Impact of SGLT-2 Inhibitors on Biomarkers of Heart Failure
Abstract
1. Introduction
2. Cardiovascular Disease
3. Diabetes Mellitus
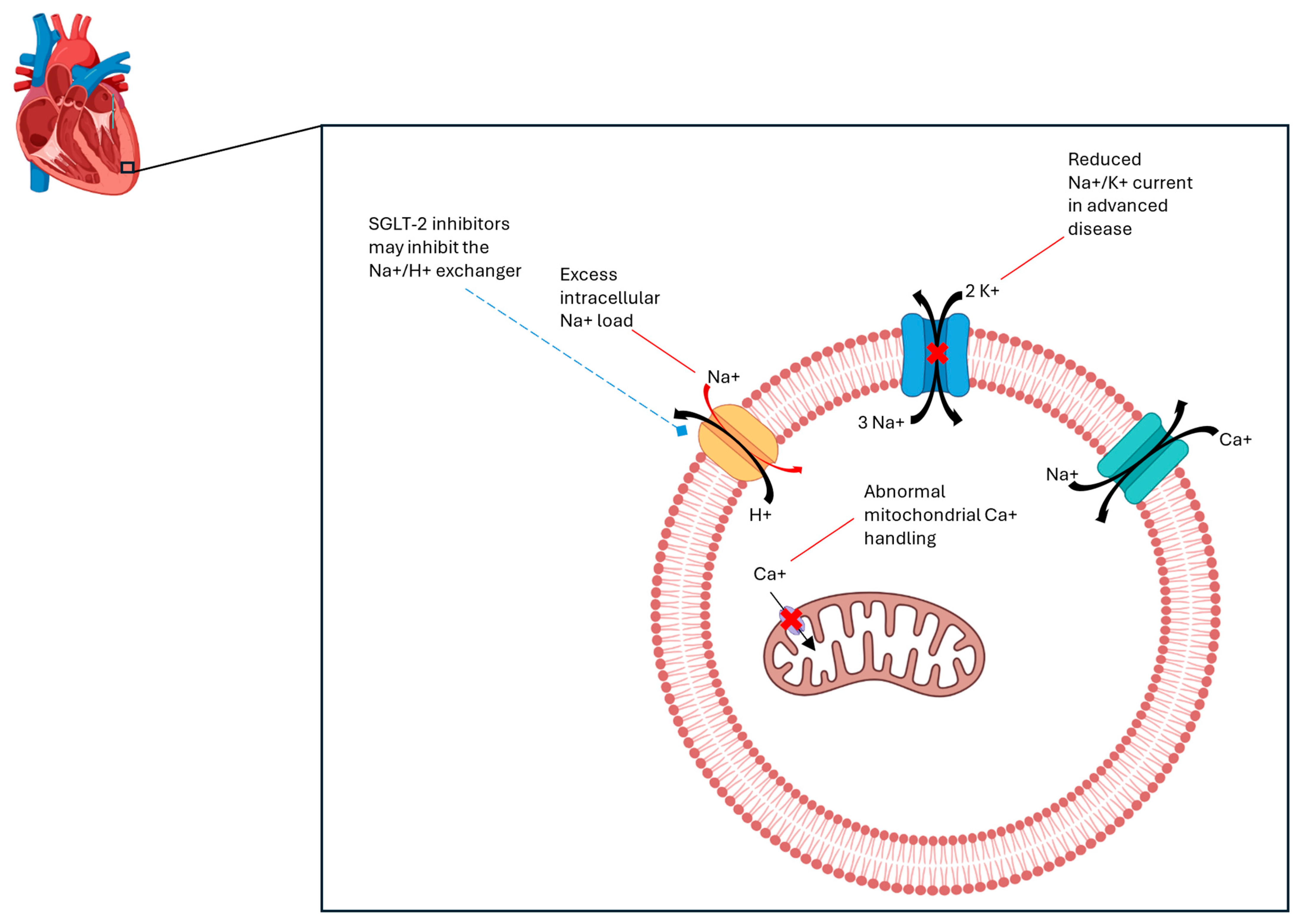
4. Direct Mechanism of Action of SGLT-2 Inhibitors
5. Indirect Effects on Cardiovascular Physiology
5.1. Reduction in Edema
5.2. Ventricular Remodeling
5.3. Cardiac Energy Metabolism
5.4. Inflammation
5.5. Sympathetic Inhibition
5.6. Improved Peripheral Vascular Function
5.7. Reduced Albuminuria
6. Cardiac Biomarkers
6.1. Troponins
6.2. Troponin Levels in Heart Failure
6.3. Troponins and SGLT-2 Inhibitors
6.4. Natriuretic Peptides
6.5. Natriuretic Peptides and SGLT-2 Inhibitors
6.6. Galectin-3
6.7. Soluble Suppression of Tumorigenicity 2
6.8. Biomarkers of Inflammation
7. Clinical Implications
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| ANP | Atrial natriuretic peptide |
| ATP | Adenosine triphosphate |
| BNP | B-type natriuretic peptide |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| Gal-3 | Galectin-3 |
| HF | Heart failure |
| HFmrEF | HF with mildly reduced ejection fraction |
| HFpEF | HF with preserved ejection fraction |
| HFrEF | HF with reduced ejection fraction |
| hs-cTn | High-sensitivity troponin assay |
| IL | Interleukin |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| RAAS | Renin–angiotensin–aldosterone system |
| ROS | Reactive oxygen species |
| SGLT-2 | Sodium–glucose cotransporter 2 |
| sST2 | Soluble suppression of tumorigenicity-2 |
| TNF-α | Tumor necrosis factor alpha |
| T2DM | Type 2 diabetes mellitus |
References
- Kelsey, M.D.; Nelson, A.J.; Green, J.B.; Granger, C.B.; Peterson, E.D.; McGuire, D.K.; Pagidipati, N.J. Guidelines for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2022, 79, 1849–1857. [Google Scholar] [CrossRef]
- Joynt Maddox, K.E.; Elkind, M.S.V.; Aparicio, H.J.; Commodore-Mensah, Y.; de Ferranti, S.D.; Dowd, W.N.; Hernandez, A.F.; Khavjou, O.; Michos, E.D.; Palaniappan, L.; et al. Forecasting the Burden of Cardiovascular Disease and Stroke in the United States Through 2050-Prevalence of Risk Factors and Disease: A Presidential Advisory From the American Heart Association. Circulation 2024, 150, e65–e88. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global burden of cardiovascular diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, zwae28. [Google Scholar] [CrossRef]
- Gyldenkerne, C.; Kahlert, J.; Thrane, P.G.; Olesen, K.K.W.; Mortensen, M.B.; Sorensen, H.T.; Thomsen, R.W.; Maeng, M. 2-Fold More Cardiovascular Disease Events Decades Before Type 2 Diabetes Diagnosis: A Nationwide Registry Study. J. Am. Coll. Cardiol. 2024, 84, 2251–2259. [Google Scholar] [CrossRef]
- Puteh, S.E.W.; Kamarudin, N.; Hussein, Z.; Adam, N.; Shahari, M.R. Cost of cardiovascular disease events in patients with and without type 2 diabetes and factors influencing cost: A retrospective cohort study. BMC Public Health 2024, 24, 2003. [Google Scholar] [CrossRef]
- Yankah, R.K.; Anku, E.K.; Eligar, V. Sodium-Glucose Cotransporter-2 Inhibitors and Cardiovascular Protection Among Patients With Type 2 Diabetes Mellitus: A Systematic Review. J. Diabetes Res. 2024, 2024, 9985836. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Docherty, K.F. SGLT2 Inhibitors in Heart Failure: Iron Mobilization at the Heart of the Matter. J. Am. Coll. Cardiol. 2025, 85, 1771–1773. [Google Scholar] [CrossRef]
- Dabour, M.S.; George, M.Y.; Daniel, M.R.; Blaes, A.H.; Zordoky, B.N. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Pastena, P.; Frye, J.T.; Ho, C.; Goldschmidt, M.E.; Kalogeropoulos, A.P. Ischemic cardiomyopathy: Epidemiology, pathophysiology, outcomes, and therapeutic options. Heart Fail. Rev. 2024, 29, 287–299. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, H.; Song, J. Accurate Classification of Non-ischemic Cardiomyopathy. Curr. Cardiol. Rep. 2023, 25, 1299–1317. [Google Scholar] [CrossRef]
- Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br. Heart J. 1980, 44, 672–673. [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Bohm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [PubMed]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L.; et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019, 140, e294–e324. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L., Jr. Heart Failure With Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Panchal, K.; Lawson, C.; Chandramouli, C.; Lam, C.; Khunti, K.; Zaccardi, F. Diabetes and risk of heart failure in people with and without cardiovascular disease: Systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2024, 207, 111054. [Google Scholar] [CrossRef] [PubMed]
