Prenatal Delta-9-Tetrahydrocannabinol Exposure Induces Transcriptional Alterations in Dopaminergic System with Associated Electrophysiological Dysregulation in the Prefrontal Cortex of Adolescent Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Treatments
2.1.1. Drugs
2.1.2. Animals and Treatments
2.2. Molecular Biology Analysis
2.2.1. Gene Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.2.2. DNA Methylation Analysis by Pyrosequencing
2.2.3. miRNAs Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.3. Electrophysiology
Electrophysiological Recordings
2.4. Immunohistochemistry
2.4.1. Tissue Preparation
2.4.2. Reaction Protocol, Image Acquisition, and Density Analysis
2.5. Statistical Analysis
3. Results
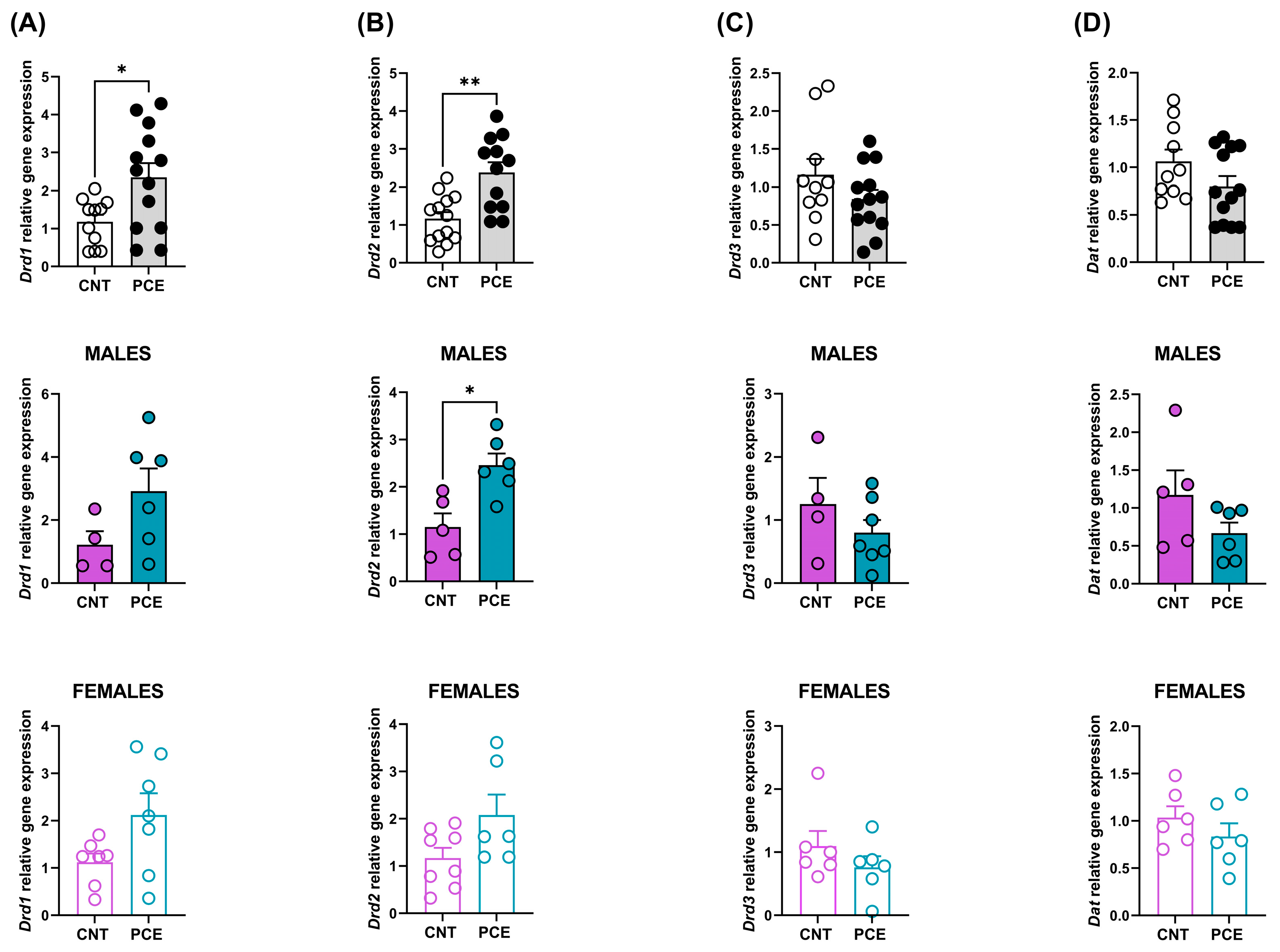
3.1. PCE Alters Drd1 and Drd2 Gene Expression in Prefrontal Cortex of Adolescent Offspring
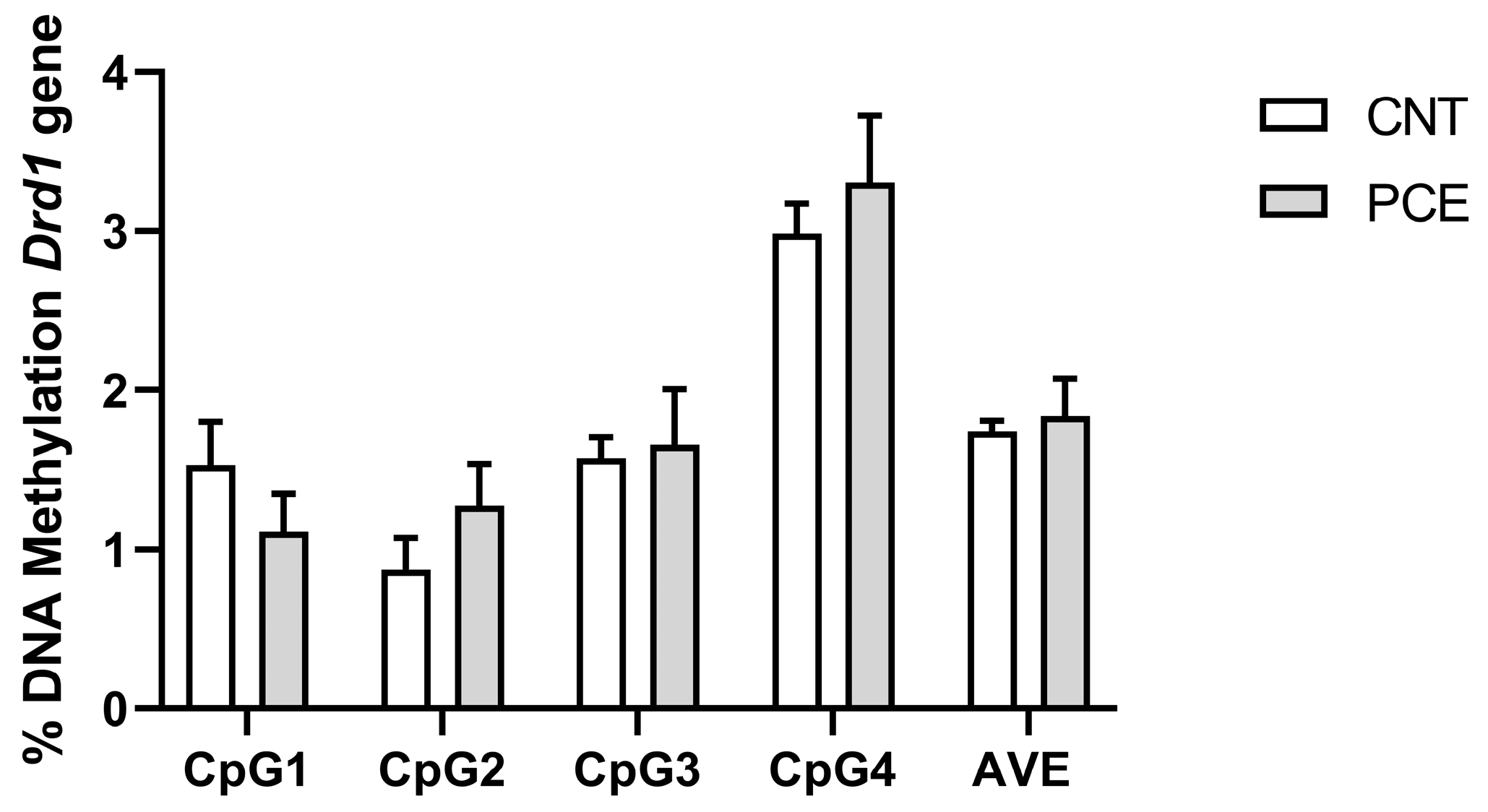
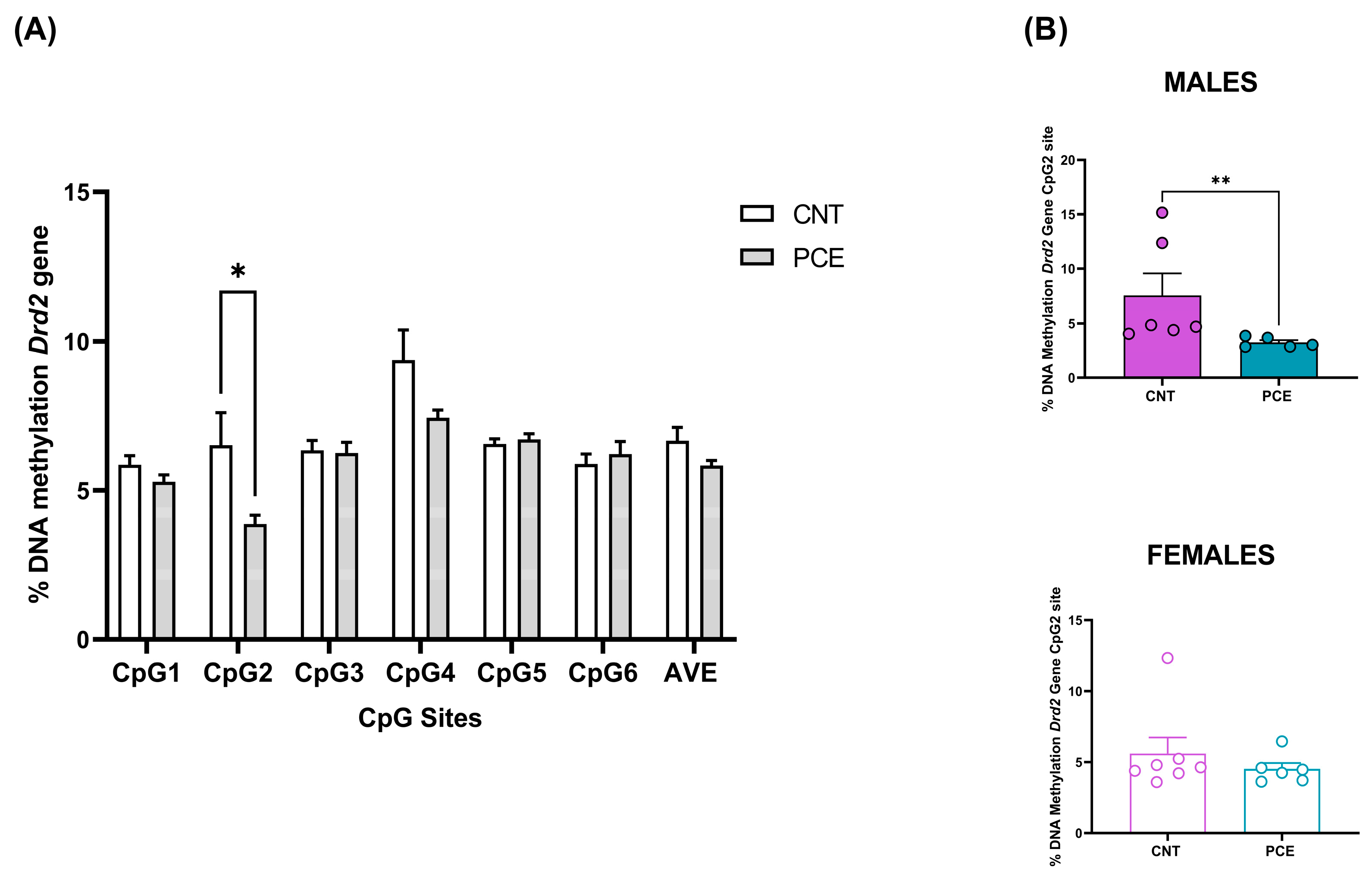
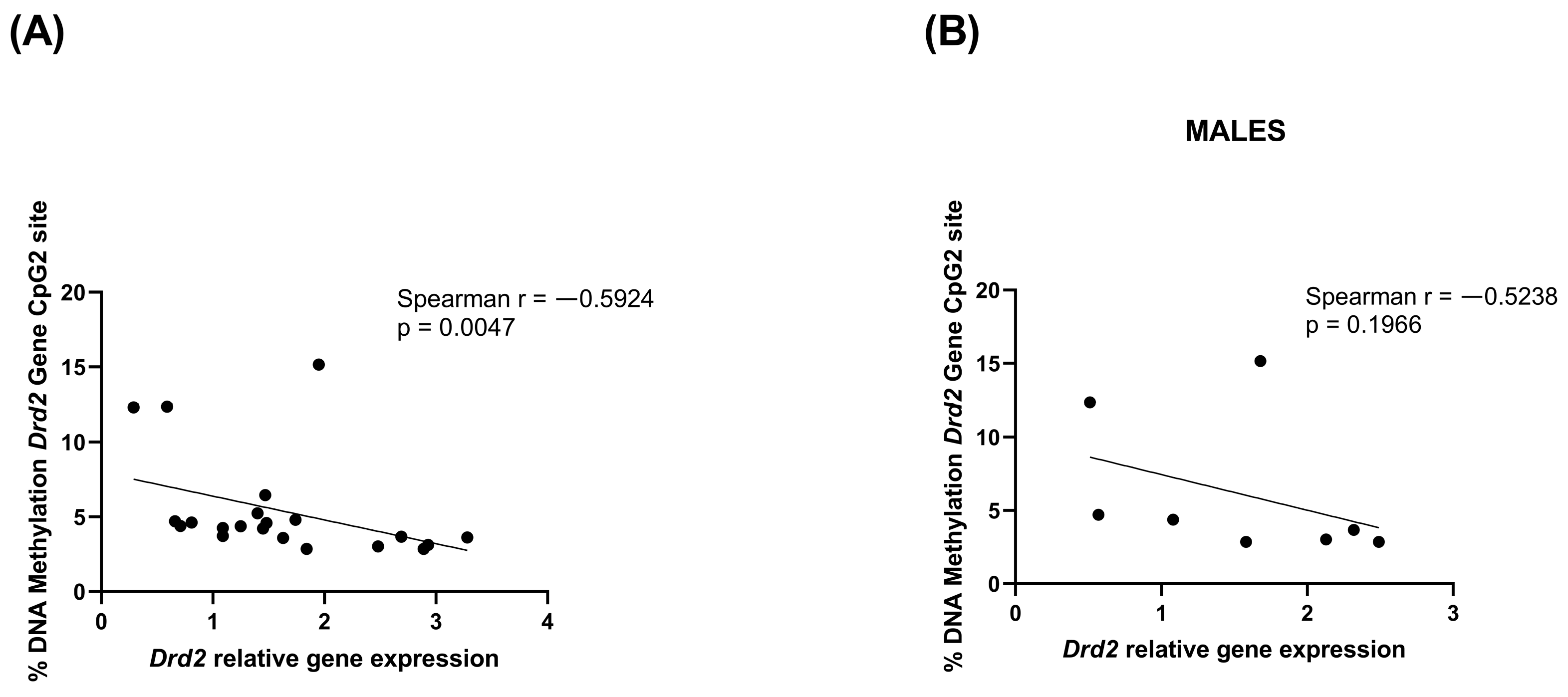
