Cytokinesis in Suspension: A Distinctive Trait of Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Synchronization
2.3. Live-Cell Imaging
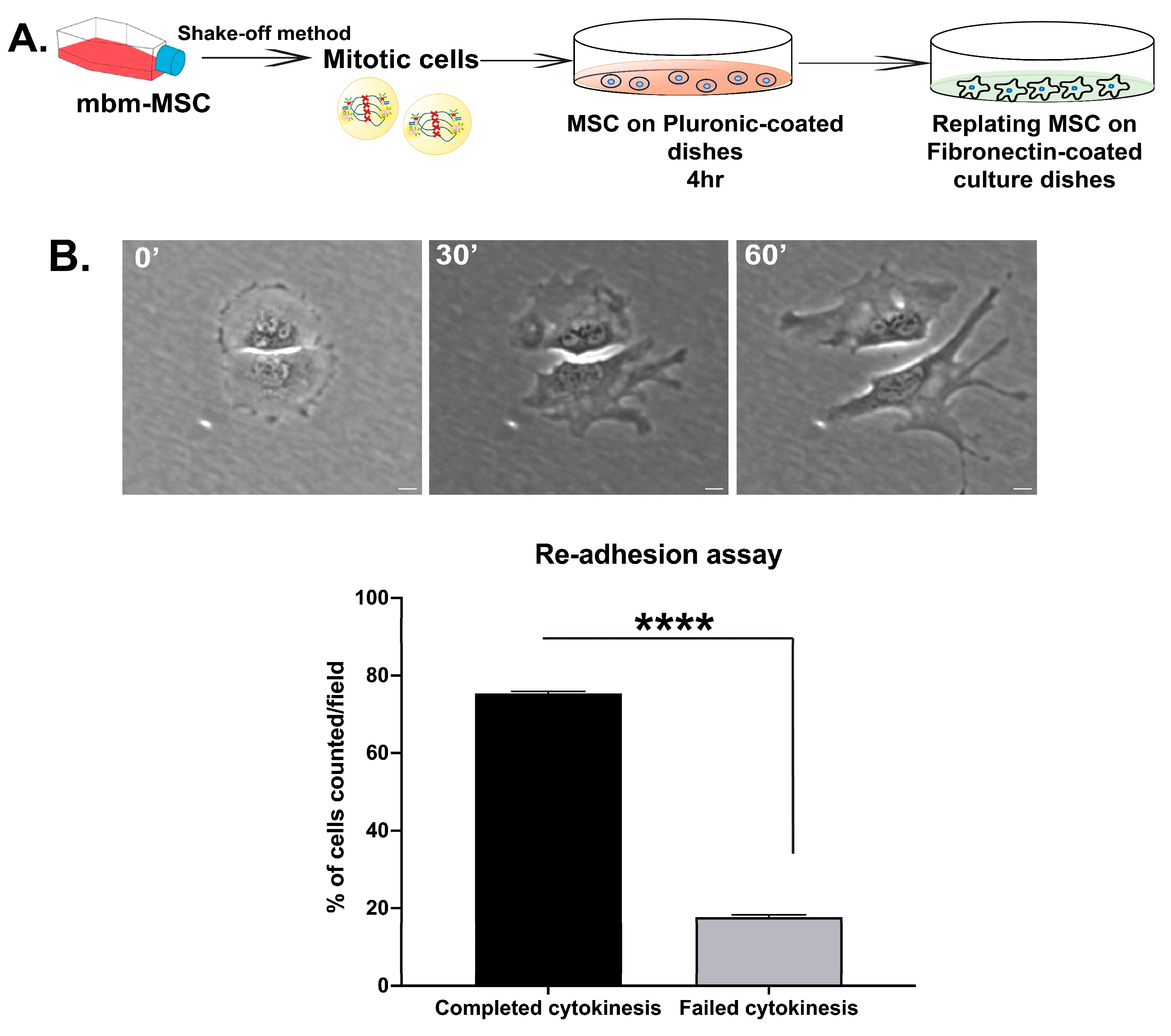
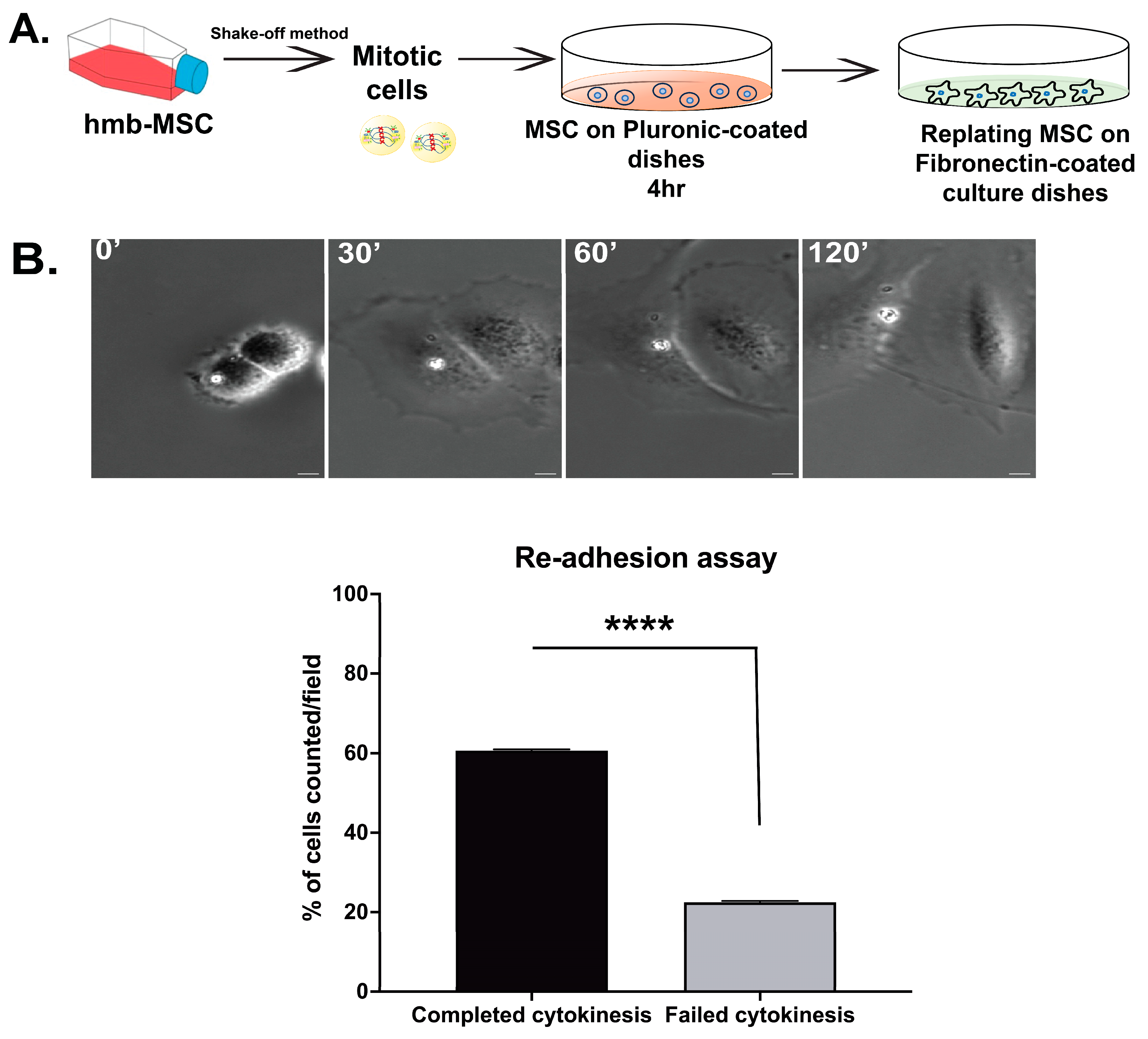
2.4. Re-Adhesion Assay
2.5. Immunofluorescence Staining
2.6. Quantification of Cytokinesis Completion and Failure
2.7. Statistical Analysis
3. Results
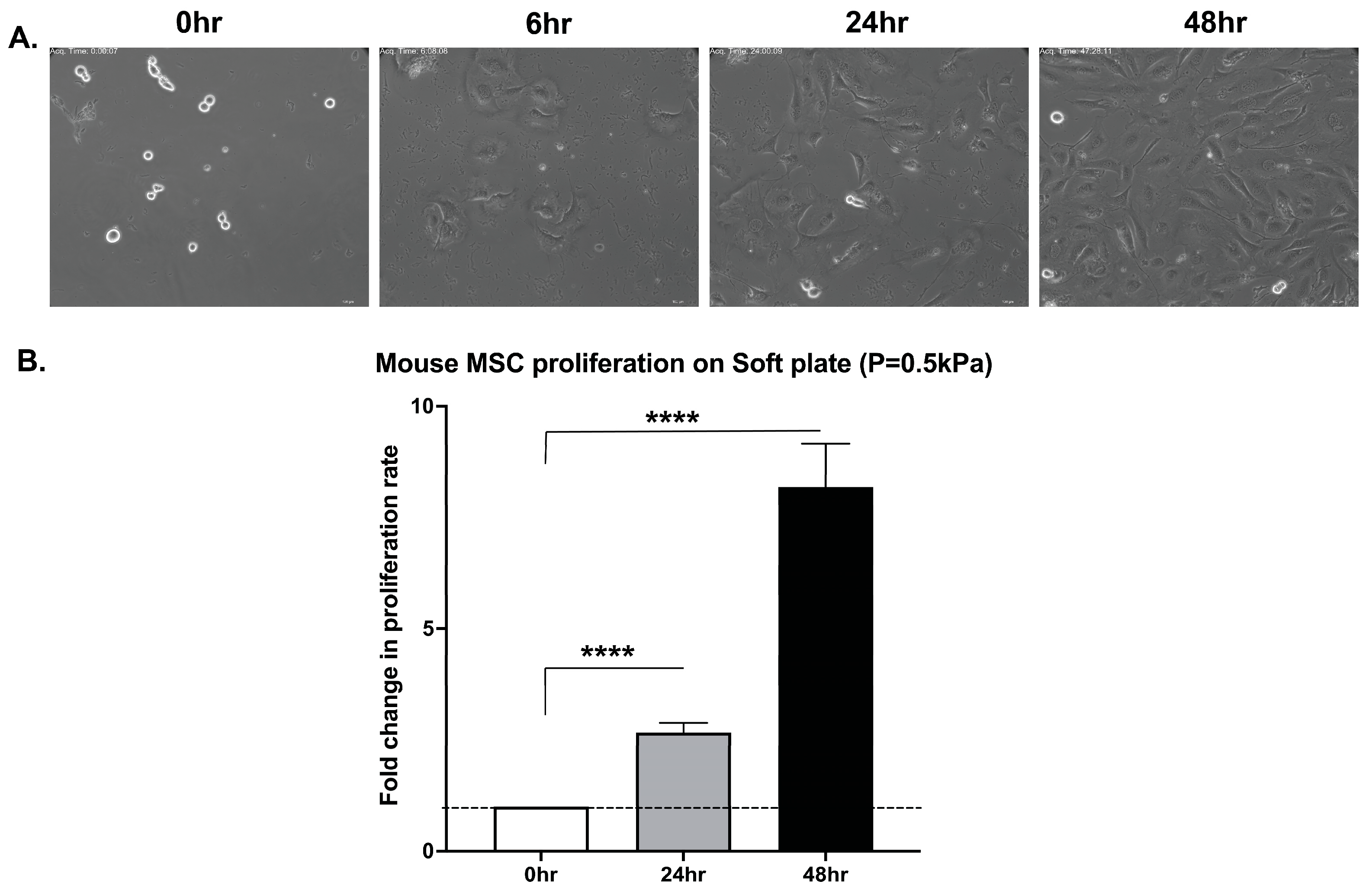
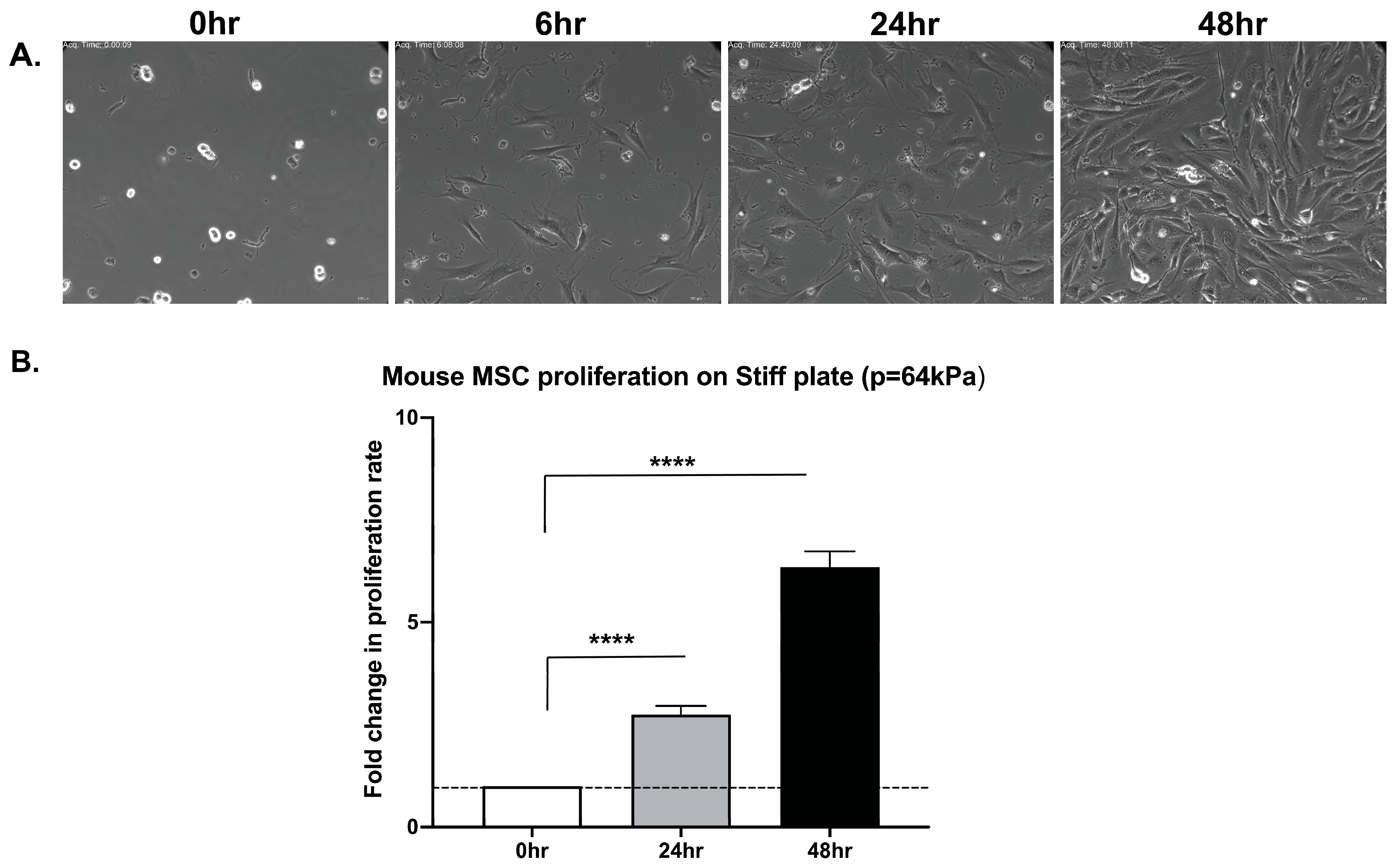
3.1. Primary Mouse Bone Marrow MSCs Undergo Cytokinesis Independent of Matrix Stiffness
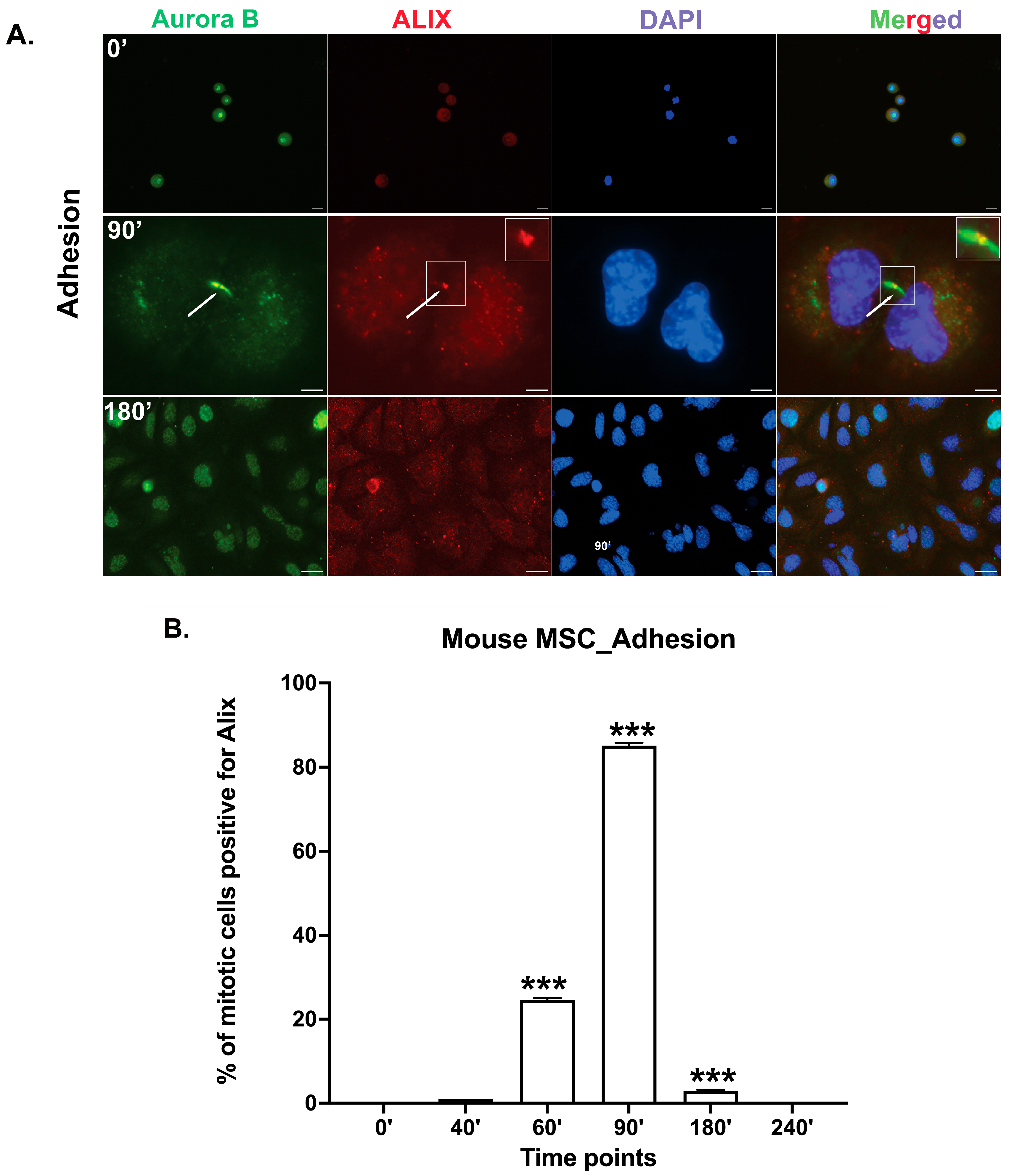
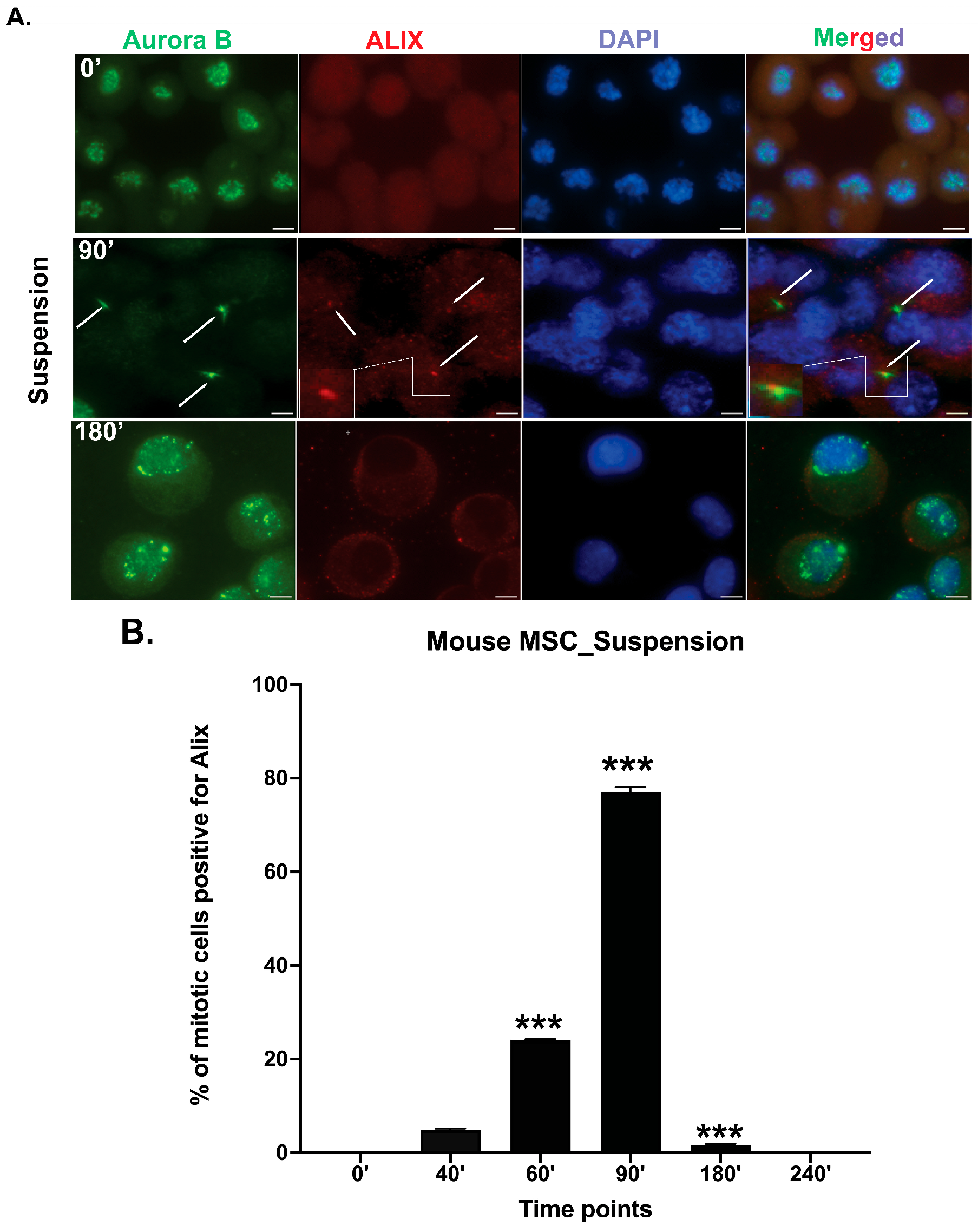
3.2. Midbody Maturation and Abscission Occur in Mbm-MSCs Without Adhesion to ECM
3.3. Abscission Completes in Suspension-Cultured Mbm-MSCs as Revealed by Re-Adhesion Assay
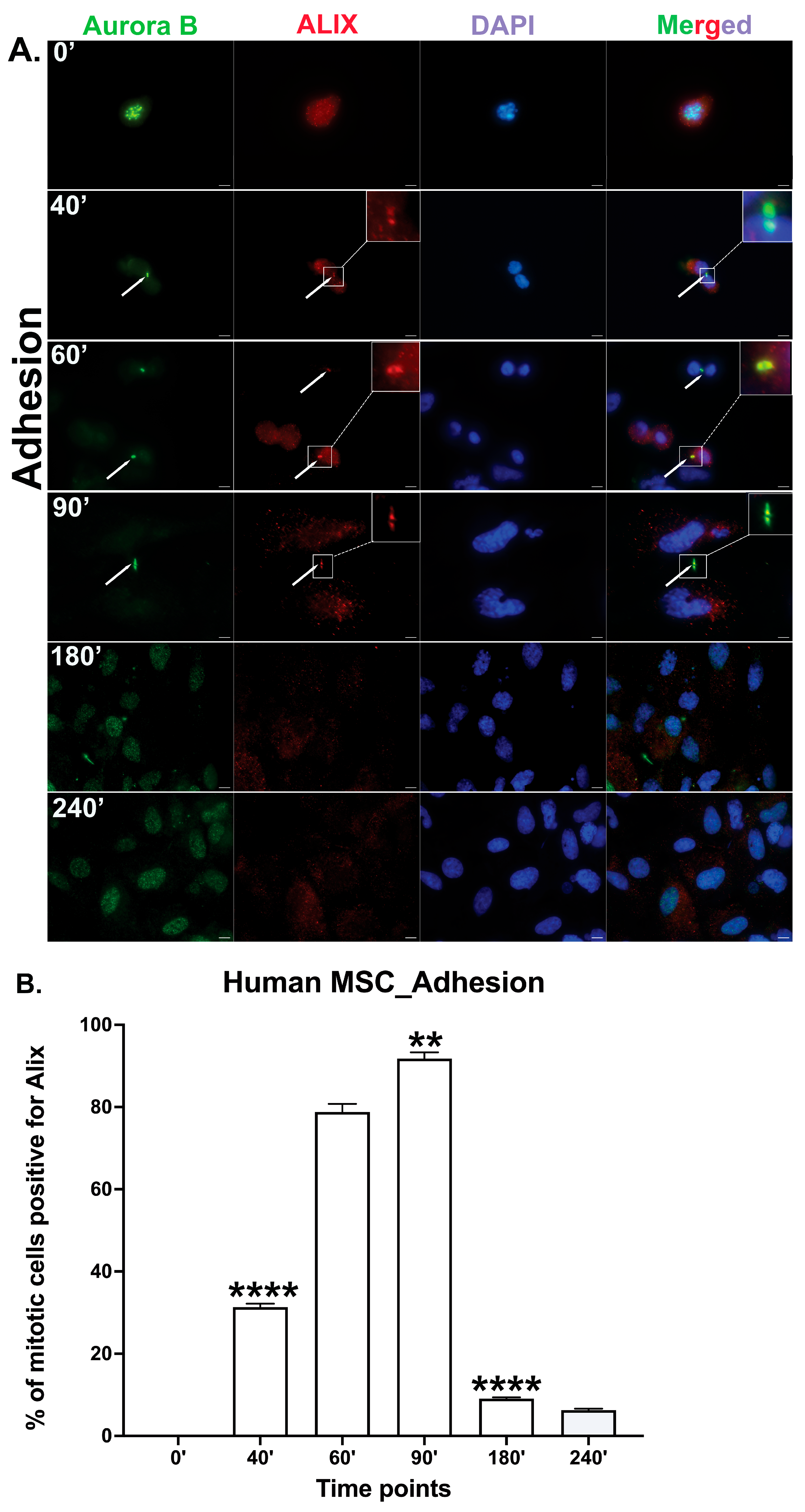
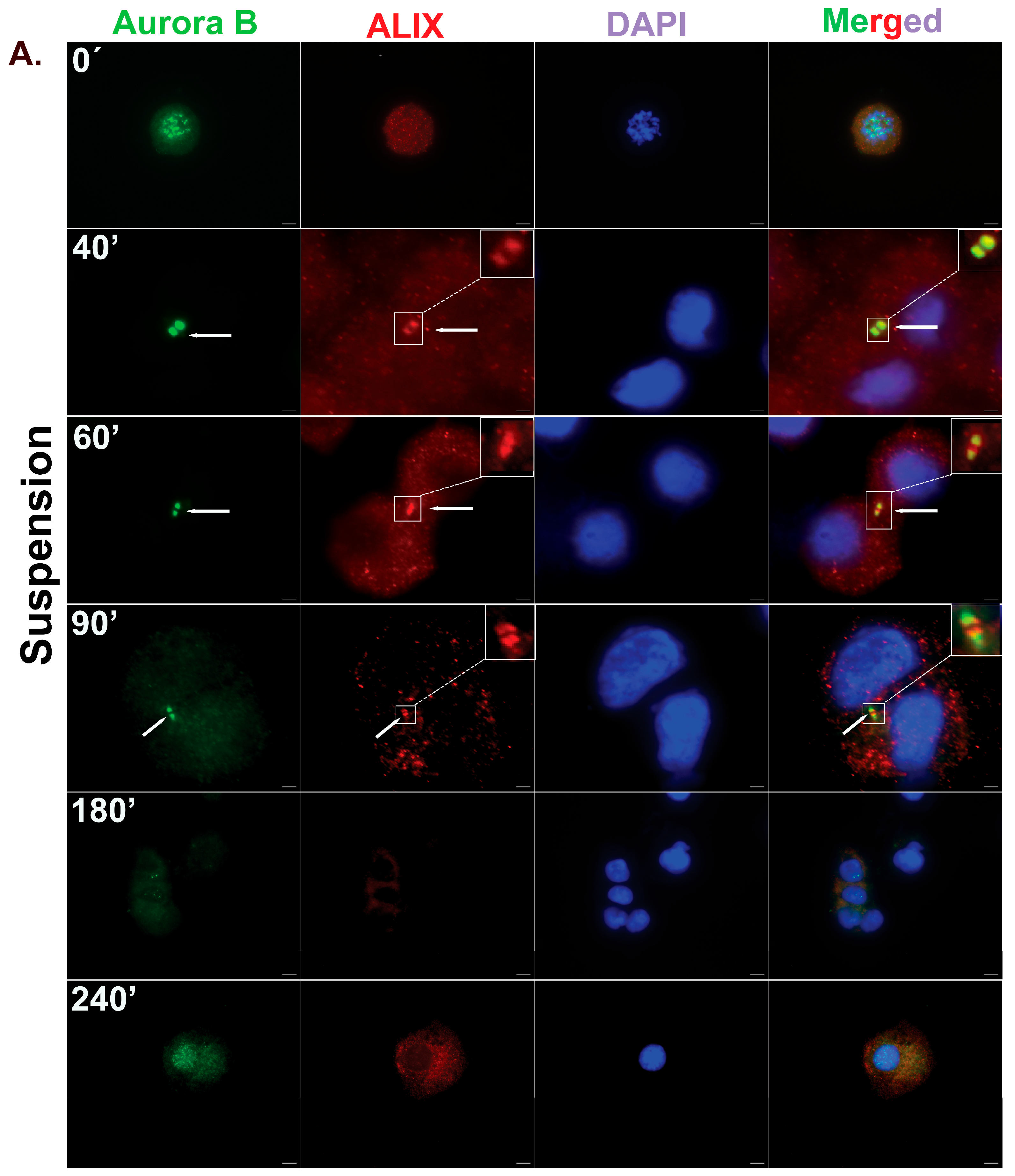
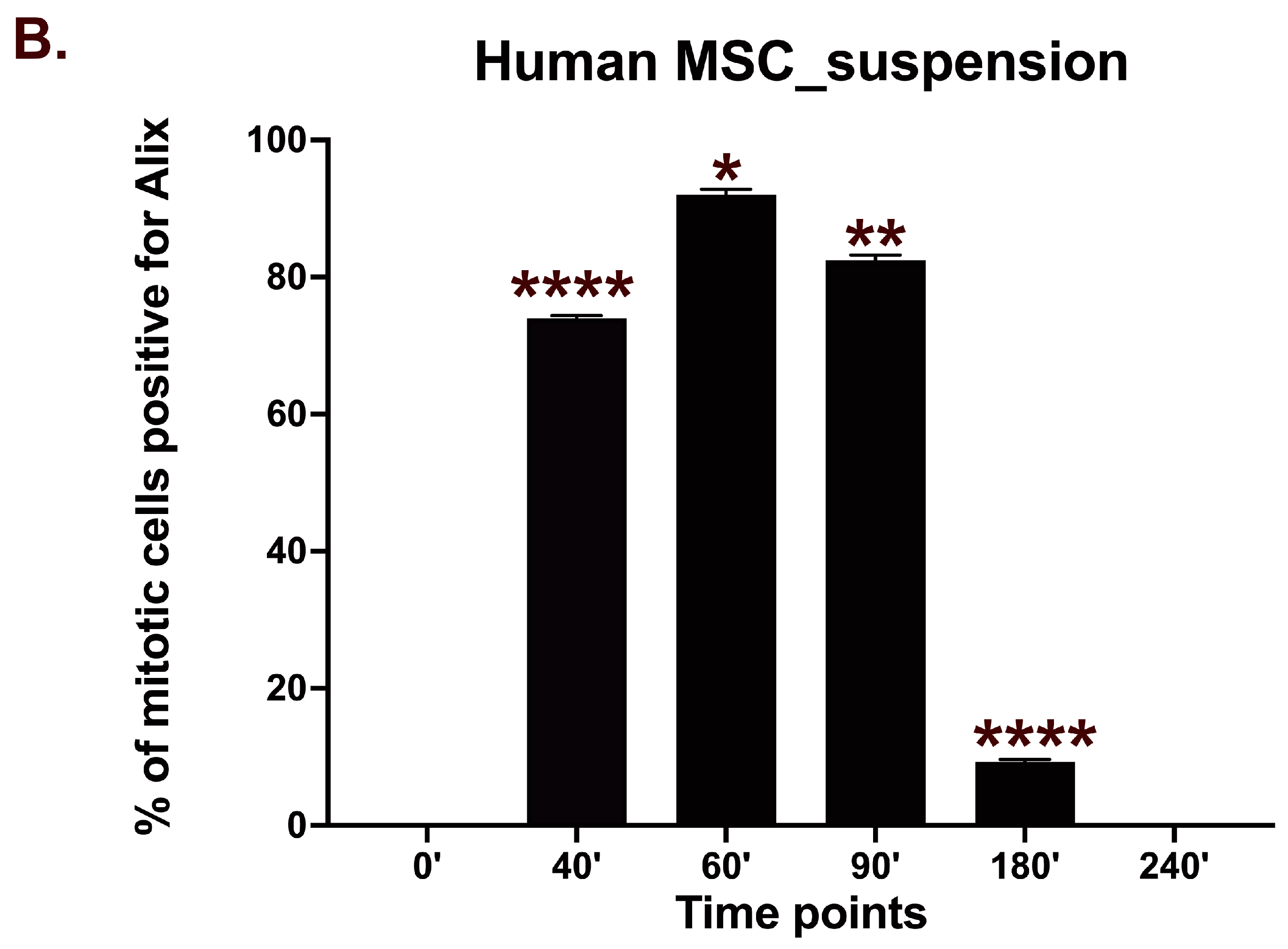
3.4. Human Bone Marrow MSCs Complete Cytokinesis in Suspension Independently of Adhesion
3.5. Re-Adhesion Assay Confirms Abscission Completion in Suspension-Cultured Hbm-MSCs
4. Discussion
5. Conclusions
- (1)
