Reduced mtDNA Copy Number Links to Vascular Calcification and Restores After Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Biochemical Measurements
2.3. Clinical Information and Health Assessments
2.4. Cardiovascular Assessments
2.5. Quantitative PCR (qPCR) with TaqMan® Probes
2.6. Measurement of ccf-DNA
2.7. Statistical Analysis
3. Results
3.1. Patient Disposition
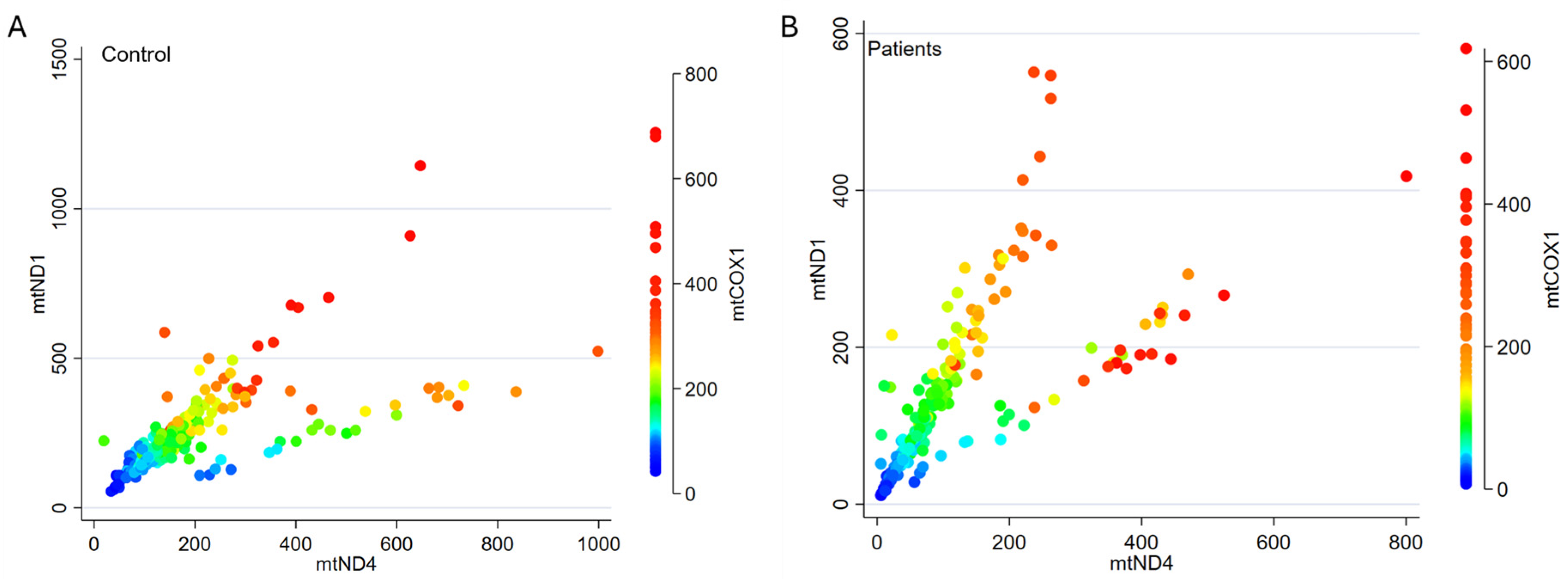
3.2. mtDNA Genes Show Similar Distribution
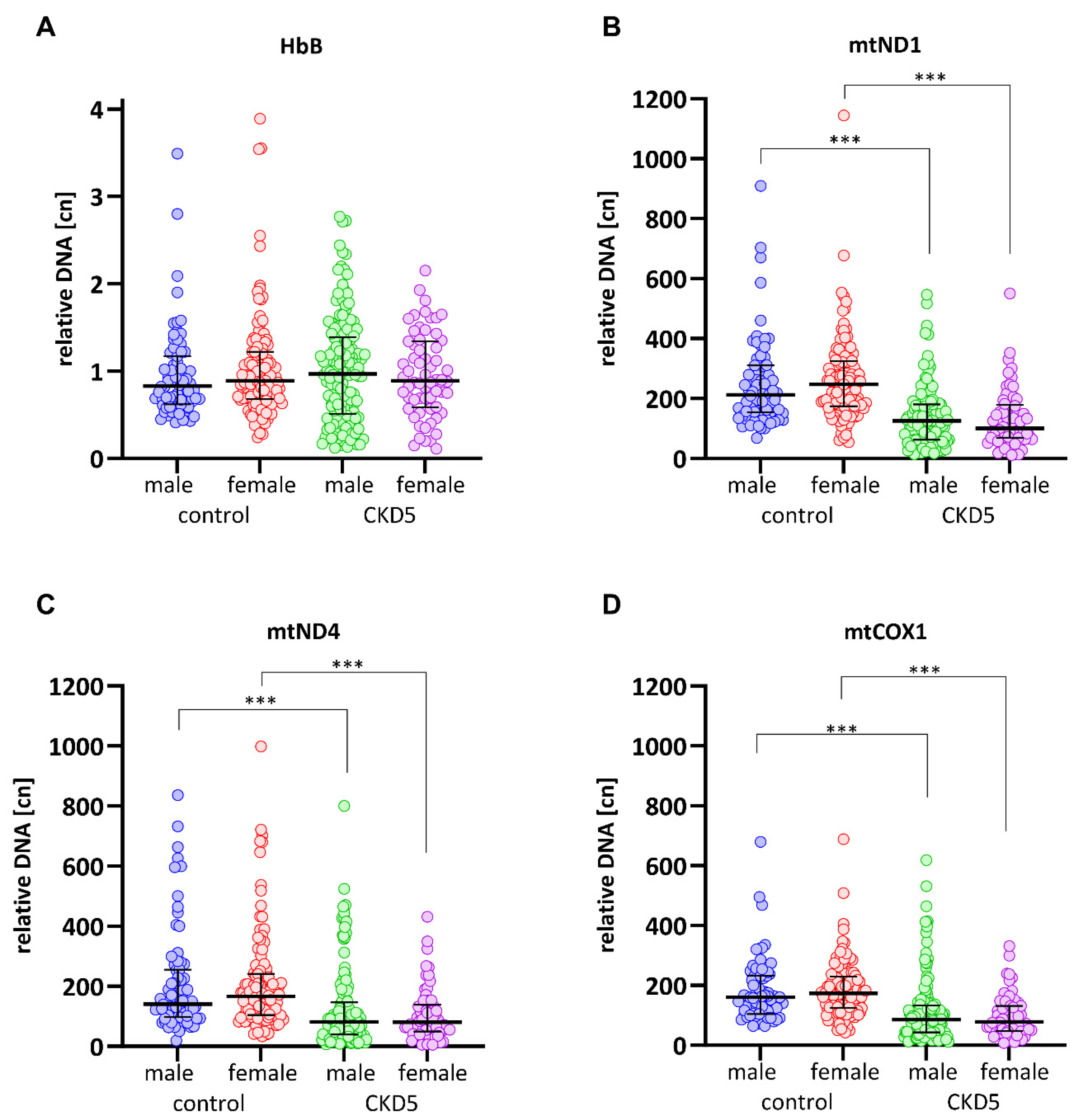
3.3. mtDNA-cn Is Significantly Lower in CKD5 Patients than in Controls
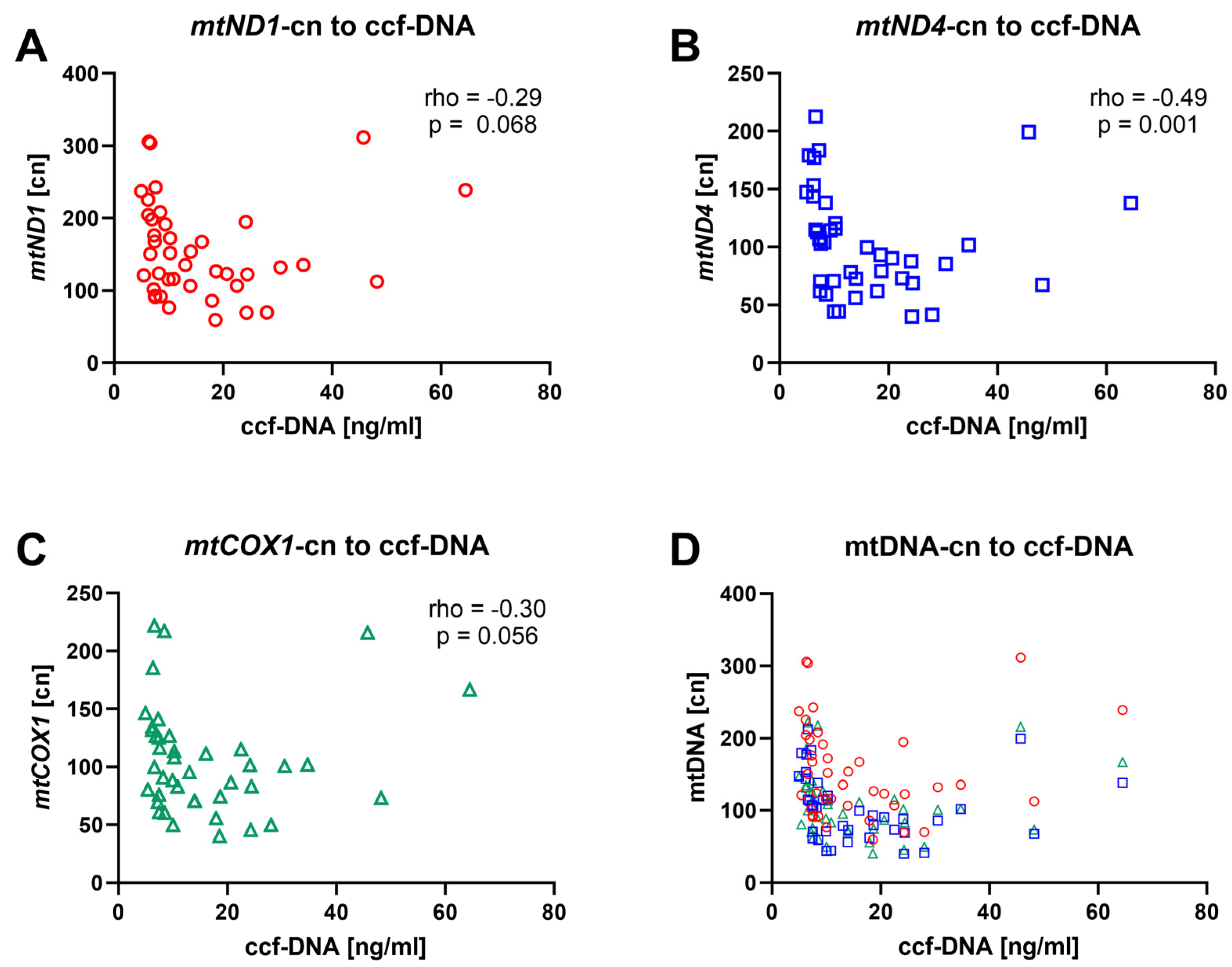
3.4. mtDNA-cn Is Inversely Correlated with ccf-mtDNA
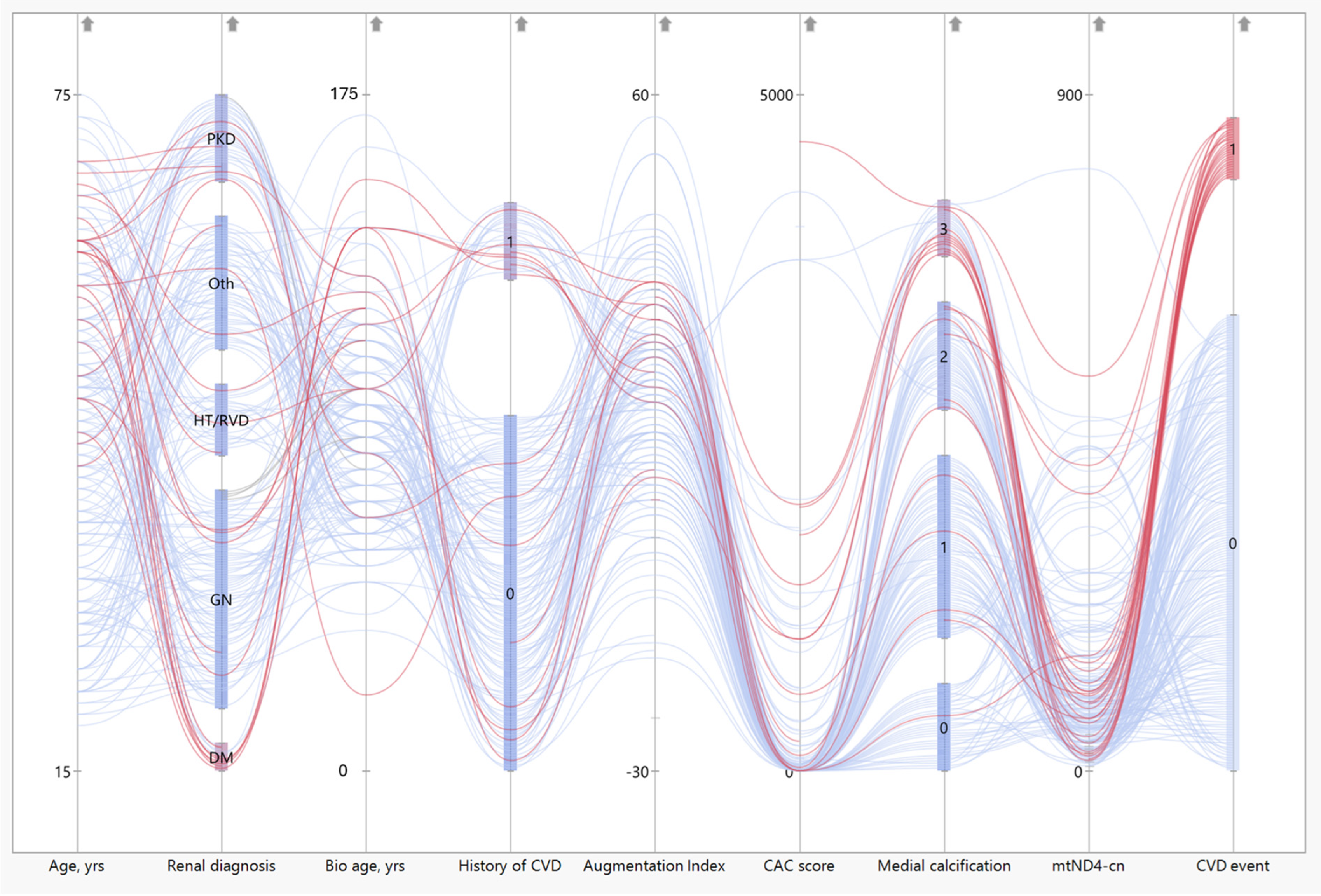
3.5. CAC Score, Medial Calcification, and Biological Age by SAF Correlate to mtDNA-cn
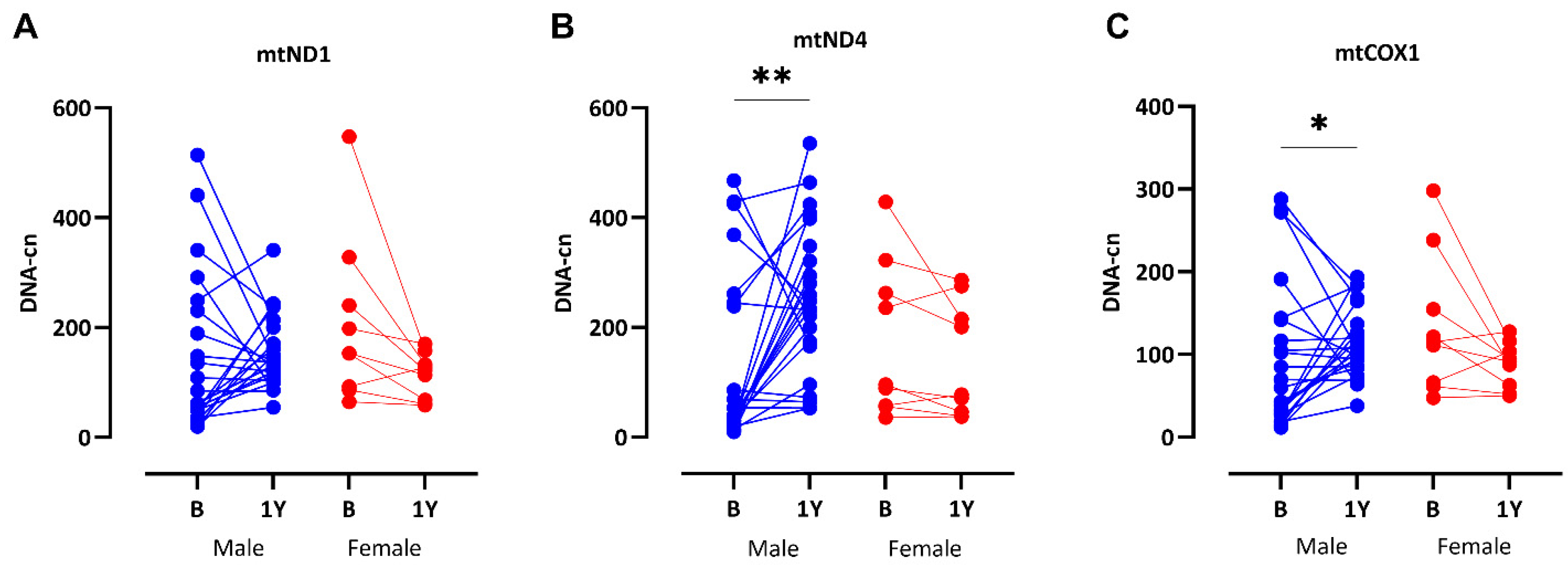
3.6. Significant Recovery of mtDNA-cn After RTx
3.7. Patients with CVD Event Have Low mtND4-cn Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Augmentation pressure |
| BMI | Body mass index |
| CAC | Coronary artery calcification |
| Ccf- | Circulatory cell-free |
| CKD | Chronic kidney disease |
| CVD | Cardiovascular disease |
| DAMP | Damage-associated molecular pattern |
| DM | Diabetes mellitus |
| eGFR | Estimated glomerular filtration rate |
| EVA | Early vascular aging |
| FRS | Framingham cardiovascular disease risk score |
| HbB | Hemoglobin beta |
| HDL-c | High-density lipoprotein cholesterol |
| HGS | Handgrip strength |
| IgAN | IgA Nephropathy |
| LD | Living donor |
| LDL-c | Low-density lipoprotein cholesterol |
| mtDNA-cn | Mitochondrial DNA copy number |
| OXPHOS | Oxidative phosphorylation |
| PEW | Protein-energy wasting |
| ROS | Reactive oxygen species |
| RTx | Renal transplantation |
| SAF | Skin autofluorescence |
| SASP | Senescence-associated secretory phenotype |
| SBP | Systolic blood pressure |
| SGA | Subjective global assessment of nutritional status |
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Huang, J.C.; Su, H.M.; Chiu, Y.W.; Chang, J.M.; Hwang, S.J.; Chen, H.C. Prognostic Cardiovascular Markers in Chronic Kidney Disease. Kidney Blood Press. Res. 2018, 43, 1388–1407. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.G.; Boutouyrie, P.; Nilsson, P.M.; Laurent, S. Early Vascular Ageing (EVA): Definitions and Clinical Applicability. Curr. Hypertens. Rev. 2017, 13, 8–15. [Google Scholar] [CrossRef]
