Multi-Omics Perspectives on Testicular Aging: Unraveling Germline Dysregulation, Niche Dysfunction, and Epigenetic Remodeling
Abstract
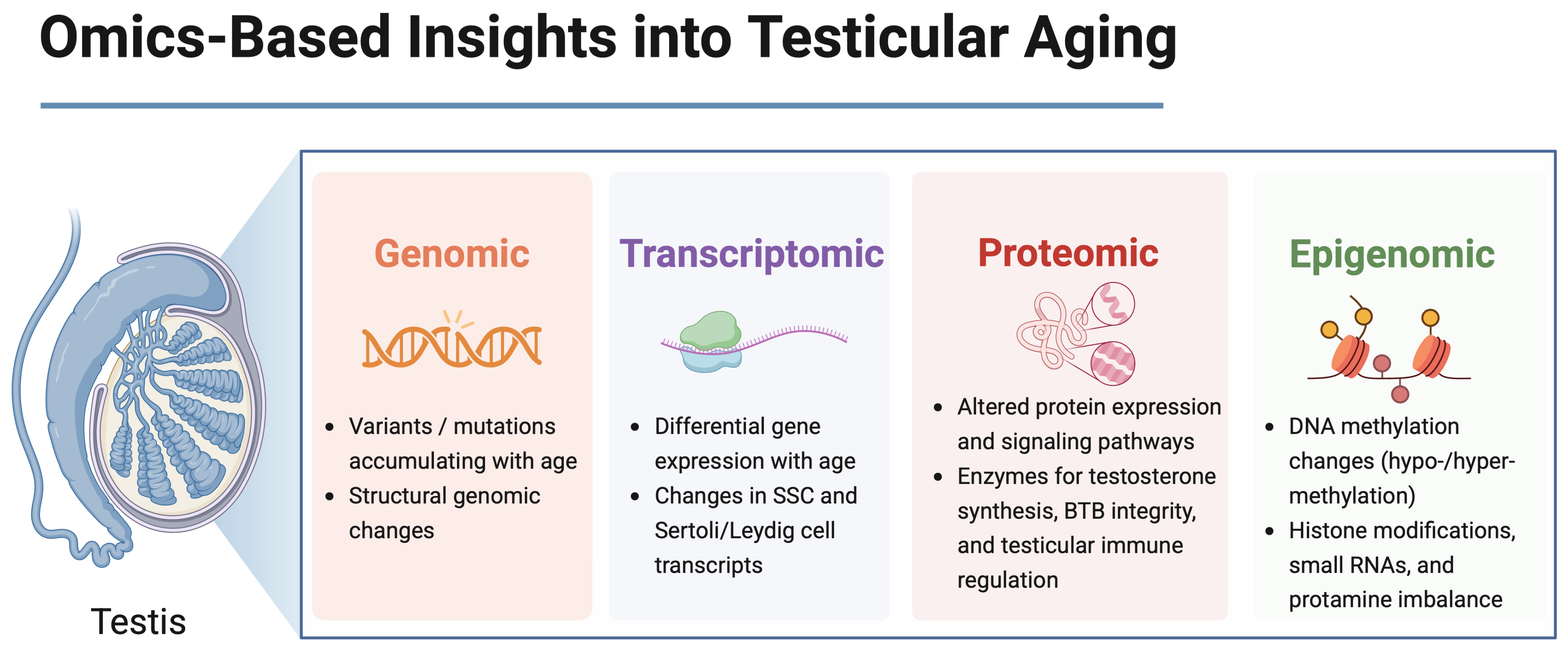
1. Introduction
2. Age-Related Gene Expression Changes in Germ Cells
3. Sertoli Cell Aging and Transcriptomic Alterations
4. Leydig Cell Aging and Steroidogenic Decline
5. Changes in the Testicular Niche and Intercellular Signaling with Age
6. Epigenetic Changes in the Aging Male Germline
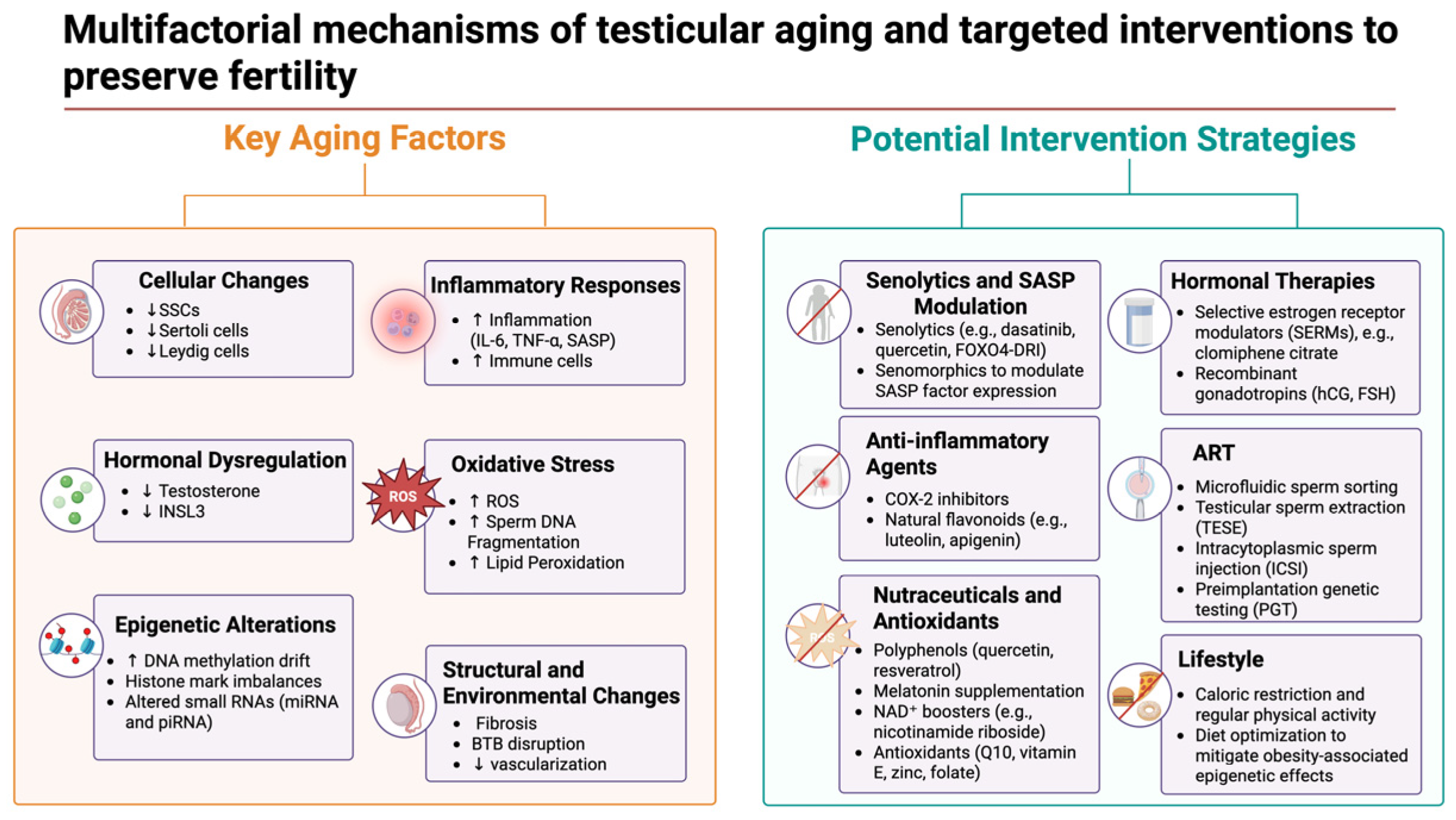
7. Translational and Clinical Implications
7.1. Pharmacological and Nutraceutical Interventions
7.2. Hormonal Modulation Strategies
7.3. Advanced Reproductive Technologies for Older Men
7.4. Linking Omics Findings to Translational Strategies
8. Future Research Directions
8.1. Reversibility of Epigenetic Alterations
8.2. Causal vs. Correlative Changes in Testicular Aging
8.3. Interventions to Prevent or Delay Sperm Aging
8.4. Preserving and Restoring SSC and Niche Function
8.5. Transgenerational Implications of Male Germline Aging
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawa, S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef]
- Aitken, R.J. Male reproductive ageing: A radical road to ruin. Hum. Reprod. 2023, 38, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zikopoulos, A.; Vrachnis, D.; Skentou, C.; Symeonidis, E.N.; Dimitriadis, F.; Stavros, S.; Chrisofos, M.; Sofikitis, N.; Vrachnis, N.; et al. Advanced Paternal Age in Focus: Unraveling Its Influence on Assisted Reproductive Technology Outcomes. J. Clin. Med. 2024, 13, 2731. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Martin, A.; Nistal, M.; Amat, P. Testicular involution in elderly men: Comparison of histologic quantitative studies with hormone patterns. Fertil. Steril. 1987, 47, 671–679. [Google Scholar] [CrossRef]
- Neaves, W.B.; Johnson, L.; Porter, J.C.; Parker, C.R., Jr.; Petty, C.S. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J. Clin. Endocrinol. Metab. 1984, 59, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Ehmcke, J.; Joshi, B.; Hergenrother, S.D.; Schlatt, S. Aging does not affect spermatogenic recovery after experimentally induced injury in mice. Reproduction 2007, 133, 75–83. [Google Scholar] [CrossRef]
- Wang, C.; Sinha Hikim, A.P.; Lue, Y.H.; Leung, A.; Baravarian, S.; Swerdloff, R.S. Reproductive aging in the Brown Norway rat is characterized by accelerated germ cell apoptosis and is not altered by luteinizing hormone replacement. J. Androl. 1999, 20, 509–518. [Google Scholar] [CrossRef]
- Urbanski, H.F.; Noriega, N.C.; Lemos, D.R.; Kohama, S.G. Gene expression profiling in the rhesus macaque: Experimental design considerations. Methods 2009, 49, 26–31. [Google Scholar] [CrossRef][Green Version]
