Histology and Immunohistochemistry of Adipose Tissue: A Scoping Review on Staining Methods and Their Informative Value
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Manual Reference Search
2.3. Literature Selection
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
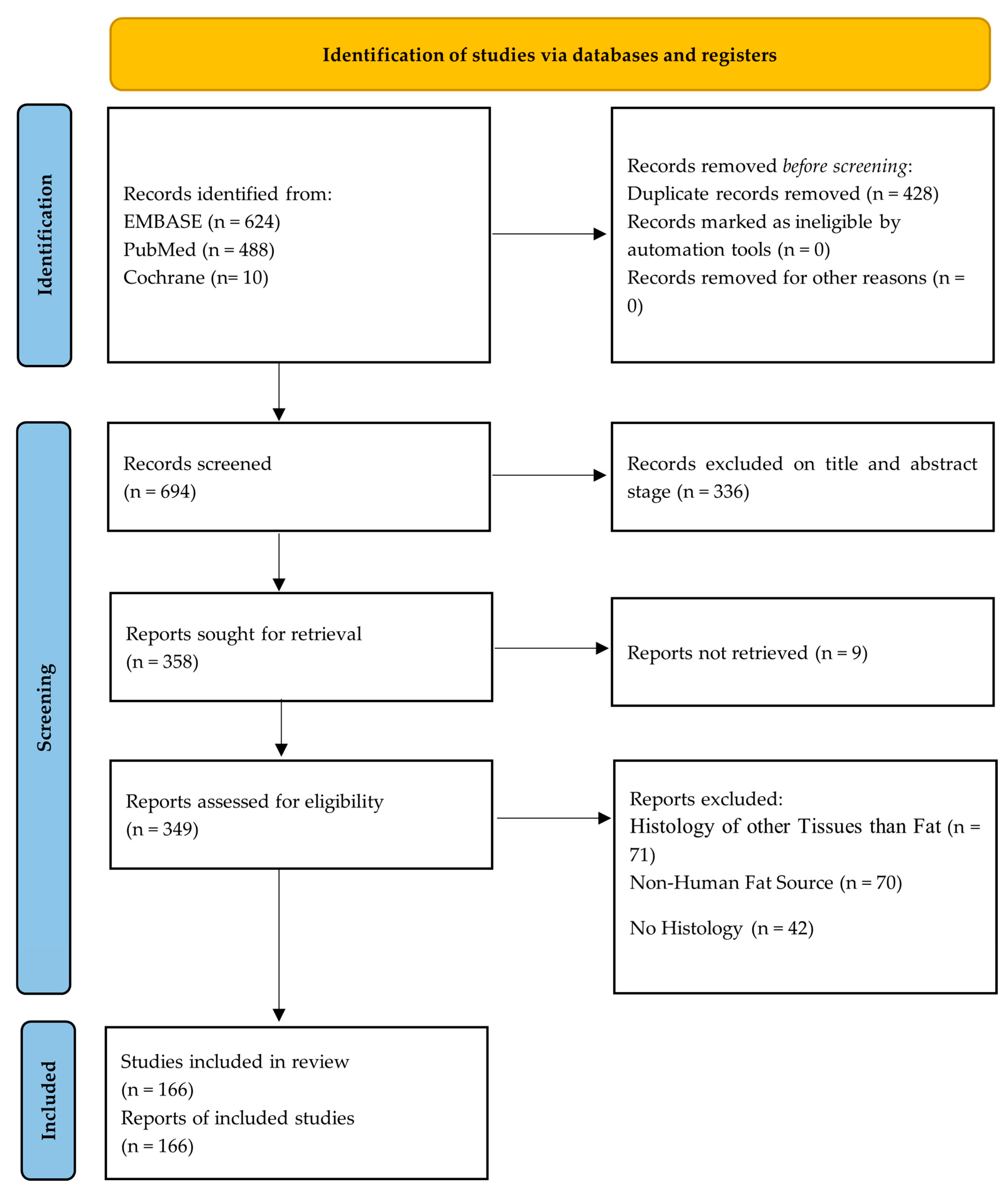
3.1. Prisma Flow Diagram
3.2. Study Selection
3.3. Patient and Animal Sample Characteristics
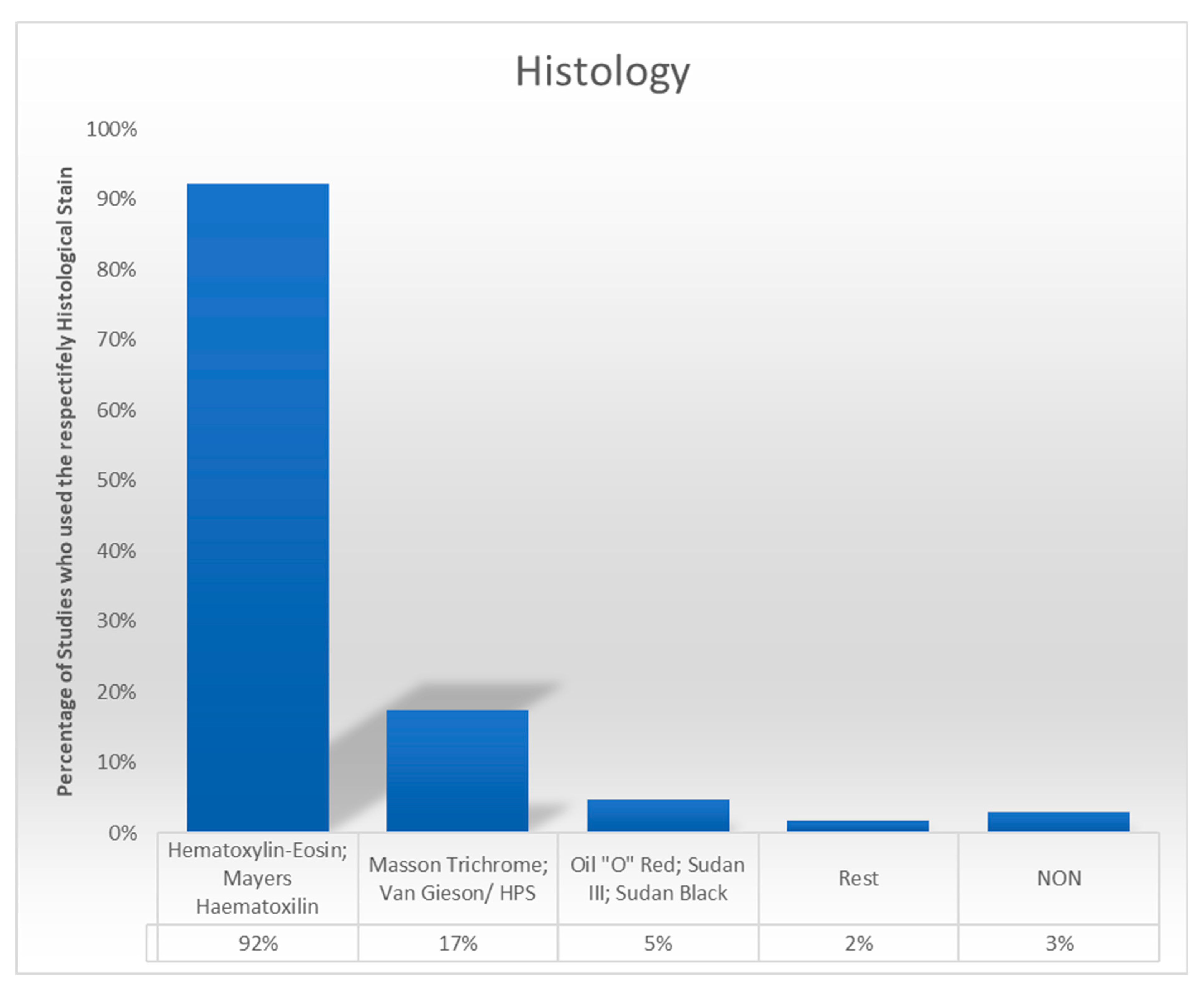
3.4. Histological Staining Techniques
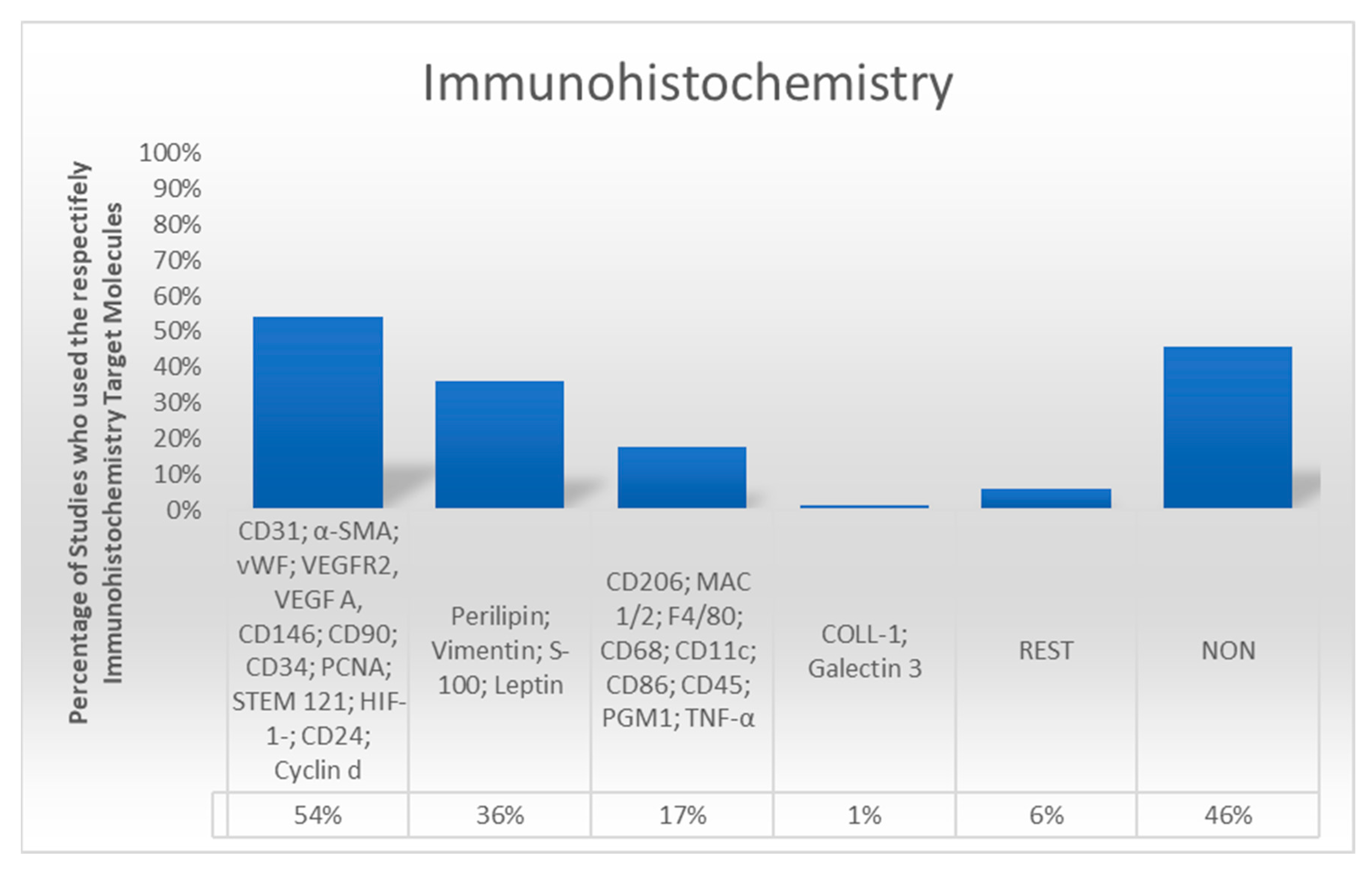
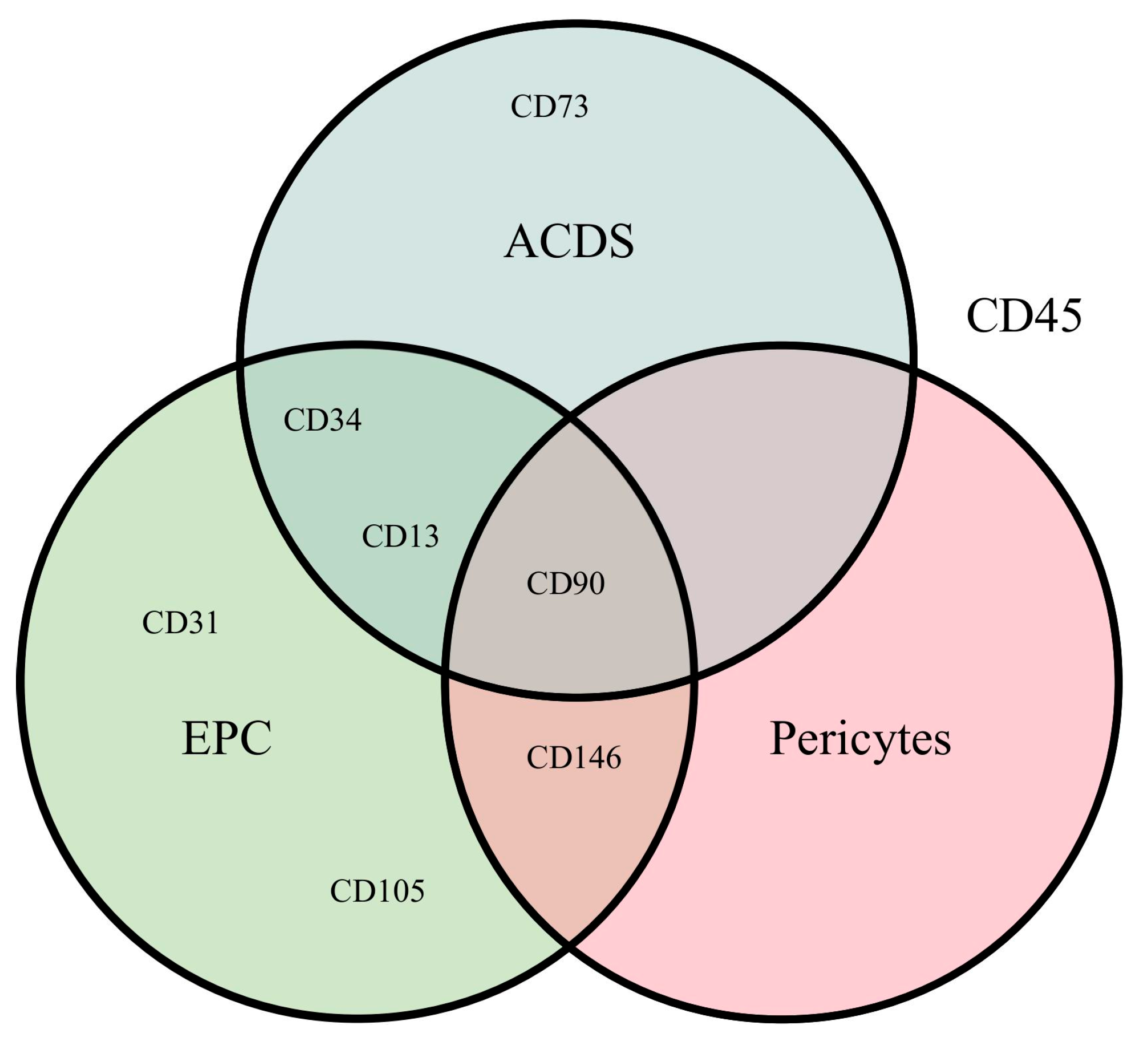
3.5. Immunohistochemical Analysis
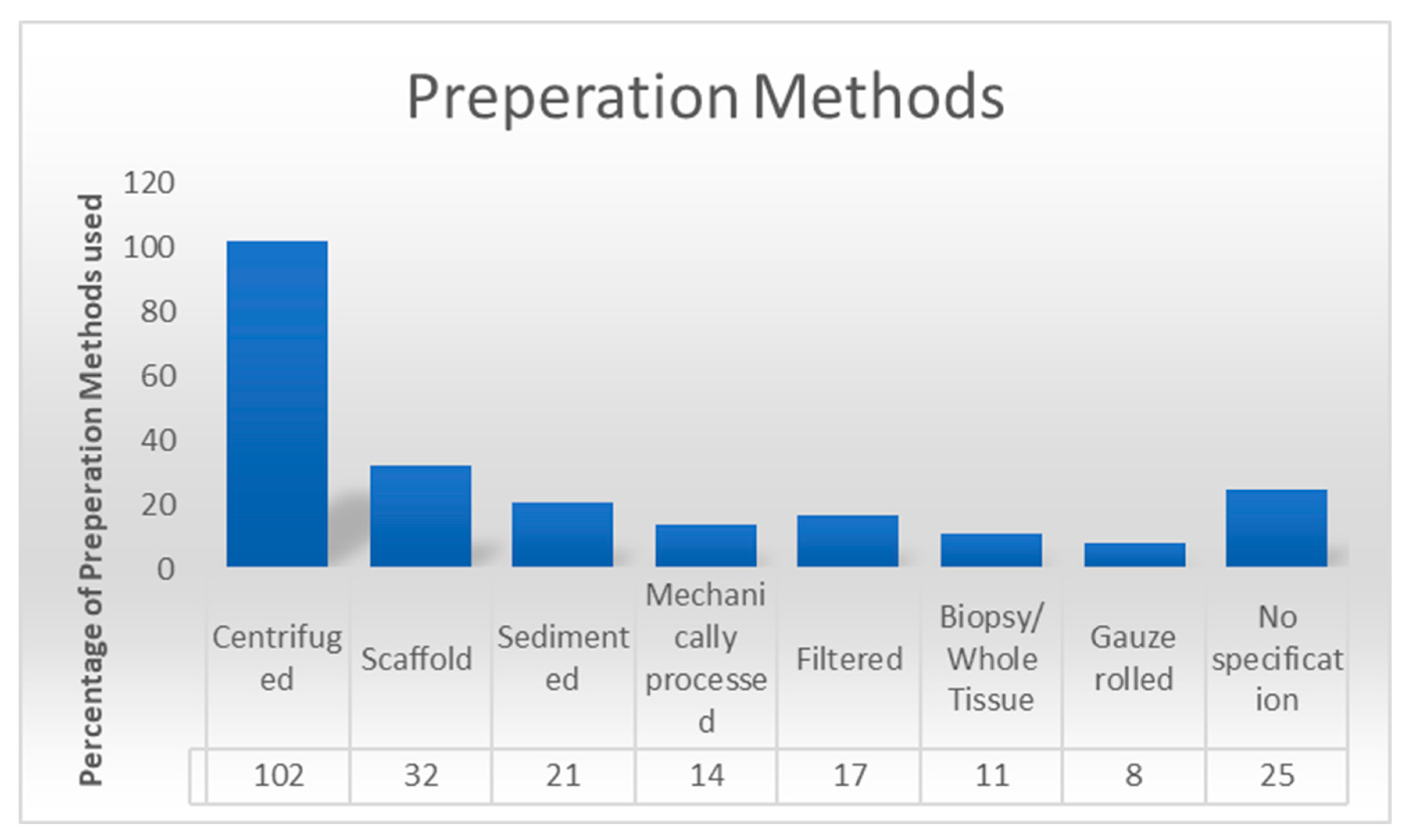
3.6. Preparation Methods
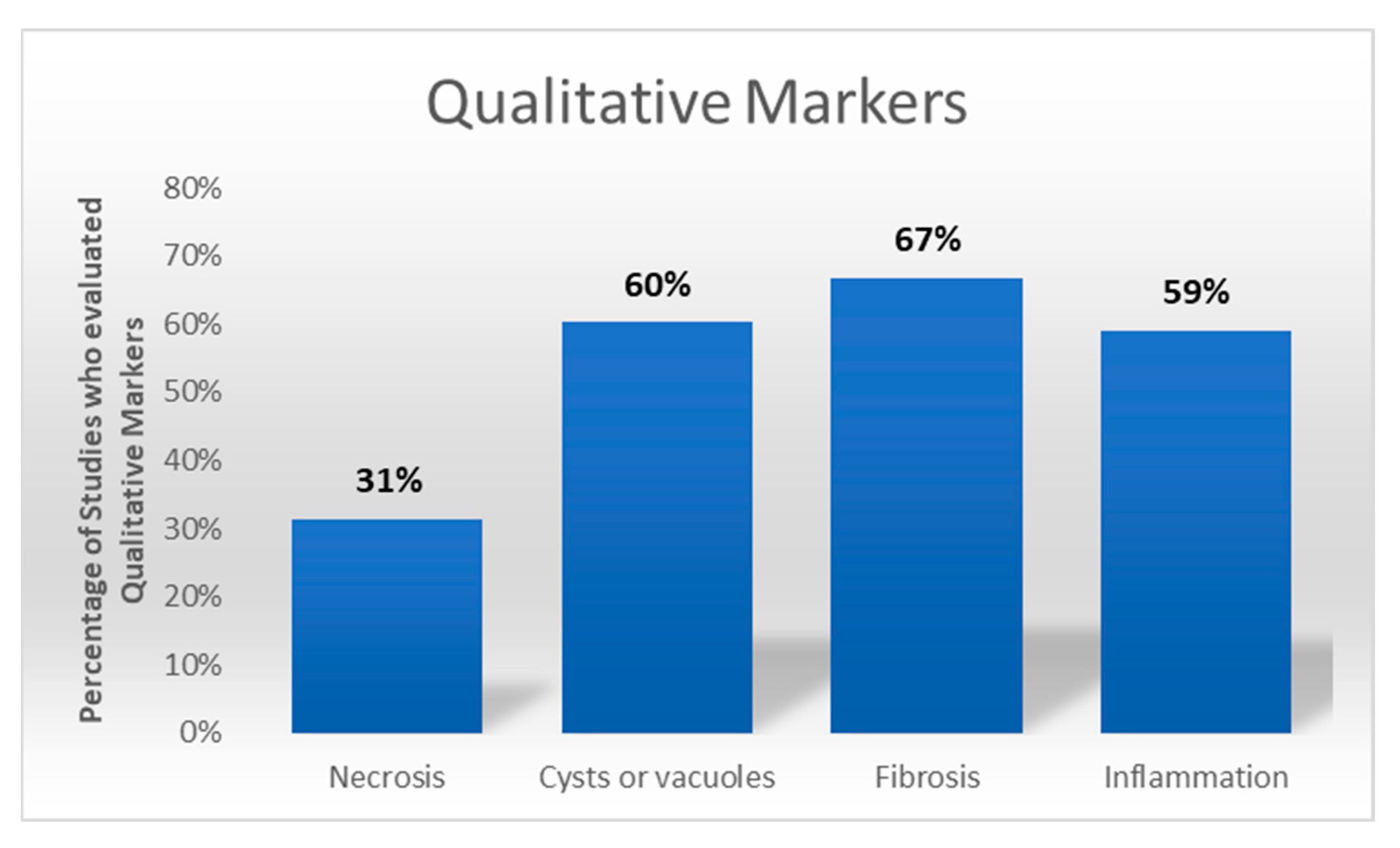
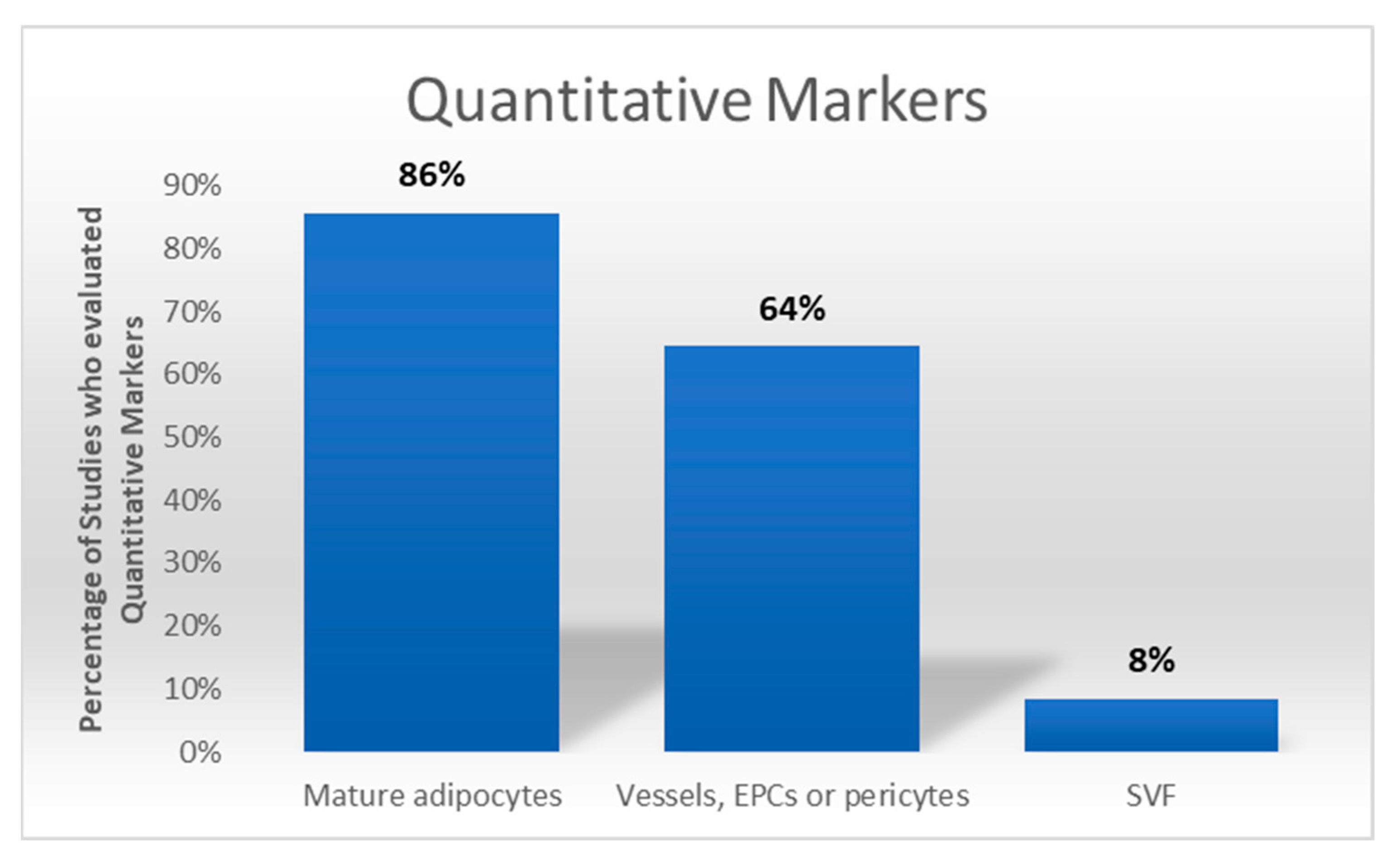
3.7. Qualitative Markers, Semiquantitative Analysis, and Quantitative Markers
3.8. Author Expertise
3.9. Reporting and Methodological Gaps
4. Discussion
4.1. Staining in Histology
4.2. Staining in Immunohistochemistry
4.3. Recommendations and Operative Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aronowitz, J.A.; Oheb, D.; Cai, N.; Pekcan, A.; Winterhalter, B. Esthetic Surgery Applications for Adipose-Derived Stem Cells. In Regenerative Medicine; Springer: Cham, Germany, 2023; pp. 265–271. ISBN 978-3-030-75516-4. [Google Scholar]
- Dong, L.; Li, X.; Leng, W.; Guo, Z.; Cai, T.; Ji, X.; Xu, C.; Zhu, Z.; Lin, J. Adipose stem cells in tissue regeneration and repair: From bench to bedside. Regen. Ther. 2023, 24, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Stachura, A.; Paskal, W.; Pawlik, W.; Mazurek, M.J.; Jaworowski, J. The Use of Adipose-Derived Stem Cells (ADSCs) and Stromal Vascular Fraction (SVF) in Skin Scar Treatment—A Systematic Review of Clinical Studies. J. Clin. Med. 2021, 10, 3637. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Fu, R.; Liu, L.; Lu, F. Stromal vascular fraction (SVF) cells enhance long-term survival of autologous fat grafting through the facilitation of M2 macrophages. Cell Biol. Int. 2013, 37, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lu, B.; Zhou, R.; Gao, W. Research progress of mechanisms of fat necrosis after autologous fat grafting: A review. Medicine 2023, 102, e33220. [Google Scholar] [CrossRef]
- Yoshimura, K.; Coleman, S.R. Complications of Fat Grafting. Clin. Plast. Surg. 2015, 42, 383–388. [Google Scholar] [CrossRef]
- Chen, X.; Deng, Z.; Feng, J.; Chang, Q.; Lu, F.; Yuan, Y. Necroptosis in Macrophage Foam Cells Promotes Fat Graft Fibrosis in Mice. Front. Cell Dev. Biol. 2021, 9, 651360. [Google Scholar] [CrossRef]
- Jia, X.; Chai, Y.; Zhu, J.; Zhang, X.; Jiang, C.; Yin, N.; Li, F. Enhancing Fat Graft Survival via Upregulating Autophagy of Adipocytes. Aesthetic Plast. Surg. 2024, 48, 1807–1816. [Google Scholar] [CrossRef]
- Genç, İ.G.; Fındıkçıoğlu, K.; Sadioğlu, A.; Erdal, A.I.; Özkoçer, S.E.; Elmas, Ç. The Effect of Ultrasonic Liposuction Energy Levels on Fat Graft Viability. Aesthetic Plast. Surg. 2022, 46, 2509–2516. [Google Scholar] [CrossRef]
- Säljö, K.; Orrhult, L.S.; Apelgren, P.; Markstedt, K.; Kölby, L.; Gatenholm, P. Successful engraftment, vascularization, and In vivo survival of 3D-bioprinted human lipoaspirate-derived adipose tissue. Bioprinting 2020, 17, e00065. [Google Scholar] [CrossRef]
- Merrifield, B.A.; Chang, A.; Hostetter, G.; Komorowska-Timek, E. Volume Retention, Metabolism, and Cellular Composition of Human Fat Xenografts. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e1869. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Witman, N.; Yan, D.; Zhang, S.; Zhou, M.; Yan, Y.; Yao, Q.; Ding, F.; Yan, B.; Wang, H.; et al. Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model. Stem Cell Res. Ther. 2020, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, S.; Ni, B.; Lin, Y. Improvement in autologous human fat transplant survival with SVF plus VEGF–PLA nano-sustained release microspheres. Cell Biol. Int. 2014, 38, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Thitilertdecha, P.; Lohsiriwat, V.; Poungpairoj, P.; Tantithavorn, V.; Onlamoon, N. Extensive Characterization of Mesenchymal Stem Cell Marker Expression on Freshly Isolated and In Vitro Expanded Human Adipose-Derived Stem Cells from Breast Cancer Patients. Stem Cells Int. 2020, 2020, 8237197. [Google Scholar] [CrossRef]
- Prantl, L.; Brix, E.; Kempa, S.; Felthaus, O.; Eigenberger, A.; Brébant, V.; Anker, A.; Strauss, C. Facial Rejuvenation with Concentrated Lipograft—A 12 Month Follow-Up Study. Cells 2021, 10, 594. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Reinhard, R.; Siegmund, A.; Heumann, K.; Felthaus, O. Cell-Enriched Lipotransfer (CELT) Improves Tissue Regeneration and Rejuvenation without Substantial Manipulation of the Adipose Tissue Graft. Cells 2022, 11, 3159. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Klein, S.; Limm, K.; Oefner, P.J.; Schratzenstaller, T.; Felthaus, O. Shear Force Processing of Lipoaspirates for Stem Cell Enrichment Does Not Affect Secretome of Human Cells Detected by Mass Spectrometry In Vitro. Plast. Reconstr. Surg. 2020, 146, 749e–758e. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Brix, E.; Kempa, S.; Baringer, M.; Felthaus, O. Adipose Tissue-Derived Stem Cell Yield Depends on Isolation Protocol and Cell Counting Method. Cells 2021, 10, 1113. [Google Scholar] [CrossRef]
- Eigenberger, A.; Felthaus, O.; Schratzenstaller, T.; Haerteis, S.; Utpatel, K.; Prantl, L. The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells. Cells 2022, 11, 2543. [Google Scholar] [CrossRef]
- De Haan, K.; Zhang, Y.; Zuckerman, J.E.; Liu, T.; Sisk, A.E.; Diaz, M.F.P.; Jen, K.-Y.; Nobori, A.; Liou, S.; Zhang, S.; et al. Deep learning-based transformation of H&E stained tissues into special stains. Nat. Commun. 2021, 12, 4884. [Google Scholar] [CrossRef]
- Bai, B.; Yang, X.; Li, Y.; Zhang, Y.; Pillar, N.; Ozcan, A. Deep learning-enabled virtual histological staining of biological samples. Light Sci. Appl. 2023, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Condé-Green, A.; Baptista, L.S.; De Amorin, N.F.G.; De Oliveira, E.D.; Da Silva, K.R.; Pedrosa, C.D.S.G.; Borojevic, R.; Pitanguy, I. Effects of Centrifugation on Cell Composition and Viability of Aspirated Adipose Tissue Processed for Transplantation. Aesthet. Surg. J. 2010, 30, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Condé-Green, A.; Gontijo De Amorim, N.F.; Pitanguy, I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: A comparative study. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.V.T.; Hong, K.Y.; Jin, X.; Chang, H. Histological Comparison of Nanofat and Lipoconcentrate: Enhanced Effects of Lipoconcentrate on Adipogenesis and Angiogenesis. Aesthetic Plast. Surg. 2024, 48, 752–763. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Adanali, G.; Erdogan, B.; Turegun, M.; Tuncel, A.; Gencaga, S.; Albayrak, L. A New, T-Shaped Adaptor for Easy, Quick and Efficient Fat Harvesting During Liposuction. Aesthetic Plast. Surg. 2002, 26, 340–344. [Google Scholar] [CrossRef]
- Adem, S.; Abbas, D.B.; Lavin, C.V.; Fahy, E.J.; Griffin, M.; Diaz Deleon, N.M.; Borrelli, M.R.; Mascharak, S.; Shen, A.H.; Patel, R.A.; et al. Decellularized Adipose Matrices Can Alleviate Radiation-Induced Skin Fibrosis. Adv. Wound Care 2022, 11, 524–536. [Google Scholar] [CrossRef]
- Afanas’eva, D.S.; Gushchina, M.B.; Borzenok, S.A. Comparison of Morphology of Adipose Body of the Orbit and Subcutaneous Fat in Humans. Bull. Exp. Biol. Med. 2018, 164, 394–396. [Google Scholar] [CrossRef]
- Agostini, T.; Lazzeri, D.; Pini, A.; Marino, G.; Li Quattrini, A.; Bani, D.; Dini, M. Wet and Dry Techniques for Structural Fat Graft Harvesting: Histomorphometric and Cell Viability Assessments of Lipoaspirated Samples. Plast. Reconstr. Surg. 2012, 130, 331e–339e. [Google Scholar] [CrossRef]
- Ansorge, H.; Garza, J.R.; McCormack, M.C.; Leamy, P.; Roesch, S.; Barere, A.; Connor, J. Autologous Fat Processing Via the Revolve System: Quality and Quantity of Fat Retention Evaluated in an Animal Model. Aesthet. Surg. J. 2014, 34, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, P. Immunohistochemical Expression of S-100 Protein in Human Embryonal Fat Cells. Cells Tissues Organs 2001, 169, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bach-Mortensen, N.; Rømert, P.; Ballegaard, S. Transplantation of Human Adipose Tissue to Nude Mice. Acta Pathol. Microbiol. Scand. 1976, 84, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.C.; Song, J.S.; Bae, S.H.; Kim, J.H. Effects of Human Adipose-Derived Stem Cells and Stromal Vascular Fraction on Cryopreserved Fat Transfer. Dermatol. Surg. 2015, 41, 605–614. [Google Scholar] [CrossRef]
