Wnt/PKC Signaling Inhibits Sensory Hair Cell Formation in the Developing Mammalian Cochlea
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and In Situ Hybridization
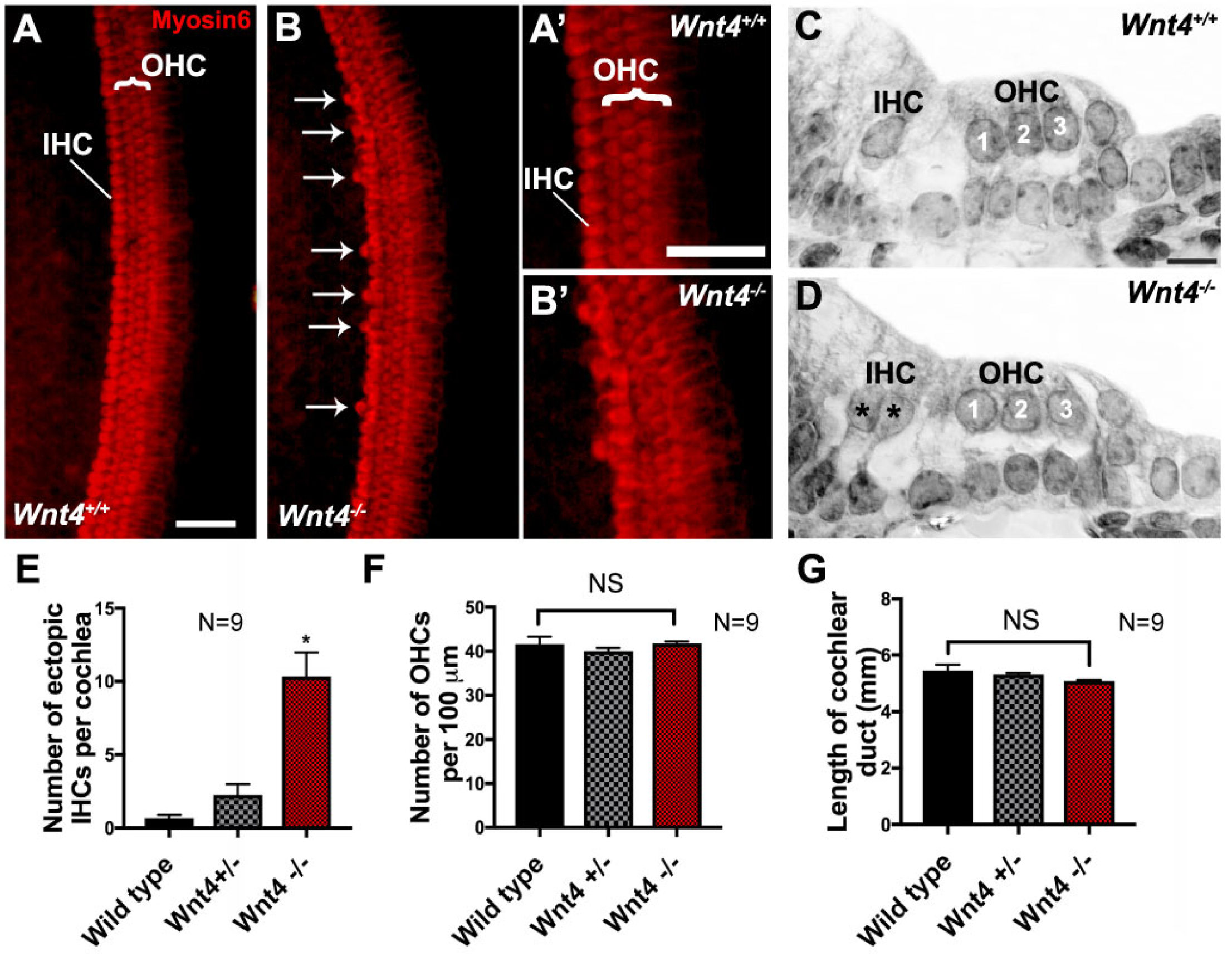
2.2. Analysis of Wnt4 Mutants
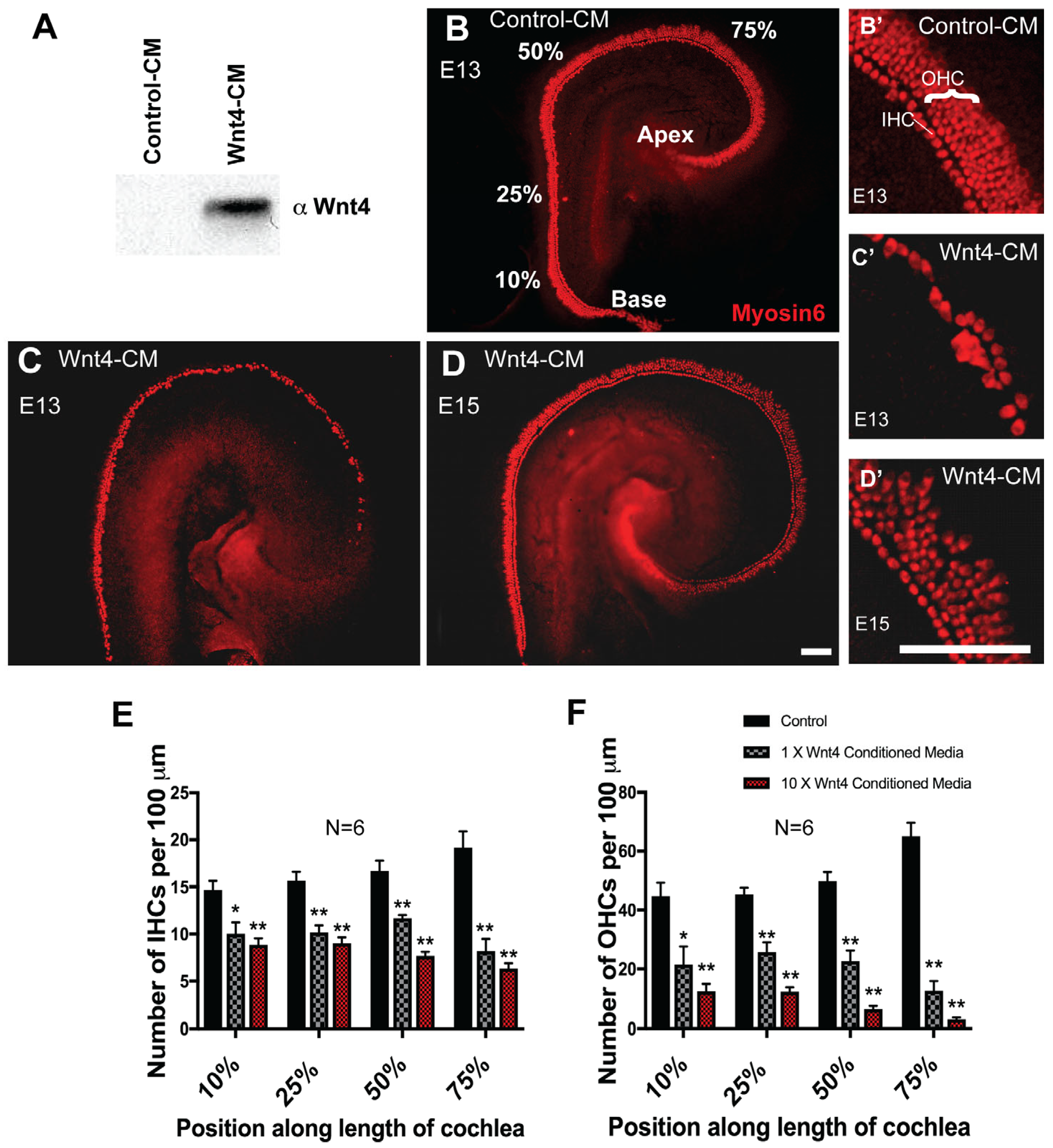
2.3. Organotypic Explant Cochlear Cultures
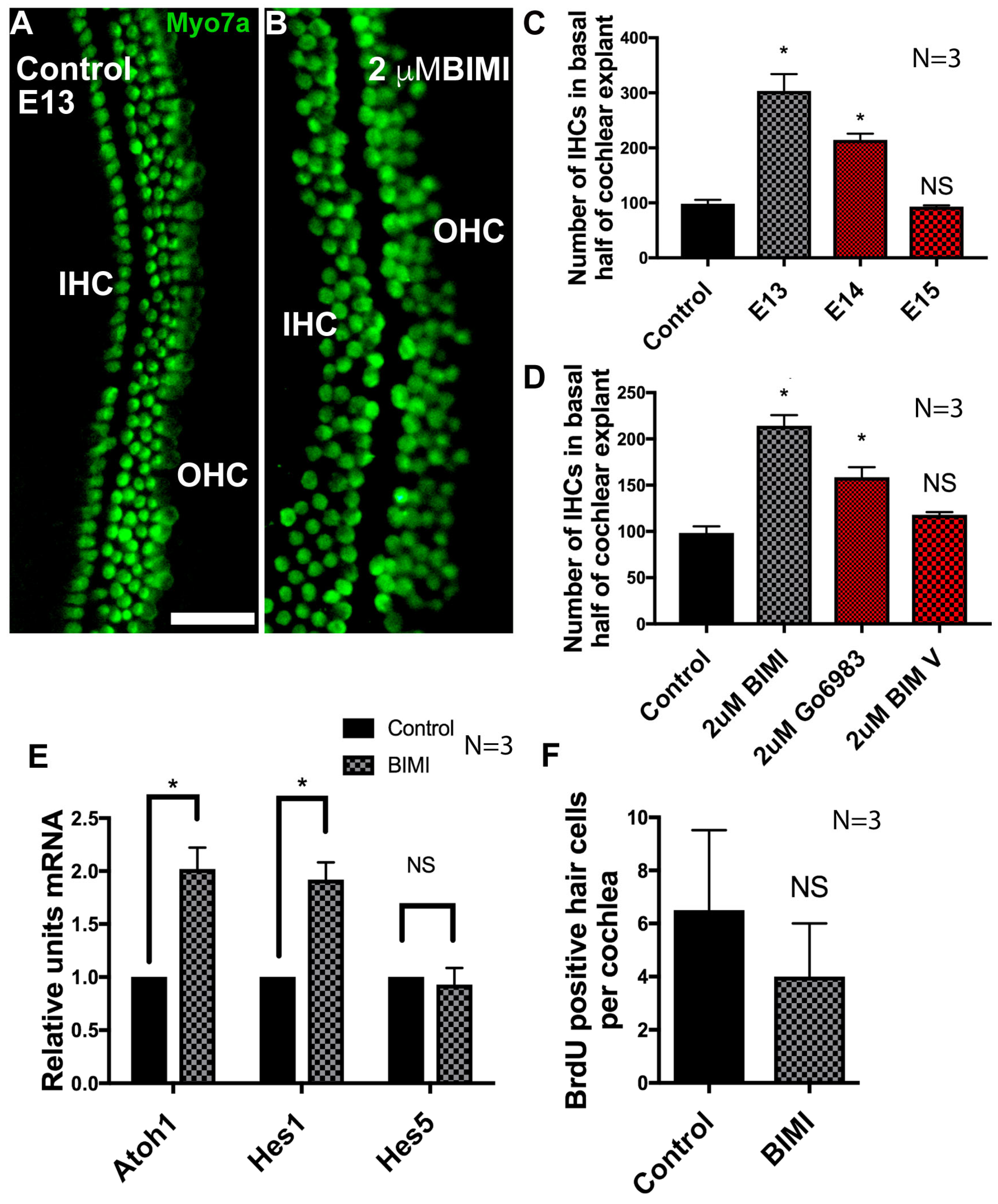
2.4. Modulation of Wnt Signaling
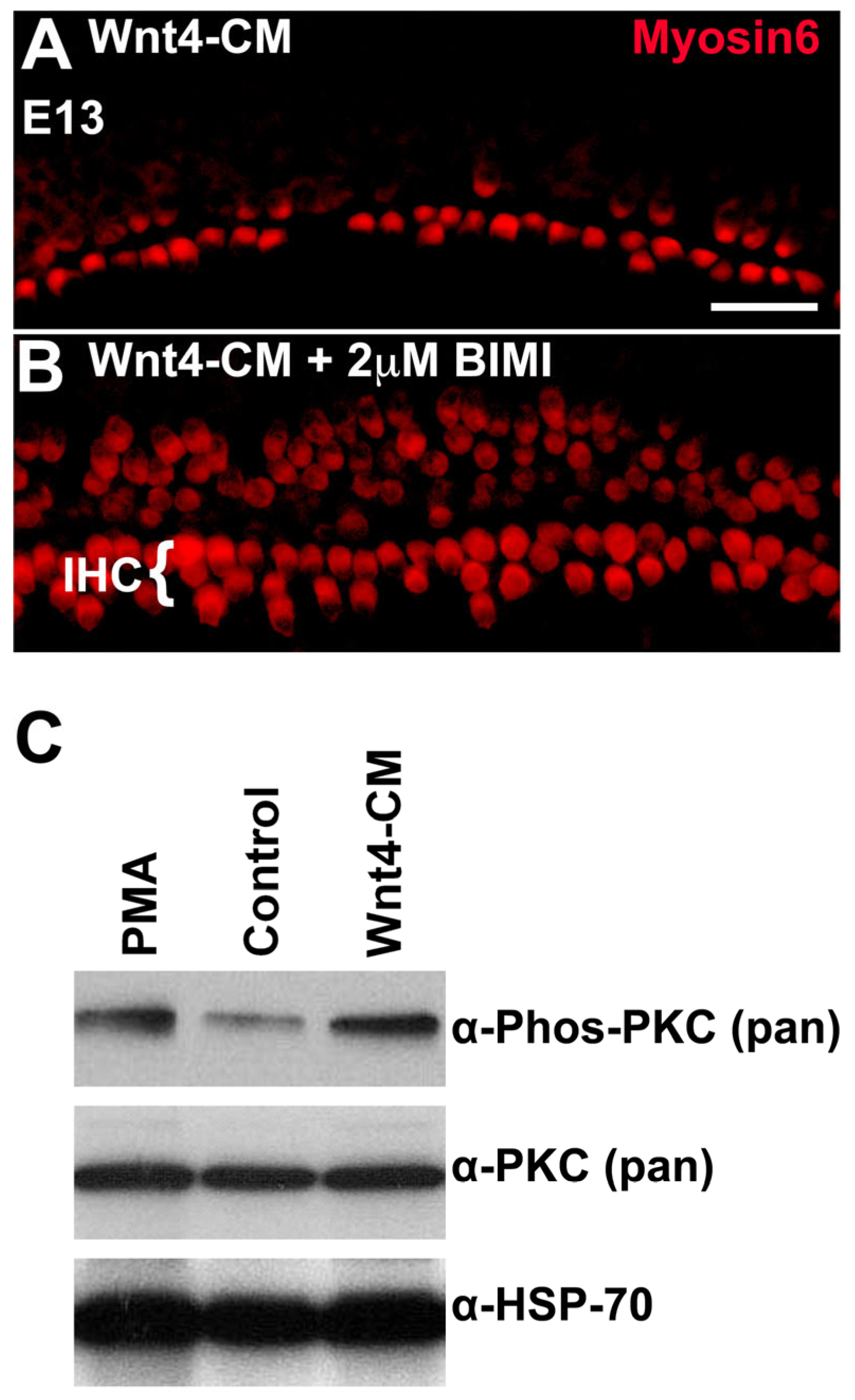
2.5. PKC Activation Assay
2.6. Real Time PCR
2.7. Site-Directed Mutagenesis
2.8. Transfection
2.9. Statistical Analysis
3. Results
3.1. Wnt4 Is Expressed in the Embryonic Cochlea
3.2. Wnt4 Mutants Contain Extra IHCs in the Cochlea
3.3. Wnt4 Inhibits Hair Cell Formation In Vitro
3.4. Specificity of Wnt4-Mediated Signaling
3.5. Calcium Chelation Results in Ectopic IHCs
3.6. Inhibition of PKC Induces Ectopic IHCs
3.7. PKC Is Phosphorylated in Response to the Addition of Wnt4 in the Embryonic Cochlea
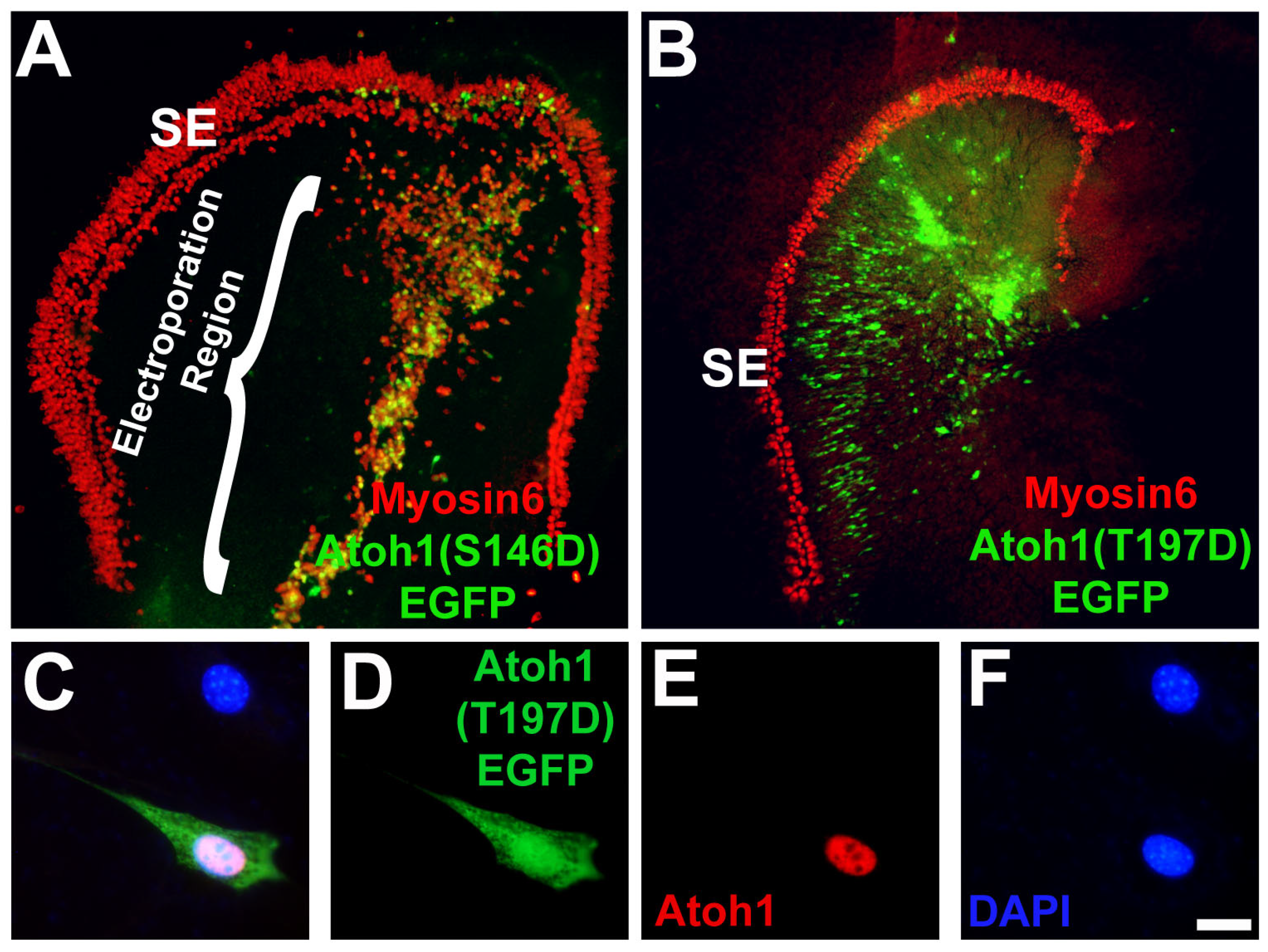
3.8. Modification of a Phospho-Serine Site on Atoh1 Inhibits Hair Cell Formation
4. Discussion
4.1. Wnt/PKC Modulates Sensory/Nonsensory Cell Fate Decisions in the Greater Epithelial Ridge
4.2. Specificity of Wnt Signaling
4.3. Protein Kinase C Regulates Cell Fate in the Cochlear Duct
4.4. Post-Translational Modification of Atoh1
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| SE | Sensory Epithelium |
| SEM | Standard Error of the Mean |
| BIM I | Bisindolymaleimide |
| PMA | Phorbol 12-myristate 13-acetate |
| bHLH | Basic helix-loop-helix |
| Atoh1 | Atonal bHLH transcription factor |
| OC | Organ of Corti |
| HC | Hair Cell |
| IHC | Inner Hair Cell |
| OHC | Outer Hair Cell |
| BSA | Bovine Serum Albumin |
| E | Embryonic Day |
| H&E | Hematoxylin & Eosin |
| RM | Reissner’s Membrane |
| WT | Wildtype |
| P | Postnatal Day |
| NS | Not Significant |
| SEM | Standard Error of the Mean |
| CM | Conditioned Media |
| PCP | Planar Cell Polarity |
| Ca2+ | Calcium |
| GER | Greater Epithelial Ridge |
| LER | Lateral Epithelial Ridge |
References
- Driver, E.C.; Kelley, M.W. Development of the cochlea. Development 2020, 147, dev162263. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.W. Regulation of cell fate in the sensory epithelia of the inner ear. Nat. Rev. Neurosci. 2006, 7, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.W.; Driver, E.C.; Puligilla, C. Regulation of cell fate and patterning in the developing mammalian cochlea. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K.; Fekete, D.M. Shaping sound in space: The regulation of inner ear patterning. Development 2012, 139, 245–257. [Google Scholar] [CrossRef]
- Webber, J.L.; Garcia-Anoveros, J. Precision patterning: How inner hair cells “hop” to it. Sci. Adv. 2023, 9, eadg8662. [Google Scholar] [CrossRef]
- Barald, K.F.; Kelley, M.W. From placode to polarization: New tunes in inner ear development. Development 2004, 131, 4119–4130. [Google Scholar] [CrossRef]
- Katsunuma, S.; Togashi, H.; Kuno, S.; Fujita, T.; Nibu, K.I. Hearing loss in mice with disruption of auditory epithelial patterning in the cochlea. Front. Cell Dev. Biol. 2022, 10, 1073830. [Google Scholar] [CrossRef]
- Brown, A.S.; Rakowiecki, S.M.; Li, J.Y.H.; Epstein, D.J. The cochlear sensory epithelium derives from Wnt responsive cells in the dorsomedial otic cup. Dev. Biol. 2015, 399, 177–187. [Google Scholar] [CrossRef]