- Sitzmann, B.D.; Urbanski, H.F.; Ottinger, M.A. Aging in male primates: Reproductive decline, effects of calorie restriction and future research potential. Age 2008, 30, 157–168. [Google Scholar] [CrossRef]
- Benayoun, B.A.; Pollina, E.A.; Singh, P.P.; Mahmoudi, S.; Harel, I.; Casey, K.M.; Dulken, B.W.; Kundaje, A.; Brunet, A. Remodeling of epigenome and transcriptome landscapes with aging in mice reveals widespread induction of inflammatory responses. Genome Res. 2019, 29, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.M.; Seieroe, K.; Pakkenberg, B. The total number of Leydig and Sertoli cells in the testes of men across various age groups—A stereological study. J. Anat. 2015, 226, 175–179. [Google Scholar] [CrossRef]
- Mularoni, V.; Esposito, V.; Di Persio, S.; Vicini, E.; Spadetta, G.; Berloco, P.; Fanelli, F.; Mezzullo, M.; Pagotto, U.; Pelusi, C.; et al. Age-related changes in human Leydig cell status. Hum. Reprod. 2020, 35, 2663–2676. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Alves, M.G.; Oliveira, P.F.; Fardilha, M. Testicular Aging: An Overview of Ultrastructural, Cellular, and Molecular Alterations. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 860–871. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, P.; Chen, W.; Chen, J.; Liu, C.; Zhang, H. Testicular fibrosis pathology, diagnosis, pathogenesis, and treatment: A perspective on related diseases. Andrology 2024, 1–11. [Google Scholar] [CrossRef]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, Y.; Ma, Y.; Lin, W.; Zhang, H.; Zhao, J. Exploring the Mechanisms of Testicular Aging: Advances in Biomarker Research. Aging Dis. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Sayed, R.K.A.; Mokhtar, D.M.; Fernandez-Ortiz, M.; Fernandez-Martinez, J.; Aranda-Martinez, P.; Escames, G.; Acuna-Castroviejo, D. Lack of retinoid acid receptor-related orphan receptor alpha accelerates and melatonin supplementation prevents testicular aging. Aging 2020, 12, 12648–12668. [Google Scholar] [CrossRef]
- Kaltsas, A.; Markou, E.; Kyrgiafini, M.A.; Zikopoulos, A.; Symeonidis, E.N.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Oxidative-Stress-Mediated Epigenetic Dysregulation in Spermatogenesis: Implications for Male Infertility and Offspring Health. Genes 2025, 16, 93. [Google Scholar] [CrossRef]
- Han, G.; Hong, S.H.; Lee, S.J.; Hong, S.P.; Cho, C. Transcriptome Analysis of Testicular Aging in Mice. Cells 2021, 10, 2895. [Google Scholar] [CrossRef]
- Fice, H.E.; Robaire, B. Aging affects gene expression in spermatids of Brown Norway rats. Exp. Gerontol. 2023, 173, 112086. [Google Scholar] [CrossRef]