- Hobson, S.; Arefin, S.; Witasp, A.; Hernandez, L.; Kublickiene, K.; Shiels, P.G.; Stenvinkel, P. Accelerated Vascular Aging in Chronic Kidney Disease: The Potential for Novel Therapies. Circ. Res. 2023, 132, 950–969. [Google Scholar] [CrossRef]
- Bellasi, A.; Di Lullo, L.; Russo, D.; Ciarcia, R.; Magnocavallo, M.; Lavalle, C.; Ratti, C.; Fusaro, M.; Cozzolino, M.; Di Iorio, B.R. Predictive Value of Measures of Vascular Calcification Burden and Progression for Risk of Death in Incident to Dialysis Patients. J. Clin. Med. 2021, 10, 376. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.P.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients with Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef]
- Erlandsson, H.; Qureshi, A.R.; Ripsweden, J.; Haugen Lofman, I.; Soderberg, M.; Wennberg, L.; Lundgren, T.; Bruchfeld, A.; Brismar, T.B.; Stenvinkel, P. Scoring of medial arterial calcification predicts cardiovascular events and mortality after kidney transplantation. J. Intern. Med. 2022, 291, 813–823. [Google Scholar] [CrossRef]
- Rao, S.; Weng, M.; Lian, R.; Zhuo, Y.; Lin, J.; You, D.; Cui, J.; Chen, Y.; Wan, J. Correlation between coronary calcification and cardiac structure in non-dialysis patients with chronic kidney disease. ESC Heart Fail. 2024, 12, 199–210. [Google Scholar] [CrossRef]
- Ward, L.J.; Laucyte-Cibulskiene, A.; Hernandez, L.; Ripsweden, J.; Collaborators, G.-F.; Stenvinkel, P.; Kublickiene, K. Coronary artery calcification and aortic valve calcification in patients with kidney failure: A sex-disaggregated study. Biol. Sex Differ. 2023, 14, 48. [Google Scholar] [CrossRef]
- Mukai, H.; Dai, L.; Chen, Z.; Lindholm, B.; Ripsweden, J.; Brismar, T.B.; Heimburger, O.; Barany, P.; Qureshi, A.R.; Soderberg, M.; et al. Inverse J-shaped relation between coronary arterial calcium density and mortality in advanced chronic kidney disease. Nephrol. Dial. Transplant. 2020, 35, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Lumpuy-Castillo, J.; Amador-Martinez, I.; Diaz-Rojas, M.; Lorenzo, O.; Pedraza-Chaverri, J.; Sanchez-Lozada, L.G.; Aparicio-Trejo, O.E. Role of mitochondria in reno-cardiac diseases: A study of bioenergetics, biogenesis, and GSH signaling in disease transition. Redox Biol. 2024, 76, 103340. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, C.M.; Falkenberg, M.; Larsson, N.G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160. [Google Scholar] [CrossRef]