- Baker, S.B.; Cohen, M.; Kuo, L.; Johnson, M.; Al-Attar, A.; Zukowska, Z. The Role of the Neuropeptide Y2 Receptor in Liporemodeling: Neuropeptide Y–Mediated Adipogenesis and Adipose Graft Maintenance. Plast. Reconstr. Surg. 2009, 123, 486–492. [Google Scholar] [CrossRef]
- Bauer, C.A.; Valentino, J.; Hoffman, H.T. Long-Term Result of Vocal Cord Augmentation with Autogenous Fat. Ann. Otol. Rhinol. Laryngol. 1995, 104, 871–874. [Google Scholar] [CrossRef]
- Bellas, E.; Panilaitis, B.J.B.; Glettig, D.L.; Kirker-Head, C.A.; Yoo, J.J.; Marra, K.G.; Rubin, J.P.; Kaplan, D.L. Sustained volume retention in vivo with adipocyte and lipoaspirate seeded silk scaffolds. Biomaterials 2013, 34, 2960–2968. [Google Scholar] [CrossRef]
- Bi, X.; Wu, W.; Zou, J.; Zhao, J.; Lin, Z.; Li, Y.; Lu, F.; Gao, J.; Li, B.; Dong, Z. Attenuated angiogenesis and macrophage infiltration during adipose tissue regeneration in a megavolume human fat grafting nude mouse model. Plast. Reconstr. Surg. 2025, 155, 491–503. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Patel, R.A.; Blackshear, C.; Vistnes, S.; Diaz Deleon, N.M.; Adem, S.; Shen, A.H.; Sokol, J.; Momeni, A.; Nguyen, D.; et al. CD34+CD146+ adipose-derived stromal cells enhance engraftment of transplanted fat. Stem Cells Transl. Med. 2020, 9, 1389–1400. [Google Scholar] [CrossRef]
- Bryant, M.S.; Bremer, A.M.; Nguyen, T.Q. Autogeneic Fat Transplants in the Epidural Space in Routine Lumbar Spine Surgery. Neurosurgery 1983, 13, 351–366. [Google Scholar] [CrossRef]
- Chai, Y.; Jia, X.; Zhu, J.; Jiang, C.; Yin, N.; Li, F. Increased Fat Graft Survival by Promoting Adipocyte Dedifferentiation. Aesthet. Surg. J. 2023, 43, NP213–NP222. [Google Scholar] [CrossRef] [PubMed]
- Chajchir, A.; Benzaquen, I. Fat-grafting injection for soft-tissue augmentation. Plast. Reconstr. Surg. 1989, 84, 921–934, discussion 935. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Tang, S.; He, J.; Li, X.; Peng, G.; Zhang, H.; Chen, J.; Chen, L.; Chen, X. Small extracellular vesicles from human adipose-derived mesenchymal stromal cells: A potential promoter of fat graft survival. Stem Cell Res. Ther. 2021, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chai, Y.; Yin, B.; Zhang, X.; Han, X.; Cai, L.; Yin, N.; Li, F. Washing Lipoaspirate Improves Fat Graft Survival in Nude Mice. Aesthetic Plast. Surg. 2022, 46, 923–936. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, J.; Xu, S.; Wu, M.; Xue, C.; Wu, M.; Lv, C.; Wang, Y. Adipose-Derived Stem Cells Exosomes Improve Fat Graft Survival by Promoting Prolipogenetic Abilities through Wnt/β-Catenin Pathway. Stem Cells Int. 2022, 2022, 5014895. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Luan, J. Oral Administration of Lutein Improves Fat Graft Survival by Alleviating Oxidative Stress in Mice. Aesthet. Surg. J. 2024, 44, NP906–NP921. [Google Scholar] [CrossRef]
- Chia, H.-L.; Woo, E.; Por, Y.-C.; Ma, D.-R.; Chang, K.; Mok, J.; Kua, J.; Yeow, V. Adipocyte and preadipocyte viability in autologous fat grafts: Comparing the water jet-assisted liposuction (WAL) and Coleman techniques. Eur. J. Plast. Surg. 2015, 38, 183–188. [Google Scholar] [CrossRef]
- Chung, N.N.; Ransom, R.C.; Blackshear, C.P.; Irizarry, D.M.; Yen, D.; Momeni, A.; Lee, G.K.; Nguyen, D.H.; Longaker, M.T.; Wan, D.C. Fat Grafting into Younger Recipients Improves Volume Retention in an Animal Model. Plast. Reconstr. Surg. 2019, 143, 1067–1075. [Google Scholar] [CrossRef]
- Cicione, C.; Di Taranto, G.; Barba, M.; Isgrò, M.A.; D’Alessio, A.; Cervelli, D.; Sciarretta, F.V.; Pelo, S.; Michetti, F.; Lattanzi, W. In Vitro Validation of a Closed Device Enabling the Purification of the Fluid Portion of Liposuction Aspirates. Plast. Reconstr. Surg. 2016, 137, 1157–1167. [Google Scholar] [CrossRef]
- Cicione, C.; Vadalà, G.; Di Giacomo, G.; Tilotta, V.; Ambrosio, L.; Russo, F.; Zampogna, B.; Cannata, F.; Papalia, R.; Denaro, V. Micro-fragmented and nanofat adipose tissue derivatives: In vitro qualitative and quantitative analysis. Front. Bioeng. Biotechnol. 2023, 11, 911600. [Google Scholar] [CrossRef]
- Condé-Green, A.; Wu, I.; Graham, I.; Chae, J.J.; Drachenberg, C.B.; Singh, D.P.; Holton, L.; Slezak, S.; Elisseeff, J. Comparison of 3 Techniques of Fat Grafting and Cell-Supplemented Lipotransfer in Athymic Rats. Aesthet. Surg. J. 2013, 33, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.O.; Rophael, J.; Morrison, W.A.; Vashi, A.V.; Mitchell, G.M.; Penington, A.J. Effect of local, long-term delivery of platelet-derived growth factor (PDGF) on injected fat graft survival in severe combined immunodeficient (SCID) mice. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pu, L.L.Q. The Search for a Useful Method for the Optimal Cryopreservation of Adipose Aspirates: Part I. In Vitro Study. Aesthet. Surg. J. 2009, 29, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pu, L.L.Q. The Search for a Useful Method for the Optimal Cryopreservation of Adipose Aspirates: Part II. In Vivo Study. Aesthet. Surg. J. 2010, 30, 451–456. [Google Scholar] [CrossRef]
- Davis, K.; Rasko, Y.; Oni, G.; Bills, J.; Geissler, P.; Kenkel, J.M. Comparison of Adipocyte Viability and Fat Graft Survival in an Animal Model Using a New Tissue Liquefaction Liposuction Device vs Standard Coleman Method for Harvesting. Aesthet. Surg. J. 2013, 33, 1175–1185. [Google Scholar] [CrossRef]
- Debald, M.; Pech, T.; Kaiser, C.; Keyver-Paik, M.-D.; Walgenbach-Bruenagel, G.; Kalff, J.C.; Kuhn, W.; Walgenbach, K.J. Lipofilling effects after breast cancer surgery in post-radiation patients: An analysis of results and algorithm proposal. Eur. J. Plast. Surg. 2017, 40, 447–454. [Google Scholar] [CrossRef]
- Deleon, N.M.D.; Adem, S.; Lavin, C.V.; Abbas, D.B.; Griffin, M.; King, M.E.; Borrelli, M.R.; Patel, R.A.; Fahy, E.J.; Lee, D.; et al. Angiogenic CD34+CD146+ adipose-derived stromal cells augment recovery of soft tissue after radiotherapy. J. Tissue Eng. Regen. Med. 2021, 15, 1105–1117. [Google Scholar] [CrossRef]
- Dimitroulis, G. Macroscopic and Histologic Analysis of Abdominal Dermis-Fat Grafts Retrieved from Human Temporomandibular Joints. J. Oral Maxillofac. Surg. 2011, 69, 2329–2333. [Google Scholar] [CrossRef]
- Dobran, M.; Brancorsini, D.; Della Costanza, M.; Liverotti, V.; Mancini, F.; Nasi, D.; Iacoangeli, M.; Scerrati, M. Epidural scarring after lumbar disc surgery: Equivalent scarring with/without free autologous fat grafts. Surg. Neurol. Int. 2017, 8, 169. [Google Scholar] [CrossRef]
- Dong, X.; Premaratne, I.; Gadjiko, M.; Berri, N.; Spector, J.A. Improving fat transplantation survival and vascularization with adenovirus E4+ endothelial cell-assisted lipotransfer. Cells Tissues Organs 2022, 212, 341–351. [Google Scholar] [CrossRef]
- Eskalen, A.; Işık, E.; Ozdemir, I.; Keskin, I.; Keskin, M.; Karacaoglan, N. Evaluation of Perilipin Expression in Centrifuged Fat Grafts on Different Revolutions Per Minute and Duration Combinations. Aesthetic Plast. Surg. 2024, 49, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Fang, M.; Li, J.; Solari, M.G.; Wu, D.; Tan, W.; Wang, Y.; Yang, X.; Lei, S. A Novel Fat Making Strategy with Adipose-Derived Progenitor Cell-Enriched Fat Improves Fat Graft Survival. Aesthet. Surg. J. 2021, 41, NP1228–NP1236. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.E.H.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L.Q. The Viability of Autologous Fat Grafts Harvested with the LipiVage System: A Comparative Study. Ann. Plast. Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Filson, S.A.; Keren, A.; Goldstein, N.; Ullmann, Y. The Opposite Expected Effect of p38 Inhibitors on Fat Graft Survival. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e806. [Google Scholar] [CrossRef]
