A Novel Modulator of Resistance for Oxaliplatin-Based Therapy for Colorectal Cancer: The ESCRT Family Member VPS4A
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
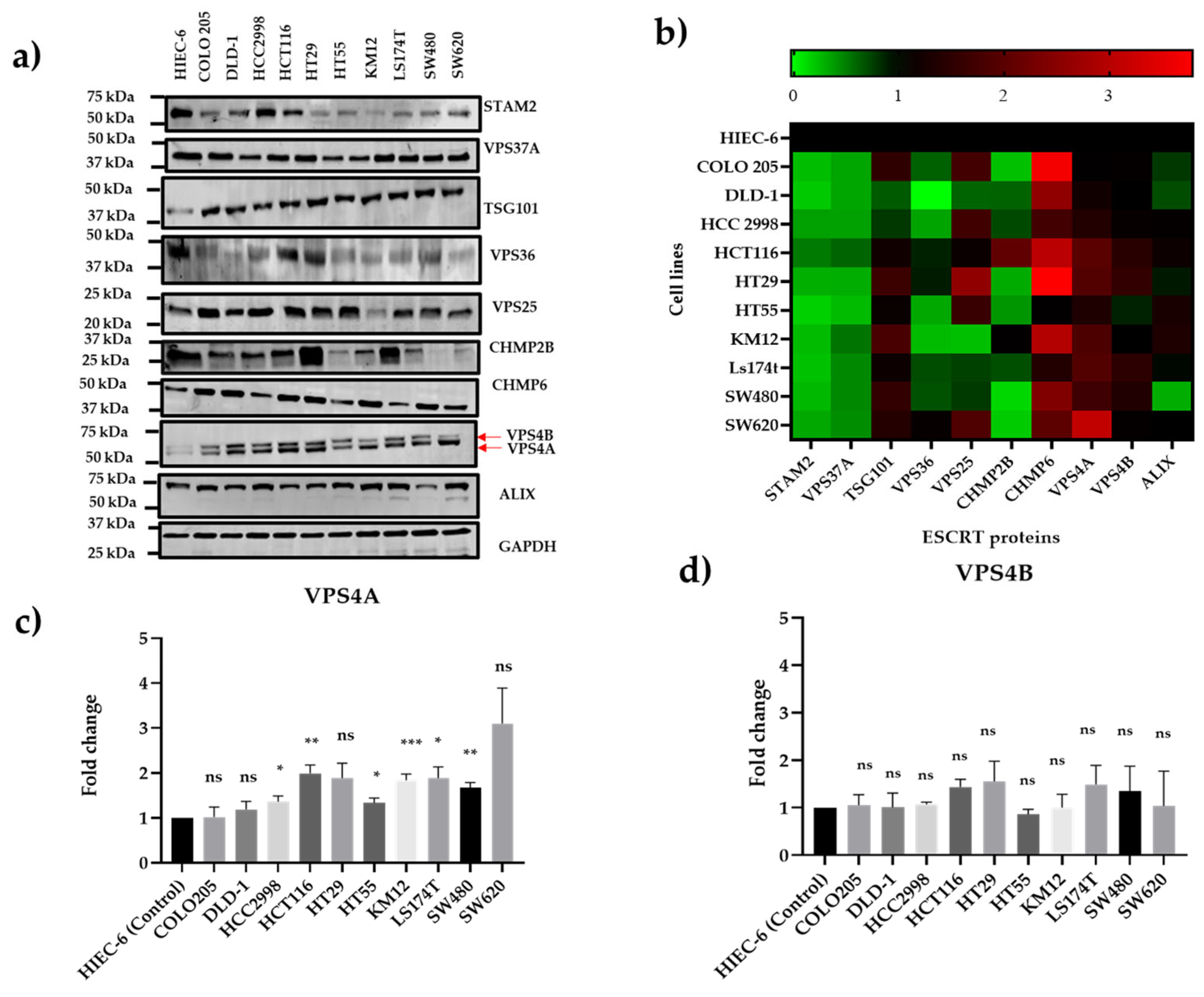
2.2. Immunoblotting
2.3. Clinical Material
2.4. Immunohistochemistry
2.5. Microscopy, Image Capture, and Analysis
2.6. Cell Knockdown
2.7. EGFR Assay
2.8. Development of Oxaliplatin-Resistant Human CRC Sublines
2.9. Chemosensitivity Assay
2.10. RNA Sequencing
2.11. Statistical Analysis
3. Results
3.1. VPS4A Is Highly Expressed Across a Panel of CRC Cell Lines
3.2. VPS4A Overexpressed in CRC Samples
3.3. Knockdown of VPS4A Expression Leads to Loss of ESCRT Function
3.4. Reduction of VPS4A Expression Sensitizes Cells to Exposure to Oxaliplatin and Other Commonly Used Treatments for CRC
3.5. VPS4A Expression Is Altered in Oxa-Resistant Cell Lines
3.6. Aloperine, an Inhibitor of VPS4A, Modulates Resistance to Oxaliplatin in Both Wild-Type and Oxa-Resistant Cell Lines
3.7. Decreased Expression of the Drug Efflux Transporter MRP2 (ABCC2) as a Result of the Knockdown of VPS4A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dazio, G.; Epistolio, S.; Frattini, M.; Saletti, P. Recent and Future Strategies to Overcome Resistance to Targeted Therapies and Immunotherapies in Metastatic Colorectal Cancer. J. Clin. Med. 2022, 11, 7523. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.; Manogaran, P. Mechanisms and Strategies to Overcome Drug Resistance in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 1988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers 2022, 14, 2928. [Google Scholar] [CrossRef]
- Arnesano, F.; Natile, G. Interference between copper transport systems and platinum drugs. Semin. Cancer Biol. 2021, 76, 173–188. [Google Scholar] [CrossRef]
- Marini, M.; Titiz, M.; Souza Monteiro de Araújo, D.; Geppetti, P.; Nassini, R.; De Logu, F. TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches. Biomolecules 2023, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Stefańska, K.; Józkowiak, M.; Angelova Volponi, A.; Shibli, J.A.; Golkar-Narenji, A.; Antosik, P.; Bukowska, D.; Piotrowska-Kempisty, H.; Mozdziak, P.; Dzięgiel, P.; et al. The Role of Exosomes in Human Carcinogenesis and Cancer Therapy-Recent Findings from Molecular and Clinical Research. Cells 2023, 12, 356. [Google Scholar] [CrossRef]
- Hurley, J.H. ESCRTs are everywhere. Embo J. 2015, 34, 2398–2407. [Google Scholar] [CrossRef]
- Stuffers, S.; Brech, A.; Stenmark, H. ESCRT proteins in physiology and disease. Exp. Cell Res. 2009, 315, 1619–1626. [Google Scholar] [CrossRef]
