C. Inflammatory Myopathies in Childhood. Neuromuscul. Disord. 2021, 31, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Gallay, L.; Mouchiroud, G.; Chazaud, B. Interferon-Signature in Idiopathic Inflammatory Myopathies. Curr. Opin. Rheumatol. 2019, 31, 634–642. [Google Scholar] [CrossRef]
- Ward, J.M.; Ambatipudi, M.; O’Hanlon, T.P.; Smith, M.A.; de Los Reyes, M.; Schiffenbauer, A.; Rahman, S.; Zerrouki, K.; Miller, F.W.; Sanjuan, M.A.; et al. Shared and Distinctive Transcriptomic and Proteomic Pathways in Adult and Juvenile Dermatomyositis. Arthritis Rheumatol. 2023, 75, 2014–2026. [Google Scholar] [CrossRef]
- Wilkinson, M.G.L.; Moulding, D.; McDonnell, T.C.R.; Orford, M.; Wincup, C.; Ting, J.Y.J.; Otto, G.W.; Restuadi, R.; Kelberman, D.; Papadopoulou, C.; et al. Role of CD14+ Monocyte-Derived Oxidised Mitochondrial DNA in the Inflammatory Interferon Type 1 Signature in Juvenile Dermatomyositis. Ann. Rheum. Dis. 2023, 82, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Besançon, A.; Brochard, K.; Dupic, L.; Gitiaux, C.; Delville, M.; Krid, S.; Quartier, P.; Saire, E.; Salomon, R.; Talbotec, C.; et al. Presentations and Outcomes of Juvenile Dermatomyositis Patients Admitted to Intensive Care Units. Rheumatology 2017, 56, 1814–1816. [Google Scholar] [CrossRef]
- Gitiaux, C.; De Antonio, M.; Aouizerate, J.; Gherardi, R.K.; Guilbert, T.; Barnerias, C.; Bodemer, C.; Brochard-Payet, K.; Quartier, P.; Musset, L.; et al. Vasculopathy-Related Clinical and Pathological Features Are Associated with Severe Juvenile Dermatomyositis. Rheumatology 2016, 55, 470–479. [Google Scholar] [CrossRef]
- Gitiaux, C.; Kostallari, E.; Lafuste, P.; Authier, F.-J.; Christov, C.; Gherardi, R.K. Whole Microvascular Unit Deletions in Dermatomyositis. Ann. Rheum. Dis. 2012, 72, 445–452. [Google Scholar] [CrossRef]
- Mammen, A.L.; Allenbach, Y.; Stenzel, W.; Benveniste, O.; ENMC 239th Workshop Study Group. 239th ENMC International Workshop: Classification of Dermatomyositis, Amsterdam, The Netherlands, 14–16 December 2018. Neuromuscul. Disord. NMD 2020, 30, 70–92. [Google Scholar] [CrossRef]
- Varsani, H.; Charman, S.C.; Li, C.K.; Marie, S.K.N.; Amato, A.A.; Banwell, B.; Bove, K.E.; Corse, A.M.; Emslie-Smith, A.M.; Jacques, T.S.; et al. Validation of a Score Tool for Measurement of Histological Severity in Juvenile Dermatomyositis and Association with Clinical Severity of Disease. Ann. Rheum. Dis. 2013, 74, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Huang, F.; Atta, L.; Sahoo, A.; Fan, J. Reference-Free Cell Type Deconvolution of Multi-Cellular Pixel-Resolution Spatially Resolved Transcriptomics Data. Nat. Commun. 2022, 13, 2339. [Google Scholar] [CrossRef]