- Macheda, M.L.; Sun, W.W.; Kugathasan, K.; Hogan, B.M.; Bower, N.I.; Halford, M.M.; Zhang, Y.F.; Jacques, B.E.; Lieschke, G.J.; Dabdoub, A.; et al. The Wnt receptor Ryk plays a role in mammalian planar cell polarity signaling. J. Biol. Chem. 2012, 287, 29312–29323. [Google Scholar] [CrossRef]
- Dabdoub, A.; Donohue, M.J.; Brennan, A.; Wolf, V.; Montcouquiol, M.; Sassoon, D.A.; Hseih, J.C.; Rubin, J.S.; Salinas, P.C.; Kelley, M.W. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 2003, 130, 2375–2384. [Google Scholar] [CrossRef]
- Huyghe, A.; Van den Ackerveken, P.; Sacheli, R.; Prevot, P.P.; Thelen, N.; Renauld, J.; Thiry, M.; Delacroix, L.; Nguyen, L.; Malgrange, B. MicroRNA-124 Regulates Cell Specification in the Cochlea through Modulation of Sfrp4/5. Cell Rep. 2015, 13, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Jacques, B.E.; Puligilla, C.; Weichert, R.M.; Ferrer-Vaquer, A.; Hadjantonakis, A.K.; Kelley, M.W.; Dabdoub, A. A dual function for canonical Wnt/beta-catenin signaling in the developing mammalian cochlea. Development 2012, 139, 4395–4404. [Google Scholar] [CrossRef] [PubMed]
- Munnamalai, V.; Fekete, D.M. Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development 2016, 143, 4003–4015. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Jones, C.; Rzadzinska, A.; Mark, S.; Zhang, X.; Steel, K.P.; Dai, X.; Chen, P. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 2007, 306, 121–133. [Google Scholar] [CrossRef]
- Shi, F.; Hu, L.; Jacques, B.E.; Mulvaney, J.F.; Dabdoub, A.; Edge, A.S. β-Catenin is required for hair-cell differentiation in the cochlea. J. Neurosci. 2014, 34, 6470–6479. [Google Scholar] [CrossRef]
- Zak, M.; van Oort, T.; Hendriksen, F.G.; Garcia, M.I.; Vassart, G.; Grolman, W. LGR4 and LGR5 Regulate Hair Cell Differentiation in the Sensory Epithelium of the Developing Mouse Cochlea. Front. Cell Neurosci. 2016, 10, 186. [Google Scholar] [CrossRef]
- Jansson, L.; Kim, G.S.; Cheng, A.G. Making sense of Wnt signaling-linking hair cell regeneration to development. Front. Cell Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef]
- May-Simera, H.; Kelley, M.W. Planar cell polarity in the inner ear. Curr. Top. Dev. Biol. 2012, 101, 111–140. [Google Scholar]
- Stevens, C.B.; Davies, A.L.; Battista, S.; Lewis, J.H.; Fekete, D.M. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev. Biol. 2003, 261, 149–164. [Google Scholar] [CrossRef]
- Riccomagno, M.M.; Takada, S.; Epstein, D.J. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes. Dev. 2005, 19, 1612–1623. [Google Scholar] [CrossRef]
- Ohyama, T.; Mohamed, O.A.; Taketo, M.M.; Dufort, D.; Groves, A.K. Wnt signals mediate a fate decision between otic placode and epidermis. Development 2006, 133, 865–875. [Google Scholar] [CrossRef]
- Mulvaney, J.F.; Thompkins, C.; Noda, T.; Nishimura, K.; Sun, W.W.; Lin, S.Y.; Coffin, A.; Dabdoub, A. Kremen1 regulates mechanosensory hair cell development in the mammalian cochlea and the zebrafish lateral line. Sci. Rep. 2016, 6, 31668. [Google Scholar] [CrossRef] [PubMed]
- Warchol, M.E.; Montcouquiol, M. Maintained expression of the planar cell polarity molecule Vangl2 and reformation of hair cell orientation in the regenerating inner ear. J. Assoc. Res. Otolaryngol. 2010, 11, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Landin Malt, A.; Clancy, S.; Hwang, D.; Liu, A.; Smith, C.; Smith, M.; Hatley, M.; Clemens, C.; Lu, X. Non-Canonical Wnt Signaling Regulates Cochlear Outgrowth and Planar Cell Polarity via Gsk3beta Inhibition. Front. Cell Dev. Biol. 2021, 9, 649830. [Google Scholar] [CrossRef]