- Kauppila, J.H.K.; Baines, H.L.; Bratic, A.; Simard, M.L.; Freyer, C.; Mourier, A.; Stamp, C.; Filograna, R.; Larsson, N.G.; Greaves, L.C.; et al. A Phenotype-Driven Approach to Generate Mouse Models with Pathogenic mtDNA Mutations Causing Mitochondrial Disease. Cell Rep. 2016, 16, 2980–2990. [Google Scholar] [CrossRef]
- Fazzini, F.; Lamina, C.; Raftopoulou, A.; Koller, A.; Fuchsberger, C.; Pattaro, C.; Del Greco, F.M.; Dottelmayer, P.; Fendt, L.; Fritz, J.; et al. Association of mitochondrial DNA copy number with metabolic syndrome and type 2 diabetes in 14 176 individuals. J. Intern. Med. 2021, 290, 190–202. [Google Scholar] [CrossRef]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.G. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
- Tewari, S.R.; Kirk, G.D.; Arking, D.E.; Astemborski, J.; Newcomb, C.; Piggott, D.A.; Mehta, S.; Lucas, G.M.; Sun, J. Mitochondrial DNA copy number is associated with incident chronic kidney disease and proteinuria in the AIDS linked to the intravenous experience cohort. Sci. Rep. 2023, 13, 18406. [Google Scholar] [CrossRef]
- Malik, A.N. Mitochondrial DNA—Novel mechanisms of kidney damage and potential biomarker. Curr. Opin. Nephrol. Hypertens. 2023, 32, 528–536. [Google Scholar] [CrossRef]
- Mengel-From, J.; Thinggaard, M.; Dalgard, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef]
- Sundquist, K.; Sundquist, J.; Palmer, K.; Memon, A.A. Role of mitochondrial DNA copy number in incident cardiovascular diseases and the association between cardiovascular disease and type 2 diabetes: A follow-up study on middle-aged women. Atherosclerosis 2022, 341, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sidore, C.; Butler, T.J.; Wing, M.K.; Qian, Y.; Meirelles, O.; Busonero, F.; Tsoi, L.C.; Maschio, A.; Angius, A.; et al. Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools. PLoS Genet. 2015, 11, e1005306. [Google Scholar] [CrossRef]
- van Leeuwen, N.; Beekman, M.; Deelen, J.; van den Akker, E.B.; de Craen, A.J.; Slagboom, P.E.; t Hart, L.M. Low mitochondrial DNA content associates with familial longevity: The Leiden Longevity Study. Age 2014, 36, 9629. [Google Scholar] [CrossRef] [PubMed]
- Tin, A.; Grams, M.E.; Ashar, F.N.; Lane, J.A.; Rosenberg, A.Z.; Grove, M.L.; Boerwinkle, E.; Selvin, E.; Coresh, J.; Pankratz, N.; et al. Association between Mitochondrial DNA Copy Number in Peripheral Blood and Incident CKD in the Atherosclerosis Risk in Communities Study. J. Am. Soc. Nephrol. 2016, 27, 2467–2473. [Google Scholar] [CrossRef]