- Young, J.B.; Dunlap, M.E.; Pfeffer, M.A.; Probstfield, J.L.; Cohen-Solal, A.; Dietz, R.; Granger, C.B.; Hradec, J.; Kuch, J.; McKelvie, R.S.; et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: Results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004, 110, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.D.; Lin, J.; Mahoney, T.; Ume, N.; Yang, G.; Gabbay, R.A.; ElSayed, N.A.; Bannuru, R.R. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care 2024, 47, 26–43. [Google Scholar] [CrossRef]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef]
- Battiprolu, P.K.; Lopez-Crisosto, C.; Wang, Z.V.; Nemchenko, A.; Lavandero, S.; Hill, J.A. Diabetic cardiomyopathy and metabolic remodeling of the heart. Life Sci. 2013, 92, 609–615. [Google Scholar] [CrossRef]
- Teshima, Y.; Takahashi, N.; Nishio, S.; Saito, S.; Kondo, H.; Fukui, A.; Aoki, K.; Yufu, K.; Nakagawa, M.; Saikawa, T. Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Circ. J. 2014, 78, 300–306. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Turgay Yildirim, O.; Yildirir, A.; Sade, L.E.; Has Hasirci, S.; Kozan, H.; Ozcalik, E.; Okyay, K.; Bal, U.A.; Aydinalp, A.; Muderrisoglu, H. Is there a relationship between resistin levels and left ventricular end-diastolic pressure? Anatol. J. Cardiol. 2018, 19, 267–272. [Google Scholar] [CrossRef]
- Potenza, M.A.; Addabbo, F.; Montagnani, M. Vascular actions of insulin with implications for endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E568–E577. [Google Scholar] [CrossRef]
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.E.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc. Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef]
- Vedin, O.; Lam, C.S.P.; Koh, A.S.; Benson, L.; Teng, T.H.K.; Tay, W.T.; Braun, O.O.; Savarese, G.; Dahlstrom, U.; Lund, L.H. Significance of Ischemic Heart Disease in Patients With Heart Failure and Preserved, Midrange, and Reduced Ejection Fraction: A Nationwide Cohort Study. Circ. Heart Fail. 2017, 10, e003875. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Hochadel, M.; Frankenstein, L.; Bruder, O.; Pauschinger, M.; Hambrecht, R.; von Scheidt, W.; Pfister, O.; Hartmann, A.; Maier, L.S.; et al. The role of diabetes in cardiomyopathies of different etiologies-Characteristics and 1-year follow-up results of the EVITA-HF registry. PLoS ONE 2020, 15, e0234260. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Banerjee, S.K. SGLT inhibitors: A novel target for diabetes. Pharm. Pat. Anal. 2013, 2, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Nespoux, J.; Vallon, V. Renal effects of SGLT2 inhibitors: An update. Curr. Opin. Nephrol. Hypertens. 2020, 29, 190–198. [Google Scholar] [CrossRef]
- Chao, E.C.; Henry, R.R. SGLT2 inhibition--a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef]
- Tuttle, K.R. Back to the Future: Glomerular Hyperfiltration and the Diabetic Kidney. Diabetes 2017, 66, 14–16. [Google Scholar] [CrossRef]
- Billing, A.M.; Kim, Y.C.; Gullaksen, S.; Schrage, B.; Raabe, J.; Hutzfeldt, A.; Demir, F.; Kovalenko, E.; Lasse, M.; Dugourd, A.; et al. Metabolic Communication by SGLT2 Inhibition. Circulation 2024, 149, 860–884. [Google Scholar] [CrossRef]
- Saha, S.; Fang, X.; Green, C.D.; Das, A. mTORC1 and SGLT2 Inhibitors-A Therapeutic Perspective for Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 15078. [Google Scholar] [CrossRef]
- Safaie, N.; Masoumi, S.; Alizadeh, S.; Mirzajanzadeh, P.; Nejabati, H.R.; Hajiabbasi, M.; Alivirdiloo, V.; Basmenji, N.C.; Derakhshi Radvar, A.; Majidi, Z.; et al. SGLT2 inhibitors and AMPK: The road to cellular housekeeping? Cell Biochem. Funct. 2024, 42, e3922. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Murad, M.H.; Arcelus, J.I.; Dager, W.E.; Dunn, A.S.; Fargo, R.A.; Levy, J.H.; Samama, C.M.; Shah, S.H.; et al. Perioperative Management of Antithrombotic Therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest 2022, 162, e207–e243. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Fu, Y.; Patel, R.; Darshi, M.; Crespo-Masip, M.; Huang, W.; Song, P.; Freeman, B.; Kim, Y.C.; Soleimani, M.; et al. A role for tubular Na(+)/H(+) exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol. Ren. Physiol. 2020, 319, F712–F728. [Google Scholar] [CrossRef]
- Verissimo, T.; Dalga, D.; Arnoux, G.; Sakhi, I.; Faivre, A.; Auwerx, H.; Bourgeois, S.; Paolucci, D.; Gex, Q.; Rutkowski, J.M.; et al. PCK1 is a key regulator of metabolic and mitochondrial functions in renal tubular cells. Am. J. Physiol. Ren. Physiol. 2023, 324, F532–F543. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, T.; Faivre, A.; Rinaldi, A.; Lindenmeyer, M.; Delitsikou, V.; Veyrat-Durebex, C.; Heckenmeyer, C.; Fernandez, M.; Berchtold, L.; Dalga, D.; et al. Decreased Renal Gluconeogenesis Is a Hallmark of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2022, 33, 810–827. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Cardiac Implantable Electronic Device Management [corrected]. Anesthesiology 2020, 132, 225–252.