- Xie, N.; Landin Malt, A.; Adylkhan, A.; Rodeman, N.; Moraes Borges, R.; Hwang, D.; Liu, A.; Smith, C.; Hogan, A.; Lu, X. Wnt7b acts in concert with Wnt5a to regulate tissue elongation and planar cell polarity via noncanonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2024, 121, e2405217121. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Han, Y.; An, W.; Wang, X.; Chen, F.; Lu, J.; Meng, Y.; Li, Y.; Wang, Y.; Li, J.; et al. Wnt signalling facilitates neuronal differentiation of cochlear Frizzled10-positive cells in mouse cochlea via glypican 6 modulation. Cell Commun. Signal. 2025, 23, 50. [Google Scholar] [CrossRef]
- Najarro, E.H.; Huang, J.; Jacobo, A.; Quiruz, L.A.; Grillet, N.; Cheng, A.G. Dual regulation of planar polarization by secreted Wnts and Vangl2 in the developing mouse cochlea. Development 2020, 147, dev191981. [Google Scholar] [CrossRef]
- Landin Malt, A.; Hogan, A.K.; Smith, C.D.; Madani, M.S.; Lu, X. Wnts regulate planar cell polarity via heterotrimeric G protein and PI3K signaling. J. Cell Biol. 2020, 219, e201912071. [Google Scholar] [CrossRef]
- Jansson, L.; Ebeid, M.; Shen, J.W.; Mokhtari, T.E.; Quiruz, L.A.; Ornitz, D.M.; Huh, S.H.; Cheng, A.G. beta-Catenin is required for radial cell patterning and identity in the developing mouse cochlea. Proc. Natl. Acad. Sci. USA 2019, 116, 21054–21060. [Google Scholar] [CrossRef]
- Ellis, K.; Driver, E.C.; Okano, T.; Lemons, A.; Kelley, M.W. GSK3 regulates hair cell fate in the developing mammalian cochlea. Dev. Biol. 2019, 453, 191–205. [Google Scholar] [CrossRef]
- Munnamalai, V.; Fekete, D.M. Wnt signaling during cochlear development. Semin. Cell Dev. Biol. 2013, 24, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Z.; Shi, X.; Zong, Y.; Sun, Y. The Effects of Viral Infections on the Molecular and Signaling Pathways Involved in the Development of the PAOs. Viruses 2024, 16, 1342. [Google Scholar] [CrossRef] [PubMed]
- Dabdoub, A.; Kelley, M.W. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J. Neurobiol. 2005, 64, 446–457. [Google Scholar] [CrossRef]
- Daudet, N.; Ripoll, C.; Moles, J.P.; Rebillard, G. Expression of members of Wnt and Frizzled gene families in the postnatal rat cochlea. Brain Res. Mol. Brain Res. 2002, 105, 98–107. [Google Scholar] [CrossRef]
- Geng, R.; Noda, T.; Mulvaney, J.F.; Lin, V.Y.; Edge, A.S.; Dabdoub, A. Comprehensive Expression of Wnt Signaling Pathway Genes during Development and Maturation of the Mouse Cochlea. PLoS ONE 2016, 11, e0148339. [Google Scholar] [CrossRef]
- Mulvaney, J.F.; Yatteau, A.; Sun, W.W.; Jacques, B.; Takubo, K.; Suda, T.; Yamada, W.; Dabdoub, A. Secreted factor R-Spondin 2 is involved in refinement of patterning of the mammalian cochlea. Dev. Dyn. 2013, 242, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Sienknecht, U.J.; Fekete, D.M. Comprehensive Wnt-related gene expression during cochlear duct development in chicken. J. Comp. Neurol. 2008, 510, 378–395. [Google Scholar] [CrossRef]
- Soma, K.; Shiomi, K.; Keino-Masu, K.; Masu, M. Expression of mouse Coiled-coil-DIX1 (Ccd1), a positive regulator of Wnt signaling, during embryonic development. Gene Expr. Patterns 2006, 6, 325–330. [Google Scholar] [CrossRef]
- Noda, T.; Oki, S.; Kitajima, K.; Harada, T.; Komune, S.; Meno, C. Restriction of Wnt signaling in the dorsal otocyst determines semicircular canal formation in the mouse embryo. Dev. Biol. 2012, 362, 83–93. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Dabdoub, A.; Smallwood, P.M.; Williams, J.; Woods, C.; Kelley, M.W.; Jiang, L.; Tasman, W.; Zhang, K.; et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004, 116, 883–895. [Google Scholar] [CrossRef]