- Yu, G.; He, Q.-Y. ReactomePA: An R/Bioconductor Package for Reactome Pathway Analysis and Visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Chaigne, B.; Clary, G.; Le Gall, M.; Dumoitier, N.; Fernandez, C.; Lofek, S.; Chafey, P.; Moinzadeh, P.; Krieg, T.; Denton, C.P.; et al. Proteomic Analysis of Human Scleroderma Fibroblasts Response to Transforming Growth Factor-ß. Proteom. Clin. Appl. 2019, 13, e1800069. [Google Scholar] [CrossRef]
- Foroutan, M.; Bhuva, D.D.; Lyu, R.; Horan, K.; Cursons, J.; Davis, M.J. Single Sample Scoring of Molecular Phenotypes. BMC Bioinform. 2018, 19, 404. [Google Scholar] [CrossRef]
- Bhuva, D.D.; Cursons, J.; Davis, M.J. Stable Gene Expression for Normalisation and Single-Sample Scoring. Nucleic Acids Res. 2020, 48, e113. [Google Scholar] [CrossRef] [PubMed]
- John, C.R.; Watson, D.; Russ, D.; Goldmann, K.; Ehrenstein, M.; Pitzalis, C.; Lewis, M.; Barnes, M. M3C: Monte Carlo Reference-Based Consensus Clustering. Sci. Rep. 2020, 10, 1816. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Derfoul, A.; Pak, K.; Miller, F.W.; Milisenda, J.C.; Grau-Junyent, J.M.; Selva-O’Callaghan, A.; Carrion-Ribas, C.; Paik, J.J.; et al. Machine Learning Algorithms Reveal Unique Gene Expression Profiles in Muscle Biopsies from Patients with Different Types of Myositis. Ann. Rheum. Dis. 2020, 79, 1234–1242. [Google Scholar] [CrossRef]
- Kim, H.; Gunter-Rahman, F.; McGrath, J.A.; Lee, E.; de Jesus, A.A.; Targoff, I.N.; Huang, Y.; O’Hanlon, T.P.; Tsai, W.L.; Gadina, M.; et al. Expression of Interferon-Regulated Genes in Juvenile Dermatomyositis versus Mendelian Autoinflammatory Interferonopathies. Arthritis Res. Ther. 2020, 22, 69. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Muñoz-Braceras, S.; Casal-Dominguez, M.; Pak, K.; Torres-Ruiz, J.; Musai, J.; Dell’Orso, S.; Naz, F.; Islam, S.; Gutierrez-Cruz, G.; et al. Pathological Autoantibody Internalisation in Myositis. Ann. Rheum. Dis. 2024, 83, 1549–1560. [Google Scholar] [CrossRef]
- Chen, X.; Lian, D.; Zeng, H. Single-Cell Profiling of Peripheral Blood and Muscle Cells Reveals Inflammatory Features of Juvenile Dermatomyositis. Front. Cell Dev. Biol. 2023, 11, 1166017. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, A.; Halasz, L.; Oleksak, D.; Wei, X.; Nagy, G.; Tzerpos, P.; Conrad, T.; Hammers, D.W.; Sweeney, H.L.; Nagy, L. Spatiotemporal Transcriptomic Mapping of Regenerative Inflammation in Skeletal Muscle Reveals a Dynamic Multilayered Tissue Architecture. J. Clin. Investig. 2024, 134, e173858. [Google Scholar] [CrossRef] [PubMed]
- Heezen, L.G.M.; Abdelaal, T.; van Putten, M.; Aartsma-Rus, A.; Mahfouz, A.; Spitali, P. Spatial Transcriptomics Reveal Markers of Histopathological Changes in Duchenne Muscular Dystrophy Mouse Models. Nat. Commun. 2023, 14, 4909. [Google Scholar] [CrossRef]
- Lequain, H.; Dégletagne, C.; Streichenberger, N.; Valantin, J.; Simonet, T.; Schaeffer, L.; Sève, P.; Leblanc, P. Spatial Transcriptomics Reveals Signatures of Histopathological Changes in Muscular Sarcoidosis. Cells 2023, 12, 2747. [Google Scholar] [CrossRef] [PubMed]