- Silva Dos Santos, D.; Polidoro, J.Z.; Borges-Junior, F.A.; Girardi, A.C.C. Cardioprotection conferred by sodium-glucose cotransporter 2 inhibitors: A renal proximal tubule perspective. Am. J. Physiol. Cell Physiol. 2020, 318, C328–C336. [Google Scholar] [CrossRef]
- O’Hara, D.V.; Lam, C.S.P.; McMurray, J.J.V.; Yi, T.W.; Hocking, S.; Dawson, J.; Raichand, S.; Januszewski, A.S.; Jardine, M.J. Applications of SGLT2 inhibitors beyond glycaemic control. Nat. Rev. Nephrol. 2024, 20, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef]
- Muskiet, M.H.A.; Heerspink, H.J.L.; van Raalte, D.H. SGLT2 inhibitors: Expanding their Empire beyond diabetes. Lancet Diabetes Endocrinol. 2021, 9, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Zhang, Y.; Yan, B. Mechanisms of Protective Effects of SGLT2 Inhibitors in Cardiovascular Disease and Renal Dysfunction. Curr. Top. Med. Chem. 2019, 19, 1818–1849. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.K.; Ghosh, G.C.; Gupta, M.; Bandyopadhyay, D.; Akhtar, T.; Deedwania, P.; Lavie, C.J.; Fonarow, G.C.; Aneja, A. Sodium Glucose Co-transporter 2 Inhibitors and Heart Failure. Am. J. Cardiol. 2019, 124, 1790–1796. [Google Scholar] [CrossRef]
- Pandey, A.K.; Bhatt, D.L.; Pandey, A.; Marx, N.; Cosentino, F.; Pandey, A.; Verma, S. Mechanisms of benefits of sodium-glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction. Eur. Heart J. 2023, 44, 3640–3651. [Google Scholar] [CrossRef]
- Hernandez, M.; Sullivan, R.D.; McCune, M.E.; Reed, G.L.; Gladysheva, I.P. Sodium-Glucose Cotransporter-2 Inhibitors Improve Heart Failure with Reduced Ejection Fraction Outcomes by Reducing Edema and Congestion. Diagnostics 2022, 12, 989. [Google Scholar] [CrossRef]
- Andreadou, I.; Efentakis, P.; Balafas, E.; Togliatto, G.; Davos, C.H.; Varela, A.; Dimitriou, C.A.; Nikolaou, P.E.; Maratou, E.; Lambadiari, V.; et al. Empagliflozin Limits Myocardial Infarction in Vivo and Cell Death in Vitro: Role of STAT3, Mitochondria, and Redox Aspects. Front. Physiol. 2017, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Biegus, J.; Fudim, M.; Salah, H.M.; Heerspink, H.J.L.; Voors, A.A.; Ponikowski, P. Sodium-glucose cotransporter-2 inhibitors in heart failure: Potential decongestive mechanisms and current clinical studies. Eur. J. Heart Fail. 2023, 25, 1526–1536. [Google Scholar] [CrossRef]
- Jensen, J.; Omar, M.; Kistorp, C.; Tuxen, C.; Gustafsson, I.; Kober, L.; Gustafsson, F.; Faber, J.; Malik, M.E.; Fosbol, E.L.; et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): A prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2021, 9, 106–116. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Zinman, B.; Fitchett, D.; Wanner, C.; Ferrannini, E.; Schumacher, M.; Schmoor, C.; Ohneberg, K.; Johansen, O.E.; George, J.T.; et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018, 41, 356–363. [Google Scholar] [CrossRef]
- Pabel, S.; Wagner, S.; Bollenberg, H.; Bengel, P.; Kovacs, A.; Schach, C.; Tirilomis, P.; Mustroph, J.; Renner, A.; Gummert, J.; et al. Empagliflozin directly improves diastolic function in human heart failure. Eur. J. Heart Fail. 2018, 20, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Ishida, N.; Ibi, M.; Saito, M.; Sanbe, A.; Shimojo, H.; Suzuki, S.; Koepsell, H.; Takeishi, Y.; Morino, Y.; et al. Chronic Pressure Overload Induces Cardiac Hypertrophy and Fibrosis via Increases in SGLT1 and IL-18 Gene Expression in Mice. Int. Heart J. 2018, 59, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Sillje, H.H.W.; Oberdorf-Maass, S.U.; Schouten, E.M.; Pavez Giani, M.G.; Hillebrands, J.L.; van Goor, H.; van Veldhuisen, D.J.; de Boer, R.A.; Westenbrink, B.D. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur. J. Heart Fail. 2019, 21, 862–873. [Google Scholar] [CrossRef]
- Lahnwong, C.; Palee, S.; Apaijai, N.; Sriwichaiin, S.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc. Diabetol. 2020, 19, 91. [Google Scholar] [CrossRef]
- Uriel, N.; Sayer, G.; Annamalai, S.; Kapur, N.K.; Burkhoff, D. Mechanical Unloading in Heart Failure. J. Am. Coll. Cardiol. 2018, 72, 569–580. [Google Scholar] [CrossRef]
- Shiou, Y.L.; Huang, I.C.; Lin, H.T.; Lee, H.C. High fat diet aggravates atrial and ventricular remodeling of hypertensive heart disease in aging rats. J. Formos. Med. Assoc. 2018, 117, 621–631. [Google Scholar] [CrossRef]
- Yerra, V.G.; Batchu, S.N.; Kabir, G.; Advani, S.L.; Liu, Y.; Siddiqi, F.S.; Connelly, K.A.; Advani, A. Empagliflozin Disrupts a Tnfrsf12a-Mediated Feed Forward Loop That Promotes Left Ventricular Hypertrophy. Cardiovasc. Drugs Ther. 2022, 36, 619–632. [Google Scholar] [CrossRef]
- Nagoshi, T.; Yoshimura, M.; Rosano, G.M.; Lopaschuk, G.D.; Mochizuki, S. Optimization of cardiac metabolism in heart failure. Curr. Pharm. Des. 2011, 17, 3846–3853. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose transporters in healthy heart and in cardiac disease. Int. J. Cardiol. 2017, 230, 70–75. [Google Scholar] [CrossRef]
- Kappel, B.A.; Lehrke, M.; Schutt, K.; Artati, A.; Adamski, J.; Lebherz, C.; Marx, N. Effect of Empagliflozin on the Metabolic Signature of Patients With Type 2 Diabetes Mellitus and Cardiovascular Disease. Circulation 2017, 136, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr.; Veech, R.L. Ketoacids? Good medicine? Trans. Am. Clin. Clim. Climatol. Assoc. 2003, 114, 149–161, discussion 162–163. [Google Scholar]