- The capacity to perform cytokinesis independently of adhesion is a trait that distinguishes MSCs from fibroblasts.
- (2)
- While the ability to form single-cell-derived spheres is a functional characteristic of some stem cells including cancer stem cells, but not for MSCs, the capacity for adhesion-independent cytokinesis may offer a more general trait of stemness.
- (3)
- MSCs proliferate well on compliant surfaces and do not exhibit the stress seen on rigid surfaces.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal stem cell |
| ALIX | Apoptosis-linked gene 2-interacting protein |
| mbm | Mouse bone marrow |
| hbm | Human bone marrow |
References
- Walma, D.A.C.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef] [PubMed]
- Vachon, P.H. Integrin signaling, cell survival, and anoikis: Distinctions, differences, and differentiation. J. Signal Transduct. 2011, 2011, 738137. [Google Scholar] [CrossRef]
- Moreno-Layseca, P.; Streuli, C.H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Dontu, G.; Wicha, M.S. Survival of mammary stem cells in suspension culture: Implications for stem cell biology and neoplasia. J. Mammary Gland Biol. Neoplasia 2005, 10, 75–86. [Google Scholar] [CrossRef]
- Cole, A.J.; Fayomi, A.P.; Anyaeche, V.I.; Bai, S.; Buckanovich, R.J. An evolving paradigm of cancer stem cell hierarchies: Therapeutic implications. Theranostics 2020, 10, 3083–3098. [Google Scholar] [CrossRef] [PubMed]
- Kamranvar, S.A.; Rani, B.; Johansson, S. Cell Cycle Regulation by Integrin-Mediated Adhesion. Cells 2022, 11, 2521. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Shackleton, M.; Sabel, M.S.; Fullen, D.R.; Johnson, T.M.; Morrison, S.J. Efficient tumour formation by single human melanoma cells. Nature 2008, 456, 593–598. [Google Scholar] [CrossRef]
- Gupta, P.B.; Chaffer, C.L.; Weinberg, R.A. Cancer stem cells: Mirage or reality? Nat. Med. 2009, 15, 1010–1012. [Google Scholar] [CrossRef]
- Lens, S.M.A.; Medema, R.H. Cytokinesis defects and cancer. Nat. Rev. Cancer 2019, 19, 32–45. [Google Scholar] [CrossRef]
- Rani, B.; Gupta, D.K.; Johansson, S.; Kamranvar, S.A. Contribution of integrin adhesion to cytokinetic abscission and genomic integrity. Front. Cell Dev. Biol. 2022, 10, 1048717. [Google Scholar] [CrossRef]