- Jones, C.; Qian, D.; Kim, S.M.; Li, S.; Ren, D.; Knapp, L.; Sprinzak, D.; Avraham, K.B.; Matsuzaki, F.; Chi, F.; et al. Ankrd6 is a mammalian functional homolog of Drosophila planar cell polarity gene diego and regulates coordinated cellular orientation in the mouse inner ear. Dev. Biol. 2014, 395, 62–72. [Google Scholar] [CrossRef]
- Rakowiecki, S.; Epstein, D.J. Divergent roles for Wnt/beta-catenin signaling in epithelial maintenance and breakdown during semicircular canal formation. Development 2013, 140, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Xia, A.; Wang, T.; Jan, T.A.; Hayashi, T.; Bermingham-McDonogh, O.; Cheng, A.G. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 2011, 12, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A.; Jiang, K.; Kempfle, J.S.; Mizutari, K.; Simmons, C.L.; Bieber, R.; Adams, J.; Edge, A.S. TAK1 expression in the cochlea: A specific marker for adult supporting cells. J. Assoc. Res. Otolaryngol. 2011, 12, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.A.; Chai, R.; Sayyid, Z.N.; Cheng, A.G. Isolating LacZ-expressing cells from mouse inner ear tissues using flow cytometry. J. Vis. Exp. 2011, 23, e3432. [Google Scholar]
- Samarajeewa, A.; Lenz, D.R.; Xie, L.; Chiang, H.; Kirchner, R.; Mulvaney, J.F.; Edge, A.S.B.; Dabdoub, A. Transcriptional response to Wnt activation regulates the regenerative capacity of the mammalian cochlea. Development 2018, 145, dev166579. [Google Scholar] [CrossRef]
- Deans, M.R. Planar cell polarity signaling guides cochlear innervation. Dev. Biol. 2022, 486, 1–4. [Google Scholar] [CrossRef]
- Brisken, C.; Heineman, A.; Chavarria, T.; Elenbaas, B.; Tan, J.; Dey, S.K.; McMahon, J.A.; McMahon, A.P.; Weinberg, R.A. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000, 14, 650–654. [Google Scholar] [CrossRef]
- Heikkila, M.; Peltoketo, H.; Leppaluoto, J.; Ilves, M.; Vuolteenaho, O.; Vainio, S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology 2002, 143, 4358–4365. [Google Scholar] [CrossRef]
- Jeays-Ward, K.; Hoyle, C.; Brennan, J.; Dandonneau, M.; Alldus, G.; Capel, B.; Swain, A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 2003, 130, 3663–3670. [Google Scholar] [CrossRef]
- Stark, K.; Vainio, S.; Vassileva, G.; McMahon, A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 1994, 372, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Vainio, S.; Heikkila, M.; Kispert, A.; Chin, N.; McMahon, A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999, 397, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pan, Y.; Ji, J.; Xu, Y.; Zhang, Q.; Qin, L. Roles and action mechanisms of WNT4 in cell differentiation and human diseases: A review. Cell Death Discov. 2021, 7, 287. [Google Scholar] [CrossRef]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef]
- Luna-Ulloa, L.B.; Hernandez-Maqueda, J.G.; Santoyo-Ramos, P.; Castaneda-Patlan, M.C.; Robles-Flores, M. Protein kinase C zeta is a positive modulator of canonical Wnt signaling pathway in tumoral colon cell lines. Carcinogenesis 2011, 32, 1615–1624. [Google Scholar] [CrossRef]
- Qin, K.; Yu, M.; Fan, J.; Wang, H.; Zhao, P.; Zhao, G.; Zeng, W.; Chen, C.; Wang, Y.; Wang, A.; et al. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. 2024, 11, 103–134. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, M.; Sheldahl, L.C.; Park, M.; Miller, J.R.; Moon, R.T. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000, 16, 279–283. [Google Scholar] [CrossRef]