- Wischnewski, S.; Thäwel, T.; Ikenaga, C.; Kocharyan, A.; Lerma-Martin, C.; Zulji, A.; Rausch, H.-W.; Brenner, D.; Thomas, L.; Kutza, M.; et al. Cell Type Mapping of Inflammatory Muscle Diseases Highlights Selective Myofiber Vulnerability in Inclusion Body Myositis. Nat. Aging 2024, 4, 969–983. [Google Scholar] [CrossRef]
- Abad, C.; Pinal-Fernandez, I.; Guillou, C.; Bourdenet, G.; Drouot, L.; Cosette, P.; Giannini, M.; Debrut, L.; Jean, L.; Bernard, S.; et al. IFNγ Causes Mitochondrial Dysfunction and Oxidative Stress in Myositis. Nat. Commun. 2024, 15, 5403. [Google Scholar] [CrossRef]
- Kleino, I.; Frolovaitė, P.; Suomi, T.; Elo, L.L. Computational Solutions for Spatial Transcriptomics. Comput. Struct. Biotechnol. J. 2022, 20, 4870–4884. [Google Scholar] [CrossRef]
- Huang, C.; Zhong, Q.; Lian, W.; Kang, T.; Hu, J.; Lei, M. Ankrd1 Inhibits the FAK/Rho-GTPase/F-Actin Pathway by Downregulating ITGA6 Transcriptional to Regulate Myoblast Functions. J. Cell. Physiol. 2024, 239, e31359. [Google Scholar] [CrossRef]
- Boskovic, S.; Marín Juez, R.; Stamenkovic, N.; Radojkovic, D.; Stainier, D.Y.; Kojic, S. The Stress Responsive Gene Ankrd1a Is Dynamically Regulated during Skeletal Muscle Development and Upregulated Following Cardiac Injury in Border Zone Cardiomyocytes in Adult Zebrafish. Gene 2021, 792, 145725. [Google Scholar] [CrossRef]
- Arimura, T.; Bos, J.M.; Sato, A.; Kubo, T.; Okamoto, H.; Nishi, H.; Harada, H.; Koga, Y.; Moulik, M.; Doi, Y.L.; et al. Cardiac Ankyrin Repeat Protein Gene (ANKRD1) Mutations in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 334–342. [Google Scholar] [CrossRef]
- Piroddi, N.; Pesce, P.; Scellini, B.; Manzini, S.; Ganzetti, G.S.; Badi, I.; Menegollo, M.; Cora, V.; Tiso, S.; Cinquetti, R.; et al. Myocardial Overexpression of ANKRD1 Causes Sinus Venosus Defects and Progressive Diastolic Dysfunction. Cardiovasc. Res. 2020, 116, 1458–1472. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, L.; Ghosh, S.; Di Cicco, E.; Isma, J.; Tavernari, D.; Samarkina, A.; Ostano, P.; Youssef, M.K.; Simon, C.; Dotto, G.P. ANKRD1 Is a Mesenchymal-Specific Driver of Cancer-Associated Fibroblast Activation Bridging Androgen Receptor Loss to AP-1 Activation. Nat. Commun. 2024, 15, 1038. [Google Scholar] [CrossRef]
- Han, S.; Guo, J.; Kong, C.; Li, J.; Lin, F.; Zhu, J.; Wang, T.; Chen, Q.; Liu, Y.; Hu, H.; et al. ANKRD1 Aggravates Renal Ischaemia–reperfusion Injury via Promoting TRIM25-Mediated Ubiquitination of ACSL3. Clin. Transl. Med. 2024, 14, e70024. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Hong, Y.; Krol, P.; Al Obaidi, M.; Pilkington, C.; Wedderburn, L.R.; Brogan, P.A.; Eleftheriou, D. The Vasculopathy of Juvenile Dermatomyositis: Endothelial Injury, Hypercoagulability, and Increased Arterial Stiffness. Arthritis Rheumatol. 2021, 73, 1253–1266. [Google Scholar] [CrossRef]