- Ozugergin, I.; Piekny, A. Diversity is the spice of life: An overview of how cytokinesis regulation varies with cell type. Front. Cell Dev. Biol. 2022, 10, 1007614. [Google Scholar] [CrossRef] [PubMed]
- La Torre, M.; Burla, R.; Saggio, I. Preserving Genome Integrity: Unveiling the Roles of ESCRT Machinery. Cells 2024, 13, 1307. [Google Scholar] [CrossRef] [PubMed]
- Kamranvar, S.A.; Gupta, D.K.; Huang, Y.; Gupta, R.K.; Johansson, S. Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody. Oncotarget 2016, 7, 30820–30830. [Google Scholar] [CrossRef] [PubMed]
- Addi, C.; Presle, A.; Fremont, S.; Cuvelier, F.; Rocancourt, M.; Milin, F.; Schmutz, S.; Chamot-Rooke, J.; Douche, T.; Duchateau, M.; et al. The Flemmingsome reveals an ESCRT-to-membrane coupling via ALIX/syntenin/syndecan-4 required for completion of cytokinesis. Nat. Commun. 2020, 11, 1941. [Google Scholar] [CrossRef]
- Pust, S.; Brech, A.; Wegner, C.S.; Stenmark, H.; Haglund, K. Vesicle-mediated transport of ALIX and ESCRT-III to the intercellular bridge during cytokinesis. Cell. Mol. Life Sci. 2023, 80, 235. [Google Scholar] [CrossRef]
- Christ, L.; Wenzel, E.M.; Liestol, K.; Raiborg, C.; Campsteijn, C.; Stenmark, H. ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J. Cell Biol. 2016, 212, 499–513. [Google Scholar] [CrossRef]