- Fisher, C.; Grahovac, T.L.; Schafer, M.E.; Shippert, R.D.; Marra, K.G.; Rubin, J.P. Comparison of Harvest and Processing Techniques for Fat Grafting and Adipose Stem Cell Isolation. Plast. Reconstr. Surg. 2013, 132, 351–361. [Google Scholar] [CrossRef]
- Girard, A.-C.; Mirbeau, S.; Gence, L.; Hivernaud, V.; Delarue, P.; Hulard, O.; Festy, F.; Roche, R. Effect of Washes and Centrifugation on the Efficacy of Lipofilling with or Without Local Anesthetic. Plast. Reconstr. Surg.-Glob. Open 2015, 3, e496. [Google Scholar] [CrossRef]
- Ha, K.-Y.; Park, H.; Park, S.-H.; Lee, B.-I.; Ji, Y.-H.; Kim, T.-Y.; Yoon, E.-S. The Relationship of a Combination of Human Adipose Tissue-Derived Stem Cells and Frozen Fat with the Survival Rate of Transplanted Fat. Arch. Plast. Surg. 2015, 42, 677–685. [Google Scholar] [CrossRef]
- Hamed, S.; Egozi, D.; Kruchevsky, D.; Teot, L.; Gilhar, A.; Ullmann, Y. Erythropoietin Improves the Survival of Fat Tissue after Its Transplantation in Nude Mice. PLoS ONE 2010, 5, e13986. [Google Scholar] [CrossRef]
- Hamed, S.; Ben-Nun, O.; Egozi, D.; Keren, A.; Malyarova, N.; Kruchevsky, D.; Gilhar, A.; Ullmann, Y. Treating Fat Grafts with Human Endothelial Progenitor Cells Promotes Their Vascularization and Improves Their Survival in Diabetes Mellitus. Plast. Reconstr. Surg. 2012, 130, 801–811. [Google Scholar] [CrossRef]
- Harris, W.M.; Plastini, M.; Kappy, N.; Ortiz, T.; Chang, S.; Brown, S.; Carpenter, J.P.; Zhang, P. Endothelial Differentiated Adipose-Derived Stem Cells Improvement of Survival and Neovascularization in Fat Transplantation. Aesthet. Surg. J. 2019, 39, 220–232. [Google Scholar] [CrossRef]
- He, Y.; Yu, X.; Chen, Z.; Li, L. Stromal vascular fraction cells plus sustained release VEGF/Ang-1-PLGA microspheres improve fat graft survival in mice. J. Cell. Physiol. 2019, 234, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, F.; Zhang, Y.; Tan, P.; Li, Q.; Cheng, C. Concentrated ultrasound-processed fat (CUPF): More than a mechanically emulsified graft. J. Plast. Reconstr. Aesthet. Surg. 2023, 83, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Herly, M.; Ørholt, M.; Glovinski, P.V.; Pipper, C.B.; Broholm, H.; Poulsgaard, L.; Fugleholm, K.; Thomsen, C.; Drzewiecki, K.T. Quantifying Long-Term Retention of Excised Fat Grafts: A Longitudinal, Retrospective Cohort Study of 108 Patients Followed for Up to 8.4 Years. Plast. Reconstr. Surg. 2017, 139, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Hersant, B.; Bouhassira, J.; SidAhmed-Mezi, M.; Vidal, L.; Keophiphath, M.; Chheangsun, B.; Niddam, J.; Bosc, R.; Nezet, A.L.; Meningaud, J.-P.; et al. Should platelet-rich plasma be activated in fat grafts? An animal study. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 681–690. [Google Scholar] [CrossRef]
- Hivernaud, V.; Lefourn, B.; Robard, M.; Guicheux, J.; Weiss, P. Autologous fat grafting: A comparative study of four current commercial protocols. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 248–256. [Google Scholar] [CrossRef]
- Ho, C.; Zheng, D.; Sun, J.; Wen, D.; Wu, S.; Yu, L.; Gao, Y.; Zhang, Y.; Li, Q. LRG-1 promotes fat graft survival through the RAB31-mediated inhibition of hypoxia-induced apoptosis. J. Cell. Mol. Med. 2022, 26, 3153–3168. [Google Scholar] [CrossRef]
- Hoareau, L.; Bencharif, K.; Girard, A.-C.; Gence, L.; Delarue, P.; Hulard, O.; Festy, F.; Roche, R. Effect of centrifugation and washing on adipose graft viability: A new method to improve graft efficiency. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 712–719. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Lai, C.-Y.; Liu, J.-W.; Yu, Y.-Y.; Chang, F.C.-S.; Huang, J.-J. Fate of Fat Grafting In Vivo and In Vitro: Does the Suction-Assisted Lipectomy Device Matter? Aesthet. Surg. J. 2021, 41, NP1323–NP1336. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Wang, M.; Tian, W.; Wang, H. Concentrated Growth Factor Enhanced Fat Graft Survival: A Comparative Study. Dermatol. Surg. 2018, 44, 976–984. [Google Scholar] [CrossRef]
- Huang, H.; Feng, S.; Zhang, W.; Li, W.; Xu, P.; Wang, X.; Ai, A. Bone marrow mesenchymal stem cell-derived extracellular vesicles improve the survival of transplanted fat grafts. Mol. Med. Rep. 2017, 16, 3069–3078. [Google Scholar] [CrossRef]
- Ichikawa, K.; Miyasaka, M.; Tanaka, R.; Tanino, R.; Mizukami, K.; Wakaki, M. Histologic evaluation of the pulsed Nd:YAG laser for laser lipolysis. Lasers Surg. Med. 2005, 36, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, R.; Jayakumar, R.; Iyer, S. Injectable Pectin–Alginate Hydrogels for Improving Vascularization and Adipogenesis of Human Fat Graft. J. Funct. Biomater. 2023, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Li, M.; Duan, W.; Dong, Y.; Wang, Y. Improvement of the Survival of Human Autologous Fat Transplantation by Adipose-Derived Stem-Cells-Assisted Lipotransfer Combined with bFGF. Sci. World J. 2015, 2015, 968057. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, F.; Chen, Y.; Fang, J.; Luo, S.; Wang, H. Exosomes Derived from Human Adipose-Derived Stem Cells Cannot Distinctively Promote Graft Survival in Cryopreservation Fat Grafting. Aesthetic Plast. Surg. 2023, 47, 2117–2129. [Google Scholar] [CrossRef]
- Jin, S.; Yang, Z.; Han, X.; Li, F. Blood Impairs Viability of Fat Grafts and Adipose Stem Cells: Importance of Washing in Fat Processing. Aesthet. Surg. J. 2021, 41, 86–97. [Google Scholar] [CrossRef]
- Jung, J.A.; Kim, Y.W.; Cheon, Y.W.; Kang, S.R. Effects of the Diabetic Condition on Grafted Fat Survival: An Experimental Study Using Streptozotocin-Induced Diabetic Rats. Arch. Plast. Surg. 2014, 41, 241–247. [Google Scholar] [CrossRef]
- Kakudo, N.; Tanaka, Y.; Morimoto, N.; Ogawa, T.; Kushida, S.; Hara, T.; Kusumoto, K. Adipose-derived regenerative cell (ADRC)-enriched fat grafting: Optimal cell concentration and effects on grafted fat characteristics. J. Transl. Med. 2013, 11, 254. [Google Scholar] [CrossRef]
- Kamel, A.H.; Kamal, A.; Abou-Elghait, A.T. A quantitative analysis of the effects of different harvesting, preparation, and injection methods on the integrity of fat cells. Eur. J. Plast. Surg. 2014, 37, 469–478. [Google Scholar] [CrossRef]
- Kanamori, M.; Kawaguchi, Y.; Ohmori, K.; Kimura, T.; Tsuji, H.; Matsui, H. The Fate of Autogenous Free-Fat Grafts After Posterior Lumbar Surgery. Spine 2001, 26, 2264–2270. [Google Scholar] [CrossRef]
- Kelmendi-Doko, A.; Rubin, J.P.; Klett, K.; Mahoney, C.; Wang, S.; Marra, K.G. Controlled dexamethasone delivery via double-walled microspheres to enhance long-term adipose tissue retention. J. Tissue Eng. 2017, 8, 2041731417735402. [Google Scholar] [CrossRef]
- Kelmendi-Doko, A.; Marra, K.G.; Vidic, N.; Tan, H.; Rubin, J.P. Adipogenic Factor-Loaded Microspheres Increase Retention of Transplanted Adipose Tissue. Tissue Eng. Part A 2014, 20, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Khater, R.; Atanassova, P.; Anastassov, Y.; Pellerin, P.; Martinot-Duquennoy, V. Clinical and Experimental Study of Autologous Fat Grafting After Processing by Centrifugation and Serum Lavage. Aesthetic Plast. Surg. 2009, 33, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kijima, Y.; Yoshinaka, H.; Hirata, M.; Umekita, Y.; Sohda, M.; Koriyama, C.; Mizoguchi, T.; Arima, H.; Nakajo, A.; Ishigami, S.; et al. Clinical and pathologic evaluation of implanted free dermal fat grafts after breast cancer surgery: A retrospective analysis. Surgery 2012, 151, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Min, H.J.; Choi, R.J.; Lee, D.H.; Cheon, Y.W. Insulin Promotes Adipose-Derived Stem Cell Differentiation after Fat Grafting. Plast. Reconstr. Surg. 2018, 142, 927–938. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, D.-S.; Ha, H.-J.; Jung, J.-W.; Baek, S.-W.; Baek, S.H.; Kim, T.-H.; Lee, J.C.; Hwang, E.; Han, D.K. Fat Graft with Allograft Adipose Matrix and Magnesium Hydroxide-Incorporated PLGA Microspheres for Effective Soft Tissue Reconstruction. Tissue Eng. Regen. Med. 2022, 19, 553–563. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, Y.J.; Kim, Y.W.; Cheon, Y.W. Heating pretreatment of the recipient site enhances survival of transplanted fat in a mouse model. Plast. Reconstr. Surg. 2023, 152, 787–795. [Google Scholar] [CrossRef]
- Kim, J.; Tran, V.V.T.; Hong, K.Y.; Chang, H. Effect of Injectable Acellular Adipose Matrix on Soft Tissue Reconstruction in a Murine Model. Aesthetic Plast. Surg. 2024, 48, 2210–2219. [Google Scholar] [CrossRef]