- Dong, F.; Ping, P.; Ma, Y.; Chen, X.F. Application of single-cell RNA sequencing on human testicular samples: A comprehensive review. Int. J. Biol. Sci. 2023, 19, 2167–2197. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zuo, Y.; Zhang, C.; Sun, G.; Jing, Y.; Lei, J.; Ma, S.; Sun, S.; Lu, H.; Cai, Y.; et al. A single-nucleus transcriptomic atlas of primate testicular aging reveals exhaustion of the spermatogonial stem cell reservoir and loss of Sertoli cell homeostasis. Protein Cell 2023, 14, 888–907. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Suzuki, S.; Nakagawa, T.; Kamo, Y.; Kanouchi, M.; Fujita, M.; Hattori, M.; Suzuki, A.; Tanemura, K.; Yoshida, S.; et al. Age-Dependent Clonal Expansion of Non-Sperm-Forming Spermatogonial Stem Cells in Mouse Testes. Aging Cell 2025, e70019. [Google Scholar] [CrossRef]
- Cui, L.; Nie, X.; Guo, Y.; Ren, P.; Guo, Y.; Wang, X.; Li, R.; Hotaling, J.M.; Cairns, B.R.; Guo, J. Single-cell transcriptomic atlas of the human testis across the reproductive lifespan. Nat. Aging 2025, 5, 658–674. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Li, Y.; Xu, Z.; Ma, M.; Chen, H.; Chen, P.; Lv, L.; Shang, X.; Liu, G. Single-cell transcriptomics identifies senescence-associated secretory phenotype (SASP) features of testicular aging in human. Aging 2024, 16, 3350–3362. [Google Scholar] [CrossRef]
- Bernhardt, L.; Dittrich, M.; Prell, A.; Potabattula, R.; Drummer, C.; Behr, R.; Hahn, T.; Schorsch, M.; Muller, T.; Haaf, T. Age-related methylation changes in the human sperm epigenome. Aging 2023, 15, 1257–1278. [Google Scholar] [CrossRef] [PubMed]
- De Sena Brandine, G.; Aston, K.I.; Jenkins, T.G.; Smith, A.D. Global effects of identity and aging on the human sperm methylome. Clin. Epigenetics 2023, 15, 127. [Google Scholar] [CrossRef]
- Nie, X.; Munyoki, S.K.; Sukhwani, M.; Schmid, N.; Missel, A.; Emery, B.R.; DonorConnect; Stukenborg, J.B.; Mayerhofer, A.; Orwig, K.E.; et al. Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev. Cell 2022, 57, 1160–1176.e5. [Google Scholar] [CrossRef]
- Alfano, M.; Tascini, A.S.; Pederzoli, F.; Locatelli, I.; Nebuloni, M.; Giannese, F.; Garcia-Manteiga, J.M.; Tonon, G.; Amodio, G.; Gregori, S.; et al. Aging, inflammation and DNA damage in the somatic testicular niche with idiopathic germ cell aplasia. Nat. Commun. 2021, 12, 5205. [Google Scholar] [CrossRef]
- Paoli, D.; Pecora, G.; Pallotti, F.; Faja, F.; Pelloni, M.; Lenzi, A.; Lombardo, F. Cytological and molecular aspects of the ageing sperm. Hum. Reprod. 2019, 34, 218–227. [Google Scholar] [CrossRef]