- Komine, A.; Abe, M.; Saeki, T.; Terakawa, T.; Uchida, C.; Uchida, T. Establishment of adipose-derived mesenchymal stem cell lines from a p53-knockout mouse. Biochem. Biophys. Res. Commun. 2012, 426, 468–474. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Qian, H.; Le Blanc, K.; Sigvardsson, M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J. Biol. Chem. 2012, 287, 25795–25807. [Google Scholar] [CrossRef]
- Qian, H.; Badaloni, A.; Chiara, F.; Stjernberg, J.; Polisetti, N.; Nihlberg, K.; Consalez, G.G.; Sigvardsson, M. Molecular characterization of prospectively isolated multipotent mesenchymal progenitors provides new insight into the cellular identity of mesenchymal stem cells in mouse bone marrow. Mol. Cell. Biol. 2013, 33, 661–677. [Google Scholar] [CrossRef]
- Fox, M.H.; Read, R.A.; Bedford, J.S. Comparison of synchronized Chinese hamster ovary cells obtained by mitotic shake-off, hydroxyurea, aphidicolin, or methotrexate. Cytometry 1987, 8, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Acebron, I.; Righetto, R.D.; Schoenherr, C.; de Buhr, S.; Redondo, P.; Culley, J.; Rodriguez, C.F.; Daday, C.; Biyani, N.; Llorca, O.; et al. Structural basis of Focal Adhesion Kinase activation on lipid membranes. EMBO J. 2020, 39, e104743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, J.; Bromberger, T.; Holly, A.; Lu, F.; Liu, H.; Sun, K.; Klapproth, S.; Hirbawi, J.; Byzova, T.V.; et al. Structure of Rap1b bound to talin reveals a pathway for triggering integrin activation. Nat. Commun. 2017, 8, 1744. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Sambandamoorthy, S.; Mathew-Steiner, S.; Varney, S.; Zuidema, J.M.; Gilbert, R.J.; Van De Water, L.; LaFlamme, S.E. Matrix compliance and the regulation of cytokinesis. Biol. Open 2015, 4, 885–892. [Google Scholar] [CrossRef]
- Carmena, M.; Wheelock, M.; Funabiki, H.; Earnshaw, W.C. The chromosomal passenger complex (CPC): From easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 2012, 13, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Kamranvar, S.A.; Du, J.; Liu, L.; Johansson, S. Septin and Ras regulate cytokinetic abscission in detached cells. Cell Div. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.T.; Ingram, P.; Bai, S.; O’Hayer, P.; Chung, J.; Yoon, E.; Jackson, T.; Buckanovich, R.J. Sampling from single-cell observations to predict tumor cell growth in-vitro and in-vivo. Oncotarget 2017, 8, 111176–111189. [Google Scholar] [CrossRef]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97. [Google Scholar] [CrossRef]
- Budeus, B.; Unger, K.; Hess, J.; Sentek, H.; Klein, D. Comparative computational analysis to distinguish mesenchymal stem cells from fibroblasts. Front. Immunol. 2023, 14, 1270493. [Google Scholar] [CrossRef]
- Chippalkatti, R.; Abankwa, D. Promotion of cancer cell stemness by Ras. Biochem. Soc. Trans. 2021, 49, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Munoz, N.; Tsai, A.C.; Logan, T.M.; Ma, T. Metabolic Reconfiguration Supports Reacquisition of Primitive Phenotype in Human Mesenchymal Stem Cell Aggregates. Stem Cells 2017, 35, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Zhang, M.; Tao, E.; Wang, J.; Wei, N.; Lu, Y.; Liu, Q.; Hao, K.; Zhou, F.; Wang, G. Pharmacokinetic characteristics of mesenchymal stem cells in translational challenges. Signal Transduct. Target. Ther. 2024, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef]