- Kirkham, J.C.; Lee, J.H.; Medina, M.A.; McCormack, M.C.; Randolph, M.A.; Austen, W.G. The Impact of Liposuction Cannula Size on Adipocyte Viability. Ann. Plast. Surg. 2012, 69, 479–481. [Google Scholar] [CrossRef]
- Ko, M.-S.; Jung, J.-Y.; Shin, I.-S.; Choi, E.-W.; Kim, J.-H.; Kang, S.K.; Ra, J.C. Effects of Expanded Human Adipose Tissue-Derived Mesenchymal Stem Cells on the Viability of Cryopreserved Fat Grafts in the Nude Mouse. Int. J. Med. Sci. 2011, 8, 231–238. [Google Scholar] [CrossRef][Green Version]
- Kokai, L.E.; Jones, T.L.; Silowash, R.; Theisen, B.; DiBernardo, G.; Lu, A.; Yi, B.; Marra, K.G.; Rubin, J.P. Optimization and Standardization of the Immunodeficient Mouse Model for Assessing Fat Grafting Outcomes. Plast. Reconstr. Surg. 2017, 140, 1185–1194. [Google Scholar] [CrossRef]
- Kuwahara, K.; Gladstone, H.B.; Gupta, V.; Kireev, V.; Neel, V.; Moy, R.L. Rupture of fat cells using laser-generated ultra short stress waves. Lasers Surg. Med. 2003, 32, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kirkham, J.C.; McCormack, M.C.; Nicholls, A.M.; Randolph, M.A.; Austen, W.G. The Effect of Pressure and Shear on Autologous Fat Grafting. Plast. Reconstr. Surg. 2013, 131, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xiao, R. A Study of the Effect of Platelet-Rich Plasma on Outcomes After Aspirated Human Fat Grafting with Experimental Design. J. Craniofac. Surg. 2020, 31, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-W.; Liao, W.-C.; Wu, S.-H.; Ma, H. Cryopreservation of Fat Tissue and Application in Autologous Fat Graft: In Vitro and In Vivo Study. Aesthetic Plast. Surg. 2012, 36, 714–722. [Google Scholar] [CrossRef]
- Li, K.; Gao, J.; Zhang, Z.; Li, J.; Cha, P.; Liao, Y.; Wang, G.; Lu, F. Selection of Donor Site for Fat Grafting and Cell Isolation. Aesthetic Plast. Surg. 2013, 37, 153–158. [Google Scholar] [CrossRef]
- Li, F.; Guo, W.; Li, K.; Yu, M.; Tang, W.; Wang, H.; Tian, W. Improved Fat Graft Survival by Different Volume Fractions of Platelet-Rich Plasma and Adipose-Derived Stem Cells. Aesthet. Surg. J. 2015, 35, 319–333. [Google Scholar] [CrossRef]
- Li, Y.; Mou, S.; Xiao, P.; Li, G.; Li, J.; Tong, J.; Wang, J.; Yang, J.; Sun, J.; Wang, Z. Delayed two steps PRP injection strategy for the improvement of fat graft survival with superior angiogenesis. Sci. Rep. 2020, 10, 5231. [Google Scholar] [CrossRef]
- Li, B.; Quan, Y.; He, Y.; He, Y.; Lu, F.; Liao, Y.; Cai, J. A Preliminary Exploratory Study of Autologous Fat Transplantation in Breast Augmentation with Different Fat Transplantation Planes. Front. Surg. 2022, 9, 895674. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Q.; Li, Y.; Yang, F.; Ke, C.; Li, T.; Li, L.; Cai, Z. Dexmedetomidine regulates the anti-oxidation and autophagy of adipose-derived stromal cells under H2O2-induced oxidative stress through Nrf2/p62 pathway and improves the retention rate of autologous fat transplantation. Front. Pharmacol. 2024, 15, 1453938. [Google Scholar] [CrossRef]
- Li, Z.; Qi, J.; Fu, S.; Luan, J.; Wang, Q. Effects of nanographene oxide on adipose-derived stem cell cryopreservation. Cell Tissue Bank. 2024, 25, 805–830. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Dong, Z.; Liang, J.; Li, S.; Han, W.; Cui, T.; Liu, H. Glutathione supplementation improves fat graft survival by inhibiting ferroptosis via the SLC7A11/GPX4 axis. Stem Cell Res. Ther. 2024, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, G.; Jin, W.; Wu, H.; Liu, N.; Zhen, Y.; An, Y. Poloxamer 188 washing of lipoaspirate improves fat graft survival: A comparative study in nude mice. J. Plast. Reconstr. Aesthet. Surg. 2024, 95, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Loder, S.; Wang, S.; Amurgis, C.; DeSanto, M.; Stavros, A.G.; Patadji, S.; Olevian, D.; Lee, P.; Guerrero, D.; Gusenoff, J.A.; et al. Active Vitamin D3 (Calcitriol) Increases Adipose Graft Retention in a Xenograft Model. Aesthet. Surg. J. 2023, 43, NP449–NP465. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, J.; Gao, J.; Ogawa, R.; Ou, C.; Yang, B.; Fu, B. Improvement of the Survival of Human Autologous Fat Transplantation by Using VEGF-Transfected Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2009, 124, 1437–1446. [Google Scholar] [CrossRef]
- Luan, A.; Zielins, E.R.; Wearda, T.; Atashroo, D.A.; Blackshear, C.P.; Raphel, J.; Brett, E.A.; Flacco, J.; Alyono, M.C.; Momeni, A.; et al. Dynamic Rheology for the Prediction of Surgical Outcomes in Autologous Fat Grafting. Plast. Reconstr. Surg. 2017, 140, 517–524. [Google Scholar] [CrossRef]
- Luo, X.; Cao, W.; Xu, H.; Wang, L.; Zhang, Z.; Lu, Y.; Jin, X.; Ren, X.; He, J.; Fu, M.; et al. Coimplanted Endothelial Cells Improve Adipose Tissue Grafts’ Survival by Increasing Vascularization. J. Craniofac. Surg. 2015, 26, 358–364. [Google Scholar] [CrossRef]
- Lv, T.; Gu, Y.; Bi, J.; Kang, N.; Yang, Z.; Fu, X.; Wang, Q.; Yan, L.; Liu, X.; Cao, Y.; et al. Fructose 1,6-Bisphosphate as a Protective Agent for Experimental Fat Grafting. Stem Cells Transl. Med. 2019, 8, 606–616. [Google Scholar] [CrossRef]
- Major, G.; Longoni, A.; Simcock, J.; Magon, N.J.; Harte, J.; Bathish, B.; Kemp, R.; Woodfield, T.; Lim, K.S. Clinical Applicability of Visible Light-Mediated Cross-linking for Structural Soft Tissue Reconstruction. Adv. Sci. 2023, 10, 2300538. [Google Scholar] [CrossRef]
- Martin-Ferrer, S. Failure of Autologous Fat Grafts to Prevent Postoperative Epidural Fibrosis in Surgery of the Lumbar Spine. Neurosurgery 1989, 24, 718–721. [Google Scholar] [CrossRef]
- Massiah, G.; De Palma, G.; Negri, A.; Mele, F.; Loisi, D.; Paradiso, A.V.; Ressa, C.M. Cryopreservation of adipose tissue with and without cryoprotective agent addition for breast lipofilling: A cytological and histological study. Cryobiology 2021, 103, 141–146. [Google Scholar] [CrossRef]
- Mecott, G.A.; Gonzalez-Cantu, C.M.; Moreno-Peña, P.J.; Flores, P.P.; Castro-Govea, Y.; De Oca-Luna, R.M.; Perez-Trujillo, J.J.; Garcia-Perez, M.M. Effect of Diameter and Fenestration Area of the Liposuction Cannula on the Viability of the Adipocytes. Aesthetic Plast. Surg. 2022, 46, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Nguyen, J.T.; McCormack, M.M.; Randolph, M.A.; Austen, W.G. A high-throughput model for fat graft assessment. Lasers Surg. Med. 2009, 41, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Nguyen, J.T.; Kirkham, J.C.; Lee, J.H.; McCormack, M.C.; Randolph, M.A.; Austen, W.G. Polymer Therapy: A Novel Treatment to Improve Fat Graft Viability. Plast. Reconstr. Surg. 2011, 127, 2270–2282. [Google Scholar] [CrossRef] [PubMed]
- Minn, K.-W.; Min, K.-H.; Chang, H.; Kim, S.; Heo, E.-J. Effects of Fat Preparation Methods on the Viabilities of Autologous Fat Grafts. Aesthetic Plast. Surg. 2010, 34, 626–631. [Google Scholar] [CrossRef]
- Mojallal, A.; Lequeux, C.; Shipkov, C.; Rifkin, L.; Rohrich, R.; Duclos, A.; Brown, S.; Damour, O. Stem Cells, Mature Adipocytes, and Extracellular Scaffold: What Does Each Contribute to Fat Graft Survival? Aesthetic Plast. Surg. 2011, 35, 1061–1072. [Google Scholar] [CrossRef]
- Nelissen, X.; Licciardi, S.; Nizet, C.; Delay, E.; Roche, R. Comparative Analysis of a New Automatic System and Four Existing Techniques for Autologous Fat Grafting. Plast. Reconstr. Surg.-Glob. Open 2023, 11, e5349. [Google Scholar] [CrossRef]
- Nguyen, P.S.A.; Desouches, C.; Gay, A.M.; Hautier, A.; Magalon, G. Development of micro-injection as an innovative autologous fat graft technique: The use of adipose tissue as dermal filler. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1692–1699. [Google Scholar] [CrossRef]
- Nicoli, F.; Chilgar, R.M.; Sapountzis, S.; Lazzeri, D.; Sze Wei, M.Y.; Ciudad, P.; Nicoli, M.; Lim, S.Y.; Chen, P.-Y.; Constantinides, J.; et al. Lymphedema Fat Graft: An Ideal Filler for Facial Rejuvenation. Arch. Plast. Surg. 2014, 41, 588–593. [Google Scholar] [CrossRef][Green Version]
- Nie, F.; Ding, P.; Zhang, C.; Zhao, Z.; Bi, H. Extracellular vesicles derived from lipoaspirate fluid promote fat graft survival. Adipocyte 2021, 10, 293–309. [Google Scholar] [CrossRef]