- Liu, C.; Peng, H.; Yu, J.; Luo, P.; Xiong, C.; Chen, H.; Fan, H.; Ma, Y.; Ou, W.; Zhang, S.; et al. Impaired ketogenesis in Leydig Cells drives testicular aging. Nat. Commun. 2025, 16, 4224. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Mori, H.; Endo, T.; Ishikawa-Yamauchi, Y.; Motooka, D.; Emori, C.; Ikawa, M. Age-related decline in spermatogenic activity accompanied with endothelial cell senescence in male mice. iScience 2023, 26, 108456. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Yamamoto, T.; Toh, H.; Kazuki, Y.; Kazuki, K.; Imoto, J.; Ikeo, K.; Oshima, M.; Shirahige, K.; Iwama, A.; et al. Aging of spermatogonial stem cells by Jnk-mediated glycolysis activation. Proc. Natl. Acad. Sci. USA 2019, 116, 16404–16409. [Google Scholar] [CrossRef]
- Luo, D.; Qi, X.; Xu, X.; Yang, L.; Yu, C.; Guan, Q. Involvement of p38 MAPK in Leydig cell aging and age-related decline in testosterone. Front. Endocrinol. 2023, 14, 1088249. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- Xia, K.; He, S.; Luo, P.; Dong, L.; Gao, F.; Chen, X.; Ye, Y.; Gao, Y.; Ma, Y.; Zhang, Y.; et al. Transcriptomic landscape and potential therapeutic targets for human testicular aging revealed by single-cell RNA sequencing. bioRxiv 2022. [CrossRef]
- Xia, K.; Luo, P.; Yu, J.; He, S.; Dong, L.; Gao, F.; Chen, X.; Ye, Y.; Gao, Y.; Ma, Y.; et al. Single-cell RNA sequencing reveals transcriptomic landscape and potential targets for human testicular ageing. Hum. Reprod. 2024, 39, 2189–2209. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.; Suvorov, A.; Pilsner, J.R.; Krawetz, S.A.; Sergeyev, O. Age-associated epigenetic changes in mammalian sperm: Implications for offspring health and development. Hum. Reprod. Update 2023, 29, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Santos, M.A.S.; Fardilha, M. Age-Dependent Alterations in Semen Parameters and Human Sperm MicroRNA Profile. Biomedicines 2023, 11, 2923. [Google Scholar] [CrossRef]
- Kyrgiafini, M.-A.; Kaltsas, A.; Chatziparasidou, A.; Mamuris, Z. The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval—A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 3537. [Google Scholar] [CrossRef]
- Miyahara, K.; Tatehana, M.; Kikkawa, T.; Osumi, N. Investigating the impact of paternal aging on murine sperm miRNA profiles and their potential link to autism spectrum disorder. Sci. Rep. 2023, 13, 20608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yue, Q.; Xie, J.; Zhang, S.; He, W.; Bai, S.; Tian, S.; Zhang, Y.; Xiong, M.; Sun, Z.; et al. Rapamycin-mediated mTOR inhibition impairs silencing of sex chromosomes and the pachytene piRNA pathway in the mouse testis. Aging 2019, 11, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zikopoulos, A.; Kojovic, V.