- Soto-Mota, A.; Norwitz, N.G.; Clarke, K. Why a d-beta-hydroxybutyrate monoester? Biochem. Soc. Trans. 2020, 48, 51–59. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4, e124079. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Korzh, S.; Videja, M.; Vilks, K.; Cirule, H.; Kuka, J.; Dambrova, M.; Liepinsh, E. Empagliflozin Protects Cardiac Mitochondrial Fatty Acid Metabolism in a Mouse Model of Diet-Induced Lipid Overload. Cardiovasc. Drugs Ther. 2020, 34, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Mudaliar, S.; Alloju, S.; Henry, R.R. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care 2016, 39, 1115–1122. [Google Scholar] [CrossRef]
- La Grotta, R.; Frige, C.; Matacchione, G.; Olivieri, F.; de Candia, P.; Ceriello, A.; Prattichizzo, F. Repurposing SGLT-2 Inhibitors to Target Aging: Available Evidence and Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 12325. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A "Thrifty Substrate" Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef]
- Packer, M. Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics. Am. J. Kidney Dis. 2021, 77, 280–286. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Otsu, K. Translation of hemodynamic stress to sterile inflammation in the heart. Trends Endocrinol. Metab. 2013, 24, 546–553. [Google Scholar] [CrossRef]
- Fedak, P.W.; Verma, S.; Weisel, R.D.; Li, R.K. Cardiac remodeling and failure: From molecules to man (Part I). Cardiovasc. Pathol. 2005, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Grubic Rotkvic, P.; Cigrovski Berkovic, M.; Bulj, N.; Rotkvic, L. Minireview: Are SGLT2 inhibitors heart savers in diabetes? Heart Fail. Rev. 2020, 25, 899–905. [Google Scholar] [CrossRef]
- Floras, J.S.; Ponikowski, P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur. Heart J. 2015, 36, 1974–1982b. [Google Scholar] [CrossRef]
- Ramchandra, R.; Hood, S.G.; Xing, D.; Lambert, G.W.; May, C.N. Mechanisms underlying the increased cardiac norepinephrine spillover in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H340–H347. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic. Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Jordan, J.; Tank, J.; Heusser, K.; Heise, T.; Wanner, C.; Heer, M.; Macha, S.; Mattheus, M.; Lund, S.S.; Woerle, H.J.; et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J. Am. Soc. Hypertens. 2017, 11, 604–612. [Google Scholar] [CrossRef]
- Malone, A.F.; Reddan, D.N. Pulse pressure. Why is it important? Perit. Dial. Int. 2010, 30, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Khan, S.A.; Wong, N.D.; Larson, M.G.; Levy, D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation 1999, 100, 354–360. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Vasan, R.S.; Keyes, M.J.; Parise, H.; Wang, T.J.; Larson, M.G.; D’Agostino, R.B., Sr.; Kannel, W.B.; Levy, D.; Benjamin, E.J. Pulse pressure and risk of new-onset atrial fibrillation. JAMA 2007, 297, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, T.; Spizzo, I.; Liu, H.; Hu, Y.; Simpson, R.W.; Widdop, R.E.; Dear, A.E. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: A potential mechanism for inhibition of atherogenesis. Diabetes Vasc. Dis. Res. 2018, 15, 64–73. [Google Scholar] [CrossRef]
- Mancini, S.J.; Boyd, D.; Katwan, O.J.; Strembitska, A.; Almabrouk, T.A.; Kennedy, S.; Palmer, T.M.; Salt, I.P. Canagliflozin inhibits interleukin-1beta-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci. Rep. 2018, 8, 5276. [Google Scholar] [CrossRef]
- Hess, D.A.; Terenzi, D.C.; Trac, J.Z.; Quan, A.; Mason, T.; Al-Omran, M.; Bhatt, D.L.; Dhingra, N.; Rotstein, O.D.; Leiter, L.A.; et al. SGLT2 Inhibition with Empagliflozin Increases Circulating Provascular Progenitor Cells in People with Type 2 Diabetes Mellitus. Cell Metab. 2019, 30, 609–613. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Farag, Y.M.K.; Durthaler, J. Albuminuria: An Underappreciated Risk Factor for Cardiovascular Disease. J. Am. Heart Assoc. 2024, 13, e030131. [Google Scholar] [CrossRef]
- Selvaraj, S.; Claggett, B.; Shah, S.J.; Anand, I.; Rouleau, J.L.; Desai, A.S.; Lewis, E.F.; Pitt, B.; Sweitzer, N.K.; Pfeffer, M.A.; et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: An analysis of the TOPCAT trial. Eur. J. Heart Fail. 2018, 20, 483–490. [Google Scholar] [CrossRef]
- Shuvy, M.; Zwas, D.R.; Lotan, C.; Keren, A.; Gotsman, I. Albuminuria: Associated With Heart Failure Severity and Impaired Clinical Outcomes. Can. J. Cardiol. 2020, 36, 527–534. [Google Scholar] [CrossRef]
- Cherney, D.; Lund, S.S.; Perkins, B.A.; Groop, P.H.; Cooper, M.E.; Kaspers, S.; Pfarr, E.; Woerle, H.J.; von Eynatten, M. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 2016, 59, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liang, B.; Li, J.; Zhang, X.; Chen, H.; Sun, J.; Zhang, Z. Dapagliflozin alleviates advanced glycation end product induced podocyte injury through AMPK/mTOR mediated autophagy pathway. Cell. Signal. 2022, 90, 110206. [Google Scholar] [CrossRef]
- Durcan, E.; Ozkan, S.; Saygi, H.I.; Dincer, M.T.; Korkmaz, O.P.; Sahin, S.; Karaca, C.; Sulu, C.; Bakir, A.; Ozkaya, H.M.; et al. Effects of SGLT2 inhibitors on patients with diabetic kidney disease: A preliminary study on the basis of podocyturia. J. Diabetes 2022, 14, 236–246. [Google Scholar] [CrossRef]
- Cassis, P.; Locatelli, M.; Cerullo, D.; Corna, D.; Buelli, S.; Zanchi, C.; Villa, S.; Morigi, M.; Remuzzi, G.; Benigni, A.; et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018, 3, e98720. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [PubMed]