3.2. PCE Alters Drd2 DNA Methylation in Prefrontal Cortex of Adolescent Offspring
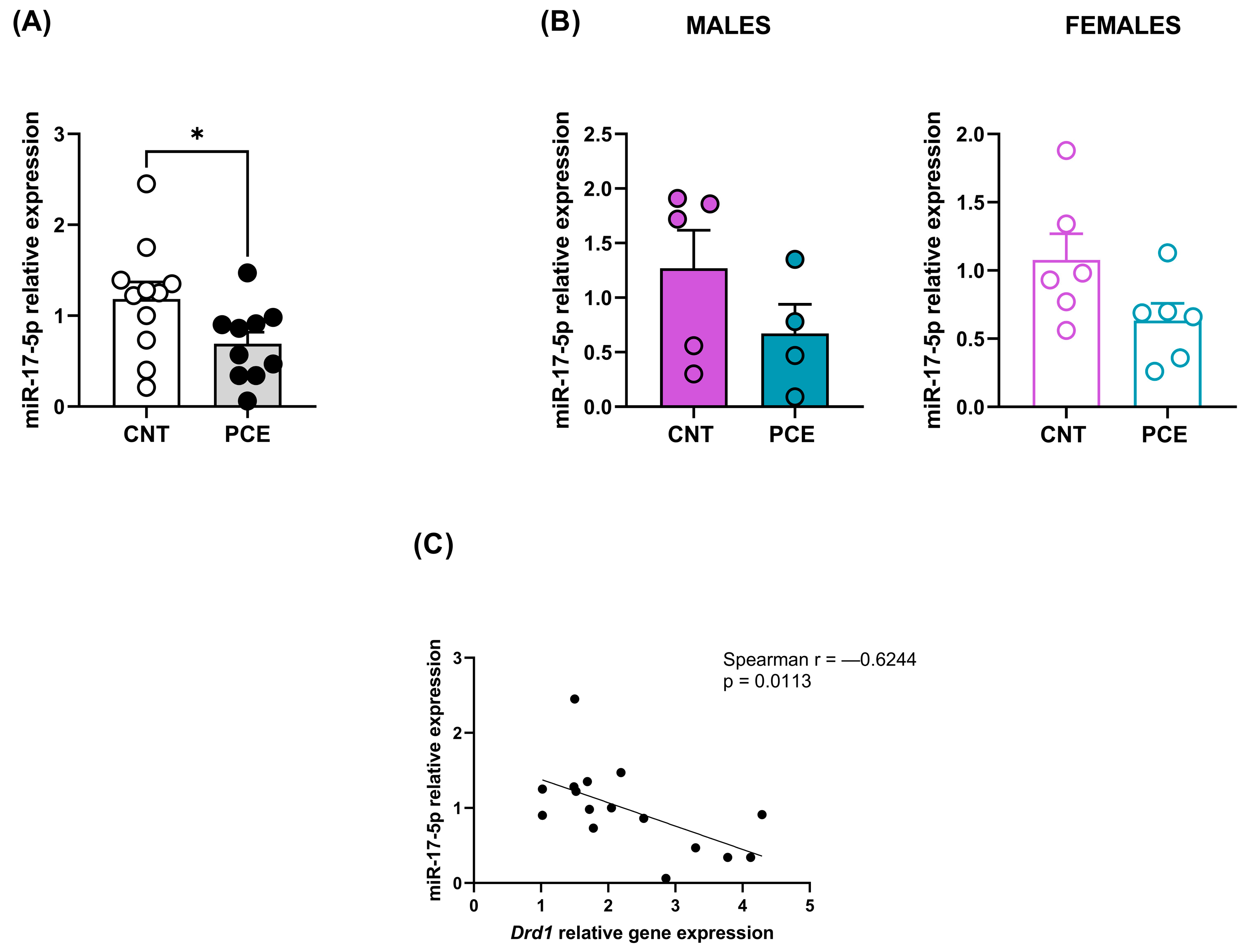
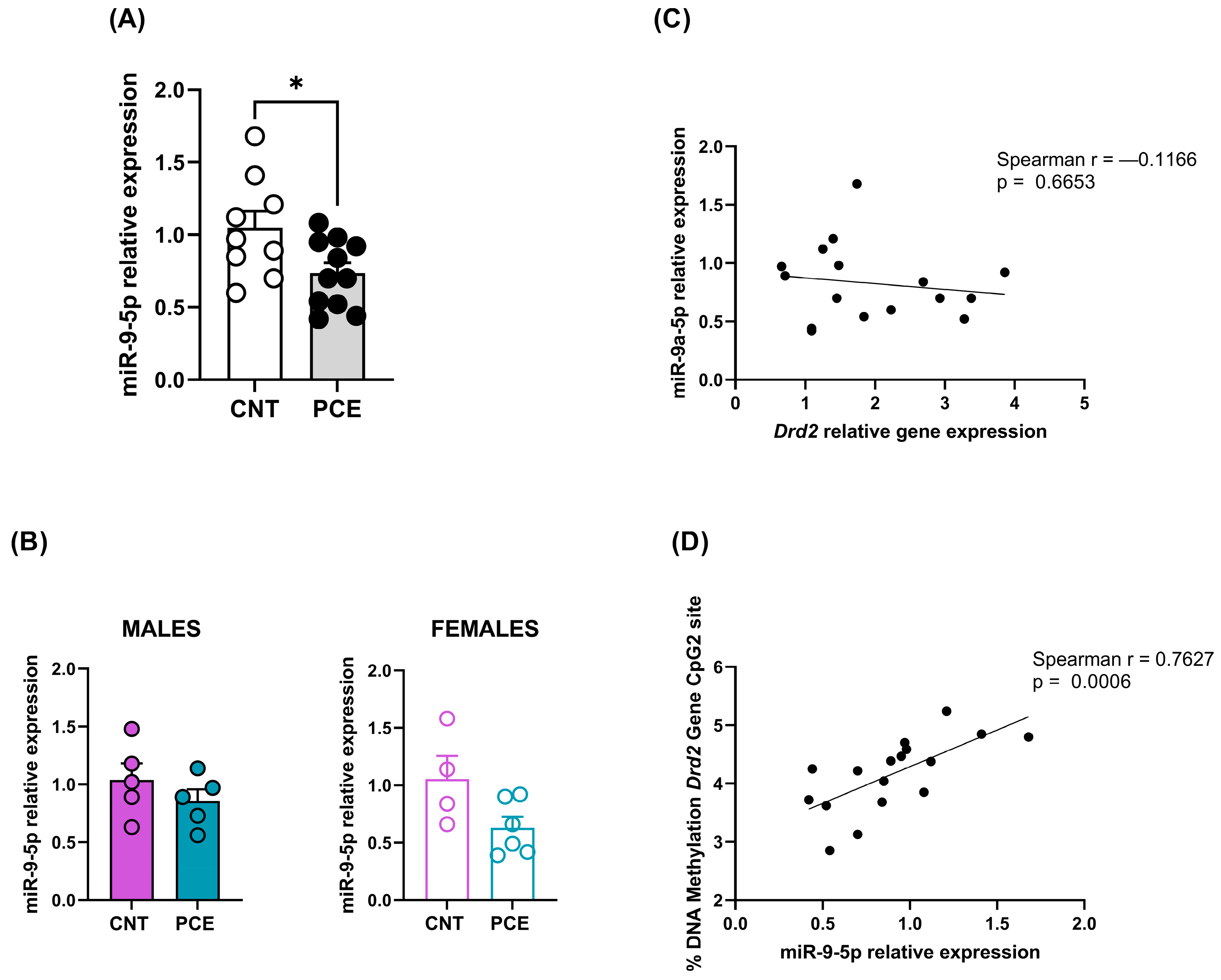
3.3. PCE Alters Selective miRNAs Expression, Targeting Drd1 and Drd2, in Prefrontal Cortex of Adolescent Offspring
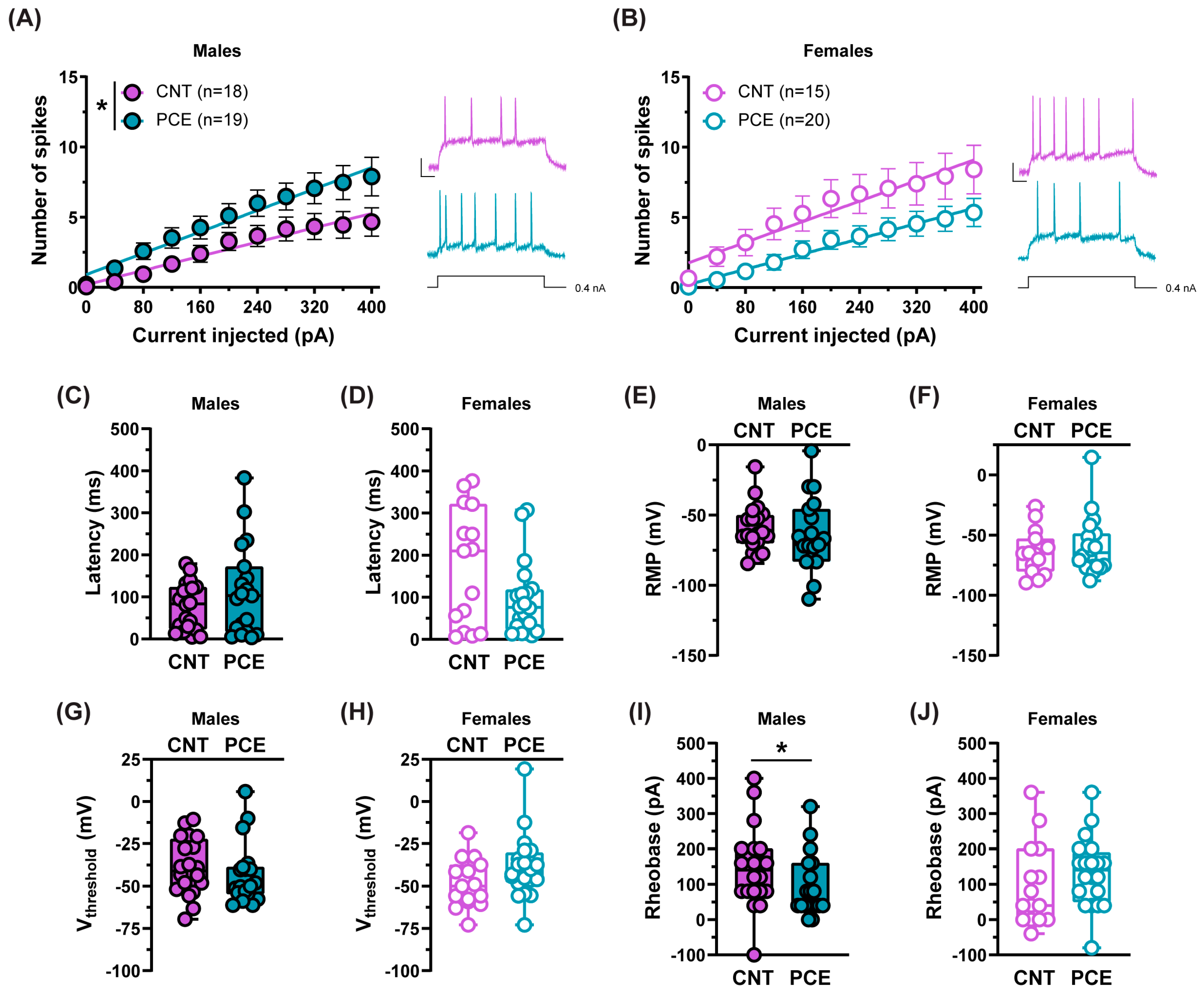
3.4. PCE Alters the Excitability of Medial Prefrontal Cortex Pyramidal Neurons in Adolescent Offspring
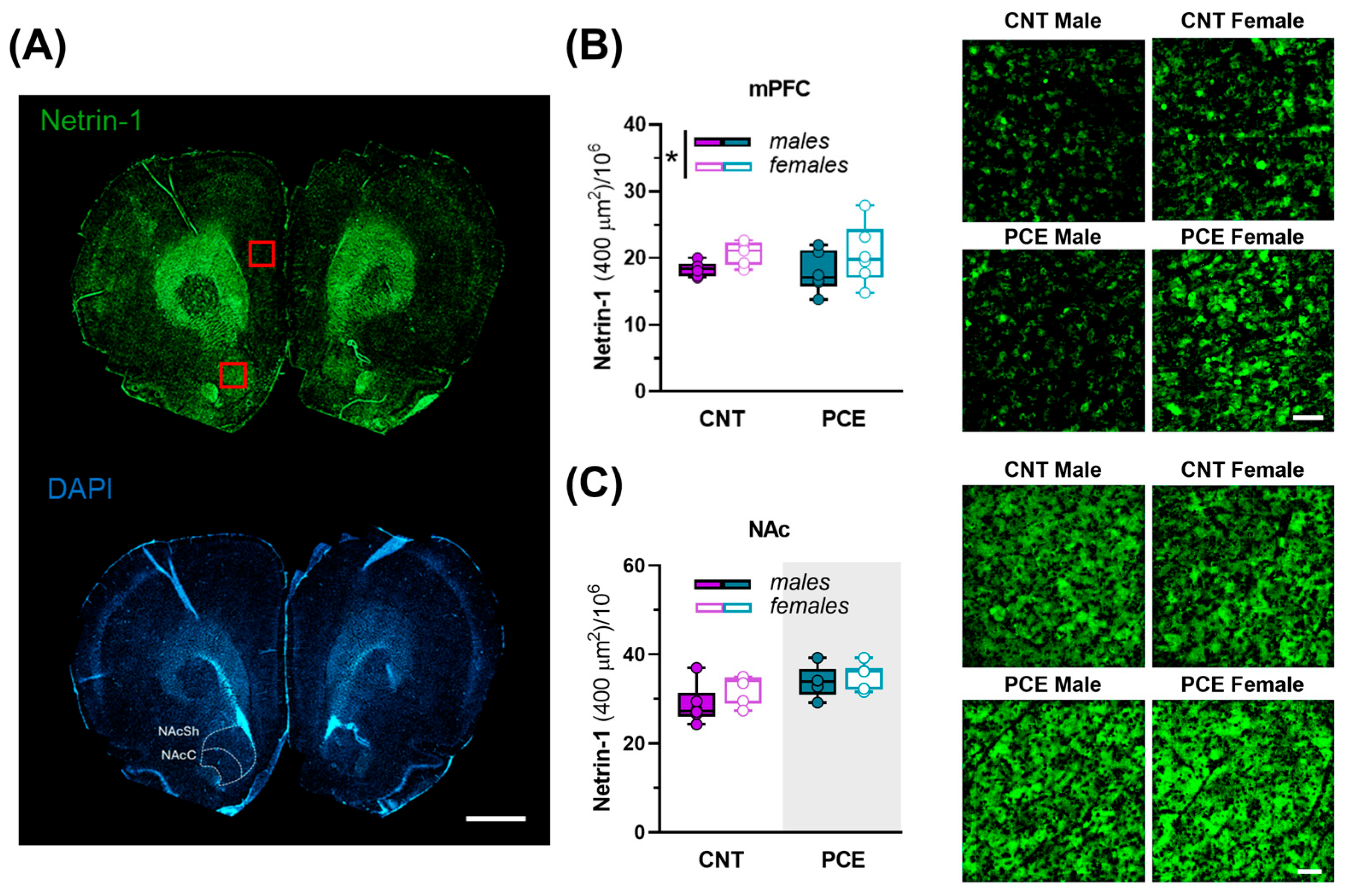
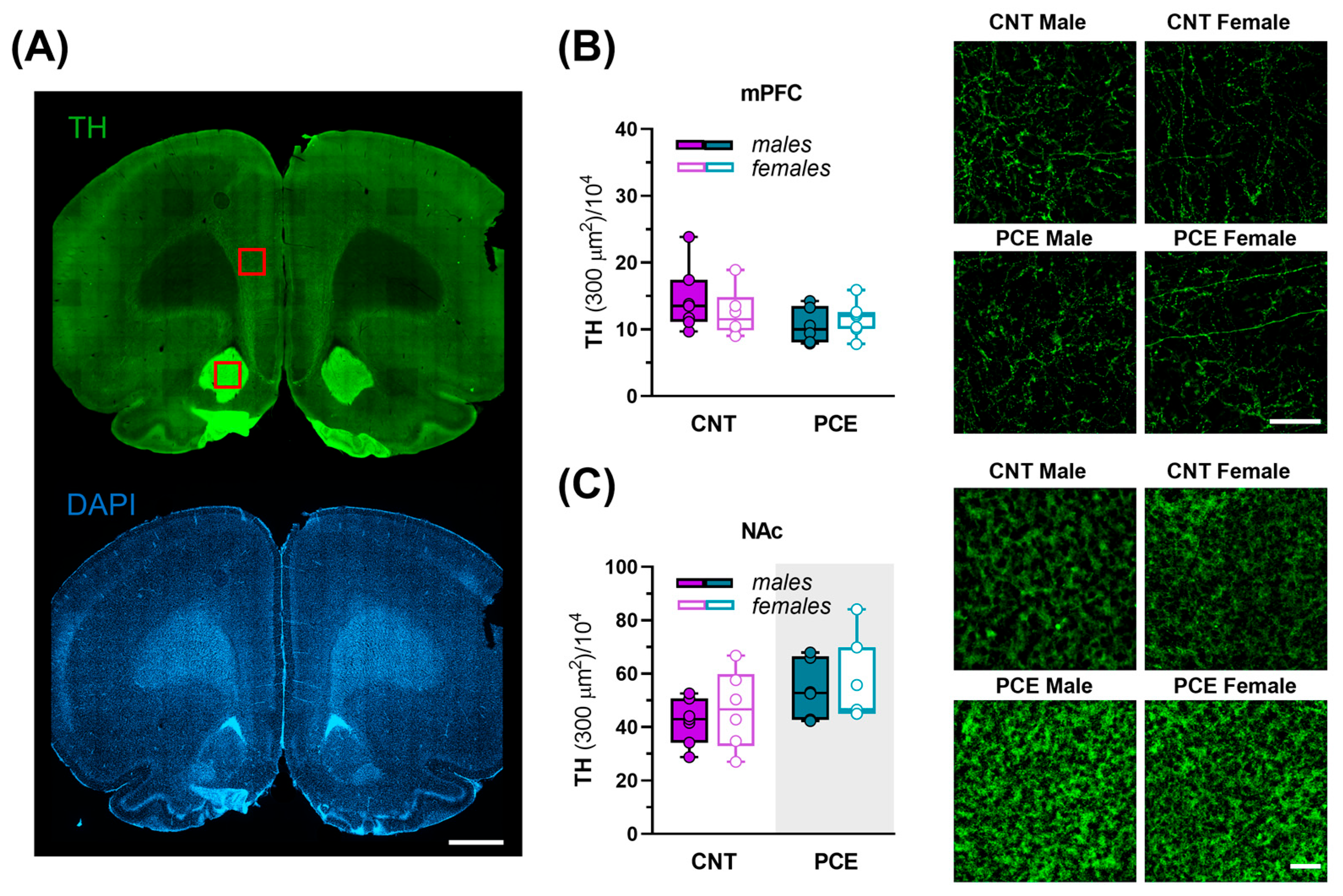
3.5. Sex-Specific PCE Effects on Netrin-1 and TH Immunoreactivity in the Medial Prefrontal Cortex and Nucleus Accumbens of Adolescent Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18S | 18s ribosomal RNA |
| 2-AG | 2-arachidonoylglycerol |
| β-ACT | Beta Actin |
| aCSF | Artificial cerebrospinal fluid |
| AEA | Anandamide |
| Cnr1 | Cannabinoid Receptor Type1 |
| Cnr2 | Cannabinoid receptor Type2 |
| Dagl-α | Diacylglycerol Lipase Alpha |
| Dat | Dopamine Transporter |
| Dcc | Deleted in Colorectal Cancer |
| DLPFC | Dorsolateral Prefrontal Cortex |
| Drd1 | Dopamine D1 receptor |
| Drd2 | Dopamine D2 receptor |
| Drd3 | Dopamine D3 receptor |
| ECS | Endocannabinoid system |
| Faah | Fatty Acid Amide Hydrolase |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| GD | Gestational day |
| Magl | Monoglyceride Lipase |
| MAM | Methylazoxymethanol acetate |
| mPFC | Medial prefrontal cortex |
| NAc | Nucleus accumbens |
| NAcC | Nucleus accumbens core |
| NacSh | Nucleus accumbens shell |
| Nape-Pld | N-Acylphosphatidylethanolamide-Phospholipase D |
| PCE | Prenatal cannabis exposure |
| PND | Postnatal day |
| PFC | Prefrontal cortex |
| SCZ | Schizophrenia |
| TH | Tyrosine hydroxylase |
| THC | Delta-9-tetrahydrocannabinol |
| Trpv1 | Transient Receptor Potential Cation Channel Subfamily V Member 1 |
References
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2021: Trends and Developments; Publications Office: Luxembourg, 2021.