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M.; Zachariou, A. Paternal Contributions to Recurrent Pregnancy Loss: Mechanisms, Biomarkers, and Therapeutic Approaches. Medicina 2024, 60, 1920. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Kimura, R.; Kobayashi, H.; Oki, S.; Kikkawa, T.; Mai, L.; Koike, K.; Mochizuki, K.; Inada, H.; Matsui, Y.; et al. Paternal age affects offspring via an epigenetic mechanism involving REST/NRSF. EMBO Rep. 2021, 22, e51524. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Broberg, D.S.; Aston, K.I.; Carrell, D.T.; Jenkins, T.G. The combined effect of obesity and aging on human sperm DNA methylation signatures: Inclusion of BMI in the paternal germ line age prediction model. Sci. Rep. 2020, 10, 15409. [Google Scholar] [CrossRef]
- Elias-Llumbet, A.; Lira, S.; Manterola, M. Male aging in germ cells: What are we inheriting? Genet. Mol. Biol. 2025, 47, e20240052. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Chen, H.; Lv, L.; Yao, J.; Zhang, M.; Xia, K.; Feng, X.; Li, Y.; Liang, X.; et al. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging 2020, 12, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Ficca, M.L.; Zwerdling, A.E.; Swanson, C.A.; Tucker, A.G.; Lopez, S.A.; Wandersee, M.K.; Warner, G.M.; Thompson, K.L.; Chini, C.C.S.; Chen, H.; et al. Low NAD+ Levels Are Associated with a Decline of Spermatogenesis in Transgenic ANDY and Aging Mice. Front. Endocrinol. 2022, 13, 896356. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Li, H.; Wei, C.; Zhu, Z.; Zhao, Y.; Zhu, J.; Lei, M.; Sun, Y.; Yang, Q. Nicotinamide Riboside Supplementation Alleviates Testicular Aging Induced by Disruption of Qprt-Dependent NAD+ De Novo Synthesis in Mice. Aging Cell 2025, e70004. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Lahimer, M.; Capelle, S.; Lefranc, E.; Bosquet, D.; Kazdar, N.; Ledu, A.; Agina, M.; Cabry, R.; BenKhalifa, M. Micronutrient-Antioxidant Therapy and Male Fertility Improvement During ART Cycles. Nutrients 2025, 17, 324. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, M.E.; Calandra, R.S.; Rossi, S.P.; Bartke, A.; Frungieri, M.B. Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad. Cells 2021, 10, 3114. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.L.; Dyson, M.T.; Eimerl, S.; Orly, J.; Hutson, J.C.; Stocco, D.M. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology 2005, 146, 4202–4208. [Google Scholar] [CrossRef]