- Banerjee, S.K.; McGaffin, K.R.; Pastor-Soler, N.M.; Ahmad, F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc. Res. 2009, 84, 111–118. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Hirayama, B.A.; Loo, D.D.; Chaptal, V.; Abramson, J.; Wright, E.M. Bridging the gap between structure and kinetics of human SGLT1. Am. J. Physiol. Cell Physiol. 2012, 302, C1293–C1305. [Google Scholar] [CrossRef]
- Despa, S.; Islam, M.A.; Weber, C.R.; Pogwizd, S.M.; Bers, D.M. Intracellular Na(+) concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation 2002, 105, 2543–2548. [Google Scholar] [CrossRef]
- Despa, S. Myocyte [Na(+)](i) Dysregulation in Heart Failure and Diabetic Cardiomyopathy. Front. Physiol. 2018, 9, 1303. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wust, R.C.; Fiolet, J.W.; Stienen, G.J.; Coronel, R.; Zuurbier, C.J. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Piek, A.; Du, W.; de Boer, R.A.; Sillje, H.H.W. Novel heart failure biomarkers: Why do we fail to exploit their potential? Crit. Rev. Clin. Lab. Sci. 2018, 55, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Filippatos, G.; Nieminen, M.; Gheorghiade, M. Troponin elevation in patients with heart failure: On behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur. Heart J. 2012, 33, 2265–2271. [Google Scholar] [CrossRef]
- Wettersten, N.; Maisel, A. Role of Cardiac Troponin Levels in Acute Heart Failure. Card. Fail. Rev. 2015, 1, 102–106. [Google Scholar] [CrossRef]
- Maisel, A.; Mueller, C.; Adams, K., Jr.; Anker, S.D.; Aspromonte, N.; Cleland, J.G.; Cohen-Solal, A.; Dahlstrom, U.; DeMaria, A.; Di Somma, S.; et al. State of the art: Using natriuretic peptide levels in clinical practice. Eur. J. Heart Fail. 2008, 10, 824–839. [Google Scholar] [CrossRef]
- Hu, X.J.; Sun, X.G.; Cheng, J.Y.; Ma, J. The Predictive Role of Cardiac Troponin Elevation Ratio Combined With Heart Function Index Model in the Prognosis of Non-ST-Segment Elevation Myocardial Infarction Patients. Cardiol. Res. 2024, 15, 246–252. [Google Scholar] [CrossRef]
- Gajardo, A.I.J.; Ferriere-Steinert, S.; Valenzuela Jimenez, J.; Heskia Araya, S.; Kouyoumdjian Carvajal, T.; Ramos-Rojas, J.; Medel, J.N. Early high-sensitivity troponin elevation and short-term mortality in sepsis: A systematic review with meta-analysis. Crit. Care 2025, 29, 76. [Google Scholar] [CrossRef]
- Kociol, R.D.; Pang, P.S.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M.; Felker, G.M. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J. Am. Coll. Cardiol. 2010, 56, 1071–1078. [Google Scholar] [CrossRef]
- Arenja, N.; Reichlin, T.; Drexler, B.; Oshima, S.; Denhaerynck, K.; Haaf, P.; Potocki, M.; Breidthardt, T.; Noveanu, M.; Stelzig, C.; et al. Sensitive cardiac troponin in the diagnosis and risk stratification of acute heart failure. J. Intern. Med. 2012, 271, 598–607. [Google Scholar] [CrossRef]
- Demir, M.; Kanadasi, M.; Akpinar, O.; Donmez, Y.; Avkarogullari, M.; Alhan, C.; Inal, T.; San, M.; Usal, A.; Demirtas, M. Cardiac troponin T as a prognostic marker in patients with heart failure: A 3-year outcome study. Angiology 2007, 58, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L., Jr.; Vergaro, G.; Ripoli, A.; Latini, R.; Masson, S.; Magnoli, M.; Anand, I.S.; Cohn, J.N.; Tavazzi, L.; et al. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation 2018, 137, 286–297. [Google Scholar] [CrossRef]
- Sherwood, M.W.; Kristin Newby, L. High-sensitivity troponin assays: Evidence, indications, and reasonable use. J. Am. Heart Assoc. 2014, 3, e000403. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, H.; Koibuchi, N.; Hasegawa, Y.; Ogawa, H.; Kim-Mitsuyama, S. Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc. Diabetol. 2016, 15, 157. [Google Scholar] [CrossRef]
- Lahnwong, C.; Chattipakorn, S.C.; Chattipakorn, N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc. Diabetol. 2018, 17, 101. [Google Scholar] [CrossRef]
- Tanajak, P.; Sa-Nguanmoo, P.; Sivasinprasasn, S.; Thummasorn, S.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J. Endocrinol. 2018, 236, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Irace, C.; Casciaro, F.; Scavelli, F.B.; Oliverio, R.; Cutruzzola, A.; Cortese, C.; Gnasso, A. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc. Diabetol. 2018, 17, 52. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Dekkers, C.C.J.; Barbour, S.J.; Cattran, D.; Abdul Gafor, A.H.; Greasley, P.J.; Laverman, G.D.; Lim, S.K.; Di Tanna, G.L.; Reich, H.N.; et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): A randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020, 8, 582–593. [Google Scholar] [CrossRef]
- Phrommintikul, A.; Wongcharoen, W.; Kumfu, S.; Jaiwongkam, T.; Gunaparn, S.; Chattipakorn, S.; Chattipakorn, N. Effects of dapagliflozin vs vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: A randomised study. Br. J. Clin. Pharmacol. 2019, 85, 1337–1347. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Morrow, D.A.; Mosenzon, O.; Goodrich, E.L.; Jarolim, P.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.; et al. Relationship between baseline cardiac biomarkers and cardiovascular death or hospitalization for heart failure with and without sodium-glucose co-transporter 2 inhibitor therapy in DECLARE-TIMI 58. Eur. J. Heart Fail. 2021, 23, 1026–1036. [Google Scholar] [CrossRef]