- Fazzini, F.; Lamina, C.; Fendt, L.; Schultheiss, U.T.; Kotsis, F.; Hicks, A.A.; Meiselbach, H.; Weissensteiner, H.; Forer, L.; Krane, V.; et al. Mitochondrial DNA copy number is associated with mortality and infections in a large cohort of patients with chronic kidney disease. Kidney Int. 2019, 96, 480–488. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Luo, N.; Li, Z.; Mao, H.; Zhou, Y. Mitochondrial DNA copy number in peripheral blood of IgA nephropathy: A cross-sectional study. Ren. Fail. 2023, 45, 2182133. [Google Scholar] [CrossRef]
- Banoth, B.; Cassel, S.L. Mitochondria in innate immune signaling. Transl. Res. 2018, 202, 52–68. [Google Scholar] [CrossRef]
- Mihm, S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int. J. Mol. Sci. 2018, 19, 3104. [Google Scholar] [CrossRef]
- Cushen, S.C.; Ricci, C.A.; Bradshaw, J.L.; Silzer, T.; Blessing, A.; Sun, J.; Zhou, Z.; Scroggins, S.M.; Santillan, M.K.; Santillan, D.A.; et al. Reduced Maternal Circulating Cell-Free Mitochondrial DNA Is Associated with the Development of Preeclampsia. J. Am. Heart Assoc. 2022, 11, e021726. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Olauson, H.; Witasp, A.; Haarhaus, M.; Brandenburg, V.; Wernerson, A.; Lindholm, B.; Soderberg, M.; Wennberg, L.; Nordfors, L.; et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015, 88, 1356–1364. [Google Scholar] [CrossRef]
- Neytchev, O.; Erlandsson, H.; Witasp, A.; Nordfors, L.; Qureshi, A.R.; Iseri, K.; Morohoshi, H.; Selman, C.; Ebert, T.; Kublickiene, K.; et al. Epigenetic clocks indicate that kidney transplantation and not dialysis mitigate the effects of renal ageing. J. Intern. Med. 2024, 295, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.R.; Alvestrand, A.; Danielsson, A.; Divino-Filho, J.C.; Gutierrez, A.; Lindholm, B.; Bergstrom, J. Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int. 1998, 53, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Svedberg, O.; Lindholm, B.; Dai, L.; Heimburger, O.; Barany, P.; Anderstam, B.; Stenvinkel, P.; Qureshi, A.R. Skin autofluorescence, arterial stiffness and Framingham risk score as predictors of clinical outcome in chronic kidney disease patients: A cohort study. Nephrol. Dial. Transplant. 2019, 34, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Filograna, R.; Koolmeister, C.; Upadhyay, M.; Pajak, A.; Clemente, P.; Wibom, R.; Simard, M.L.; Wredenberg, A.; Freyer, C.; Stewart, J.B.; et al. Modulation of mtDNA copy number ameliorates the pathological consequences of a heteroplasmic mtDNA mutation in the mouse. Sci. Adv. 2019, 5, eaav9824. [Google Scholar] [CrossRef]