- Mattiotti, A.; Prakash, S.; Barnett, P.; van den Hoff, M.J.B. Follistatin-like 1 in Development and Human Diseases. Cell. Mol. Life Sci. 2018, 75, 2339–2354. [Google Scholar] [CrossRef]
- Maruyama, S.; Nakamura, K.; Papanicolaou, K.N.; Sano, S.; Shimizu, I.; Asaumi, Y.; van den Hoff, M.J.; Ouchi, N.; Recchia, F.A.; Walsh, K. Follistatin-like 1 Promotes Cardiac Fibroblast Activation and Protects the Heart from Rupture. EMBO Mol. Med. 2016, 8, 949–966. [Google Scholar] [CrossRef]
- Ouchi, N.; Oshima, Y.; Ohashi, K.; Higuchi, A.; Ikegami, C.; Izumiya, Y.; Walsh, K. Follistatin-like 1, a Secreted Muscle Protein, Promotes Endothelial Cell Function and Revascularization in Ischemic Tissue through a Nitric-Oxide Synthase-Dependent Mechanism. J. Biol. Chem. 2008, 283, 32802–32811. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Tanaka, M.; Usui, T.; Kawabata, D.; Shiomi, A.; Iguchi-Hashimoto, M.; Shimizu, M.; Yukawa, N.; Yoshifuji, H.; Nojima, T.; et al. Follistatin-Related Protein/Follistatin-like 1 Evokes an Innate Immune Response via CD14 and Toll-like Receptor 4. FEBS Lett. 2012, 586, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Chaly, Y.; Fu, Y.; Marinov, A.; Hostager, B.; Yan, W.; Campfield, B.; Kellum, J.A.; Bushnell, D.; Wang, Y.; Vockley, J.; et al. Follistatin-like Protein 1 Enhances NLRP3 Inflammasome-Mediated IL-1β Secretion from Monocytes and Macrophages. Eur. J. Immunol. 2014, 44, 1467–1479. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Jiang, D.; Dong, Y.; Liu, Y.; Zhang, S.; Guo, J.; Qi, C.; Zhao, C.; Jiang, F.; et al. Targeting FSTL1 for Multiple Fibrotic and Systemic Autoimmune Diseases. Mol. Ther. 2021, 29, 347–364. [Google Scholar] [CrossRef]
- Hou, C.; Durrleman, C.; Periou, B.; Barnerias, C.; Bodemer, C.; Desguerre, I.; Quartier, P.; Melki, I.; Rice, G.I.; Rodero, M.P.; et al. From Diagnosis to Prognosis: Revisiting the Meaning of Muscle ISG15 Overexpression in Juvenile Inflammatory Myopathies. Arthritis Rheumatol. 2021, 73, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Rodero, M.P.; Pelleau, S.; Welfringer-Morin, A.; FJDM Study Group; Duffy, D.; Melki, I.; Bader-Meunier, B.; FJDM Study Group. Onset and Relapse of Juvenile Dermatomyositis Following Asymptomatic SARS-CoV-2 Infection. J. Clin. Immunol. 2022, 42, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; El-Hallak, M.; Dedeoglu, F.; Zurakowski, D.; Fuhlbrigge, R.C.; Sundel, R.P. Complete and Sustained Remission of Juvenile Dermatomyositis Resulting from Aggressive Treatment. Arthritis Rheum. 2009, 60, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, K.; Ujiie, H.; Yokozeki, M.; Tsukinaga, I.; Ito, M.; Shikano, T.; Suzuki, A.; Tozawa, Y.; Kobayashi, I. Recurrence of Juvenile Dermatomyositis 8 Years after Remission. JAAD Case Rep. 2017, 3, 29–32. [Google Scholar] [CrossRef]