- Nie, M.; Tian, Y.; Xiao, Y.; Lei, S.; Wu, D. Enhancing high-quality fat survival: A novel strategy using cell-free fat extract. FASEB J. 2024, 38, e23733. [Google Scholar] [CrossRef]
- Niechajev, I.; Śevćuk, O. Long-Term Results of Fat Transplantation: Clinical and Histologic Studies. Plast. Reconstr. Surg. 1994, 94, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Niţă, A.C.; Jianu, D.M.; Florescu, I.P.; Filipescu, M.; Cobani, O.; Jianu, S.A.; Chiriţă, D.A.; Bold, A. The synergy between lasers and adipose tissues surgery in cervicofacial rejuvenation: Histopathological aspects. Rom. J. Morphol Embryol 2013, 54, 1039–1043. [Google Scholar] [PubMed]
- Oh, D.S.; Cheon, Y.W.; Jeon, Y.R.; Lew, D.H. Activated Platelet-Rich Plasma Improves Fat Graft Survival in Nude Mice: A Pilot Study. Dermatol. Surg. 2011, 37, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Olenczak, J.B.; Seaman, S.A.; Lin, K.Y.; Pineros-Fernandez, A.; Davis, C.E.; Salopek, L.S.; Peirce, S.M.; Cottler, P.S. Effects of Collagenase Digestion and Stromal Vascular Fraction Supplementation on Volume Retention of Fat Grafts. Ann. Plast. Surg. 2017, 78, S335–S342. [Google Scholar] [CrossRef]
- Paik, K.J.; Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Luan, A.; Walmsley, G.G.; Momeni, A.; Vistnes, S.; Gurtner, G.C.; et al. Studies in Fat Grafting: Part V. Cell-Assisted Lipotransfer to Enhance Fat Graft Retention Is Dose Dependent. Plast. Reconstr. Surg. 2015, 136, 67–75. [Google Scholar] [CrossRef]
- Palumbo, P.; Miconi, G.; Cinque, B.; La Torre, C.; Lombardi, F.; Zoccali, G.; Orsini, G.; Leocata, P.; Giuliani, M.; Cifone, M.G. In Vitro Evaluation of Different Methods of Handling Human Liposuction Aspirate and Their Effect on Adipocytes and Adipose Derived Stem Cells. J. Cell. Physiol. 2015, 230, 1974–1981. [Google Scholar] [CrossRef]
- Park, E.; Kim, H.; Kim, M.; Oh, H. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J. Cosmet. Dermatol. 2013, 12, 240–243. [Google Scholar] [CrossRef]
- Park, T.H.; Choi, W.Y.; Lee, J.H.; Lee, W.J. Micronized Cross-Linked Human Acellular Dermal Matrices: An Effective Scaffold for Collagen Synthesis and Promising Material for Tissue Augmentation. Tissue Eng. Regen. Med. 2017, 14, 517–523. [Google Scholar] [CrossRef]
- Pelosi, M.; Testet, E.; Le Lay, S.; Dugail, I.; Tang, X.; Mabilleau, G.; Hamel, Y.; Madrange, M.; Blanc, T.; Odent, T.; et al. Normal human adipose tissue functions and differentiation in patients with biallelic LPIN1 inactivating mutations. J. Lipid Res. 2017, 58, 2348–2364. [Google Scholar] [CrossRef]
- Philips, B.J.; Grahovac, T.L.; Valentin, J.E.; Chung, C.W.; Bliley, J.M.; Pfeifer, M.E.; Roy, S.B.; Dreifuss, S.; Kelmendi-Doko, A.; Kling, R.E.; et al. Prevalence of Endogenous CD34+ Adipose Stem Cells Predicts Human Fat Graft Retention in a Xenograft Model. Plast. Reconstr. Surg. 2013, 132, 845–858. [Google Scholar] [CrossRef]
- Por, Y.-C.; Yeow, V.K.-L.; Louri, N.; Lim, T.K.-H.; Kee, I.; Song, I.-C. Platelet-rich plasma has no effect on increasing free fat graft survival in the nude mouse. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.L.Q.; Coleman, S.R.; Cui, X.; Ferguson, R.E.H.; Vasconez, H.C. Autologous Fat Grafts Harvested and Refined by the Coleman Technique: A Comparative Study. Plast. Reconstr. Surg. 2008, 122, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.L.Q.; Coleman, S.R.; Cui, X.; Ferguson, R.E.H.; Vasconez, H.C. Cryopreservation of Autologous Fat Grafts Harvested with the Coleman Technique. Ann. Plast. Surg. 2010, 64, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Viganò, M.; Torretta, E.; Perucca Orfei, C.; Colombini, A.; Tremolada, C.; Gelfi, C.; De Girolamo, L. Characterization of Microfragmented Adipose Tissue Architecture, Mesenchymal Stromal Cell Content and Release of Paracrine Mediators. J. Clin. Med. 2022, 11, 2231. [Google Scholar] [CrossRef]
- Ramon, Y.; Shoshani, O.; Peled, I.J.; Gilhar, A.; Carmi, N.; Fodor, L.; Risin, Y.; Ullmann, Y. Enhancing the Take of Injected Adipose Tissue by a Simple Method for Concentrating Fat Cells. Plast. Reconstr. Surg. 2005, 115, 197–201. [Google Scholar] [CrossRef]
- Reddy, R.; Iyer, S.; Sharma, M.; Vijayaraghavan, S.; Kishore, P.; Mathew, J.; Unni, A.K.K.; Reshmi, P.; Sharma, R.; Prasad, C. Effect of external volume expansion on the survival of fat grafts. Indian J. Plast. Surg. 2016, 49, 151–158. [Google Scholar] [CrossRef]
- Sesé, B.; Sanmartín, J.M.; Ortega, B.; Llull, R. Human Stromal Cell Aggregates Concentrate Adipose Tissue Constitutive Cell Population by In Vitro DNA Quantification Analysis. Plast. Reconstr. Surg. 2020, 146, 1285–1293. [Google Scholar] [CrossRef]
- Sheng, L.; Yu, Z.; Li, S.; Cao, W. Long-term volume retention after fat processing with cotton gauze rolling and centrifugation: A comparative study in nude mice. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 4290–4296. [Google Scholar] [CrossRef]
- Shoshani, O.; Shupak, A.; Ullmann, Y.; Ramon, Y.; Gilhar, A.; Kehat, I.; Peled, I.J. The Effect of Hyperbaric Oxygenation on the Viability of Human Fat Injected into Nude Mice. Plast. Reconstr. Surg. 2000, 106, 1390–1396. [Google Scholar] [CrossRef]
- Shoshani, O.; Ullmann, Y.; Shupak, A.; Ramon, Y.; Gilhar, A.; Kehat, I.; Peled, I.J. The Role of Frozen Storage in Preserving Adipose Tissue Obtained by Suction-Assisted Lipectomy for Repeated Fat Injection Procedures. Dermatol. Surg. 2001, 27, 645–647. [Google Scholar] [CrossRef]
- Shoshani, O.; Livne, E.; Armoni, M.; Shupak, A.; Berger, J.; Ramon, Y.; Fodor, L.; Gilhar, A.; Peled, I.J.; Ullmann, Y. The Effect of Interleukin-8 on the Viability of Injected Adipose Tissue in Nude Mice. Plast. Reconstr. Surg. 2005, 115, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Skorobac Asanin, V.; Sopta, J. Lower Leg Augmentation with Fat Grafting, MRI and Histological Examination. Aesthetic Plast. Surg. 2017, 41, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Adams, W.P.; Lipschitz, A.H.; Chau, B.; Sorokin, E.; Rohrich, R.J.; Brown, S.A. Autologous Human Fat Grafting: Effect of Harvesting and Preparation Techniques on Adipocyte Graft Survival. Plast. Reconstr. Surg. 2006, 117, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-M. Salvianolic Acid B Reduces the Inflammation of Fat Grafts by Inhibiting the NF-Kb Signalling Pathway in Macrophages. Aesthetic Surg. J. 2023, 43, NP372–NP390. [Google Scholar] [CrossRef]
- Szychta, P.; Kuczynski, M.; Dzieniecka, M. Histological Properties of Adipose Tissue as an Autologous Tissue Filler Harvested from Different Donor Areas and Impact of Centrifugation. Plast. Reconstr. Surg.-Glob. Open 2024, 12, e5912. [Google Scholar] [CrossRef]
- Teng, S.-C.; Li, L.-T.; Chen, S.-G.; Chen, T.-M.; Liao, C.-H.; Fang, H.-W. Dose-Dependent Effects of Adipose Tissue-Derived Stromal Vascular Fraction Cells on Angiogenesis and Fibrosis in Human Fat Grafts. Biomed. Eng. Appl. Basis Commun. 2014, 26, 1450045. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, P.; Wang, Y.; Geng, C.; Han, X.; Ma, J.; Li, F.; Cai, L. The Effect of Liposuction Cannula Diameter on Fat Retention—Based on a Rheological Simulation. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e2021. [Google Scholar] [CrossRef]
- Ullmann, Y.; Shoshani, O.; Fodor, A.; Ramon, Y.; Carmi, N.; Eldor, L.; Gilhar, A. Searching for the Favorable Donor Site for Fat Injection: In Vivo Study Using the Nude Mice Model. Dermatol. Surg. 2006, 31, 1304–1307. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Gostelie, O.F.E.; Vonk, L.A.; De Bruijn, J.J.; Van Der Lei, B.; Harmsen, M.C.; Stevens, H.P. Fractionation of Adipose Tissue Procedure with a Disposable One-Hole Fractionator. Aesthet. Surg. J. 2020, 40, NP194–NP201. [Google Scholar] [CrossRef]
- Von Heimburg, D.; Pallua, N. Two-Year Histological Outcome of Facial Lipofilling. Ann. Plast. Surg. 2001, 46, 644–646. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Xiong, L.; Yang, J. Influence of Repeated Aspiration on Viability of Fat Grafts: A Comparative Study. Aesthet. Surg. J. 2015, 35, NP248–NP260. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, W.; Gundogan, B.; Moscoso, A.V.; Orgill, D.P.; Giatsidis, G. Delayed Postconditioning with External Volume Expansion Improves Survival of Adipose Tissue Grafts in a Murine Model. Plast. Reconstr. Surg. 2019, 143, 99e–110e. [Google Scholar] [CrossRef] [PubMed]