- Muratoglu, S.; Akarca Dizakar, O.S.; Keskin Aktan, A.; Omeroglu, S.; Akbulut, K.G. The protective role of melatonin and curcumin in the testis of young and aged rats. Andrologia 2019, 51, e13203. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Paul, K.H.; Tripathi, U.; Minamino, T.; Tchkonia, T.; Kirkland, J.L. Targeting Cell Senescence and Senolytics: Novel Interventions for Age-Related Endocrine Dysfunction. Endocr. Rev. 2024, 45, 655–675. [Google Scholar] [CrossRef]
- Salonia, A.; Rastrelli, G.; Hackett, G.; Seminara, S.B.; Huhtaniemi, I.T.; Rey, R.A.; Hellstrom, W.J.G.; Palmert, M.R.; Corona, G.; Dohle, G.R.; et al. Paediatric and adult-onset male hypogonadism. Nat. Rev. Dis. Primers 2019, 5, 38. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Mskhalaya, G.; Zitzmann, M.; Corona, G.; Arver, S.; Kalinchenko, S.; Tishova, Y.; Morgentaler, A. Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men. Aging Male 2021, 24, 119–138. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zachariou, A.; Dimitriadis, F.; Chrisofos, M.; Sofikitis, N. Empirical Treatments for Male Infertility: A Focus on Lifestyle Modifications and Medicines. Diseases 2024, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Ide, V.; Vanderschueren, D.; Antonio, L. Treatment of Men with Central Hypogonadism: Alternatives for Testosterone Replacement Therapy. Int. J. Mol. Sci. 2020, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.M.; Sharma, D.; Kavoussi, P.K.; Smith, R.P.; Costabile, R. Clomiphene Citrate for the Treatment of Hypogonadism. Sex. Med. Rev. 2019, 7, 272–276. [Google Scholar] [CrossRef]
- Pelusi, C.; Giagulli, V.A.; Baccini, M.; Fanelli, F.; Mezzullo, M.; Fazzini, A.; Bianchi, N.; Carbone, M.D.; De Pergola, G.; Mastroroberto, M.; et al. Clomiphene citrate effect in obese men with low serum testosterone treated with metformin due to dysmetabolic disorders: A randomized, double-blind, placebo-controlled study. PLoS ONE 2017, 12, e0183369. [Google Scholar] [CrossRef]
- Kliesch, S.; Behre, H.M.; Nieschlag, E. Recombinant human follicle-stimulating hormone and human chorionic gonadotropin for induction of spermatogenesis in a hypogonadotropic male. Fertil. Steril. 1995, 63, 1326–1328. [Google Scholar] [CrossRef]
- Pyo, Y.; Kwon, K.H. Aging, testosterone and male fertility therapy: A review. J. Men’s Health 2024, 20, 1–10. [Google Scholar]
- Escude-Logares, L.; Serrano-Novillo, C.; Uroz, L.; Galindo, A.; Marquez, C. Advanced Paternal Age: A New Indicator for the Use of Microfluidic Devices for Sperm DNA Fragmentation Selection. J. Clin. Med. 2024, 13, 457. [Google Scholar] [CrossRef]
- Pacheco, A.; Blanco, A.; Bronet, F.; Cruz, M.; Garcia-Fernandez, J.; Garcia-Velasco, J.A. Magnetic-Activated Cell Sorting (MACS): A Useful Sperm-Selection Technique in Cases of High Levels of Sperm DNA Fragmentation. J. Clin. Med. 2020, 9, 3976. [Google Scholar] [CrossRef] [PubMed]