- Martin, C.E.; Longinaker, N.; Mark, K.; Chisolm, M.S.; Terplan, M. Recent Trends in Treatment Admissions for Marijuana Use during Pregnancy. J. Addict. Med. 2015, 9, 99–104. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice Committee Opinion No. 637: Marijuana Use During Pregnancy and Lactation. Obs. Gynecol. 2015, 126, 234–238. [CrossRef] [PubMed]
- Beatty, J.R.; Svikis, D.S.; Ondersma, S.J. Prevalence and Perceived Financial Costs of Marijuana versus Tobacco Use among Urban Low-Income Pregnant Women. J. Addict. Res. Ther. 2012, 3, 1000135. [Google Scholar] [CrossRef] [PubMed]
- Passey, M.E.; Sanson-Fisher, R.W.; D’Este, C.A.; Stirling, J.M. Tobacco, Alcohol and Cannabis Use during Pregnancy: Clustering of Risks. Drug Alcohol. Depend. 2014, 134, 44–50. [Google Scholar] [CrossRef]
- Moore, D.G.; Turner, J.D.; Parrott, A.C.; Goodwin, J.E.; Fulton, S.E.; Min, M.O.; Fox, H.C.; Braddick, F.M.; Axelsson, E.L.; Lynch, S.; et al. During Pregnancy, Recreational Drug-Using Women Stop Taking Ecstasy (3,4-Methylenedioxy-N-Methylamphetamine) and Reduce Alcohol Consumption, but Continue to Smoke Tobacco and Cannabis: Initial Findings from the Development and Infancy Study. J. Psychopharmacol. 2010, 24, 1403–1410. [Google Scholar] [CrossRef]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Sarovar, V.; Tucker, L.-Y.; Avalos, L.A.; Alexeeff, S.; Conway, A.; Armstrong, M.A.; Weisner, C.; Campbell, C.I.; Goler, N. Trends in Marijuana Use among Pregnant Women with and without Nausea and Vomiting in Pregnancy, 2009–2016. Drug Alcohol Depend. 2019, 196, 66–70. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Sarovar, V.; Tucker, L.-Y.; Conway, A.; Alexeeff, S.; Weisner, C.; Armstrong, M.A.; Goler, N. Self-Reported Daily, Weekly, and Monthly Cannabis Use Among Women Before and During Pregnancy. JAMA Network Open 2019, 2, e196471. [Google Scholar] [CrossRef]
- Brown, Q.L.; Sarvet, A.L.; Shmulewitz, D.; Martins, S.S.; Wall, M.M.; Hasin, D.S. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. JAMA 2017, 317, 207–209. [Google Scholar] [CrossRef]
- Spindle, T.R.; Bonn-Miller, M.O.; Vandrey, R. Changing Landscape of Cannabis: Novel Products, Formulations, and Methods of Administration. Curr. Opin. Psychol. 2019, 30, 98–102. [Google Scholar] [CrossRef]
- Skelton, K.R.; Hecht, A.A.; Benjamin-Neelon, S.E. Recreational Cannabis Legalization in the US and Maternal Use during the Preconception, Prenatal, and Postpartum Periods. Int. J. Environ. Res. Public Health 2020, 17, 909. [Google Scholar] [CrossRef] [PubMed]
- Mehmedic, Z.; Chandra, S.; Slade, D.; Denham, H.; Foster, S.; Patel, A.S.; Ross, S.A.; Khan, I.A.; ElSohly, M.A. Potency Trends of Δ9-THC and Other Cannabinoids in Confiscated Cannabis Preparations from 1993 to 2008. J. Forensic Sci. 2010, 55, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.; Gryczynski, J.; Axenfeld, E.; Schwartz, R.P.; Terplan, M. Pregnant Women’s Current and Intended Cannabis Use in Relation to Their Views Toward Legalization and Knowledge of Potential Harm. J. Addict. Med. 2017, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Borodovsky, J.T.; Crosier, B.S.; Lee, D.C.; Sargent, J.D.; Budney, A.J. Smoking, Vaping, Eating: Is Legalization Impacting the Way People Use Cannabis? Int. J. Drug Policy 2016, 36, 141–147. [Google Scholar] [CrossRef]
- Chandra, S.; Radwan, M.M.; Majumdar, C.G.; Church, J.C.; Freeman, T.P.; ElSohly, M.A. New Trends in Cannabis Potency in USA and Europe during the Last Decade (2008–2017). Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 5–15. [Google Scholar] [CrossRef]
- Hutchings, D.E.; Martin, B.R.; Gamagaris, Z.; Miller, N.; Fico, T. Plasma Concentrations of Delta-9-Tetrahydrocannabinol in Dams and Fetuses Following Acute or Multiple Prenatal Dosing in Rats. Life Sci. 1989, 44, 697–701. [Google Scholar] [CrossRef]
- Bernick, S.J.; Kane, S. Drug Transfer to the Fetus and to the Breastfeeding Infant: What Do We Know? Curr. Drug Deliv. 2012, 9, 350–355. [Google Scholar] [CrossRef]
- Fantasia, H.C. Pharmacologic Implications of Marijuana Use During Pregnancy. Nurs. Women’s Health 2017, 21, 217–223. [Google Scholar] [CrossRef]
- Jaques, S.C.; Kingsbury, A.; Henshcke, P.; Chomchai, C.; Clews, S.; Falconer, J.; Abdel-Latif, M.E.; Feller, J.M.; Oei, J.L. Cannabis, the Pregnant Woman and Her Child: Weeding out the Myths. J. Perinatol. 2014, 34, 417–424. [Google Scholar] [CrossRef]
- Evanski, J.M.; Zundel, C.G.; Baglot, S.L.; Desai, S.; Gowatch, L.C.; Ely, S.L.; Sadik, N.; Lundahl, L.H.; Hill, M.N.; Marusak, H.A. The First “Hit” to the Endocannabinoid System? Associations Between Prenatal Cannabis Exposure and Frontolimbic White Matter Pathways in Children. Biol. Psychiatry Glob. Open Sci. 2024, 4, 11–18. [Google Scholar] [CrossRef]
- Fried, P.A. Marihuana Use by Pregnant Women: Neurobehavioral Effects in Neonates. Drug Alcohol. Depend. 1980, 6, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Fried, P.A.; Watkinson, B. 12- and 24-Month Neurobehavioural Follow-up of Children Prenatally Exposed to Marihuana, Cigarettes and Alcohol. Neurotoxicol. Teratol. 1988, 10, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Fried, P.A.; Watkinson, B.; Gray, R. Differential Effects on Cognitive Functioning in 9- to 12-Year Olds Prenatally Exposed to Cigarettes and Marihuana. Neurotoxicol. Teratol. 1998, 20, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Fried, P.A.; Hogan, M.J.; Cameron, I. Effects of Prenatal Marijuana on Visuospatial Working Memory: An fMRI Study in Young Adults. Neurotoxicol. Teratol. 2006, 28, 286–295. [Google Scholar] [CrossRef]
- Smith, A.M.; Mioduszewski, O.; Hatchard, T.; Byron-Alhassan, A.; Fall, C.; Fried, P.A. Prenatal Marijuana Exposure Impacts Executive Functioning into Young Adulthood: An fMRI Study. Neurotoxicol. Teratol. 2016, 58, 53–59. [Google Scholar] [CrossRef]
- Day, N.; Sambamoorthi, U.; Taylor, P.; Richardson, G.; Robles, N.; Jhon, Y.; Scher, M.; Stoffer, D.; Cornelius, M.; Jasperse, D. Prenatal Marijuana Use and Neonatal Outcome. Neurotoxicol. Teratol. 1991, 13, 329–334. [Google Scholar] [CrossRef]
- Goldschmidt, L.; Richardson, G.A.; Cornelius, M.D.; Day, N.L. Prenatal Marijuana and Alcohol Exposure and Academic Achievement at Age 10. Neurotoxicol. Teratol. 2004, 26, 521–532. [Google Scholar] [CrossRef]
- Hofman, A.; Jaddoe, V.W.V.; Mackenbach, J.P.; Moll, H.A.; Snijders, R.F.M.; Steegers, E.A.P.; Verhulst, F.C.; Witteman, J.C.M.; Büller, H.A. Growth, Development and Health from Early Fetal Life until Young Adulthood: The Generation R Study. Paediatr. Perinat. Epidemiol. 2004, 18, 61–72. [Google Scholar] [CrossRef]
- Jaddoe, V.W.V.; van Duijn, C.M.; Franco, O.H.; van der Heijden, A.J.; van IIzendoorn, M.H.; de Jongste, J.C.; van der Lugt, A.; Mackenbach, J.P.; Moll, H.A.; Raat, H.; et al. The Generation R Study: Design and Cohort Update 2012. Eur. J. Epidemiol. 2012, 27, 739–756. [Google Scholar] [CrossRef]
- Willford, J.A.; Goldschmidt, L.; De Genna, N.M.; Day, N.L.; Richardson, G.A. A Longitudinal Study of the Impact of Marijuana on Adult Memory Function: Prenatal, Adolescent, and Young Adult Exposures. Neurotoxicol. Teratol. 2021, 84, 106958. [Google Scholar] [CrossRef]
- El Marroun, H.; Tiemeier, H.; Franken, I.H.A.; Jaddoe, V.W.V.; van der Lugt, A.; Verhulst, F.C.; Lahey, B.B.; White, T. Prenatal Cannabis and Tobacco Exposure in Relation to Brain Morphology: A Prospective Neuroimaging Study in Young Children. Biol. Psychiatry 2016, 79, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Murnan, A.W.; Keim, S.A.; Yeates, K.O.; Boone, K.M.; Sheppard, K.W.; Klebanoff, M.A. Behavioral and Cognitive Differences in Early Childhood Related to Prenatal Marijuana Exposure. J. Appl. Dev. Psychol. 2021, 77, 101348. [Google Scholar] [CrossRef] [PubMed]
- Cioffredi, L.-A.; Anderson, H.; Loso, H.; East, J.; Nguyen, P.; Garavan, H.; Potter, A. Prenatal Cannabis Exposure Predicts Attention Problems, without Changes on fMRI in Adolescents. Neurotoxicol. Teratol. 2022, 91, 107089. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Jew, C.P.; Lu, H.-C. Lasting Impacts of Prenatal Cannabis Exposure and the Role of Endogenous Cannabinoids in the Developing Brain. Future Neurol. 2011, 6, 459–480. [Google Scholar] [CrossRef]
- Huizink, A.C. Prenatal Cannabis Exposure and Infant Outcomes: Overview of Studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 52, 45–52. [Google Scholar] [CrossRef]
- McLemore, G.L.; Richardson, K.A. Data from Three Prospective Longitudinal Human Cohorts of Prenatal Marijuana Exposure and Offspring Outcomes from the Fetal Period through Young Adulthood. Data Brief. 2016, 9, 753–757. [Google Scholar] [CrossRef]