- de Bold, A.J.; Borenstein, H.B.; Veress, A.T.; Sonnenberg, H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981, 28, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Carnovali, M.; Mastromarino, V. The natriuretic peptides system in the pathophysiology of heart failure: From molecular basis to treatment. Clin. Sci. 2016, 130, 57–77. [Google Scholar] [CrossRef]
- Sudoh, T.; Minamino, N.; Kangawa, K.; Matsuo, H. Brain natriuretic peptide-32: N-terminal six amino acid extended form of brain natriuretic peptide identified in porcine brain. Biochem. Biophys. Res. Commun. 1988, 155, 726–732. [Google Scholar] [CrossRef]
- Yan, W.; Wu, F.; Morser, J.; Wu, Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 8525–8529. [Google Scholar] [CrossRef] [PubMed]
- de Bold, A.J.; Bruneau, B.G.; Kuroski de Bold, M.L. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc. Res. 1996, 31, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Trung, M.L.; Tridetti, J.; Ancion, A.; Oury, C.; Lancellotti, P. [Natriuretic peptides in heart failure]. Rev. Med. Liege 2020, 75, 644–648. [Google Scholar]
- Harris, P.J.; Thomas, D.; Morgan, T.O. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 1987, 326, 697–698. [Google Scholar] [CrossRef]
- Wijeyaratne, C.N.; Moult, P.J. The effect of alpha human atrial natriuretic peptide on plasma volume and vascular permeability in normotensive subjects. J. Clin. Endocrinol. Metab. 1993, 76, 343–346. [Google Scholar]
- Zeidel, M.L. Renal actions of atrial natriuretic peptide: Regulation of collecting duct sodium and water transport. Annu. Rev. Physiol. 1990, 52, 747–759. [Google Scholar] [CrossRef]
- Atlas, S.A.; Volpe, M.; Sosa, R.E.; Laragh, J.H.; Camargo, M.J.; Maack, T. Effects of atrial natriuretic factor on blood pressure and the renin-angiotensin-aldosterone system. Fed. Proc. 1986, 45, 2115–2121. [Google Scholar]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef]
- Hayashi, D.; Kudoh, S.; Shiojima, I.; Zou, Y.; Harada, K.; Shimoyama, M.; Imai, Y.; Monzen, K.; Yamazaki, T.; Yazaki, Y.; et al. Atrial natriuretic peptide inhibits cardiomyocyte hypertrophy through mitogen-activated protein kinase phosphatase-1. Biochem. Biophys. Res. Commun. 2004, 322, 310–319. [Google Scholar] [CrossRef]
- Yoshimura, M.; Yasue, H.; Okumura, K.; Ogawa, H.; Jougasaki, M.; Mukoyama, M.; Nakao, K.; Imura, H. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993, 87, 464–469. [Google Scholar] [CrossRef]
- Daniels, L.B.; Maisel, A.S. Natriuretic peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Marin-Grez, M.; Fleming, J.T.; Steinhausen, M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 1986, 324, 473–476. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. In cGMP: Generators, Effectors and Therapeutic Implications; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 341–366. [Google Scholar]
- Bracamonte, J.H.; Watkins, L.; Pat, B.; Dell’Italia, L.J.; Saucerman, J.J.; Holmes, J.W. Contributions of mechanical loading and hormonal changes to eccentric hypertrophy during volume overload: A Bayesian analysis using logic-based network models. PLoS Comput. Biol. 2025, 21, e1012390. [Google Scholar] [CrossRef] [PubMed]
- Ibebuogu, U.N.; Gladysheva, I.P.; Houng, A.K.; Reed, G.L. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ. Heart Fail. 2011, 4, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Osman, E.E.A.; Rehemtulla, A.; Neamati, N. Why All the Fury over Furin? J. Med. Chem. 2022, 65, 2747–2784. [Google Scholar] [CrossRef]
- Harlid, S.; Myte, R.; Van Guelpen, B. The Metabolic Syndrome, Inflammation, and Colorectal Cancer Risk: An Evaluation of Large Panels of Plasma Protein Markers Using Repeated, Prediagnostic Samples. Mediat. Inflamm. 2017, 2017, 4803156. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Turpeinen, H.; Seppala, I.; Lyytikainen, L.P.; Raitoharju, E.; Hutri-Kahonen, N.; Levula, M.; Oksala, N.; Waldenberger, M.; Klopp, N.; Illig, T.; et al. A genome-wide expression quantitative trait loci analysis of proprotein convertase subtilisin/kexin enzymes identifies a novel regulatory gene variant for FURIN expression and blood pressure. Hum. Genet. 2015, 134, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Tang, J.N.; Han, L.; Liu, X.D.; Shen, Y.L.; Zhang, C.Y.; Liu, X.B. Elevated FURIN levels in predicting mortality and cardiovascular events in patients with acute myocardial infarction. Metabolism 2020, 111, 154323. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Barsotti, E.; Clerico, A.; Muscelli, E. Renal Handling of Ketones in Response to Sodium-Glucose Cotransporter 2 Inhibition in Patients With Type 2 Diabetes. Diabetes Care 2017, 40, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Butler, J.; Jarolim, P.; Sattar, N.; Vijapurkar, U.; Desai, M.; Davies, M.J. Effects of Canagliflozin on Cardiovascular Biomarkers in Older Adults With Type 2 Diabetes. J. Am. Coll. Cardiol. 2017, 70, 704–712. [Google Scholar] [CrossRef]
- Køber, L.; Docherty, K.; Inzucchi, S.E.; Jhund, P.; Kosiborod, M.; Langkilde, A.M.; Martinez, F.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.; et al. Dapagliflozin Improves Outcomes Irrespective of Nt-Probnp Concentration in Patients with Hfref: An Analysis of the Dapa-Hf Trial. JACC 2020, 75 (Suppl. S1), 675. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef]