- Ambar, R.F.; Agarwal, A.; Majzoub, A.; Vij, S.; Tadros, N.N.; Cho, C.L.; Parekh, N.; Borges, E.; Glina, S. The Use of Testicular Sperm for Intracytoplasmic Sperm Injection in Patients with High Sperm DNA Damage: A Systematic Review. World J. Men’s Health 2021, 39, 391–398. [Google Scholar] [CrossRef]
- Wang, S.; Huo, T.; Lu, M.; Zhao, Y.; Zhang, J.; He, W.; Chen, H. Recent Advances in Aging and Immunosenescence: Mechanisms and Therapeutic Strategies. Cells 2025, 14, 499. [Google Scholar] [CrossRef]
- Costa-Junior, J.M.; Ferreira, S.M.; Kurauti, M.A.; Bernstein, D.L.; Ruano, E.G.; Kameswaran, V.; Schug, J.; Freitas-Dias, R.; Zoppi, C.C.; Boschero, A.C.; et al. Paternal Exercise Improves the Metabolic Health of Offspring via Epigenetic Modulation of the Germline. Int. J. Mol. Sci. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Akhatova, A.; Jones, C.; Coward, K.; Yeste, M. How do lifestyle and environmental factors influence the sperm epigenome? Effects on sperm fertilising ability, embryo development, and offspring health. Clin. Epigenetics 2025, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.Y.; Orwig, K.E.; Oatley, J.M.; Avarbock, M.R.; Brinster, R.L. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 2006, 24, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Sanou, I.; van Maaren, J.; Eliveld, J.; Lei, Q.; Meissner, A.; de Melker, A.A.; Hamer, G.; van Pelt, A.M.M.; Mulder, C.L. Spermatogonial Stem Cell-Based Therapies: Taking Preclinical Research to the Next Level. Front. Endocrinol. 2022, 13, 850219. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Z.; Wang, G.; Wang, H.; Zhou, Y.; Zhao, X.; Cheng, C.Y.; Qiao, Y.; Sun, F. Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discov. 2021, 7, 101. [Google Scholar] [CrossRef]
| Study (First Author, Year) | Population (Species, Sample Size) | Age Groups/Time Points | Methodology | Sample/Cell Type | Main Findings |
|---|---|---|---|---|---|
| Cui et al., 2025 [25] | Humans (n = 35) | 21–69 y | scRNA-seq | Whole testis |
|
| He 2024 [26] | Humans (n = 23) | Young: <60 y (n = 14) Old: ≥60 y (n = 9) | scRNA-seq + CellCall + SASP gene profiling | Leydig cells |
|
| Bernhardt 2023 [27] | Humans (n = 73) | 25.8–50.4 y | RRBS + validation via pyrosequencing | Sperm |
|
| De Sena Brandine et al., 2023 [28] | Humans (n = 10) | Samples 10–18 years apart | WGBS + mixed-effects modeling | Sperm |
|
| Nie et al., 2022 [29] | Humans (n = 12) | 17–22 y vs. 62–76 y | Single-cell RNA-seq + CellChat + validation assays | Whole testis |
|