- Huang, L.J.; Zhan, S.T.; Pan, Y.Q.; Bao, W.; Yang, Y. The role of Vps4 in cancer development. Front. Oncol. 2023, 13, 1203359. [Google Scholar] [CrossRef]
- Chen, X.R.; Tan, X.Y.; Zhang, Z.L.; Yuan, J.S.; Song, W.Q. ESCRT may function as a tumor biomarker, transitioning from pan-cancer analysis to validation within breast cancer. Front. Immunol. 2025, 16, 1531940. [Google Scholar] [CrossRef]
- Manteghi, S.; Gingras, M.C.; Kharitidi, D.; Galarneau, L.; Marques, M.; Yan, M.; Cencic, R.; Robert, F.; Paquet, M.; Witcher, M.; et al. Haploinsufficiency of the ESCRT Component HD-PTP Predisposes to Cancer. Cell Rep. 2016, 15, 1893–1900. [Google Scholar] [CrossRef]
- Sadler, J.B.A.; Wenzel, D.M.; Strohacker, L.K.; Guindo-Martínez, M.; Alam, S.L.; Mercader, J.M.; Torrents, D.; Ullman, K.S.; Sundquist, W.I.; Martin-Serrano, J. A cancer-associated polymorphism in ESCRT-III disrupts the abscission checkpoint and promotes genome instability. Proc. Natl. Acad. Sci. USA 2018, 115, E8900–E8908. [Google Scholar] [CrossRef]
- Behan, F.M.; Iorio, F.; Picco, G.; Gonçalves, E.; Beaver, C.M.; Migliardi, G.; Santos, R.; Rao, Y.; Sassi, F.; Pinnelli, M.; et al. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature 2019, 568, 511–516. [Google Scholar] [CrossRef]
- McDonald, E.R.; de Weck, A.; Schlabach, M.R.; Billy, E.; Mavrakis, K.J.; Hoffman, G.R.; Belur, D.; Castelletti, D.; Frias, E.; Gampa, K.; et al. Project DRIVE: A Compendium of Cancer Dependencies and Synthetic Lethal Relationships Uncovered by Large-Scale, Deep RNAi Screening. Cell 2017, 170, 577–592.e510. [Google Scholar] [CrossRef]
- Szymańska, E.; Nowak, P.; Kolmus, K.; Cybulska, M.; Goryca, K.; Derezińska-Wołek, E.; Szumera-Ciećkiewicz, A.; Brewińska-Olchowik, M.; Grochowska, A.; Piwocka, K.; et al. Synthetic lethality between VPS4A and VPS4B triggers an inflammatory response in colorectal cancer. EMBO Mol. Med. 2020, 12, e10812. [Google Scholar] [CrossRef]
- Bernareggi, D.; Xie, Q.; Prager, B.C.; Yun, J.; Cruz, L.S.; Pham, T.V.; Kim, W.; Lee, X.; Coffey, M.; Zalfa, C.; et al. CHMP2A regulates tumor sensitivity to natural killer cell-mediated cytotoxicity. Nat. Commun. 2022, 13, 1899. [Google Scholar] [CrossRef]
- Hattori, T.; Takahashi, Y.; Chen, L.; Tang, Z.; Wills, C.A.; Liang, X.; Wang, H.G. Targeting the ESCRT-III component CHMP2A for noncanonical Caspase-8 activation on autophagosomal membranes. Cell Death Differ. 2021, 28, 657–670. [Google Scholar] [CrossRef]