- Leotta, C.; Hernandez, L.; Tothova, L.; Arefin, S.; Ciceri, P.; Cozzolino, M.G.; Barany, P.; Chromek, M.; Stenvinkel, P.; Kublickiene, K. Levels of Cell-Free DNA in Kidney Failure Patients before and after Renal Transplantation. Cells 2023, 12, 2774. [Google Scholar] [CrossRef]
- Chen, R.Y.; Shi, J. Evaluation of the CKD-EPI 2021 creatinine equation using laboratory data: Considerations for practice changes among clinical laboratories in British Columbia, Canada. Clin. Biochem. 2024, 123, 110686. [Google Scholar] [CrossRef]
- Kanazashi, Y.; Maejima, K.; Johnson, T.A.; Sasagawa, S.; Jikuya, R.; Hasumi, H.; Matsumoto, N.; Maekawa, S.; Obara, W.; Nakagawa, H. Mitochondrial DNA Variants at Low-Level Heteroplasmy and Decreased Copy Numbers in Chronic Kidney Disease (CKD) Tissues with Kidney Cancer. Int. J. Mol. Sci. 2023, 24, 7212. [Google Scholar] [CrossRef]
- Canadas-Garre, M.; Maqueda, J.J.; Banos-Jaime, B.; Hill, C.; Skelly, R.; Cappa, R.; Brennan, E.; Doyle, R.; Godson, C.; Maxwell, A.P.; et al. Mitochondrial related variants associated with cardiovascular traits. Front. Physiol. 2024, 15, 1395371. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. Landmark Ed. 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.V.; Hajizadeh, S.; Holme, E.; Jonsson, I.M.; Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 2004, 75, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Bartoli-Leonard, F.; Zimmer, J.; Sonawane, A.R.; Perez, K.; Turner, M.E.; Kuraoka, S.; Pham, T.; Li, F.; Aikawa, M.; Singh, S.; et al. NLRP3 Inflammasome Activation in Peripheral Arterial Disease. J. Am. Heart Assoc. 2023, 12, e026945. [Google Scholar] [CrossRef]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early vascular ageing in chronic kidney disease: Impact of inflammation, vitamin K, senescence and genomic damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef]
- Shiels, P.G.; McGlynn, L.M.; MacIntyre, A.; Johnson, P.C.; Batty, G.D.; Burns, H.; Cavanagh, J.; Deans, K.A.; Ford, I.; McConnachie, A.; et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS ONE 2011, 6, e22521. [Google Scholar] [CrossRef]