- Zhong, C.; Chrzanowska-Wodnicka, M.; Brown, J.; Shaub, A.; Belkin, A.M.; Burridge, K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 1998, 141, 539–551. [Google Scholar] [CrossRef]
- Wennerberg, K.; Lohikangas, L.; Gullberg, D.; Pfaff, M.; Johansson, S.; Fassler, R. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J. Cell Biol. 1996, 132, 227–238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, B.; Qian, H.; Johansson, S. Cytokinesis in Suspension: A Distinctive Trait of Mesenchymal Stem Cells. Cells 2025, 14, 932. https://doi.org/10.3390/cells14120932
Rani B, Qian H, Johansson S. Cytokinesis in Suspension: A Distinctive Trait of Mesenchymal Stem Cells. Cells. 2025; 14(12):932. https://doi.org/10.3390/cells14120932
Chicago/Turabian StyleRani, Bhavna, Hong Qian, and Staffan Johansson. 2025. "Cytokinesis in Suspension: A Distinctive Trait of Mesenchymal Stem Cells" Cells 14, no. 12: 932. https://doi.org/10.3390/cells14120932
APA StyleRani, B., Qian, H., & Johansson, S. (2025). Cytokinesis in Suspension: A Distinctive Trait of Mesenchymal Stem Cells. Cells, 14(12), 932. https://doi.org/10.3390/cells14120932