- Delrue, I.; Pan, Q.; Baczmanska, A.K.; Callens, B.W.; Verdoodt, L.L.M. Determination of the Selection Capacity of Antibiotics for Gene Selection. Biotechnol. J. 2018, 13, e1700747. [Google Scholar] [CrossRef]
- Munro, T.P.; Pilbrough, W.; Hughes, B.S.; Gray, P.P. 1.11—Cell Line Isolation and Design. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2011; pp. 144–153. [Google Scholar]
- Kantamneni, S.; Holman, D.; Wilkinson, K.A.; Nishimune, A.; Henley, J.M. GISP increases neurotransmitter receptor stability by down-regulating ESCRT-mediated lysosomal degradation. Neurosci. Lett. 2009, 452, 106–110. [Google Scholar] [CrossRef]
- Ortega Duran, M.; Shaheed, S.U.; Sutton, C.W.; Shnyder, S.D. A Proteomic Investigation to Discover Candidate Proteins Involved in Novel Mechanisms of 5-Fluorouracil Resistance in Colorectal Cancer. Cells 2024, 13, 342. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef]
- Nowakowska, M.; Pospiech, K.; Lewandowska, U.; Piastowska-Ciesielska, A.W.; Bednarek, A.K. Diverse effect of WWOX overexpression in HT29 and SW480 colon cancer cell lines. Tumour Biol. 2014, 35, 9291–9301. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, H.; Wang, J.; Lu, J.; Dong, Y.; Kang, Z.; Qiu, X.; Ouyang, X.; Chen, Q.; Li, J.; et al. Aloperine Suppresses Cancer Progression by Interacting with VPS4A to Inhibit Autophagosome-lysosome Fusion in NSCLC. Adv. Sci. 2024, 11, e2308307. [Google Scholar] [CrossRef]
- Hashemi, M.; Esbati, N.; Rashidi, M.; Gholami, S.; Raesi, R.; Bidoki, S.S.; Goharrizi, M.; Motlagh, Y.S.M.; Khorrami, R.; Tavakolpournegari, A.; et al. Biological landscape and nanostructural view in development and reversal of oxaliplatin resistance in colorectal cancer. Transl. Oncol. 2024, 40, 101846. [Google Scholar] [CrossRef]
- Martinez-Balibrea, E.; Martínez-Cardús, A.; Ginés, A.; Ruiz de Porras, V.; Moutinho, C.; Layos, L.; Manzano, J.L.; Bugés, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767–1776. [Google Scholar] [CrossRef]
- Myint, K.; Biswas, R.; Li, Y.; Jong, N.; Jamieson, S.; Liu, J.; Han, C.; Squire, C.; Merien, F.; Lu, J.; et al. Identification of MRP2 as a targetable factor limiting oxaliplatin accumulation and response in gastrointestinal cancer. Sci. Rep. 2019, 9, 2245. [Google Scholar] [CrossRef]
- Wei, J.R.; Lu, M.Y.; Wei, T.H.; Fleishman, J.S.; Yu, H.; Chen, X.L.; Kong, X.T.; Sun, S.L.; Li, N.G.; Yang, Y.; et al. Overcoming cancer therapy resistance: From drug innovation to therapeutics. Drug Resist. Updat. 2025, 81, 101229. [Google Scholar] [CrossRef]