- Kuhl, M.; Geis, K.; Sheldahl, L.C.; Pukrop, T.; Moon, R.T.; Wedlich, D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/beta-catenin and Wnt/Ca2+ signaling. Mech. Dev. 2001, 106, 61–76. [Google Scholar] [CrossRef]
- Tauc, H.M.; Mann, T.; Werner, K.; Pandur, P. A role for Drosophila Wnt-4 in heart development. Genesis 2012, 50, 466–481. [Google Scholar] [CrossRef]
- Cerpa, W.; Latorre-Esteves, E.; Barria, A. RoR2 functions as a noncanonical Wnt receptor that regulates NMDAR-mediated synaptic transmission. Proc. Natl. Acad. Sci. USA 2015, 112, 4797–4802. [Google Scholar] [CrossRef]
- Range, R. Specification and positioning of the anterior neuroectoderm in deuterostome embryos. Genesis 2014, 52, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, J.; MacDonald, M.L.; Kaykas, A.; Sheldahl, L.C.; Zeisler, J.; Dube, M.P.; Zhang, L.H.; Singaraja, R.R.; Guernsey, D.L.; Zheng, B.; et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet. 2002, 32, 326–330. [Google Scholar] [CrossRef]
- Jones, J.M.; Montcouquiol, M.; Dabdoub, A.; Woods, C.; Kelley, M.W. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J. Neurosci. 2006, 26, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.H.; Kaufman, M.H. The Atlas of Mouse Development; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Dabdoub, A.; Puligilla, C.; Jones, J.M.; Fritzsch, B.; Cheah, K.S.; Pevny, L.H.; Kelley, M.W. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA 2008, 105, 18396–18401. [Google Scholar] [CrossRef]
- Mulvaney, J.F.; Dabdoub, A. Long-term time lapse imaging of mouse cochlear explants. J. Vis. Exp. 2014, e52101. [Google Scholar] [CrossRef]
- Kispert, A.; Vainio, S.; McMahon, A.P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 1998, 125, 4225–4234. [Google Scholar] [CrossRef]
- Yoshino, K.; Rubin, J.S.; Higinbotham, K.G.; Uren, A.; Anest, V.; Plisov, S.Y.; Perantoni, A.O. Secreted Frizzled-related proteins can regulate metanephric development. Mech. Dev. 2001, 102, 45–55. [Google Scholar] [CrossRef]
- Haque, K.D.; Pandey, A.K.; Kelley, M.W.; Puligilla, C. Culture of embryonic mouse cochlear explants and gene transfer by electroporation. J. Vis. Exp. 2015, 52260. [Google Scholar] [CrossRef]
- Zheng, J.L.; Gao, W.Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 2000, 3, 580–586. [Google Scholar] [CrossRef]
- Mii, Y.; Taira, M. Secreted Wnt “inhibitors” are not just inhibitors: Regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev. Growth Differ. 2011, 53, 911–923. [Google Scholar] [CrossRef]
- Lee, C.S.; Buttitta, L.A.; May, N.R.; Kispert, A.; Fan, C.M. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development 2000, 127, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lescher, B.; Haenig, B.; Kispert, A. sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney. Dev. Dyn. 1998, 213, 440–451. [Google Scholar] [CrossRef]
- Lyuksyutova, A.I.; Lu, C.C.; Milanesio, N.; King, L.A.; Guo, N.; Wang, Y.; Nathans, J.; Tessier-Lavigne, M.; Zou, Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 2003, 302, 1984–1988. [Google Scholar] [CrossRef]
- Kuhl, M. The WNT/calcium pathway: Biochemical mediators, tools and future requirements. Front. Biosci. 2004, 9, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Martiny-Baron, G.; Kazanietz, M.G.; Mischak, H.; Blumberg, P.M.; Kochs, G.; Hug, H.; Marme, D.; Schachtele, C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993, 268, 9194–9197. [Google Scholar] [CrossRef] [PubMed]