- Junker, A.; Wang, J.; Gouspillou, G.; Ehinger, J.K.; Elmer, E.; Sjovall, F.; Fisher-Wellman, K.H.; Neufer, P.D.; Molina, A.J.A.; Ferrucci, L.; et al. Human studies of mitochondrial biology demonstrate an overall lack of binary sex differences: A multivariate meta-analysis. FASEB J. 2022, 36, e22146. [Google Scholar] [CrossRef]
- El-Ansary, M.; Saadi, G.; Hassaballa, M.; Zidan, M.; Abdel Fattah, W.; Kelany, A.K.; Hanna, M.O.F. Donor cell microchimerism in kidney transplantation: Implications for graft function. Int. J. Immunogenet. 2020, 47, 494–500. [Google Scholar] [CrossRef]
- Lindqvist, D.; Wolkowitz, O.M.; Picard, M.; Ohlsson, L.; Bersani, F.S.; Fernstrom, J.; Westrin, A.; Hough, C.M.; Lin, J.; Reus, V.I.; et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 2018, 43, 1557–1564. [Google Scholar] [CrossRef]
- Arefin, S.; Buchanan, S.; Hobson, S.; Steinmetz, J.; Alsalhi, S.; Shiels, P.G.; Kublickiene, K.; Stenvinkel, P. Nrf2 in early vascular ageing: Calcification, senescence and therapy. Clin. Chim. Acta 2020, 505, 108–118. [Google Scholar] [CrossRef]
- DeBarmore, B.; Longchamps, R.J.; Zhang, Y.; Kalyani, R.R.; Guallar, E.; Arking, D.E.; Selvin, E.; Young, J.H. Mitochondrial DNA copy number and diabetes: The Atherosclerosis Risk in Communities (ARIC) study. BMJ Open Diabetes Res. Care 2020, 8, e001204. [Google Scholar] [CrossRef]

| All Patients | Female Patients | Male Patients | p-Value | N Data | All Controls | Female Controls | Male Controls | p-Value | N | |
|---|---|---|---|---|---|---|---|---|---|---|
| N = 211 | N = 69 | N = 142 | N = 196 | N = 117 | N = 71 | Data | ||||
| Age (years) | 47 (32–58) | 49 (30–61) | 47 (33–55) | 0.52 | 211 | 51 (42–59) | 51 (43–61) | 48 (39–56) | <0.05 | 186 |
| Bio. age by SAF (years) | 90 (70–107) | 97 (78–111) | 86 (70–103) | 0.072 | 174 | n.a. | n.a. | n.a. | n.a. | |
| BMI, kg/m2 | 24.2 (22.2–26.7) | 24.4 (22.5–26.5) | 23.5 (21.6–27.1) | 0.34 | 200 | 24.9 (23.1–27.2) | 24.4 (22.5–27.2) | 25.2 (23.9–27.2) | 0.16 | 161 |
| Systolic BP, mmHg | 141 (130–155) | 142 (131–156) | 138 (126–154) | 0.15 | 200 | 124 (117–131) | 123 (116–131) | 125 (117–132) | 0.39 | 161 |
| Diastolic BP, mmHg | 85 (76–94) | 83 (76–90) | 86 (76–95) | 0.31 | 200 | 75 (70–80) | 74 (68–80) | 79 (71–83) | 0.014 | 161 |