- Weisz, G.M.; Gal, A. Long-term Survival of a Free Fat Graft in the Spinal Canal: A 40-Month Postlaminectomy Case Report. Clin. Orthop. 1986, 205, 204–206. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Wang, Z.; Feng, J.; Wang, J.; Xiao, X.; Lu, F.; Dong, Z. Botulinum Toxin A Improves Supramuscular Fat Graft Retention by Enhancing Angiogenesis and Adipogenesis. Dermatol. Surg. 2020, 46, 646–652. [Google Scholar] [CrossRef]
- Wu, W.; Bi, X.; Zhao, J.; Lin, Z.; Lu, F.; Dong, Z.; Li, Y. Ultra-condensed Fat: A Novel Fat Product for Volume Augmentation. Aesthetic Plast. Surg. 2023, 47, 2074–2083. [Google Scholar] [CrossRef]
- Xia, J.; Zhu, H.; Zhu, S.; Ge, J.; Wang, Z.; Lu, F.; Liao, Y.; Cai, J. Induced Beige Adipocytes Improved Fat Graft Retention by Promoting Adipogenesis and Angiogenesis. Plast. Reconstr. Surg. 2021, 148, 549–558. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, D.; Li, Q.; Chen, Y.; Lei, H.; Pu, L.L.Q. The effect of centrifugation on viability of fat grafts: An evaluation with the glucose transport test. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 482–487. [Google Scholar] [CrossRef]
- Xiong, B.-J.; Tan, Q.-W.; Chen, Y.-J.; Zhang, Y.; Zhang, D.; Tang, S.-L.; Zhang, S.; Lv, Q. The Effects of Platelet-Rich Plasma and Adipose-Derived Stem Cells on Neovascularization and Fat Graft Survival. Aesthetic Plast. Surg. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Xu, F.; Li, H.; Yin, Q.-S.; Liu, D.; Nan, H.; Zhao, P.; Liang, S. Human Breast Adipose-Derived Stem Cells Transfected with the Stromal Cell-Derived Factor-1 Receptor CXCR4 Exhibit Enhanced Viability in Human Autologous Free Fat Grafts. Cell. Physiol. Biochem. 2014, 34, 2091–2104. [Google Scholar] [CrossRef]
- Yagima Odo, M.E.; Cucé, L.C.; Odo, L.M.; Natrielli, A. Action of Sodium Deoxycholate on Subcutaneous Human Tissue: Local and Systemic Effects. Dermatol. Surg. 2007, 33, 178–189. [Google Scholar] [CrossRef]
- Yang, X.; Brower, J.P.; Kokai, L.; Gusenoff, B.R.; Gusenoff, J.A. A New Device for Autologous Small Volume Fat Grafting. Aesthet. Surg. J. 2021, 41, NP1686–NP1694. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Z.; Cai, Z.; He, Y.; Ke, C.; Wang, J.; Lin, M.; Li, L. Pluronic F-127 Hydrogel Loaded with Human Adipose-Derived Stem Cell-Derived Exosomes Improve Fat Graft Survival via HIF-1α-Mediated Enhancement of Angiogenesis. Int. J. Nanomedicine 2023, 18, 6781–6796. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, H.; Gao, Q.; Yuan, X.; Hu, Y.; Qi, Z. Enhancing Fat Transplantation Efficiency in a Mouse Model through Pretreatment of Adipose-Derived Stem Cells with RIP3 Inhibitors. Aesthetic Plast. Surg. 2024, 48, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Pan, Y.; Zhen, Y.; Zhang, L.; Zhang, X.; Shu, M.; Han, Y.; Guo, S. Enhancement of Viability of Fat Grafts in Nude Mice by Endothelial Progenitor Cells. Dermatol. Surg. 2006, 32, 1437–1443. [Google Scholar] [CrossRef]
- Yi, C.G.; Xia, W.; Zhang, L.X.; Zhen, Y.; Shu, M.G.; Han, Y.; Guo, S.Z. VEGF gene therapy for the survival of transplanted fat tissue in nude mice. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 272–278. [Google Scholar] [CrossRef]
- Yu, Q.; Cai, Y.; Huang, H.; Wang, Z.; Xu, P.; Wang, X.; Zhang, L.; Zhang, W.; Li, W. Co-Transplantation of Nanofat Enhances Neovascularization and Fat Graft Survival in Nude Mice. Aesthet. Surg. J. 2018, 38, 667–675. [Google Scholar] [CrossRef]
- Yu, P.; Zhai, Z.; Lu, H.; Jin, X.; Yang, X.; Qi, Z. Platelet-Rich Fibrin Improves Fat Graft Survival Possibly by Promoting Angiogenesis and Adipogenesis, Inhibiting Apoptosis, and Regulating Collagen Production. Aesthet. Surg. J. 2020, 40, NP530–NP545. [Google Scholar] [CrossRef]
- Yu, P.; Yang, X.; Zhai, Z.; Gao, Q.; Yang, Z.; Qi, Z. Long-Term Effects of Platelet-Rich Fibrin on Fat Graft Survival and Their Optimal Mixing Ratio. Aesthet. Surg. J. 2021, 41, NP921–NP934. [Google Scholar] [CrossRef]
- Zhan, W.; Tan, S.S.; Han, X.; Palmer, J.A.; Mitchell, G.M.; Morrison, W.A. Indomethacin Enhances Fat Graft Retention by Up-Regulating Adipogenic Genes and Reducing Inflammation. Plast. Reconstr. Surg. 2017, 139, 1093e–1104e. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Zhou, T.; Yao, Y.; Dong, Z.; Lu, F. Improved Long-Term Volume Retention of Stromal Vascular Fraction Gel Grafting with Enhanced Angiogenesis and Adipogenesis. Plast. Reconstr. Surg. 2018, 141, 676e–686e. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, B.; Wang, L.; Fu, Z.; Hu, J.; Liu, Y.; Wang, J.; He, Y. Rapid printing of 3D porous scaffolds for breast reconstruction. Bio-Des. Manuf. 2023, 6, 691–703. [Google Scholar] [CrossRef]
- Zhu, H.; Quan, Y.; Wang, J.; Jiang, S.; Lu, F.; Cai, J.; Liao, Y. Improving Low-Density Fat by Condensing Cellular and Collagen Content through a Mechanical Process: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2021, 148, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, R. Phloxine As An Histologic Stain, Especially In Combination with Hematoxylin. Stain Technol. 1947, 22, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Ceccopieri, C.; Skonieczna, J.; Madej, J.P. Modification of a haematoxylin, eosin, and natural saffron staining method for the detection of connective tissue. J. Vet. Res. 2021, 65, 125–130. [Google Scholar] [CrossRef]
- Valls, A.A.; Cosio, M.G. Periodic Acid Schiff—Hematoxylin, Phloxine, Saffron: A New Multi-Purpose Stain. J. Histotechnol. 1979, 2, 104–105. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. Microscopy: Light Microscopy and Histochemical Methods. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 715–723. ISBN 978-0-12-384953-3. [Google Scholar]
- Read, B.E. Sudan III|C22H16N4O|CID 62331-PubChem. Science 1918, 47, 562–563. [Google Scholar] [CrossRef]
- Sikkeland, J.; Jin, Y.; Saatcioglu, F. Methods to Assess Lipid Accumulation in Cancer Cells. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 542, pp. 407–423. ISBN 978-0-12-416618-9. [Google Scholar]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Péault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010, 77, 22–30. [Google Scholar] [CrossRef]
- Taqi, S.; Sami, S.; Sami, L.; Zaki, S. A review of artifacts in histopathology. J. Oral Maxillofac. Pathol. 2018, 22, 279. [Google Scholar] [CrossRef]
- Surmi, B.; Hasty, A. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef]
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef]
- Hamann, J.; Koning, N.; Pouwels, W.; Ulfman, L.H.; van Eijk, M.; Stacey, M.; Lin, H.; Gordon, S.; Kwakkenbos, M.J. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur. J. Immunol. 2007, 37, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, I.; Aso, H.; Yamaguchi, T.; Ozutsumi, K. Adipose tissue extracellular matrix: Newly organized by adipocytes during differentiation. Differentiation 1998, 63, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.J.A.; Mostaço-Guidolin, L.B. Optical Microscopy and the Extracellular Matrix Structure: A Review. Cells 2021, 10, 1760. [Google Scholar] [CrossRef]
- Rickelt, S.; Hynes, R.O. Antibodies and methods for immunohistochemistry of extracellular matrix proteins. Matrix Biol. 2018, 71, 10–27. [Google Scholar] [CrossRef]
- Börgeson, E.; Boucher, J.; Hagberg, C.E. Of mice and men: Pinpointing species differences in adipose tissue biology. Front. Cell Dev. Biol. 2022, 10, 1003118. [Google Scholar] [CrossRef]
- Bicer, A. Nanofat Grafts Enhance Tendon Healing in a Chronic Achilles Tendinopathy Rat Model. Sports Traumatol. Arthrosc. 2024, 1, 3–13. [Google Scholar] [CrossRef]
- Iyyanki, T.; Hubenak, J.; Liu, J.; Chang, E.I.; Beahm, E.K.; Zhang, Q. Harvesting Technique Affects Adipose-Derived Stem Cell Yield. Aesthet. Surg. J. 2015, 35, 467–476. [Google Scholar] [CrossRef]
- Schreml, S.; Babilas, P.; Fruth, S.; Orsó, E.; Schmitz, G.; Mueller, M.B.; Nerlich, M.; Prantl, L. Harvesting human adipose tissue-derived adult stem cells: Resection versus liposuction. Cytotherapy 2009, 11, 947–957. [Google Scholar] [CrossRef]
- Prantl, L.; Schreml, J.; Gehmert, S.; Klein, S.; Bai, X.; Zeitler, K.; Schreml, S.; Alt, E.; Gehmert, S.; Felthaus, O. Transcription Profile in Sporadic Multiple Symmetric Lipomatosis Reveals Differential Expression at the Level of Adipose Tissue–Derived Stem Cells. Plast. Reconstr. Surg. 2016, 137, 1181–1190. [Google Scholar] [CrossRef]