- Rodero, M.P.; Decalf, J.; Bondet, V.; Hunt, D.; Rice, G.I.; Werneke, S.; McGlasson, S.L.; Alyanakian, M.-A.; Bader-Meunier, B.; Barnerias, C.; et al. Detection of Interferon Alpha Protein Reveals Differential Levels and Cellular Sources in Disease. J. Exp. Med. 2017, 214, 1547–1555. [Google Scholar] [CrossRef]
| Sample ID | JDM1 | JDM2 | JDM3 Onset | JDM3 Treated |
|---|---|---|---|---|
| Age at sample, years | 13 | 5 | 13 | 15 |
| Sex | F | F | F | F |
| Disease duration at time of biopsy, months | 1 | 4 | 1 | 24 |
| Myositis specific antibody | NXP2 | 0 | NXP2 | NXP2 |
| Disease activity at time of biopsy: | ||||
| CMAS (0–52) | 2 | 30/46 | 2 | 52 |
| MMT (0–80) | 47 | NR | 38 | 80 |
| CK U/L | 3020 | 1500 | 862 | 100 |
| Characteristic skin rash | 1 | 1 | 1 | 0 |
| Gottron’s papules | 1 | 1 | 1 | 0 |
| Telangiectasiae | 1 | 0 | 1 | 0 |
| Subcutaneous limb edema | 1 | 0 | 1 | 0 |
| Skin ulcerations | 0 | 0 | 0 | 0 |
| Interstitial lung disease | 0 | 0 | 0 | 0 |
| Cardiovascular involvement | 0 | 0 | 1 | 0 |
| Gastrointestinal involvement | 0 | 0 | 1 | 0 |
| Muscle biopsy score data * | ||||
| Inflammatory domain (0–12) | 8 | 4 | 12 | 0 |
| Muscle fibre domain (0–10) | 7 | 7 | 9 | 2 |
| Vascular domain (0–3) | 3 | 1 | 3 | 0 |
| Connective tissu domain (0–2) | 2 | 1 | 2 | 1 |
| Total biopsy score (0–27) | 20 | 13 | 26 | 3 |
| Histopathology visual analogue score (VAS) (0–10) | 7 | 4 | 8 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tragin, M.; Degrelle, S.A.; Periou, B.; Bader-Meunier, B.; Barnerias, C.; Bodemer, C.; Desguerre, I.; Rodero, M.P.; Authier, F.J.; Gitiaux, C. Muscle Spatial Transcriptomic Reveals Heterogeneous Profiles in Juvenile Dermatomyositis and Persistence of Abnormal Signature After Remission. Cells 2025, 14, 939. https://doi.org/10.3390/cells14120939
Tragin M, Degrelle SA, Periou B, Bader-Meunier B, Barnerias C, Bodemer C, Desguerre I, Rodero MP, Authier FJ, Gitiaux C. Muscle Spatial Transcriptomic Reveals Heterogeneous Profiles in Juvenile Dermatomyositis and Persistence of Abnormal Signature After Remission. Cells. 2025; 14(12):939. https://doi.org/10.3390/cells14120939
Chicago/Turabian StyleTragin, Margot, Séverine A. Degrelle, Baptiste Periou, Brigitte Bader-Meunier, Christine Barnerias, Christine Bodemer, Isabelle Desguerre, Mathieu Paul Rodero, François Jérôme Authier, and Cyril Gitiaux. 2025. "Muscle Spatial Transcriptomic Reveals Heterogeneous Profiles in Juvenile Dermatomyositis and Persistence of Abnormal Signature After Remission" Cells 14, no. 12: 939. https://doi.org/10.3390/cells14120939
APA StyleTragin, M., Degrelle, S. A., Periou, B., Bader-Meunier, B., Barnerias, C., Bodemer, C., Desguerre, I., Rodero, M. P., Authier, F. J., & Gitiaux, C. (2025). Muscle Spatial Transcriptomic Reveals Heterogeneous Profiles in Juvenile Dermatomyositis and Persistence of Abnormal Signature After Remission. Cells, 14(12), 939. https://doi.org/10.3390/cells14120939