| Creatinine, µmol/L | 720 (581–877) | 598 (490–722) | 775 (622–943) | <0.001 | 211 | 69 (62–80) | 65 (58–70) | 81 (74–87) | <0.001 | 160 |
| eGFR, mL/min | 7 (5–9) | 7 (6–9) | 7 (5–8) | 0.25 | 202 | 100 (90–110) | 98 (89–110) | 103 (92–110) | 0.22 | 161 |
| Triglycerides, mmol/L | 1.3 (1.0–1.9) | 1.4 (1.0–2.0) | 1.3 (1.0–1.9) | 0.84 | 210 | 0.8 (0.7–1.3) | 0.7 (0.6–1.0) | 1.2 (0.8–1.6) | <0.001 | 146 |
| Cholesterol, mmol/L | 4.4 (3.6–5.1) | 4.8 (3.9–5.6) | 4.2 (3.5–4.9) | <0.001 | 210 | 5.2 (4.6–5.8) | 5.2 (4.5–5.8) | 5.2 (4.6–5.7) | 0.94 | 150 |
| HDL-cholesterol, mmol/L | 1.3 (1.1–1.6) | 1.6 (1.3–1.9) | 1.2 (1.0–1.5) | <0.001 | 210 | n.a. | n.a. | n.a. | n.a. | |
| S-Albumin g/L | 34 (32–37) | 33 (32–36) | 35 (32–38) | 0.081 | 209 | n.a. | n.a. | n.a. | n.a. | |
| hsCRP, mg/L | 0.9 (0.3–2.0) | 0.8 (0.3–2.2) | 0.9 (0.4–1.9) | 0.73 | 209 | n.a. | n.a. | n.a. | n.a. | |
| TMAO, μM | 51 (34–89) | 49 (32–94) | 54 (36–88) | 0.65 | 183 | n.a. | n.a. | n.a. | n.a. | |
| iPTH, ng/L | 260 (170–400) | 240 (160–420) | 270 (175–394) | 0.55 | 209 | n.a. | n.a. | n.a. | n.a. | |
| Calcium, mmol/L | 2.3 (2.2–2.4) | 2.3 (2.2–2.4) | 2.3 (2.1–2.4) | 0.61 | 209 | n.a. | n.a. | n.a. | n.a. | |
| Phosphate, mmol/L | 1.7 (1.4–2.0) | 1.6 (1.4–1.9) | 1.7 (1.3–2.1) | 0.47 | 209 | n.a. | n.a. | n.a. | n.a. | |
| Troponin-T, ng/L | 22.5 (14–38) | 20 (11–30) | 25 (16–44) | 0.006 | 202 | n.a. | n.a. | n.a. | n.a. | |
| Diabetes mellitus, n (%) | 20 (10.0) | 5 (8.2) | 15 (10.7) | 0.58 | 201 | n.a. | n.a. | n.a. | n.a. | |
| CAC > 0, n (%) | 77 (49.7) | 23 (48.9) | 54 (50.0) | 0.90 | 155 | n.a. | n.a. | n.a. | n.a. | |
| CT-total | 0 (0–126) | 0 (0–137) | 2 (0–97) | 0.84 | 159 | n.a. | n.a. | n.a. | n.a. | |
| PEW (SGA > 1), n (%) | 66 (32.7) | 24 (37.5) | 42 (30.4) | 0.32 | 202 | n.a. | n.a. | n.a. | n.a. | |
| AORTIC AIX@HR75 (%) | 20.2 (10.7–27.3) | 23.1 (13.6–28.4) | 19 (7.9–26) | 0.053 | 127 | n.a. | n.a. | n.a. | n.a. | |
| FRS (%) | 6.9 (3.2–14.8) | 4.8 (2.0–8.9) | 8.0 (3.7–16.5) | 0.001 | 201 | n.a. | n.a. | n.a. | n.a. | |
| Hand grip strength, % | 95 (78–109) | 93 (74–104) | 98 (81–111) | 0.13 | 157 | n.a. | n.a. | n.a. | n.a. |
| Patients (N = 211) | Controls (N = 196) | p-Value | |
|---|---|---|---|
| mtND1, cn | 119 (65–180) | 222 (162–316) | <0.0001 |
| mtND4, cn | 81 (40–143) | 153 (103–242) | <0.0001 |
| mtCOX1, cn | 85 (45–132) | 166 (116–232) | <0.0001 |
| HbB, cn | 1.0 (0.6–1.4) | 0.9 (0.7–1.2) | 0.687 |