- Doetzlhofer, A.; Basch, M.L.; Ohyama, T.; Gessler, M.; Groves, A.K.; Segil, N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev. Cell 2009, 16, 58–69. [Google Scholar] [CrossRef]
- Zine, A.; Aubert, A.; Qiu, J.; Therianos, S.; Guillemot, F.; Kageyama, R.; de Ribaupierre, F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 2001, 21, 4712–4720. [Google Scholar] [CrossRef]
- Basch, M.L.; Brown, R.M., 2nd; Jen, H.I.; Semerci, F.; Depreux, F.; Edlund, R.K.; Zhang, H.; Norton, C.R.; Gridley, T.; Cole, S.E.; et al. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. Elife 2016, 5, e19921. [Google Scholar] [CrossRef]
- Mulvaney, J.F.; Amemiya, Y.; Freeman, S.D.; Ladher, R.K.; Dabdoub, A. Molecular cloning and functional characterisation of chicken Atonal homologue 1: A comparison with human Atoh1. Biol. Cell 2015, 107, 41–60. [Google Scholar] [CrossRef]
- Woods, C.; Montcouquiol, M.; Kelley, M.W. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat. Neurosci. 2004, 7, 1310–1318. [Google Scholar] [CrossRef]
- Aurade, F.; Pfarr, C.M.; Lindon, C.; Garcia, A.; Primig, M.; Montarras, D.; Pinset, C. The glucocorticoid receptor and AP-1 are involved in a positive regulation of the muscle regulatory gene myf5 in cultured myoblasts. J. Cell Sci. 1997, 110 Pt 22, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Bengal, E.; Flores, O.; Rangarajan, P.N.; Chen, A.; Weintraub, H.; Verma, I.M. Positive control mutations in the MyoD basic region fail to show cooperative DNA binding and transcriptional activation in vitro. Proc. Natl. Acad. Sci. USA 1994, 91, 6221–6225. [Google Scholar] [CrossRef] [PubMed]
- Blagden, C.S.; Fromm, L.; Burden, S.J. Accelerated response of the myogenin gene to denervation in mutant mice lacking phosphorylation of myogenin at threonine 87. Mol. Cell Biol. 2004, 24, 1983–1989. [Google Scholar] [CrossRef]
- Firulli, B.A.; Howard, M.J.; McDaid, J.R.; McIlreavey, L.; Dionne, K.M.; Centonze, V.E.; Cserjesi, P.; Virshup, D.M.; Firulli, A.B. PKA, PKC, and the protein phosphatase 2A influence HAND factor function: A mechanism for tissue-specific transcriptional regulation. Mol. Cell 2003, 12, 1225–1237. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; James, G.; Heller-Harrison, R.; Czech, M.P.; Olson, E.N. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell 1992, 71, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- McGovern, M.M.; Hosamani, I.V.; Niu, Y.; Nguyen, K.Y.; Zong, C.; Groves, A.K. Expression of Atoh1, Gfi1, and Pou4f3 in the mature cochlea reprograms nonsensory cells into hair cells. Proc. Natl. Acad. Sci. USA 2024, 121, e2304680121. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, Y.; Li, Y.; Li, G.L.; Chai, R.; Li, W.; Li, H. Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea. Cell Rep. 2021, 35, 109016. [Google Scholar] [CrossRef]
- Dayaratne, M.W.; Vlajkovic, S.M.; Lipski, J.; Thorne, P.R. Kolliker’s organ and the development of spontaneous activity in the auditory system: Implications for hearing dysfunction. Biomed Res. Int. 2014, 2014, 367939. [Google Scholar] [CrossRef]
- Kolla, L.; Kelly, M.C.; Mann, Z.F.; Anaya-Rocha, A.; Ellis, K.; Lemons, A.; Palermo, A.T.; So, K.S.; Mays, J.C.; Orvis, J.; et al. Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat. Commun. 2020, 11, 2389. [Google Scholar] [CrossRef]