- Huang, T.; He, D.; Kleiner, G.; Kuluz, J.T. Neuron-like Differentiation of Adipose-Derived Stem Cells from Infant Piglets in Vitro. J. Spinal Cord Med. 2007, 30, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Suartz, C.V.; Gaiba, S.; França, J.P.D.; Aloise, A.C.; Ferreira, L.M. Adipose-derived stem cells (ADSC) in the viability of a random pattern dorsal skin flap in rats. Acta Cir. Bras. 2014, 29, 02–05. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Kotlyarova, A. Involvement of Fatty Acids and Their Metabolites in the Development of Inflammation in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1308. [Google Scholar] [CrossRef]
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Study Type | In vitro and in vivo studies with histology of fat tissue | Case reports, editorials, commentaries, reviews without original data, systematic reviews, or meta-analyses |
| Population | All human fat studies including animals transplanted with human fat | All animal fat studies |
| Intervention | Whole tissue fat | Studies focusing only on stem cells of the fat (SVF) and their histology or complex manipulated fat |
| Publication Date | All studies available (1972–2024) | - |
| Language | Published in English | Non-English language studies, unless translated versions are available |
| Peer-reviewed | Studies must be published in peer-reviewed journals | Non-peer-reviewed articles, conference abstracts, or unpublished theses |
| Autor/Year of Publication | Tissue | Analyzed Attributes | Staining Methods |
|---|---|---|---|
| Adanali et al., 2002 [28] | C | MAs | Sudan Black |
| Adem et al., 2022 [29] | S | V, I, Cy, Fi | HE, Anti-CD31 |
| Afanas’eva et al., 2018 [30] | - | MAs, V | HE |
| Agostini et al., 2012 [31] | C | MAs, N, Fi | HE, Sudan Black |
| Ansorge et al., 2014 [32] | C, S, F | MAs, V, I, Cy, Fi | HE |
| Atanassova et al., 2001 [33] | B | MAs, SVFCs | Sudan III, Anti-S-100 (S-100-protein) |
| Bach-Mortensen et al., 1976 [34] | B | MAs, V, I, Fi | Toluidine blue |
| Bae et al., 2015 [35] | C, Sc | MAs, V, I, Fi | HE, Anti-CD31 |
| Baker et al., 2009 [36] | F | MAs, V | Anti-CD31 |
| Bauer et al., 1995 [37] | B | MAs, V, I, Fi | HE |
| Bellas et al., 2013 [38] | Sc | MAs, V, I | HE, MT, Oil O Red |
| Bi et al., 2024 [39] | C | MAs, V, I, Cy, N, Fi | HE, MT, Anti-perilipin, Anti-CD31, Anti-CD206, Anti-MAC2 (Galectin-3) |
| Borrelli et al., 2020 [40] | S, Sc | MAs, V, I, Cy, N, Fi | HE, MT, Anti-perilipin, Anti-CD31, Anti-F4/80 (EGF-like module containing, mucin-like, hormone receptor-like sequence 1) |
| Bryant et al., 1983 [41] | B | MAs, V, Fi | HE |
| Chai et al., 2023 [42] | - | MAs, V, I, Cy, N, Fi | HE, Anti-perilipin, Anti-CD31, Anti-Ki67 (Marker of Proliferation Ki-67) |
| Chajchir and Benzaquen et al., 1989 [43] | - | MAs, V, Cy, Fi | HE |
| Chen et al., 2021 [44] | - | MAs, V, I, Cy, Fi | HE, Anti-CD34, Anti-Ki67, Anti-VEGF (Vascular Endothelial Growth Factor) |
| Chen et al., 2022 [45] | S, F | MAs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-CD31 |
| Chen et al., 2022 [46] | Sc | MAs, V | HE, Anti-CD31 |
| Chen et al., 2024 [47] | F | MAs, I, Cy, Fi | HE, Anti-perilipin, Anti-caspase3 |
| Chia et al., 2015 [48] | C, F | MAs, N | HE |
| Chung et al., 2019 [49] | C | MAs, V, I, Cy, Fi | HE, Anti-CD31 |
| Cicione et al., 2016 [50] | C | MAs, SVFCs | HE, Sudan III |
| Cicione et al., 2023 [51] | M, - | MAs, SVFCs | HE, Anti-PCNA (Proliferating Cell Nuclear Antigen), Anti-CD31, Anti-CD34, Anti-COLL1 (collagen) |
| Condé-Green et al., 2010 [24] | C, S | MAs, N | HE, PAS-Reaction |
| Condé-Green et al., 2010 [23] | C, S | MAs | HE, PAS-Reaction |
| Condé-Green et al., 2013 [52] | C, S, Sc | MAs, V, I, Cy, N, Fi | HE |
| Craft et al., 2009 [53] | M | MAs, I, Cy, Fi | MT, Anti-S-100 |
| Cui and Pu et al., 2009 [54] | C | MAs, N | HE |
| Cui and Pu et al., 2010 [55] | C | MAs, N, Fi | HE |
| Davis et al., 2013 [56] | C | MAs, Fi | HE |
| Debald et al., 2017 [57] | F | V, N, Fi | HE |
| Deleon et al., 2021 [58] | S | MAs, V, I, Cy, Fi | HE, Anti-CD31, Anti-perilipin |
| Dimitroulis et al., 2011 [59] | - | MAs, Cy, N | HE |
| Dobran et al., 2017 [60] | - | Fi | HE |
| Dong et al., 2022 [61] | Sc, G | MAs, V, I, N, Fi | HE, Anti-CD31, Anti-perilipin, Anti-STEM 121 (Microtubule-associated protein 1 light chain 3 alpha) |
| Eigenberger et al., 2022 [20] | C, S, M | MAs, SVFCs, V | HE |
| Eskalen et al., 2024 [62] | C | MAs | HE, Anti-perilipin |
| Fan et al., 2023 [63] | C, M | MAs, V, Fi | HE, Anti-perilipin, Anti-CD31 |
| Ferguson et al., 2008 [64] | C, F | MAs, N | HE |
| Filson et al., 2016 [65] | C | V, I, Cy, Fi | HE |
| Fisher et al., 2013 [66] | C, F, G | V | Anti-CD31 |
| Genç et al., 2022 [9] | S | MAs, I, Cy, N, Fi | HE, MT, Anti-perilipin |
| Girard et al., 2015 [67] | C, S | MAs, V, Cy, N, Fi | HE, MT |
| Ha et al., 2015 [68] | C, Sc | Cy | HE |
| Hamed et al., 2010 [69] | - | MAs, V, I, Cy, Fi | HE, Anti-CD31, Anti-CD68, Anti-VEGF, Anti-EPOR (erythropoietin receptor) |
| Hamed et al., 2012 [70] | Sc, - | MAs, V, I, Cy, Fi | HE, Mayer’s hematoxylin |
| Harris et al., 2019 [71] | Sc, - | MAs, V, I, Cy, N, Fi | HE, Anti-CD31 |
| He et al., 2019 [72] | Sc, - | MAs, V, I, Fi | HE, Anti-CD31, Anti-MAC2, Sirius Red |
| He et al., 2023 [73] | C, M | MAs, V, Cy | HE, Anti-CD31, Anti-Ki67 |
| Herly et al., 2017 [74] | - | MAs, I, Cy, N, Fi | HE, van Gieson, Anti-CD68, Anti-PGM1 (phosphoglucomutase 1), Anti-S-100 |
| Hersant et al., 2018 [75] | C | MAs, V, I, Fi | HE, Anti-perilipin, Anti-CD31, Anti-CD45 |
| Hivernaud et al., 2017 [76] | C, S, F | I, Cy, Fi | HPS |
| Ho et al., 2022 [77] | C | MAs, I, Cy, Fi | HE, Anti-perilipin, Anti-F4/80, Anti-caspase3 |
| Hoareau et al., 2013 [78] | C, S | MAs, Fi | HES, MT |
| Hsiao et al., 2021 [79] | C | MAs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-CD31, Anti-αSMA (Alpha-Smooth Muscle Actin) |
| Hu et al., 2018 [80] | C | MAs, V, Cy, Fi | HE, Anti-CD31 |
| Huang et al., 2017 [81] | S, Sc | MAs, V, Cy, Fi | HE, MT, Anti-CD31 |
| Ichikawa et al., 2005 [82] | B | MAs, N | HE |
| Janarthanan et al., 2023 [83] | C | MAs, V, I, Cy, N | HE, Anti-CD31, Anti-perilipin, Anti-vimentin |
| Jia et al., 2024 [8] | F | MAs, V, I, Cy, N, Fi | HE, Anti-perilipin, Anti-CD31 |
| Jiang et al., 2015 [84] | C, S, M, Sc, F, G, B, - | V, Fi | HE |
| Jiang et al., 2023 [85] | S | MAs, V, I, Cy, N, Fi | HE, MT, Anti-perilipin, Anti-CD31, Anti-CD206 |
| Jin et al., 2021 [86] | G | MAs, V, I, Cy, N, Fi | HE, Anti-perilipin, Anti-CD31 |
| Jung et al., 2014 [87] | C | MAs, V, I, Cy, Fi | HE, Anti-CD31 |
| Kakudo et al., 2013 [88] | Sc | MAs, V, I, Cy, Fi | HE, Anti-vWF (von Willebrand factor) |
| Kamel et al., 2014 [89] | C, F | MAs | HE |
| Kanamori et al., 2001 [90] | B | MAs, V, Cy, Fi | HE |
| Kelmendi-Doko, 2017 [91] | C | V | HE, Anti-CD31 |
| Kelmendi-Doko et al., 2014 [92] | C | V | HE, Anti-CD31 |
| Khater et al., 2009 [93] | C | MAs, SVFCs, V, I, Cy, N | HE, Anti-cyclin D1, Anti-leptin |
| Kijima et al., 2012 [94] | B | MAs, N, Fi | HE, Oil O Red |
| Kim et al., 2018 [95] | C | MAs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-VEGF |
| Kim et al., 2022 [96] | C | MAs, V | HE, Anti-CD31, Anti-perilipin |
| Kim et al., 2023 [97] | C | MAs, V, I, Cy, Fi | HE |
| Kim et al., 2024 [98] | C, Sc | MAs, V, I, Fi | HE, MT, Anti-perilipin, Anti-CD31 |
| Kirkham et al., 2012 [99] | C | MAs, I, Fi | HE |
| Ko et al., 2011 [100] | C, Sc | MAs, I, Cy, N, Fi | HE |
| Kokai et al., 2017 [101] | C | MAs, I, Cy, Fi | HE, MT, Anti-perilipin, Anti-F4/80, |
| Kuwahara et al., 2003 [102] | -, Laser | MAs, V | HE |
| Lee et al., 2013 [103] | C | MAs, I, Cy, Fi | HE |
| Lei and Xiao et al., 2020 [104] | C | MAs, V, Cy, N, Fi | HE |
| Li et al., 2012 [105] | C | MAs, I, Cy, Fi | HE |
| Li et al., 2013 [106] | C | MAs, V, I, Cy, Fi | HE |
| Li et al., 2014 [14] | Sc, - | MAs, V, I, N, Fi | HE, Anti-CD31 |
| Li et al., 2015 [107] | F | MAs, V, I, Cy, Fi | HE |
| Li et al., 2020 [108] | F | MAs, SVFCs, V, I, Cy, N, Fi | HE, Anti-perilipin, Anti-CD31, Anti-CD206 |
| Li et al., 2022 [109] | C | MAs, I, Cy | HE, Anti-perilipin |