| mtND4cn-Tertiles | Low | Medium | High | All | N | p-Value |
|---|---|---|---|---|---|---|
| N = 69 | N = 70 | N = 72 | N = 211 | |||
| mtND4, cn | 30 (18–40) | 79 (66–92) | 193 (135–337) | 81 (40–143) | 211 | <0.001 |
| Males, n (%) | 43 (62.3) | 50 (71.4) | 49 (68.1) | 142 (67.3) | 211 | 0.51 |
| Age (years) | 51 (35–60) | 49 (37–61) | 41 (30–49) | 47 (32–58) | 211 | 0.012 |
| Bio. age by SAF (years) | 99 (78–115) | 99 (78–111) | 82 (65–95) | 90 (70–107) | 174 | <0.001 |
| BMI, kg/m2 | 25.1 (22.6–27.8) | 24.3 (22–27.5) | 23.5 (22.2–26) | 24.2 (22.3–26.9) | 209 | 0.056 |
| Creatinine, µmol/L | 686 (560–829) | 684 (584–859) | 736 (598–942) | 720 (581–877) | 211 | 0.39 |
| CT-total | 6 (0–361) | 13 (0–282) | 0 (0–14) | 0 (0–126) | 159 | 0.008 |
| Media calcification score, n (%) | 177 | <0.001 | ||||
| 0 | 5 (7.2) | 11 (15.7) | 24 (33.3) | 40 (19) | ||
| 1 | 24 (34.8) | 25 (35.7) | 24 (33.3) | 73 (34.6) | ||
| 2 | 11 (15.9) | 16 (22.9) | 15 (20.8) | 42 (20.8) | ||
| 3 | 8 (11.6) | 8 (11.4) | 6 (8.3) | 22 (10.4) | ||
| FRS (%) | 9.1 (4.2–17.2) | 7.8 (4.3–19.0) | 4.6 (2.3–9.1) | 6.9 (3.2–14.8) | 201 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, A.; Qureshi, A.R.; Hernandez, L.; Wennberg, L.; Wernerson, A.; Kublickiene, K.; Shiels, P.G.; Filograna, R.; Stenvinkel, P.; Witasp, A. Reduced mtDNA Copy Number Links to Vascular Calcification and Restores After Transplantation. Cells 2025, 14, 917. https://doi.org/10.3390/cells14120917
Schwarz A, Qureshi AR, Hernandez L, Wennberg L, Wernerson A, Kublickiene K, Shiels PG, Filograna R, Stenvinkel P, Witasp A. Reduced mtDNA Copy Number Links to Vascular Calcification and Restores After Transplantation. Cells. 2025; 14(12):917. https://doi.org/10.3390/cells14120917
Chicago/Turabian StyleSchwarz, Angelina, Abdul Rashid Qureshi, Leah Hernandez, Lars Wennberg, Annika Wernerson, Karolina Kublickiene, Paul G. Shiels, Roberta Filograna, Peter Stenvinkel, and Anna Witasp. 2025. "Reduced mtDNA Copy Number Links to Vascular Calcification and Restores After Transplantation" Cells 14, no. 12: 917. https://doi.org/10.3390/cells14120917
APA StyleSchwarz, A., Qureshi, A. R., Hernandez, L., Wennberg, L., Wernerson, A., Kublickiene, K., Shiels, P. G., Filograna, R., Stenvinkel, P., & Witasp, A. (2025). Reduced mtDNA Copy Number Links to Vascular Calcification and Restores After Transplantation. Cells, 14(12), 917. https://doi.org/10.3390/cells14120917