| Alfano et al., 2021 [30] | Human testis from infertile men with idiopathic GCA (n = 3 iNOA) vs. fertile controls | 32–41 y vs. controls | scRNA-seq + histology + hormone/immune profiling | Somatic cells from whole testis tissue (Sertoli, Leydig, peritubular myoid, endothelial, stromal, immune) |
|
| Paoli 2019 [31] | Human males (n = 2626 for semen analysis; n = 40 vs. 40 for molecular) | 20–40 y vs. 50–81 y | Semen analysis, TUNEL, RT-qPCR (PRM1/2), miRNA profiling | Ejaculated sperm and seminal plasma |
|
| Liu et al., 2025 [32] | Mice (C57BL/6; n = 3 young vs. 3 old for scRNA-seq; n = 10 for BHB studies) | 2 mo vs. 24 mo | scRNA-seq, qPCR, AAV-Cre/overexpression, BHB supplementation | Leydig cells |
|
| Kawahara et al., 2025 [24] | Mice (C57BL/6J; n = 3 per age group; n = 800–1000 tubule sections) | 3–4 mo vs. 15–20 mo vs. 25–28 mo | scRNA-seq, lineage tracing, intravital imaging | Undifferentiated spermatogonia (GFRα1⁺ SSCs) |
|
| Ozawa 2023 [33] | Mice (C57BL/6J; n = 8–13 per group) | 2 mo vs. >24 mo | Histology, IHC, SA-β-gal, RNA-seq, GSC-EC co-culture | Testicular endothelial cells (ECs) |
|
| Kanatsu-Shinohara et al., 2019 [34] | Mouse SSC cultures (5M vs. 60M; n = 3–12); aged mouse and rat testes | 5 mo vs. 60 mo (in vitro); 2 y in vivo | Long-term SSC culture, transplantation, RNA-seq, ChIP-seq, metabolic assays | Cultured SSCS and SSCs from aged mice/rats |
|
| Fice & Robaire 2023 [21] | Brown Norway rats (n = 5/group; RS purity ≥88%) | 5.3 mo vs. 19.2 mo | RNA-seq (RS); GO, IPA, PCA | Round spermatids |
|
| Luo 2023 [35] | Mice (C57BL/6; n = 6/group; + p38LCKO line) | 6 mo vs. 18 mo; + HFD mice for 24 wks | ELISA, qPCR, WB, IF, IHC, SA-β-Gal, scRNA-seq (human) | Leydig cells |
|
| Huang et al., 2023 [23] | Cynomolgus monkeys (n = 4 young, n = 4 old) | 5–6 y vs. 18–21 y | snRNA-seq (70,400 nuclei); IF, IHC, SA-β-Gal, transcriptome-wide analysis | SSCs, Sertoli cells, Leydig cells |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A. Multi-Omics Perspectives on Testicular Aging: Unraveling Germline Dysregulation, Niche Dysfunction, and Epigenetic Remodeling. Cells 2025, 14, 899. https://doi.org/10.3390/cells14120899
Kaltsas A. Multi-Omics Perspectives on Testicular Aging: Unraveling Germline Dysregulation, Niche Dysfunction, and Epigenetic Remodeling. Cells. 2025; 14(12):899. https://doi.org/10.3390/cells14120899
Chicago/Turabian StyleKaltsas, Aris. 2025. "Multi-Omics Perspectives on Testicular Aging: Unraveling Germline Dysregulation, Niche Dysfunction, and Epigenetic Remodeling" Cells 14, no. 12: 899. https://doi.org/10.3390/cells14120899
APA StyleKaltsas, A. (2025). Multi-Omics Perspectives on Testicular Aging: Unraveling Germline Dysregulation, Niche Dysfunction, and Epigenetic Remodeling. Cells, 14(12), 899. https://doi.org/10.3390/cells14120899