- Tanaka, A.; Hisauchi, I.; Taguchi, I.; Sezai, A.; Toyoda, S.; Tomiyama, H.; Sata, M.; Ueda, S.; Oyama, J.I.; Kitakaze, M.; et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: A randomized trial (CANDLE). ESC Heart Fail. 2020, 7, 1585–1594. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef]
- Feng, X.; Gu, Q.; Gao, G.; Yuan, L.; Li, Q.; Zhang, Y. The plasma levels of atrial natriuretic peptide and brain natriuretic peptide in type 2 diabetes treated with sodium-glucose cotransporter-2 inhibitor. Ann. Endocrinol. 2020, 81, 476–481. [Google Scholar] [CrossRef]
- Sezai, A.; Sekino, H.; Unosawa, S.; Taoka, M.; Osaka, S.; Tanaka, M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc. Diabetol. 2019, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, V.; Manca, P.; Parisi, F.; Madaudo, C.; Sciacca, S.; Cannizzo, N.; Mule, M.; Cipriani, M.G. SGLT2 inhibitor therapy in patients with advanced heart failure and reduced ejection fraction. Curr. Probl. Cardiol. 2024, 49, 102823. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Mascha, E.J.; Yang, D.; Maheshwari, K.; Cohen, B.; Khanna, A.K.; Ruetzler, K.; Turan, A.; Sessler, D.I. Associations of Intraoperative Radial Arterial Systolic, Diastolic, Mean, and Pulse Pressures with Myocardial and Acute Kidney Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology 2020, 132, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Wan, Q.; Tian, Y.; Jia, W.; Luo, X.; Yang, T.; Shi, Y.; Gu, X.; Xu, S. Comparative study on pros and cons of sequential high-flow nasal cannula and non-invasive positive pressure ventilation immediately following early extubated patients with severe respiratory failure due to acute exacerbations of chronic obstructive pulmonary disease. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 1215–1220. [Google Scholar]
- Besler, C.; Lang, D.; Urban, D.; Rommel, K.P.; von Roeder, M.; Fengler, K.; Blazek, S.; Kandolf, R.; Klingel, K.; Thiele, H.; et al. Plasma and Cardiac Galectin-3 in Patients With Heart Failure Reflects Both Inflammation and Fibrosis: Implications for Its Use as a Biomarker. Circ. Heart Fail. 2017, 10, e003804. [Google Scholar] [CrossRef]
- Zaborska, B.; Sikora-Frac, M.; Smarz, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Galectin-3 in Heart Failure-The Diagnostic, Prognostic and Therapeutic Potential-Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 13111. [Google Scholar] [CrossRef] [PubMed]
- Khadeja Bi, A.; Santhosh, V.; Sigamani, K. Levels of Galectin-3 in Chronic Heart Failure: A Case-Control Study. Cureus 2022, 14, e28310. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, J.; Ling, Y.; Zhang, Y.; Lin, W.; Tang, L.; Liu, J.; Li, S. Diagnostic value of novel biomarkers for heart failure: A meta-analysis. Herz 2020, 45, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Fiuzat, M.; Shaw, L.K.; Clare, R.; Whellan, D.J.; Bettari, L.; Shirolkar, S.C.; Donahue, M.; Kitzman, D.W.; Zannad, F.; et al. Galectin-3 in ambulatory patients with heart failure: Results from the HF-ACTION study. Circ. Heart Fail. 2012, 5, 72–78. [Google Scholar] [CrossRef]
- Athiraman, U.; Liu, M.; Jayaraman, K.; Yuan, J.; Mehla, J.; Zipfel, G.J. Anesthetic and subanesthetic doses of isoflurane conditioning provides strong protection against delayed cerebral ischemia in a mouse model of subarachnoid hemorrhage. Brain Res. 2021, 1750, 147169. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Kaluzna-Oleksy, M.; Migaj, J.; Sawczak, F.; Krysztofiak, H.; Lesiak, M.; Straburzynska-Migaj, E. sST2 and Heart Failure-Clinical Utility and Prognosis. J. Clin. Med. 2023, 12, 3136. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Gonzalez, A.; Lupon, J. ST2 in Heart Failure. Circ. Heart Fail. 2018, 11, e005582. [Google Scholar] [CrossRef] [PubMed]
- Dieplinger, B.; Egger, M.; Gegenhuber, A.; Haltmayer, M.; Mueller, T. Analytical and clinical evaluation of a rapid quantitative lateral flow immunoassay for measurement of soluble ST2 in human plasma. Clin. Chim. Acta 2015, 451 Pt B, 310–315. [Google Scholar] [CrossRef][Green Version]
- Pascual-Figal, D.A.; Januzzi, J.L. The biology of ST2: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115, 3B–7B. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L., Jr.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Villacorta, H.; Maisel, A.S. Soluble ST2 Testing: A Promising Biomarker in the Management of Heart Failure. Arq. Bras. Cardiol. 2016, 106, 145–152. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Zamora, E.; de Antonio, M.; Galan, A.; Vila, J.; Urrutia, A.; Diez, C.; Coll, R.; Altimir, S.; Lupon, J. Soluble ST2 serum concentration and renal function in heart failure. J. Card. Fail. 2013, 19, 768–775. [Google Scholar] [CrossRef]
- Cong, Z.; Wan, T.; Wang, J.; Feng, L.; Cao, C.; Li, Z.; Wang, X.; Han, Y.; Zhou, Y.; Gao, Y.; et al. Epidemiological and clinical features of malignant hyperthermia: A scoping review. Clin. Genet. 2024, 105, 233–242. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, P.; Song, D.; Song, D.; Chen, Z.; Li, H. Growth stimulation expressed gene 2 (ST2): Clinical research and application in the cardiovascular related diseases. Front. Cardiovasc. Med. 2022, 9, 1007450. [Google Scholar] [CrossRef]
- 2021 Anesthesia Almanac. Available online: https://www.asahq.org/-/media/sites/asahq/files/public/resources/analytics-research-services/2021-anesthesia-almanac.pdf?la=en&hash=404CEC03EBEE9D7BE5E0C3C24AA59EB835A25B54 (accessed on 2 February 2025).