- Torres, C.A.; Medina-Kirchner, C.; O’Malley, K.Y.; Hart, C.L. Totality of the Evidence Suggests Prenatal Cannabis Exposure Does Not Lead to Cognitive Impairments: A Systematic and Critical Review. Front. Psychol. 2020, 11, 816. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; de Salas-Quiroga, A.; Simón Sánchez, S.; Guzmán, M. Chapter 13—Prenatal THC Exposure Interferes with the Neurodevelopmental Role of Endocannabinoid Signaling. In Cannabis and the Developing Brain; Melis, M., Manzoni, O.J.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 259–282. ISBN 978-0-12-823490-7. [Google Scholar]
- Fourrier, C.; Singhal, G.; Baune, B.T. Neuroinflammation and Cognition across Psychiatric Conditions. CNS Spectr. 2019, 24, 4–15. [Google Scholar] [CrossRef]
- Manitt, C.; Eng, C.; Pokinko, M.; Ryan, R.T.; Torres-Berrío, A.; Lopez, J.P.; Yogendran, S.V.; Daubaras, M.J.J.; Grant, A.; Schmidt, E.R.E.; et al. Dcc Orchestrates the Development of the Prefrontal Cortex during Adolescence and Is Altered in Psychiatric Patients. Transl. Psychiatry 2013, 3, e338. [Google Scholar] [CrossRef]
- Menon, V. Large-Scale Brain Networks and Psychopathology: A Unifying Triple Network Model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- McTeague, L.M.; Goodkind, M.S.; Etkin, A. Transdiagnostic Impairment of Cognitive Control in Mental Illness. J. Psychiatr. Res. 2016, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Huemer, J.; Carreon, D.M.; Jiang, Y.; Eickhoff, S.B.; Etkin, A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am. J. Psychiatry 2017, 174, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Goodkind, M.; Eickhoff, S.B.; Oathes, D.J.; Jiang, Y.; Chang, A.; Jones-Hagata, L.B.; Ortega, B.N.; Zaiko, Y.V.; Roach, E.L.; Korgaonkar, M.S.; et al. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA Psychiatry 2015, 72, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Wager, T.D.; Mechelli, A.; He, Y. Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biol. Psychiatry 2019, 85, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Avramescu, R.G.; Hernandez, G.; Flores, C. Rewiring the Future: Drugs Abused in Adolescence May Predispose to Mental Illness in Adult Life by Altering Dopamine Axon Growth. J. Neural. Transm. 2024, 131, 461–467. [Google Scholar] [CrossRef]
- Etkin, A.; Gyurak, A.; O’Hara, R. A Neurobiological Approach to the Cognitive Deficits of Psychiatric Disorders. Dialogues Clin. Neurosci. 2013, 15, 419–429. [Google Scholar] [CrossRef]
- Selemon, L.D.; Zecevic, N. Schizophrenia: A Tale of Two Critical Periods for Prefrontal Cortical Development. Transl. Psychiatry 2015, 5, e623. [Google Scholar] [CrossRef]
- Dienel, S.J.; Schoonover, K.E.; Lewis, D.A. Cognitive Dysfunction and Prefrontal Cortical Circuit Alterations in Schizophrenia: Developmental Trajectories. Biol. Psychiatry 2022, 92, 450–459. [Google Scholar] [CrossRef]
- Tendilla-Beltrán, H.; Sanchez-Islas, N.d.C.; Marina-Ramos, M.; Leza, J.C.; Flores, G. The Prefrontal Cortex as a Target for Atypical Antipsychotics in Schizophrenia, Lessons of Neurodevelopmental Animal Models. Prog. Neurobiol. 2021, 199, 101967. [Google Scholar] [CrossRef]
- Paul, S.E.; Hatoum, A.S.; Fine, J.D.; Johnson, E.C.; Hansen, I.; Karcher, N.R.; Moreau, A.L.; Bondy, E.; Qu, Y.; Carter, E.B.; et al. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry 2021, 78, 64–76. [Google Scholar] [CrossRef]
- Fine, J.D.; Moreau, A.L.; Karcher, N.R.; Agrawal, A.; Rogers, C.E.; Barch, D.M.; Bogdan, R. Association of Prenatal Cannabis Exposure With Psychosis Proneness Among Children in the Adolescent Brain Cognitive Development (ABCD) Study. JAMA Psychiatry 2019, 76, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, K.; Kushner, S.A.; Yalniz, S.; Hillegers, M.H.J.; Jaddoe, V.W.V.; Tiemeier, H.; El Marroun, H. Maternal and Paternal Cannabis Use during Pregnancy and the Risk of Psychotic-like Experiences in the Offspring. Schizophr. Res. 2018, 202, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III--the Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Jutras-Aswad, D.; DiNieri, J.A.; Harkany, T.; Hurd, Y.L. Neurobiological Consequences of Maternal Cannabis on Human Fetal Development and Its Neuropsychiatric Outcome. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 395–412. [Google Scholar] [CrossRef]
- Bara, A.; Ferland, J.-M.N.; Rompala, G.; Szutorisz, H.; Hurd, Y.L. Cannabis and Synaptic Reprogramming of the Developing Brain. Nat. Rev. Neurosci. 2021, 22, 423–438. [Google Scholar] [CrossRef]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental Consequences of Fetal Exposure to Drugs: What We Know and What We Still Must Learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef]
- Renard, J.; Norris, C.; Rushlow, W.; Laviolette, S.R. Neuronal and Molecular Effects of Cannabidiol on the Mesolimbic Dopamine System: Implications for Novel Schizophrenia Treatments. Neurosci. Biobehav. Rev. 2017, 75, 157–165. [Google Scholar] [CrossRef]
- Luján, M.Á.; Young-Morrison, R.; Aroni, S.; Katona, I.; Melis, M.; Cheer, J.F. Dynamic Overrepresentation of Accumbal Cues in Food- and Opioid-Seeking Rats after Prenatal THC Exposure. Sci. Adv. 2024, 10, eadq5652. [Google Scholar] [CrossRef]
- Peterson, C.S.; Baglot, S.L.; Sallam, N.A.; Mina, S.; Hill, M.N.; Borgland, S.L. Oral Pre- and Early Postnatal Cannabis Exposure Disinhibits Ventral Tegmental Area Dopamine Neuron Activity but Does Not Influence Cocaine Preference in Offspring in Mice. J. Neurosci. Res. 2024, 102, e25369. [Google Scholar] [CrossRef]
- Grant, K.S.; Petroff, R.; Isoherranen, N.; Stella, N.; Burbacher, T.M. Cannabis Use during Pregnancy: Pharmacokinetics and Effects on Child Development. Pharmacol. Ther. 2018, 182, 133–151. [Google Scholar] [CrossRef]
- Sagheddu, C.; Traccis, F.; Serra, V.; Congiu, M.; Frau, R.; Cheer, J.F.; Melis, M. Mesolimbic Dopamine Dysregulation as a Signature of Information Processing Deficits Imposed by Prenatal THC Exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110128. [Google Scholar] [CrossRef] [PubMed]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal Cannabis Use Alters Ventral Striatal Dopamine D2 Gene Regulation in the Offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Frau, R.; Miczán, V.; Traccis, F.; Aroni, S.; Pongor, C.I.; Saba, P.; Serra, V.; Sagheddu, C.; Fanni, S.; Congiu, M.; et al. Prenatal THC Exposure Produces a Hyperdopaminergic Phenotype Rescued by Pregnenolone. Nat. Neurosci. 2019, 22, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Valeria, S.; Francesco, T.; Sonia, A.; Laura, V.P.; Luca, C.; Marcello, S.; Roberta, L.; Patrizia, P.; Arnau, B.G.; Roberto, F.; et al. Sex-Specific Maladaptive Responses to Acute Stress upon in Utero THC Exposure Are Mediated by Dopamine. Pharmacol. Res. 2024, 210, 107536. [Google Scholar] [CrossRef]
- Hermann, H.; Marsicano, G.; Lutz, B. Coexpression of the Cannabinoid Receptor Type 1 with Dopamine and Serotonin Receptors in Distinct Neuronal Subpopulations of the Adult Mouse Forebrain. Neuroscience 2002, 109, 451–460. [Google Scholar] [CrossRef]
- Meschler, J.P.; Howlett, A.C. Signal Transduction Interactions between CB1 Cannabinoid and Dopamine Receptors in the Rat and Monkey Striatum. Neuropharmacology 2001, 40, 918–926. [Google Scholar] [CrossRef]
- Mlost, J.; Wąsik, A.; Starowicz, K. Role of Endocannabinoid System in Dopamine Signalling within the Reward Circuits Affected by Chronic Pain. Pharmacol. Res. 2019, 143, 40–47. [Google Scholar] [CrossRef]
- D’Addario, C.; Di Francesco, A.; Pucci, M.; Finazzi Agrò, A.; Maccarrone, M. Epigenetic Mechanisms and Endocannabinoid Signalling. FEBS J. 2013, 280, 1905–1917. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Ashton, J.C.; Dowie, M.J.; Glass, M. Chapter 5—The Endocannabinoid System and Human Brain Functions: Insight From Memory, Motor, and Mood Pathologies. In The Endocannabinoid System; Murillo-Rodríguez, E., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 115–186. ISBN 978-0-12-809666-6. [Google Scholar]
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.T.; Drago, F. Endocannabinoid System and Mood Disorders: Priming a Target for New Therapies. Pharmacol. Ther. 2013, 138, 18–37. [Google Scholar] [CrossRef]
- Harkany, T.; Guzmán, M.; Galve-Roperh, I.; Berghuis, P.; Devi, L.A.; Mackie, K. The Emerging Functions of Endocannabinoid Signaling during CNS Development. Trends Pharmacol. Sci. 2007, 28, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Harkany, T.; Keimpema, E.; Barabás, K.; Mulder, J. Endocannabinoid Functions Controlling Neuronal Specification during Brain Development. Mol. Cell Endocrinol. 2008, 286, S84–S90. [Google Scholar] [CrossRef] [PubMed]