- de Haan, S.; Corbat, A.A.; Cederroth, C.R.; Autrum, L.G.; Hankeova, S.; Driver, E.C.; Canlon, B.; Kelley, M.W.; Andersson, E.R. Jag1 represses Notch activation in lateral supporting cells and inhibits an outer hair cell fate in the medial cochlea. Development 2024, 151, dev202949. [Google Scholar] [CrossRef]
- Huh, S.H.; Jones, J.; Warchol, M.E.; Ornitz, D.M. Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biol. 2012, 10, e1001231. [Google Scholar] [CrossRef] [PubMed]
- Urness, L.D.; Wang, X.; Shibata, S.; Ohyama, T.; Mansour, S.L. Fgf10 is required for specification of non-sensory regions of the cochlear epithelium. Dev. Biol. 2015, 400, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Basch, M.L.; Mishina, Y.; Lyons, K.M.; Segil, N.; Groves, A.K. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 2010, 30, 15044–15051. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; Kelly, M.C.; Hoa, M.; Morell, R.J.; Kelley, M.W. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat. Commun. 2015, 6, 8557. [Google Scholar] [CrossRef]
- Simonneau, L.; Gallego, M.; Pujol, R. Comparative expression patterns of T-, N-, E-cadherins, beta-catenin, and polysialic acid neural cell adhesion molecule in rat cochlea during development: Implications for the nature of Kolliker’s organ. J. Comp. Neurol. 2003, 459, 113–126. [Google Scholar] [CrossRef]
- Waldhaus, J.; Durruthy-Durruthy, R.; Heller, S. Quantitative High-Resolution Cellular Map of the Organ of Corti. Cell Rep. 2015, 11, 1385–1399. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Yang, J.; Sun, S.; Chai, R.; Chen, Z.Y.; Li, H. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 166–171. [Google Scholar] [CrossRef]
- Franco, B.; Malgrange, B. Concise Review: Regeneration in Mammalian Cochlea Hair Cells: Help from Supporting Cells Transdifferentiation. Stem Cells 2017, 35, 551–556. [Google Scholar] [CrossRef]
- Gubbels, S.P.; Woessner, D.W.; Mitchell, J.C.; Ricci, A.J.; Brigande, J.V. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 2008, 455, 537–541. [Google Scholar] [CrossRef]
- Kelly, M.C.; Chang, Q.; Pan, A.; Lin, X.; Chen, P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J. Neurosci. 2012, 32, 6699–6710. [Google Scholar] [CrossRef]
- Kuo, B.R.; Baldwin, E.M.; Layman, W.S.; Taketo, M.M.; Zuo, J. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of beta-Catenin and Atoh1. J. Neurosci. 2015, 35, 10786–10798. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Lin, C.; Guo, L.; Wu, J.; Chen, Y.; Chai, R.; Li, W.; Li, H. Extensive Supporting Cell Proliferation and Mitotic Hair Cell Generation by In Vivo Genetic Reprogramming in the Neonatal Mouse Cochlea. J. Neurosci. 2016, 36, 8734–8745. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Scheibinger, M.; Jan, T.A.; Heller, S. Greater epithelial ridge cells are the principal organoid-forming progenitors of the mouse cochlea. Cell Rep. 2021, 34, 108646. [Google Scholar] [CrossRef] [PubMed]
- Driver, E.C.; Sillers, L.; Coate, T.M.; Rose, M.F.; Kelley, M.W. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev. Biol. 2013, 376, 86–98. [Google Scholar] [CrossRef]
- Helms, A.W.; Abney, A.L.; Ben-Arie, N.; Zoghbi, H.Y.; Johnson, J.E. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 2000, 127, 1185–1196. [Google Scholar] [CrossRef]
- Brooker, R.; Hozumi, K.; Lewis, J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 2006, 133, 1277–1286. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Xu, J.; Gridley, T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006, 2, e4. [Google Scholar] [CrossRef]