- Neggers, J.E.; Paolella, B.R.; Asfaw, A.; Rothberg, M.V.; Skipper, T.A.; Yang, A.; Kalekar, R.L.; Krill-Burger, J.M.; Dharia, N.V.; Kugener, G.; et al. Synthetic Lethal Interaction between the ESCRT Paralog Enzymes VPS4A and VPS4B in Cancers Harboring Loss of Chromosome 18q or 16q. Cell Rep. 2020, 33, 108493. [Google Scholar] [CrossRef]
- Fundora, K.A.; Zhuang, Y.; Hamamoto, K.; Wang, G.; Chen, L.; Hattori, T.; Liang, X.; Bao, L.; Vangala, V.; Tian, F.; et al. DBeQ derivative targets vacuolar protein sorting 4 functions in cancer cells and suppresses tumor growth in mice. J. Pharmacol. Exp. Ther. 2025, 392, 103524. [Google Scholar] [CrossRef]
- Dai, E.; Meng, L.; Kang, R.; Wang, X.; Tang, D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 415–421. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Huang, Y.; Chen, C.; Cai, L. ELK4 targets CHMP6 to inhibit ferroptosis and enhance malignant properties of skin cutaneous melanoma cells. Arch. Dermatol. Res. 2024, 316, 634. [Google Scholar] [CrossRef]
- Russo, P.; Malacarne, D.; Falugi, C.; Trombino, S.; O’Connor, P.M. RPR-115135, a farnesyltransferase inhibitor, increases 5-FU- cytotoxicity in ten human colon cancer cell lines: Role of p53. Int. J. Cancer 2002, 100, 266–275. [Google Scholar] [CrossRef]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef]
- Yu, X.; Riley, T.; Levine, A.J. The regulation of the endosomal compartment by p53 the tumor suppressor gene. Febs J. 2009, 276, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, X.; Feng, Y.; Liu, X.; Zhou, L.; Sui, H.; Ji, Q.; E, Q.; Chen, J.; Wu, L.; et al. Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anticancer Drugs 2017, 28, 281–288. [Google Scholar] [CrossRef]
- Biswas, R.; Bugde, P.; He, J.; Merien, F.; Lu, J.; Liu, D.X.; Myint, K.; Liu, J.; McKeage, M.; Li, Y. Transport-Mediated Oxaliplatin Resistance Associated with Endogenous Overexpression of MRP2 in Caco-2 and PANC-1 Cells. Cancers 2019, 11, 1330. [Google Scholar] [CrossRef]
- Hosokawa, M.; Tanaka, S.; Ueda, K.; Iwakawa, S.; Ogawara, K.I. Decitabine exerted synergistic effects with oxaliplatin in colorectal cancer cells with intrinsic resistance to decitabine. Biochem. Biophys. Res. Commun. 2019, 509, 249–254. [Google Scholar] [CrossRef]