- Alpár, A.; Di Marzo, V.; Harkany, T. At the Tip of an Iceberg: Prenatal Marijuana and Its Possible Relation to Neuropsychiatric Outcome in the Offspring. Biol. Psychiatry 2016, 79, e33–e45. [Google Scholar] [CrossRef] [PubMed]
- Day, N.L.; Goldschmidt, L.; Thomas, C.A. Prenatal Marijuana Exposure Contributes to the Prediction of Marijuana Use at Age 14. Addiction 2006, 101, 1313–1322. [Google Scholar] [CrossRef]
- Porath, A.J.; Fried, P.A. Effects of Prenatal Cigarette and Marijuana Exposure on Drug Use among Offspring. Neurotoxicol. Teratol. 2005, 27, 267–277. [Google Scholar] [CrossRef]
- Sonon, K.E.; Richardson, G.A.; Cornelius, J.R.; Kim, K.H.; Day, N.L. Prenatal Marijuana Exposure Predicts Marijuana Use in Young Adulthood. Neurotoxicol Teratol. 2015, 47, 10–15. [Google Scholar] [CrossRef]
- Smigielski, L.; Jagannath, V.; Rössler, W.; Walitza, S.; Grünblatt, E. Epigenetic Mechanisms in Schizophrenia and Other Psychotic Disorders: A Systematic Review of Empirical Human Findings. Mol. Psychiatry 2020, 25, 1718–1748. [Google Scholar] [CrossRef]
- Noble, A.J.; Adams, A.T.; Satsangi, J.; Boden, J.M.; Osborne, A.J. Prenatal Cannabis Exposure Is Associated with Alterations in Offspring DNA Methylation at Genes Involved in Neurodevelopment, across the Life Course. Mol Psychiatry 2024, 30, 1418–1429. [Google Scholar] [CrossRef]
- Levenson, J.M.; Sweatt, J.D. Epigenetic Mechanisms: A Common Theme in Vertebrate and Invertebrate Memory Formation. Cell Mol. Life Sci. 2006, 63, 1009–1016. [Google Scholar] [CrossRef]
- Zahir, F.R.; Brown, C.J. Epigenetic Impacts on Neurodevelopment: Pathophysiological Mechanisms and Genetic Modes of Action. Pediatr. Res. 2011, 69, 92–100. [Google Scholar] [CrossRef]
- Brown, A.S. The Environment and Susceptibility to Schizophrenia. Prog. Neurobiol. 2011, 93, 23–58. [Google Scholar] [CrossRef] [PubMed]
- Szutorisz, H.; Hurd, Y.L. Epigenetic Effects of Cannabis Exposure. Biol. Psychiatry 2016, 79, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Szutorisz, H.; Hurd, Y.L. High Times for Cannabis: Epigenetic Imprint and Its Legacy on Brain and Behavior. Neurosci. Biobehav. Rev. 2018, 85, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.T.; Szutorisz, H.; Garg, P.; Martin, Q.; Landry, J.A.; Sharp, A.J.; Hurd, Y.L. Genome-Wide DNA Methylation Profiling Reveals Epigenetic Changes in the Rat Nucleus Accumbens Associated With Cross-Generational Effects of Adolescent THC Ex-posure. Neuropsychopharmacology 2015, 40, 2993–3005. [Google Scholar] [CrossRef]
- Shorey-Kendrick, L.E.; Roberts, V.H.J.; D’Mello, R.J.; Sullivan, E.L.; Murphy, S.K.; Mccarty, O.J.T.; Schust, D.J.; Hedges, J.C.; Mitchell, A.J.; Terrobias, J.J.D.; et al. Prenatal Delta-9-Tetrahydrocannabinol Exposure Is Associated with Changes in Rhesus Macaque DNA Methylation Enriched for Autism Genes. Clin. Epigenetics 2023, 15, 104. [Google Scholar] [CrossRef]
- Ellis, R.J.; Bara, A.; Vargas, C.A.; Frick, A.L.; Loh, E.; Landry, J.; Uzamere, T.O.; Callens, J.E.; Martin, Q.; Rajarajan, P.; et al. Prenatal Δ9-Tetrahydrocannabinol Exposure in Males Leads to Motivational Disturbances Related to Striatal Epigenetic Dysreg-ulation. Biol. Psychiatry 2022, 92, 127–138. [Google Scholar] [CrossRef]
- Schrott, R.; Modliszewski, J.L.; Hawkey, A.B.; Grenier, C.; Holloway, Z.; Evans, J.; Pippen, E.; Corcoran, D.L.; Levin, E.D.; Murphy, S.K. Sperm DNA Methylation Alterations from Cannabis Extract Exposure Are Evident in Offspring. Epigenetics Chromatin 2022, 15, 33. [Google Scholar] [CrossRef]
- Schrott, R.; Rajavel, M.; Acharya, K.; Huang, Z.; Acharya, C.; Hawkey, A.; Pippen, E.; Lyerly, H.K.; Levin, E.D.; Murphy, S.K. Sperm DNA Methylation Altered by THC and Nicotine: Vulnerability of Neurodevelopmental Genes with Bivalent Chromatin. Sci. Rep. 2020, 10, 16022. [Google Scholar] [CrossRef]
- Chandra, L.C.; Kumar, V.; Torben, W.; Stouwe, C.V.; Winsauer, P.; Amedee, A.; Molina, P.E.; Mohan, M. Chronic Administration of Δ9-Tetrahydrocannabinol Induces Intestinal Anti-Inflammatory MicroRNA Expression during Acute Simian Immunodeficiency Virus Infection of Rhesus Macaques. J. Virol. 2014, 89, 1168–1181. [Google Scholar] [CrossRef]
- Hegde, V.L.; Tomar, S.; Jackson, A.; Rao, R.; Yang, X.; Singh, U.P.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Distinct MicroRNA Expression Profile and Targeted Biological Pathways in Functional Myeloid-Derived Suppressor Cells Induced by Δ9-Tetrahydrocannabinol in Vivo. J. Biol. Chem. 2013, 288, 36810–36826. [Google Scholar] [CrossRef]
- Jackson, A.R.; Nagarkatti, P.; Nagarkatti, M. Anandamide Attenuates Th-17 Cell-Mediated Delayed-Type Hypersensitivity Re-sponse by Triggering IL-10 Production and Consequent microRNA Induction. PLoS ONE 2014, 9, e93954. [Google Scholar] [CrossRef]
- Molina, P.E.; Amedee, A.; LeCapitaine, N.J.; Zabaleta, J.; Mohan, M.; Winsauer, P.; Stouwe, C.V. Cannabinoid Neuroimmune Modulation of SIV Disease. J. Neuroimmune Pharmacol. 2011, 6, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.S.; Karpf, J.A.; Chan, C.N.; Hagen, O.L.; McFarland, T.J.; Urian, J.W.; Wang, X.; Boniface, E.R.; Hakar, M.H.; Terrobias, J.J.D.; et al. Prenatal Delta-9-Tetrahydrocannabinol Exposure Alters Fetal Neurodevelopment in Rhesus Macaques. Sci. Rep. 2024, 14, 5808. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peña, A.A.; Lee, K.; Pereira, M.; Ayyash, A.; Petrik, J.J.; Hardy, D.B.; Holloway, A.C. Prenatal Exposure to Delta-9-Tetrahydrocannabinol (THC) Alters the Expression of miR-122-5p and Its Target Igf1r in the Adult Rat Ovary. Int. J. Mol. Sci. 2022, 23, 8000. [Google Scholar] [CrossRef]
- Shansky, R.M.; Lipps, J. Stress-Induced Cognitive Dysfunction: Hormone-Neurotransmitter Interactions in the Prefrontal Cortex. Front. Hum. Neurosci. 2013, 7, 123. [Google Scholar] [CrossRef]
- Kolk, S.M.; Rakic, P. Development of Prefrontal Cortex. Neuropsychopharmacology 2022, 47, 41–57. [Google Scholar] [CrossRef]
- Islam, K.U.S.; Meli, N.; Blaess, S. The Development of the Mesoprefrontal Dopaminergic System in Health and Disease. Front. Neural. Circuits 2021, 15, 746582. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Pokinko, M.; Torres-Berrío, A.; Cuesta, S.; Lambert, L.C.; Del Cid Pellitero, E.; Wodzinski, M.; Manitt, C.; Krimpenfort, P.; Kolb, B.; et al. DCC Receptors Drive Prefrontal Cortex Maturation by Determining Dopamine Axon Targeting in Adolescence. Biol. Psychiatry 2018, 83, 181–192. [Google Scholar] [CrossRef]
- Hoops, D.; Flores, C. Making Dopamine Connections in Adolescence. Trends Neurosci. 2017, 40, 709–719. [Google Scholar] [CrossRef]
- Avramescu, R.G.; Capolicchio, T.; Flores, C. Dynamic Insights into Dopamine Axon Growth in Adolescence and Its Implications for Psychiatric Risk. Curr. Opin. Behav. Sci. 2024, 59, 101435. [Google Scholar] [CrossRef]
- Tortoriello, G.; Morris, C.V.; Alpar, A.; Fuzik, J.; Shirran, S.L.; Calvigioni, D.; Keimpema, E.; Botting, C.H.; Reinecke, K.; Herdegen, T.; et al. Miswiring the Brain: Δ9-Tetrahydrocannabinol Disrupts Cortical Development by Inducing an SCG10/Stathmin-2 Degradation Pathway. EMBO J. 2014, 33, 668–685. [Google Scholar] [CrossRef] [PubMed]
- Traccis, F.; Serra, V.; Sagheddu, C.; Congiu, M.; Saba, P.; Giua, G.; Devoto, P.; Frau, R.; Cheer, J.F.; Melis, M. Prenatal THC Does Not Affect Female Mesolimbic Dopaminergic System in Preadolescent Rats. Int. J. Mol. Sci. 2021, 22, 1666. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Carta, G.; Manca, C.; Verce, M.; Everard, A.; Serra, V.; Aroni, S.; Melis, M.; Banni, S. Impact of Prenatal THC Exposure on Lipid Metabolism and Microbiota Composition in Rat Offspring. Heliyon 2024, 10, e35637. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.L.; O’Connell, M.M.; Tokarz, M.E.; Wright, M.J. Pharmacological Effects of Acute and Repeated Administration of Δ9-Tetrahydrocannabinol in Adolescent and Adult Rats. J. Pharmacol. Exp. Ther. 2007, 320, 1097–1105. [Google Scholar] [CrossRef]
- Klein, C.; Karanges, E.; Spiro, A.; Wong, A.; Spencer, J.; Huynh, T.; Gunasekaran, N.; Karl, T.; Long, L.E.; Huang, X.-F.; et al. Cannabidiol Potentiates Δ9-Tetrahydrocannabinol (THC) Behavioural Effects and Alters THC Pharmacokinetics during Acute and Chronic Treatment in Adolescent Rats. Psychopharmacology 2011, 218, 443–457. [Google Scholar] [CrossRef]
- Schwope, D.M.; Karschner, E.L.; Gorelick, D.A.; Huestis, M.A. Identification of Recent Cannabis Use: Whole-Blood and Plasma Free and Glucuronidated Cannabinoid Pharmacokinetics Following Controlled Smoked Cannabis Administration. Clin. Chem. 2011, 57, 1406–1414. [Google Scholar] [CrossRef]