| Li et al., 2024 [110] | C, Sc | SVFCs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-CD31, Anti-VEGF |
| Li et al., 2024 [111] | C, Sc | MAs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-CD31, Anti-vimentin, Anti-CD68 |
| Li et al., 2024 [112] | C | MAs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-vWF |
| Liu et al., 2024 [113] | G | MAs, V, I, Cy, N, Fi | HE, MT, Anti-perilipin, Anti-CD31 |
| Loder et al., 2023 [114] | C | MAs, V, I, Cy, Fi | HE, Anti-CD31, Anti-perilipin, Anti-HIF-1-α (Hypoxia Inducible Factor 1), Sirius Red |
| Lu et al., 2009 [115] | Sc, - | MAs, V, N, Fi | HE, Anti-CD31 |
| Luan et al., 2017 [116] | C | I, Cy, Fi | HE |
| Luo et al., 2015 [117] | Sc, - | MAs, V, Cy, Fi | HE, Anti-CD31, Anti-vWF, Anti-VEGF |
| Lv et al., 2019 [118] | C | MAs, V, I, N | HE, Anti-perilipin, Anti-vWF, Anti-CD68 |
| Major et al., 2023 [119] | - | MAs, V, Cy, Fi | HE, Anti-perilipin, Anti-CD31 |
| Martin-Ferrer et al., 1989 [120] | - | Fi | HE |
| Massiah et al., 2021 [121] | C | MAs, V | HE, Anti-perilipin, Anti-Ki67 |
| Mecott et al., 2022 [122] | - | MAs | Fluorescence microscopy |
| Medina et al., 2009 [123] | C | MAs, I, Cy, Fi | HE |
| Medina et al., 2011 [124] | C | I, Cy, Fi | HE |
| Merrifield et al., 2018 [11] | C | MAs, SVFCs, V, I, Cy | HE, Anti-perilipin, Anti-CD34, Anti-CD24, Anti-CD68, Anti-Ki67 |
| Minn et al., 2010 [125] | C, F, G | V, I, N | HE |
| Mojallal et al., 2011 [126] | C, Sc | MAs, V, Cy, Fi | HPS, Oil O Red, Anti-vimentin |
| Nelissen et al., 2023 [127] | C, S | MAs, I, Cy, Fi | HE, MT |
| Nguyen et al., 2012 [128] | C | MAs, V, N | HES, Anti-CD31 |
| Nicoli et al., 2014 [129] | S | MAs | HE, MT |
| Nie et al., 2023 [130] | - | MAs, I, Cy, Fi | HE, MT, Anti-perilipin |
| Nie et al., 2024 [131] | C | MAs, V, I, N | HE, Anti-perilipin, Anti-CD31, Anti-CD206, Anti-Ki67, Anti-CD86 |
| Niechajev and Śevćuk et al., 1994 [132] | F | MAs, I, Fi | HE |
| Niță et al., 2013 [133] | C | MAs, SVFCs, V, I, Cy, Fi | HE, Anti-DLK1(delta-like 1 homolog) |
| Oh et al., 2011 [134] | C | MAs, V, I, Cy, Fi | HE, Anti-CD31 |
| Olenczak et al., 2017 [135] | C, Sc | MAs, I, Cy, Fi | HE |
| Paik et al., 2015 [136] | C, Sc | MAs, V, I, Cy, Fi | HE, Anti-CD31 |
| Palumbo et al., 2015 [137] | C, S | MAs | HE, Anti-vimentin |
| Park et al., 2013 [138] | B | I, Cy, N, Fi | HE |
| Park et al., 2017 [139] | C, Sc | V, I, Cy, N, Fi | HE, MT |
| Pelosi et al., 2017 [140] | B | MAs, V | Anti-perilipin |
| Philips et al., 2013 [141] | C | MAs, V, Cy, Fi | HE, Anti-CD31 |
| Por et al., 2009 [142] | C | MAs, V, I, Cy, N, Fi | Oil O Red |
| Pu et al., 2008 [143] | C | MAs, N | HE |
| Pu et al., 2010 [144] | C | MAs, N | HE |
| Ragni et al., 2022 [145] | C, M | SVFCs, V | HE, Anti-CD31, Anti-CD90, Anti-CD146 |
| Ramon et al., 2005 [146] | C, F | MAs, V, I, Cy, Fi | HE |
| Reddy et al., 2016 [147] | C | MAs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-CD31, Anti-vimentin |
| Säljö et al., 2020 [10] | M, Sc | MAs, SVFCs, V, Fi | HE, Anti-CD31, Anti-CD90 |
| Sesé et al., 2020 [148] | C, M | MAs, SVFCs | HE, MT |
| Sheng et al., 2022 [149] | C, G | MAs, V, I, Cy, N, Fi | HE, Anti-CD31, Anti-CD68 |
| Shoshani et al., 2000 [150] | C | I, Cy, Fi | HE |
| Shoshani et al., 2001 [151] | C | MAs, I, Cy, Fi | HE |
| Shoshani et al., 2005 [152] | C | MAs, I, Cy, Fi | HE |
| Skorobac Asanin and Sopta et al., 2017 [153] | C | MAs, V | HE |
| Smith et al., 2006 [154] | C | MAs, I, Cy, N, Fi | HE |
| Sun et al., 2023 [155] | C | MAs, I, Cy, Fi | HE, Anti-perilipin, Anti-F4/80, Anti-CD206, Anti-MAC2, Anti-CD11c |
| Szychta et al., 2024 [156] | C | MAs, V, I, Cy, N, Fi | HE |
| Teng et al., 2014 [157] | C | MAs, V, I, Cy, N, Fi | HE |
| Tong et al., 2018 [158] | G | Cy | HE |
| Tran et al., 2024 [25] | C, M | MAs, V, I, Cy, Fi | HE, Anti-perilipin, Anti-CD31, Anti-F4/80 |
| Ullmann et al., 2006 [159] | C | MAs, V, I, Cy, N, Fi | HE |
| Van Dongen et al., 2020 [160] | C, M | MAs, SVFCs, V | MT, Anti-CD31, Anti-αSMA |
| Von Heimburg and Pallua et al., 2001 [161] | S | MAs, I, Cy, Fi | HE |
| Wang et al., 2015 [162] | F | MAs, V, I, Cy, N, Fi | HE |
| Wei et al., 2019 [163] | C | MAs, V, I, Cy, N, Fi | HE, Anti-perilipin, Anti-CD31 |
| Weisz and Gal, 1986 [164] | B | MAs, V | HE |
| Wu et al., 2020 [165] | C | MAs, V, I, Cy | HE, Anti-perilipin, Anti-CD31 |
| Wu et al., 2023 [166] | C, M | MAs, V, I, Cy | HE, Anti-perilipin, Anti-CD31, Anti-MAC2, Anti-CD206 |
| Xia et al., 2021 [167] | Sc, - | MAs, V, Cy, N | HE, Anti-perilipin, Anti-CD31, Anti-UCP1 (uncoupling protein 1) |
| Xie et al., 2010 [168] | C | MAs | HE |
| Xiong et al., 2018 [169] | C | V, Fi | HE |
| Xu et al., 2014 [170] | - | MAs, V, I, N, Fi | HE |
| Yagima Odo et al., 2007 [171] | B | I, N, Fi | HE |
| Yang et al., 2021 [172] | C, M, G | MAs | HE, Anti-perilipin |
| Yang et al., 2023 [173] | Sc, - | V, I, Cy, Fi | HE, MT, Anti-CD31, Anti-αSMA, Anti-HIF-1α, Anti-VEGF |
| Yang et al., 2024 [174] | C, Sc | MAs, V, I, Cy, Fi | HE, MT, Anti-perilipin, Anti-CD31, Anti-αSMA, Anti-MAC2 |
| Yi et al., 2006 [175] | C | MAs, V, I, Cy, Fi | HE, Anti-vWF |
| Yi et al., 2007 [176] | C | MAs, V, I, Cy, Fi | HE, Anti-vWF |
| Yu et al., 2018 [177] | S, M | MAs, V, I, Cy, Fi | HE, MT, Anti-CD31 |
| Yu et al., 2020 [178] | C | MAs, V, I, Cy, Fi | HE, MT, Anti-perilipin, Anti- αSMA |
| Yu et al., 2020 [13] | C, Sc | MAs, V, Cy, N | HE, von Kossa, Anti-perilipin, Anti-CD31, Anti-αSMA, Anti-Ki67, Anti-VEGF, Anti-ANA (antinuclear), Anti-TNFα (tumor necrosis factor α) |
| Yu et al., 2021 [179] | C | MAs, V, I, Cy, Fi | HE, MT, Anti-perilipin, Anti- αSMA |
| Zhan et al., 2017 [180] | Sc, - | MAs, V | HE, Anti-perilipin, Anti-CD31 |
| Zhang et al., 2018 [181] | C, M | MAs, SVFCs, V, I, Cy, N, Fi | HE, MT, Anti-perilipin, Anti-CD206, Anti-MAC2, Anti-HLA |
| Zhao et al., 2023 [182] | M, Sc | MAs | HE |
| Zhu et al., 2021 [183] | C, M | MAs, I, Cy, Fi | HE, Anti-perilipin |
| Tissue Preparation | Preservation of Architecture | Evaluable Histological Targets | Recommended Stains | Specific Characteristics/Limitations |
|---|---|---|---|---|
| Lipoaspirate | Low | SVF | H&E, IHC | Fragmented, non-coherent mixture; vessel and ECM structures disrupted; interpretation of inflammation and fibrosis unreliable |
| MAs | Perilipin, Oil O Red | |||
| ECM | Masson Trichrome | |||
| Vascularization/Vessel Integrity | Anti-CD31, Anti-αSMA | Enables analysis of neovascularization and graft viability | ||
| Surgically Excised Fat (including explants) | High | SVF, MAs, ECM, and Vessels | See above | The same reasons are applicable for surgically excised fat as for lipoaspirate |
| Fibrosis | Masson Trichrome, H&E-Saffron | Enables semiquantitative scoring (e.g., 0–5 scale) | ||
| Inflammation/Immune Cell Infiltration | H&E, Anti-CD68, Anti-F4/80 | |||
| Oil Cysts/Vacuoles | Oil O Red, Sudan stains | |||
| Necrosis | H&E, Anti-HIF-1α |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schimanski, T.; Loucas, R.; Loucas, M.; Felthaus, O.; Brébant, V.; Klein, S.; Anker, A.; Frank, K.; Siegmund, A.; Pagani, A.; et al. Histology and Immunohistochemistry of Adipose Tissue: A Scoping Review on Staining Methods and Their Informative Value. Cells 2025, 14, 898. https://doi.org/10.3390/cells14120898
Schimanski T, Loucas R, Loucas M, Felthaus O, Brébant V, Klein S, Anker A, Frank K, Siegmund A, Pagani A, et al. Histology and Immunohistochemistry of Adipose Tissue: A Scoping Review on Staining Methods and Their Informative Value. Cells. 2025; 14(12):898. https://doi.org/10.3390/cells14120898
Chicago/Turabian StyleSchimanski, Tom, Rafael Loucas, Marios Loucas, Oliver Felthaus, Vanessa Brébant, Silvan Klein, Alexandra Anker, Konstantin Frank, Andreas Siegmund, Andrea Pagani, and et al. 2025. "Histology and Immunohistochemistry of Adipose Tissue: A Scoping Review on Staining Methods and Their Informative Value" Cells 14, no. 12: 898. https://doi.org/10.3390/cells14120898
APA StyleSchimanski, T., Loucas, R., Loucas, M., Felthaus, O., Brébant, V., Klein, S., Anker, A., Frank, K., Siegmund, A., Pagani, A., Geis, S., Diesch, S. T., Eigenberger, A., & Prantl, L. (2025). Histology and Immunohistochemistry of Adipose Tissue: A Scoping Review on Staining Methods and Their Informative Value. Cells, 14(12), 898. https://doi.org/10.3390/cells14120898