- Sheldahl, L.C.; Park, M.; Malbon, C.C.; Moon, R.T. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol. 1999, 9, 695–698. [Google Scholar] [CrossRef]
- Bermingham, N.A.; Hassan, B.A.; Price, S.D.; Vollrath, M.A.; Ben-Arie, N.; Eatock, R.A.; Bellen, H.J.; Lysakowski, A.; Zoghbi, H.Y. Math1: An essential gene for the generation of inner ear hair cells. Science 1999, 284, 1837–1841. [Google Scholar] [CrossRef]
- Shi, F.; Cheng, Y.F.; Wang, X.L.; Edge, A.S. Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3’ enhancer. J. Biol. Chem. 2010, 285, 392–400. [Google Scholar] [CrossRef]
- Ikeda, R.; Pak, K.; Chavez, E.; Ryan, A.F. Transcription factors with conserved binding sites near ATOH1 on the POU4F3 gene enhance the induction of cochlear hair cells. Mol. Neurobiol. 2015, 51, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Hassan, B.A. Beyond proneural: Emerging functions and regulations of proneural proteins. Curr. Opin. Neurobiol. 2017, 42, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Tong, M.; Edge, A.S. Destabilization of Atoh1 by E3 Ubiquitin Ligase Huwe1 and Casein Kinase 1 Is Essential for Normal Sensory Hair Cell Development. J. Biol. Chem. 2016, 291, 21096–21109. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Fu, Y.; Pan, W.; Yu, J.; Wang, J. Atoh1 and other related key regulators in the development of auditory sensory epithelium in the mammalian inner ear: Function and interplay. Dev. Biol. 2019, 446, 133–141. [Google Scholar] [CrossRef]
- Xie, W.R.; Jen, H.I.; Seymour, M.L.; Yeh, S.Y.; Pereira, F.A.; Groves, A.K.; Klisch, T.J.; Zoghbi, H.Y. An Atoh1-S193A Phospho-Mutant Allele Causes Hearing Deficits and Motor Impairment. J. Neurosci. 2017, 37, 8583–8594. [Google Scholar] [CrossRef]
- Quan, X.J.; Yuan, L.; Tiberi, L.; Claeys, A.; De Geest, N.; Yan, J.; van der Kant, R.; Xie, W.R.; Klisch, T.J.; Shymkowitz, J.; et al. Post-translational Control of the Temporal Dynamics of Transcription Factor Activity Regulates Neurogenesis. Cell 2016, 164, 460–475. [Google Scholar] [CrossRef]



| pCLIG | pCLIG-Atoh1 | pCLIG-Atoh1 (S146D) | pCLIG-Atoh1 (T197D) |
|---|---|---|---|
| 0% HC | 98.5% HC | 97.1% HC | 0% HC |
| n = 57 | n = 204 | n = 340 | n = 195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulvaney, J.F.; Layman, E.M.; Feroze-Merzoug, F.; Abitbol, J.M.; Jones, J.M.; O’Connor, D.; Naillat, F.; Vainio, S.; Rubin, J.S.; Kelley, M.W.; et al. Wnt/PKC Signaling Inhibits Sensory Hair Cell Formation in the Developing Mammalian Cochlea. Cells 2025, 14, 888. https://doi.org/10.3390/cells14120888
Mulvaney JF, Layman EM, Feroze-Merzoug F, Abitbol JM, Jones JM, O’Connor D, Naillat F, Vainio S, Rubin JS, Kelley MW, et al. Wnt/PKC Signaling Inhibits Sensory Hair Cell Formation in the Developing Mammalian Cochlea. Cells. 2025; 14(12):888. https://doi.org/10.3390/cells14120888
Chicago/Turabian StyleMulvaney, Joanna F., Erynn M. Layman, Farhana Feroze-Merzoug, Julia M. Abitbol, Jennifer M. Jones, Dara O’Connor, Florence Naillat, Seppo Vainio, Jeffrey S. Rubin, Matthew W. Kelley, and et al. 2025. "Wnt/PKC Signaling Inhibits Sensory Hair Cell Formation in the Developing Mammalian Cochlea" Cells 14, no. 12: 888. https://doi.org/10.3390/cells14120888
APA StyleMulvaney, J. F., Layman, E. M., Feroze-Merzoug, F., Abitbol, J. M., Jones, J. M., O’Connor, D., Naillat, F., Vainio, S., Rubin, J. S., Kelley, M. W., & Dabdoub, A. (2025). Wnt/PKC Signaling Inhibits Sensory Hair Cell Formation in the Developing Mammalian Cochlea. Cells, 14(12), 888. https://doi.org/10.3390/cells14120888