- DeVuono, M.V.; Nashed, M.G.; Sarikahya, M.H.; Kocsis, A.; Lee, K.; Vanin, S.R.; Hudson, R.; Lonnee, E.P.; Rushlow, W.J.; Hardy, D.B.; et al. Prenatal Tetrahydrocannabinol and Cannabidiol Exposure Produce Sex-Specific Pathophysiological Phenotypes in the Adolescent Prefrontal Cortex and Hippocampus. Neurobiol. Dis. 2024, 199, 106588. [Google Scholar] [CrossRef]
- Bara, A.; Manduca, A.; Bernabeu, A.; Borsoi, M.; Serviado, M.; Lassalle, O.; Murphy, M.; Wager-Miller, J.; Mackie, K.; Pelis-sier-Alicot, A.-L.; et al. Sex-Dependent Effects of in Utero Cannabinoid Exposure on Cortical Function. Elife 2018, 7, e36234. [Google Scholar] [CrossRef]
- Manduca, A.; Servadio, M.; Melancia, F.; Schiavi, S.; Manzoni, O.J.; Trezza, V. Sex-specific Behavioural Deficits Induced at Early Life by Prenatal Exposure to the Cannabinoid Receptor Agonist WIN55, 212–2 Depend on mGlu5 Receptor Signalling. Br. J. Pharmacol. 2020, 177, 449–463. [Google Scholar] [CrossRef]
- D’Addario, C.; Micale, V.; Di Bartolomeo, M.; Stark, T.; Pucci, M.; Sulcova, A.; Palazzo, M.; Babinska, Z.; Cremaschi, L.; Drago, F.; et al. A Preliminary Study of Endocannabinoid System Regulation in Psychosis: Distinct Alterations of CNR1 Promoter DNA Methylation in Patients with Schizophrenia. Schizophr. Res. 2017, 188, 132–140. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Stark, T.; Maurel, O.M.; Iannotti, F.A.; Kuchar, M.; Ruda-Kucerova, J.; Piscitelli, F.; Laudani, S.; Pekarik, V.; Salomone, S.; et al. Crosstalk between the Transcriptional Regulation of Dopamine D2 and Cannabinoid CB1 Receptors in Schizophrenia: Analyses in Patients and in Perinatal Δ9-Tetrahydrocannabinol-Exposed Rats. Pharmacol. Res. 2021, 164, 105357. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, M.; Stark, T.; Di Martino, S.; Iannotti, F.A.; Ruda-Kucerova, J.; Romano, G.L.; Kuchar, M.; Laudani, S.; Palivec, P.; Piscitelli, F.; et al. The Effects of Peripubertal THC Exposure in Neurodevelopmental Rat Models of Psychopathology. Int. J. Mol. Sci. 2023, 24, 3907. [Google Scholar] [CrossRef] [PubMed]
- Lyon, E. Mutation Detection Using Fluorescent Hybridization Probes and Melting Curve Analysis. Expert. Rev. Mol. Diagn. 2001, 1, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Čerňanová, A.; Petrušová, V.; Di Martino, S.; Hodosy, J.; Drago, F.; Micale, V.; D’Addario, C. DNA Methylation at Cannabinoid Type 1 and Dopamine D2 Receptor Genes in Saliva Samples of Psychotic Subjects: Is There an Effect of Cannabis Use? Pharmacol. Res. 2024, 208, 107343. [Google Scholar] [CrossRef]
- Kasanetz, F.; Lafourcade, M.; Deroche-Gamonet, V.; Revest, J.-M.; Berson, N.; Balado, E.; Fiancette, J.-F.; Renault, P.; Piazza, P.-V.; Manzoni, O.J. Prefrontal Synaptic Markers of Cocaine Addiction-like Behavior in Rats. Mol. Psychiatry 2013, 18, 729–737. [Google Scholar] [CrossRef]
- Frau, R.; Fanni, S.; Serra, V.; Simola, N.; Godar, S.C.; Traccis, F.; Devoto, P.; Bortolato, M.; Melis, M. Dysfunctional Mesocortical Dopamine Circuit at Pre-Adolescence Is Associated to Aggressive Behavior in MAO-A Hypomorphic Mice Exposed to Early Life Stress. Neuropharmacology 2019, 159, 107517. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, Sixth Edition: Hard Cover Edition (The Rat Brain in Stereotaxic Coordinates), 6th ed.; Academic Press: Cambridge, MA, USA, 2007; ISBN 978-0-12-374121-9. [Google Scholar]
- Gee, S.; Ellwood, I.; Patel, T.; Luongo, F.; Deisseroth, K.; Sohal, V.S. Synaptic Activity Unmasks Dopamine D2 Receptor Modulation of a Specific Class of Layer V Pyramidal Neurons in Prefrontal Cortex. J. Neurosci. 2012, 32, 4959–4971. [Google Scholar] [CrossRef]
- Vosberg, D.E.; Leyton, M.; Flores, C. The Netrin-1/DCC Guidance System: Dopamine Pathway Maturation and Psychiatric Disorders Emerging in Adolescence. Mol. Psychiatry 2020, 25, 297–307. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine Neuron Systems in the Brain: An Update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Sabatini, B.L. Dopaminergic Modulation of Synaptic Transmission in Cortex and Striatum. Neuron 2012, 76, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Roeper, J. Dissecting the Diversity of Midbrain Dopamine Neurons. Trends Neurosci. 2013, 36, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.Z.; Naneix, F. The Role of Dopamine and Endocannabinoid Systems in Prefrontal Cortex Development: Adolescence as a Critical Period. Front. Neural. Circuits 2022, 16, 939235. [Google Scholar] [CrossRef] [PubMed]
- Spencer, G.; Klumperman, J.; Syed, N. Neurotransmitters and Neurodevelopment. Role of Dopamine in Neurite Outgrowth, Target Selection and Specific Synapse Formation. Perspect. Dev. Neurobiol. 1998, 5, 451–467. [Google Scholar]
- Stanwood, G.D.; Levitt, P. Prenatal Exposure to Cocaine Produces Unique Developmental and Long-Term Adaptive Changes in Dopamine D1 Receptor Activity and Subcellular Distribution. J. Neurosci. 2007, 27, 152–157. [Google Scholar] [CrossRef]
- Stanwood, G.D.; Parlaman, J.P.; Levitt, P. Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J. Comp. Neurol. 2005, 487, 270–282. [Google Scholar] [CrossRef]
- Bhide, P.G. Dopamine, Cocaine and the Development of Cerebral Cortical Cytoarchitecture: A Review of Current Concepts. Semin. Cell Dev. Biol. 2009, 20, 395–402. [Google Scholar] [CrossRef]
- Lu, H.; Lim, B.; Poo, M. Cocaine Exposure In Utero Alters Synaptic Plasticity in the Medial Prefrontal Cortex of Postnatal Rats. J. Neurosci. 2009, 29, 12664–12674. [Google Scholar] [CrossRef]
- Zhang, X.; Bearer, E.L.; Boulat, B.; Hall, F.S.; Uhl, G.R.; Jacobs, R.E. Altered Neurocircuitry in the Dopamine Transporter Knockout Mouse Brain. PLoS ONE 2010, 5, e11506. [Google Scholar] [CrossRef]
- Bellone, C.; Mameli, M.; Lüscher, C. In Utero Exposure to Cocaine Delays Postnatal Synaptic Maturation of Glutamatergic Transmission in the VTA. Nat. Neurosci. 2011, 14, 1439–1446. [Google Scholar] [CrossRef]
- Cai, Y.; Xing, L.; Yang, T.; Chai, R.; Wang, J.; Bao, J.; Shen, W.; Ding, S.; Chen, G. The Neurodevelopmental Role of Dopaminergic Signaling in Neurological Disorders. Neurosci. Lett. 2021, 741, 135540. [Google Scholar] [CrossRef] [PubMed]
- Modinos, G.; Allen, P.; Grace, A.A.; McGuire, P. Translating the MAM Model of Psychosis to Humans. Trends Neurosci. 2015, 38, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Ruda-Kucerova, J.; Iannotti, F.A.; D’Addario, C.; Di Marco, R.; Pekarik, V.; Drazanova, E.; Piscitelli, F.; Bari, M.; Babinska, Z.; et al. Peripubertal Cannabidiol Treatment Rescues Behavioral and Neurochemical Abnormalities in the MAM Model of Schizophrenia. Neuropharmacology 2019, 146, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kaalund, S.S.; Newburn, E.N.; Ye, T.; Tao, R.; Li, C.; Deep-Soboslay, A.; Herman, M.M.; Hyde, T.M.; Weinberger, D.R.; Lipska, B.K.; et al. Contrasting Changes in DRD1 and DRD2 Splice Variant Expression in Schizophrenia and Affective Disorders, and Associations with SNPs in Postmortem Brain. Mol. Psychiatry 2014, 19, 1258–1266. [Google Scholar] [CrossRef]
- Moreno, M.; Trigo, J.M.; Escuredo, L.; Rodríguez de Fonseca, F.; Navarro, M. Perinatal Exposure to Δ9-Tetrahydrocannabinol Increases Presynaptic Dopamine D2 Receptor Sensitivity: A Behavioral Study in Rats. Pharmacol. Biochem. Behav. 2003, 75, 565–575. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’his, D. The Diverse Roles of DNA Methylation in Mammalian Development and Disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Smith, Z.D.; Hetzel, S.; Meissner, A. DNA Methylation in Mammalian Development and Disease. Nat. Rev. Genet. 2025, 26, 7–30. [Google Scholar] [CrossRef]
- Castellani, C.A.; Melka, M.G.; Diehl, E.J.; Laufer, B.I.; O’Reilly, R.L.; Singh, S.M. DNA Methylation in Psychosis: Insights into Etiology and Treatment. Epigenomics 2015, 7, 67–74. [Google Scholar] [CrossRef]
- Jaffe, A.E.; Gao, Y.; Deep-Soboslay, A.; Tao, R.; Hyde, T.M.; Weinberger, D.R.; Kleinman, J.E. Mapping DNA Methylation across Development, Genotype and Schizophrenia in the Human Frontal Cortex. Nat. Neurosci. 2016, 19, 40–47. [Google Scholar] [CrossRef]
- Hannon, E.; Dempster, E.L.; Mansell, G.; Burrage, J.; Bass, N.; Bohlken, M.M.; Corvin, A.; Curtis, C.J.; Dempster, D.; Di Forti, M.; et al. DNA Methylation Meta-Analysis Reveals Cellular Alterations in Psychosis and Markers of Treatment-Resistant Schizophrenia. Elife 2021, 10, e58430. [Google Scholar] [CrossRef]
- Kiltschewskij, D.J.; Reay, W.R.; Cairns, M.J. Schizophrenia Is Associated with Altered DNA Methylation Variance. Mol. Psychiatry 2024, 30, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Yapijakis, C. Regulatory Role of MicroRNAs in Brain Development and Function. In GeNeDis 2018, Advances in Experimental Medicine and Biology; Vlamos, P., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 237–247. [Google Scholar]