- Sun, W.; Ge, Y.; Cui, J.; Yu, Y.; Liu, B. Scutellarin resensitizes oxaliplatin-resistant colorectal cancer cells to oxaliplatin treatment through inhibition of PKM2. Mol. Ther. Oncolytics 2021, 21, 87–97. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Xu, Z.; Gao, H.; Feng, W.; Li, W.; Miao, Y.; Xu, Z.; Zong, Y.; Zhao, J.; et al. KLF5 inhibition overcomes oxaliplatin resistance in patient-derived colorectal cancer organoids by restoring apoptotic response. Cell Death Dis. 2022, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Nakagawa, H.; Hagiya, Y.; Yasuoka, T.; Ishikawa, T. Transport−Metabolism Interplay: LXRα-Mediated Induction of Human ABC Transporter ABCC2 (cMOAT/MRP2) in HepG2 Cells. Mol. Pharm. 2009, 6, 1678–1688. [Google Scholar] [CrossRef] [PubMed]






| Patient ID Code | Sex | Stage T | Stage N | Stage M |
|---|---|---|---|---|
| 9 | M | 4 | 1 | 1 |
| 22 | M | 3 | 0 | 1 |
| 24 | F | 4 | 2 | 1 |
| 31 | M | 3 | 2 | 1 |
| 33 | M | 3 | 1 | 1 |
| 37 | F | 3 | 2 | 1 |
| 56 | M | 3 | 1 | 1 |
| 57 | F | 4 | 0 | 1 |
| 58 | F | 3 | 0 | 1 |
| Drug | IC50 (µM) for SW480/shVPS4A | IC50 (µM) for SW480shSCR | Fold-Difference | p Value |
|---|---|---|---|---|
| Oxaliplatin | 0.47 ± 0.05 | 1.6 ± 0.17 | 3.4 | ** |
| 5-Fluorouracil | 1.73 ± 0.07 | 2.37 ± 0.3 | 1.37 | ns |
| Irinotecan | 4.23 ± 0.93 | 6.56 ± 1.35 | 1.55 | ns |
| Drug | IC50 (µM) for KM12/shVPS4A | IC50 (µM) for KM12/shSCR | Fold-difference | p value |
| Oxaliplatin | 1.53 ± 0.12 | 2.17 ± 0.17 | 1.44 | * |
| 5-Fluorouracil | 2.17 ± 0.26 | 2.13 ± 0.43 | 1.05 | ns |
| Irinotecan | 2.50 ± 0.12 | 9.17 ± 0.44 | 3.67 | ** |
| SW480/OxaR | SW480/WT | |
|---|---|---|
| Synergy Score | 15.61 ± 1.16 | 3.00 ± 0.99 |
| Interpretation | Likely to be synergistic | Likely to be additive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrazik, N.M.; Patel, A.; Conn, A.; Sutton, C.W.; Kantamneni, S.; Shnyder, S.D. A Novel Modulator of Resistance for Oxaliplatin-Based Therapy for Colorectal Cancer: The ESCRT Family Member VPS4A. Cells 2025, 14, 929. https://doi.org/10.3390/cells14120929
Abdelrazik NM, Patel A, Conn A, Sutton CW, Kantamneni S, Shnyder SD. A Novel Modulator of Resistance for Oxaliplatin-Based Therapy for Colorectal Cancer: The ESCRT Family Member VPS4A. Cells. 2025; 14(12):929. https://doi.org/10.3390/cells14120929
Chicago/Turabian StyleAbdelrazik, Noha M., Anjana Patel, Andrew Conn, Christopher W. Sutton, Sriharsha Kantamneni, and Steven D. Shnyder. 2025. "A Novel Modulator of Resistance for Oxaliplatin-Based Therapy for Colorectal Cancer: The ESCRT Family Member VPS4A" Cells 14, no. 12: 929. https://doi.org/10.3390/cells14120929
APA StyleAbdelrazik, N. M., Patel, A., Conn, A., Sutton, C. W., Kantamneni, S., & Shnyder, S. D. (2025). A Novel Modulator of Resistance for Oxaliplatin-Based Therapy for Colorectal Cancer: The ESCRT Family Member VPS4A. Cells, 14(12), 929. https://doi.org/10.3390/cells14120929