- Januzzi, J.L., Jr.; Peacock, W.F.; Maisel, A.S.; Chae, C.U.; Jesse, R.L.; Baggish, A.L.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; van Kimmenade, R.R.; et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J. Am. Coll. Cardiol. 2007, 50, 607–613. [Google Scholar] [CrossRef]
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupon, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J. Am. Coll. Cardiol. 2018, 72, 2309–2320. [Google Scholar] [CrossRef]
- Yamamoto, M.; Seo, Y.; Ishizua, T.; Nakagawa, D.; Sato, K.; Machino-Ohtsuka, T.; Nishi, I.; Hamada-Harimura, Y.; Sai, S.; Sugano, A.; et al. Comparison of Soluble ST2, Pentraxin-3, Galectin-3, and High-Sensitivity Troponin T of Cardiovascular Outcomes in Patients With Acute Decompensated Heart Failure. J. Card. Fail. 2021, 27, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Concistre, A.; Suppa, M.; Galardo, G.; Rosa, A.; Bertazzoni, G.; Pugliese, F.; Letizia, C.; Petramala, L. Prognostic Role of sST2 in Acute Heart Failure and COVID-19 Infection-A Narrative Review on Pathophysiology and Clinical Prospective. Int. J. Mol. Sci. 2022, 23, 8230. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; Matacchione, G.; Giuliani, A.; Sabbatinelli, J.; Olivieri, F.; de Candia, P.; De Nigris, V.; Ceriello, A. Extracellular vesicle-shuttled miRNAs: A critical appraisal of their potential as nano-diagnostics and nano-therapeutics in type 2 diabetes mellitus and its cardiovascular complications. Theranostics 2021, 11, 1031–1045. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, P.; Gao, X.; Shi, S.; Li, Y.; Zhang, B.; Wu, H.; Song, Q. Association of systemic inflammatory markers with clinical adverse prognosis and outcomes in HFpEF: A systematic review and meta-analysis of cohort studies. Front. Cardiovasc. Med. 2024, 11, 1461073. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibitors on inflammation: A systematic review and meta-analysis of studies in rodents. Int. Immunopharmacol. 2022, 111, 109080. [Google Scholar] [CrossRef]
- Gohari, S.; Ismail-Beigi, F.; Mahjani, M.; Ghobadi, S.; Jafari, A.; Ahangar, H.; Gohari, S. The effect of sodium-glucose co-transporter-2 (SGLT2) inhibitors on blood interleukin-6 concentration: A systematic review and meta-analysis of randomized controlled trials. BMC Endocr. Disord. 2023, 23, 257. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Stephens, J.W. A systematic review examining the effects of sodium-glucose cotransporter-2 inhibitors (SGLT2is) on biomarkers of inflammation and oxidative stress. Diabetes Res. Clin. Pr. Pract. 2020, 168, 108368. [Google Scholar] [CrossRef]
- Sun, W.; Xing, Y.; Kong, D.; Zhang, Z.; Ma, H.; Yang, L. Meta-analysis of the effect of sodium-dependent glucose transporter 2 inhibitors on C-reactive protein in type 2 diabetes. Medicine 2022, 101, e30553. [Google Scholar] [CrossRef]
- Xu, C.; Wang, W.; Zhong, J.; Lei, F.; Xu, N.; Zhang, Y.; Xie, W. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem. Pharmacol. 2018, 152, 45–59. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Das, N.A.; Carpenter, A.J.; Belenchia, A.; Aroor, A.R.; Noda, M.; Siebenlist, U.; Chandrasekar, B.; DeMarco, V.G. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cell. Signal. 2020, 68, 109506. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt-Dirican, Z.E.; Qi, C.; Wang, Y.C.; Simm, A.; Deelen, L.; Hafiz Abbas Gasim, A.; Lewis-McDougall, F.; Ellison-Hughes, G.M. SGLT2 inhibitors as a novel senotherapeutic approach. NPJ Aging 2025, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Majid, H.; Khan, P.; Masoom, M.; Parveen, R.; Kapur, P.; Kohli, S.; Nidhi. Effect of sodium-glucose Co-transporter 2 inhibitors on MCP-1 and uromodulin levels in patients with type 2 diabetes mellitus. Clin. Epidemiol. Glob. Health 2025, 31, 101888. [Google Scholar] [CrossRef]
- Sen, T.; Koshino, A.; Neal, B.; Bijlsma, M.J.; Arnott, C.; Li, J.; Hansen, M.K.; Ix, J.H.; Heerspink, H.J.L. Mechanisms of action of the sodium-glucose cotransporter-2 (SGLT2) inhibitor canagliflozin on tubular inflammation and damage: A post hoc mediation analysis of the CANVAS trial. Diabetes Obes. Metab. 2022, 24, 1950–1956. [Google Scholar] [CrossRef]
- van der Leeuw, J.; Beulens, J.W.; van Dieren, S.; Schalkwijk, C.G.; Glatz, J.F.; Hofker, M.H.; Verschuren, W.M.; Boer, J.M.; van der Graaf, Y.; Visseren, F.L.; et al. Novel Biomarkers to Improve the Prediction of Cardiovascular Event Risk in Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2016, 5, e003048. [Google Scholar] [CrossRef]
- Taylor, K.S.; Verbakel, J.Y.; Feakins, B.G.; Price, C.P.; Perera, R.; Bankhead, C.; Pluddemann, A. Diagnostic accuracy of point-of-care natriuretic peptide testing for chronic heart failure in ambulatory care: Systematic review and meta-analysis. BMJ 2018, 361, k1450. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Khanna, N.N.; Laird, J.R.; Nicolaides, A.; Faa, G.; Johri, A.M.; Mantella, L.E.; Fernandes, J.F.E.; Teji, J.S.; et al. Artificial intelligence for cardiovascular disease risk assessment in personalised framework: A scoping review. eClinicalMedicine 2024, 73, 102660. [Google Scholar] [CrossRef]
- Merlo, A.; D’Elia, E.; Di Odoardo, L.; Sciatti, E.; Senni, M. SGLT2 inhibitors and new frontiers in heart failure treatment regardless of ejection fraction and setting. Eur. Heart J. 2025, 27 (Suppl. S1), i137–i140. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, D.; Kumar, P.; Urs, K.; Dave, S. Impact of SGLT-2 Inhibitors on Biomarkers of Heart Failure. Cells 2025, 14, 919. https://doi.org/10.3390/cells14120919
Darwish D, Kumar P, Urs K, Dave S. Impact of SGLT-2 Inhibitors on Biomarkers of Heart Failure. Cells. 2025; 14(12):919. https://doi.org/10.3390/cells14120919
Chicago/Turabian StyleDarwish, Dana, Pooja Kumar, Khushi Urs, and Siddharth Dave. 2025. "Impact of SGLT-2 Inhibitors on Biomarkers of Heart Failure" Cells 14, no. 12: 919. https://doi.org/10.3390/cells14120919
APA StyleDarwish, D., Kumar, P., Urs, K., & Dave, S. (2025). Impact of SGLT-2 Inhibitors on Biomarkers of Heart Failure. Cells, 14(12), 919. https://doi.org/10.3390/cells14120919