- Thomas, K.T.; Zakharenko, S.S. MicroRNAs in the Onset of Schizophrenia. Cells 2021, 10, 2679. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.H.; Zeier, Z.; Xi, L.; Lanz, T.A.; Deng, S.; Strathmann, J.; Willoughby, D.; Kenny, P.J.; Elsworth, J.D.; Lawrence, M.S.; et al. MicroRNA-132 Dysregulation in Schizophrenia Has Implications for Both Neurodevelopment and Adult Brain Function. Proc. Natl. Acad. Sci. USA 2012, 109, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Miyano, T.; Mikkaichi, T.; Nakamura, K.; Yoshigae, Y.; Abernathy, K.; Ogura, Y.; Kiyosawa, N. Circulating microRNA Profiles Identify a Patient Subgroup with High Inflammation and Severe Symptoms in Schizophrenia Experiencing Acute Psychosis. Int. J. Mol. Sci. 2024, 25, 4291. [Google Scholar] [CrossRef]
- Fu, X.; Baranova, A.; Cao, H.; Liu, Y.; Sun, J.; Zhang, F. miR-9-5p Deficiency Contributes to Schizophrenia. Schizophr. Res. 2023, 262, 168–174. [Google Scholar] [CrossRef]
- Fuso, A.; Raia, T.; Orticello, M.; Lucarelli, M. The Complex Interplay between DNA Methylation and miRNAs in Gene Expression Regulation. Biochimie 2020, 173, 12–16. [Google Scholar] [CrossRef]
- Marusak, H.A. The Role of Cannabinoids in Shaping Lifespan Neurodevelopment. J. Neurosci. Res. 2022, 100, 709–712. [Google Scholar] [CrossRef]
- Dembrow, N.C.; Chitwood, R.A.; Johnston, D. Projection-Specific Neuromodulation of Medial Prefrontal Cortex Neurons. J. Neurosci. 2010, 30, 16922–16937. [Google Scholar] [CrossRef]
- Robinson, S.E.; Sohal, V.S. Dopamine D2 Receptors Modulate Pyramidal Neurons in Mouse Medial Prefrontal Cortex through a Stimulatory G-Protein Pathway. J. Neurosci. 2017, 37, 10063–10073. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, X.-X.; Jin, S.-Y.; He, X.-Y.; Li, X.-W.; Yang, J.-M.; Gao, T.-M.; Chen, Y.-H. Dopamine D2 Receptors in Pyramidal Neurons in the Medial Prefrontal Cortex Regulate Social Behavior. Pharmacol. Res. 2024, 199, 107042. [Google Scholar] [CrossRef]
- Mereu, G.; Fà, M.; Ferraro, L.; Cagiano, R.; Antonelli, T.; Tattoli, M.; Ghiglieri, V.; Tanganelli, S.; Gessa, G.L.; Cuomo, V. Prenatal Exposure to a Cannabinoid Agonist Produces Memory Deficits Linked to Dysfunction in Hippocampal Long-Term Potentiation and Glutamate Release. Proc. Natl. Acad. Sci. USA 2003, 100, 4915–4920. [Google Scholar] [CrossRef] [PubMed]
- Pinky, P.D.; Bloemer, J.; Smith, W.D.; Du, Y.; Heslin, R.T.; Setti, S.E.; Pfitzer, J.C.; Chowdhury, K.; Hong, H.; Bhattacharya, S.; et al. Prenatal Cannabinoid Exposure Elicits Memory Deficits Associated with Reduced PSA-NCAM Expression, Altered Glutamatergic Signaling, and Adaptations in Hippocampal Synaptic Plasticity. Cells 2023, 12, 2525. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, R.N.; Rossignoli, M.T.; Marques, D.B.; de Sousa, B.M.; Romcy-Pereira, R.N.; Lopes-Aguiar, C.; Leite, J.P. Neuromodulation of Hippocampal-Prefrontal Cortical Synaptic Plasticity and Functional Connectivity: Implications for Neuropsychiatric Disorders. Front. Cell Neurosci. 2021, 15, 732360. [Google Scholar] [CrossRef] [PubMed]
- Sarikahya, M.H.; Cousineau, S.L.; De Felice, M.; Szkudlarek, H.J.; Wong, K.K.W.; DeVuono, M.V.; Lee, K.; Rodríguez-Ruiz, M.; Gummerson, D.; Proud, E.; et al. Prenatal THC Exposure Induces Long-Term, Sex-Dependent Cognitive Dysfunction Associated with Lipidomic and Neuronal Pathology in the Prefrontal Cortex-Hippocampal Network. Mol. Psychiatry 2023, 28, 4234–4250. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Hernandez, G.; MacGowan, D.; Popescu, C.; Nouel, D.; Cuesta, S.; Burke, S.; Savell, K.E.; Zhao, J.; Re-strepo-Lozano, J.M.; et al. Amphetamine Disrupts Dopamine Axon Growth in Adolescence by a Sex-Specific Mechanism in Mice. Nat. Commun. 2023, 14, 4035. [Google Scholar] [CrossRef]
- Restrepo-Lozano, J.M.; Pokhvisneva, I.; Wang, Z.; Patel, S.; Meaney, M.J.; Silveira, P.P.; Flores, C. Corticolimbic DCC Gene Co-Expression Networks as Predictors of Impulsivity in Children. Mol. Psychiatry 2022, 27, 2742–2750. [Google Scholar] [CrossRef]
- Flores, C. Role of Netrin-1 in the Organization and Function of the Mesocorticolimbic Dopamine System. J. Psychiatry Neurosci. 2011, 36, 296–310. [Google Scholar] [CrossRef]
- Hoops, D.; Kyne, R.F.; Salameh, S.; MacGowan, D.; Avramescu, R.G.; Ewing, E.; He, A.T.; Orsini, T.; Durand, A.; Popescu, C.; et al. The Scheduling of Adolescence with Netrin-1 and UNC5C. eLife 2023, 12, RP88261. [Google Scholar] [CrossRef]
- Torres-Berrío, A.; Hernandez, G.; Nestler, E.J.; Flores, C. The Netrin-1/DCC Guidance Cue Pathway as a Molecular Target in Depression: Translational Evidence. Biol. Psychiatry 2020, 88, 611–624. [Google Scholar] [CrossRef]
- Cuesta, S.; Nouel, D.; Reynolds, L.M.; Morgunova, A.; Torres-Berrío, A.; White, A.; Hernandez, G.; Cooper, H.M.; Flores, C. Dopamine Axon Targeting in the Nucleus Accumbens in Adolescence Requires Netrin-1. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Morris, C.V.; DiNieri, J.A.; Szutorisz, H.; Hurd, Y.L. Molecular Mechanisms of Maternal Cannabis and Cigarette Use on Human Neurodevelopment. Eur. J. Neurosci. 2011, 34, 1574–1583. [Google Scholar] [CrossRef]
- Du, J.; Li, M.; Huang, Q.; Liu, W.; Li, W.; Li, Y.; Gong, Z. The Critical Role of microRNAs in Stress Response: Therapeutic Prospect and Limitation. Pharmacol. Res. 2019, 142, 294–302. [Google Scholar] [CrossRef]
| Gene | 5′-Forward Primer-3′ | 5′-Reverse Primer-3′ | |
|---|---|---|---|
| Housekeeping | ß-Actin | AGATCAAGATCATTGCTCCTCCT | ACGCAGCTCAGTAACAGTCC |
| Gapdh | AGACAGCCGCATCTTCTTGT | CTTGCCGTGGGTAGAGTCAT | |
| 18S | ACGGACCAGAGCGAAAGCAT | TGTCAATCCTGTCCGTGTCC | |
| Endocannabinoid System | Cnr1 | TTCCACCGTAAAGACAGCCC | TCCACATCAGGCAAAAGGCC |
| Cnr2 | TTGACCGATACCTATGTCTGTGC | TGCTTTCCAGAGGACATACCC | |
| Trpv1 | ATTGAACGGCGGAACATGACG | ATCTCTTCCAGCTTCAGCG | |
| Nape-pld | TGTCCCGGGTTCCAAAGAGGAGC | ACCATCAGCGTCGCGTGTCC | |
| Dagl-α | ATTCTCTCCTTCCTCCTGC | ATTTGGGCTTGGTGCTTCG | |
| Faah | ATGGAAGTCCTCCAAGAGC | TAGAGCTTTCAGGCATAGCG | |
| Magl | ACGTGAACACCGTCCAGAAG | TTGGCAGCAAGGACCTTCAA | |
| Dopaminergic System | Drd1 | TCGAACTGTATGGTGCCCTT | AAGAATTCGCCCACCCAAAC |
| Drd2 | TACGTGCCCTTCATCGTCAC | GTGGGTACAGTTGCCCTTGA | |
| Drd3 | ATTCGGCAGTTTTCAATAAGG | GGGTGTCTCAAGGCAGTGTC | |
| Dat | AGCTACCATGCCCTATGTGG | ATCAGCACTCCAAACCCAAC | |
| Gene | Sequence | n° CpG Sites | 5′-Primers-3′ |
|---|---|---|---|
| Rn_Drd1 | ggcgtgggcgtggggagggtcggctctgat tccgagctttgggtggaacttgaggttgg | 4 | F: GGGTAGTGTTTTGGGTTAGT Biot_R: TCTCTTCAAACCAACCTCAAAT S: AGTGTTTTGGGTTAGTAG |
| Rn_Drd2 | ttcccgacgcccgaggcgcaatctgcccgtcgga | 6 | Included in the Qiagen Assay PM00586096 |
| ECS Genes | CNT (Mean ± SEM) | PCE (Mean ± SEM) | p-Values |
|---|---|---|---|
| Cnr1 | 1.34 ± 0.29 | 1.10 ± 0.27 | 0.4777 |
| Cnr2 | 1.09 ± 0.12 | 1.61 ± 0.28 | 0.2456 |
| Trpv1 | 1.11 ± 0.14 | 1.30 ± 0.12 | 0.3369 |
| Nape-pld | 1.16 ± 0.17 | 1.19 ± 0.25 | 0.7068 |
| Dagl-α | 1.13 ± 0.13 | 1.06 ± 0.17 | 0.6397 |
| Faah | 1.03 ± 0.08 | 1.08 ± 0.14 | >0.9999 |
| Magl | 1.20 ± 0.20 | 1.14 ± 0.24 | 0.7291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bartolomeo, M.; Aroni, S.; Serra, M.; Serra, V.; Martella, F.; Gilardini, F.; Melis, M.; D’Addario, C. Prenatal Delta-9-Tetrahydrocannabinol Exposure Induces Transcriptional Alterations in Dopaminergic System with Associated Electrophysiological Dysregulation in the Prefrontal Cortex of Adolescent Rats. Cells 2025, 14, 904. https://doi.org/10.3390/cells14120904
Di Bartolomeo M, Aroni S, Serra M, Serra V, Martella F, Gilardini F, Melis M, D’Addario C. Prenatal Delta-9-Tetrahydrocannabinol Exposure Induces Transcriptional Alterations in Dopaminergic System with Associated Electrophysiological Dysregulation in the Prefrontal Cortex of Adolescent Rats. Cells. 2025; 14(12):904. https://doi.org/10.3390/cells14120904
Chicago/Turabian StyleDi Bartolomeo, Martina, Sonia Aroni, Marcello Serra, Valeria Serra, Francesca Martella, Federica Gilardini, Miriam Melis, and Claudio D’Addario. 2025. "Prenatal Delta-9-Tetrahydrocannabinol Exposure Induces Transcriptional Alterations in Dopaminergic System with Associated Electrophysiological Dysregulation in the Prefrontal Cortex of Adolescent Rats" Cells 14, no. 12: 904. https://doi.org/10.3390/cells14120904
APA StyleDi Bartolomeo, M., Aroni, S., Serra, M., Serra, V., Martella, F., Gilardini, F., Melis, M., & D’Addario, C. (2025). Prenatal Delta-9-Tetrahydrocannabinol Exposure Induces Transcriptional Alterations in Dopaminergic System with Associated Electrophysiological Dysregulation in the Prefrontal Cortex of Adolescent Rats. Cells, 14(12), 904. https://doi.org/10.3390/cells14120904









