Inorganic Arsenite [As (III)] Represses Human Renal Progenitor Cell Characteristics and Induces Neoplastic-like Transformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. RNA Extraction and RT-qPCR
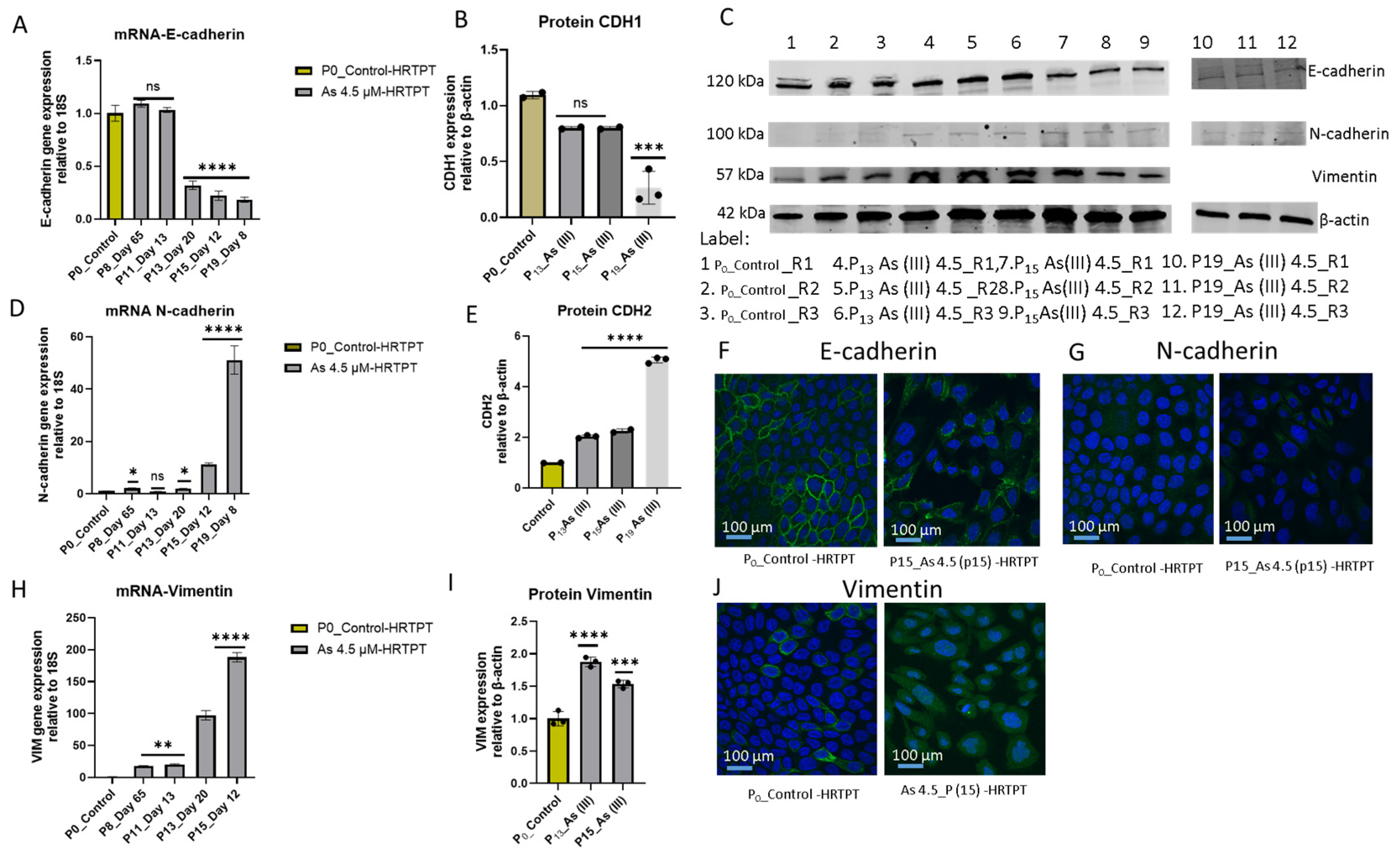
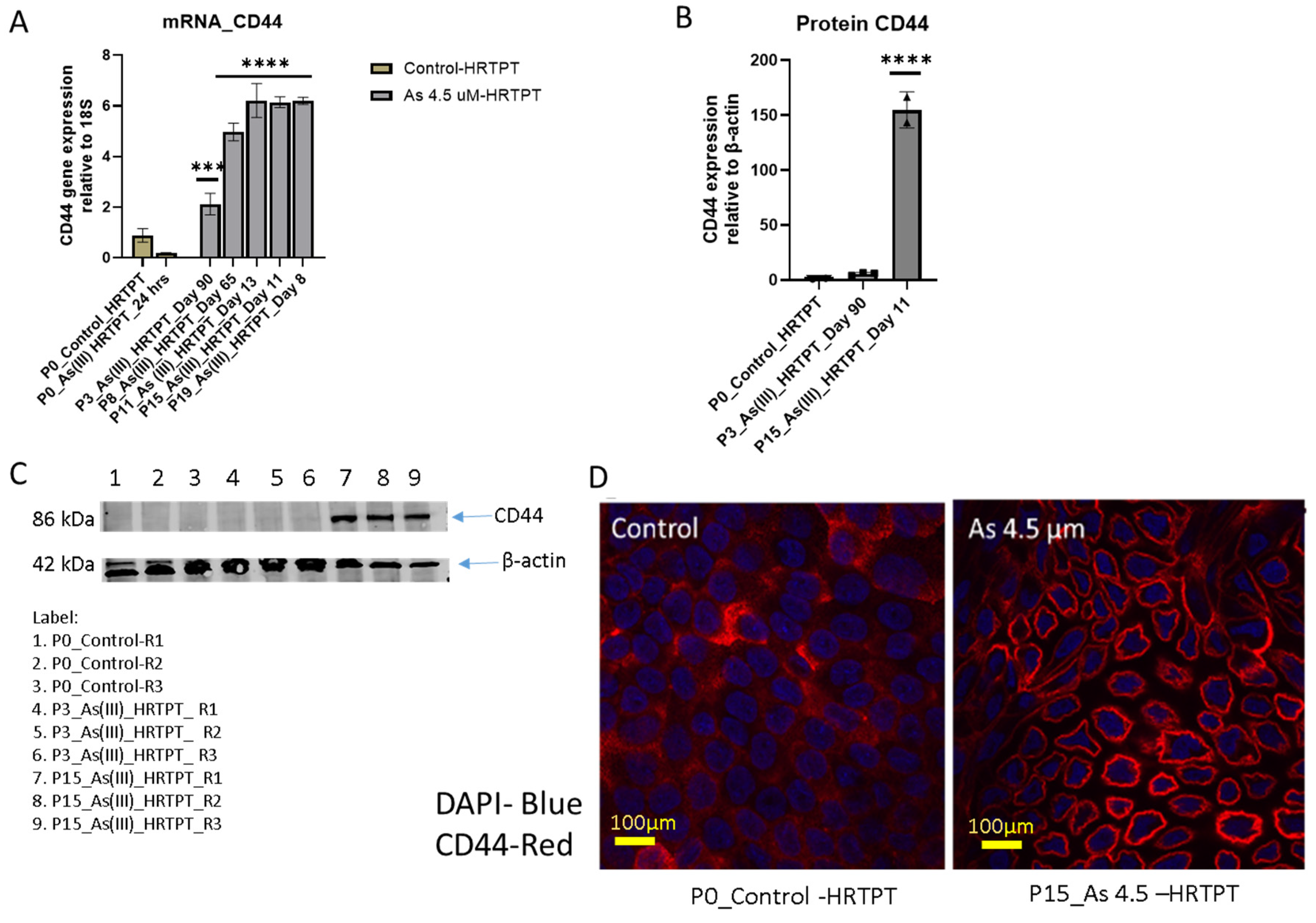
2.3. Western Blot
2.4. Capillary Electrophoresis Western
2.5. Immunofluorescence
2.6. Sphere Formation Assay
2.7. Matrigel™ Tubular Differentiation
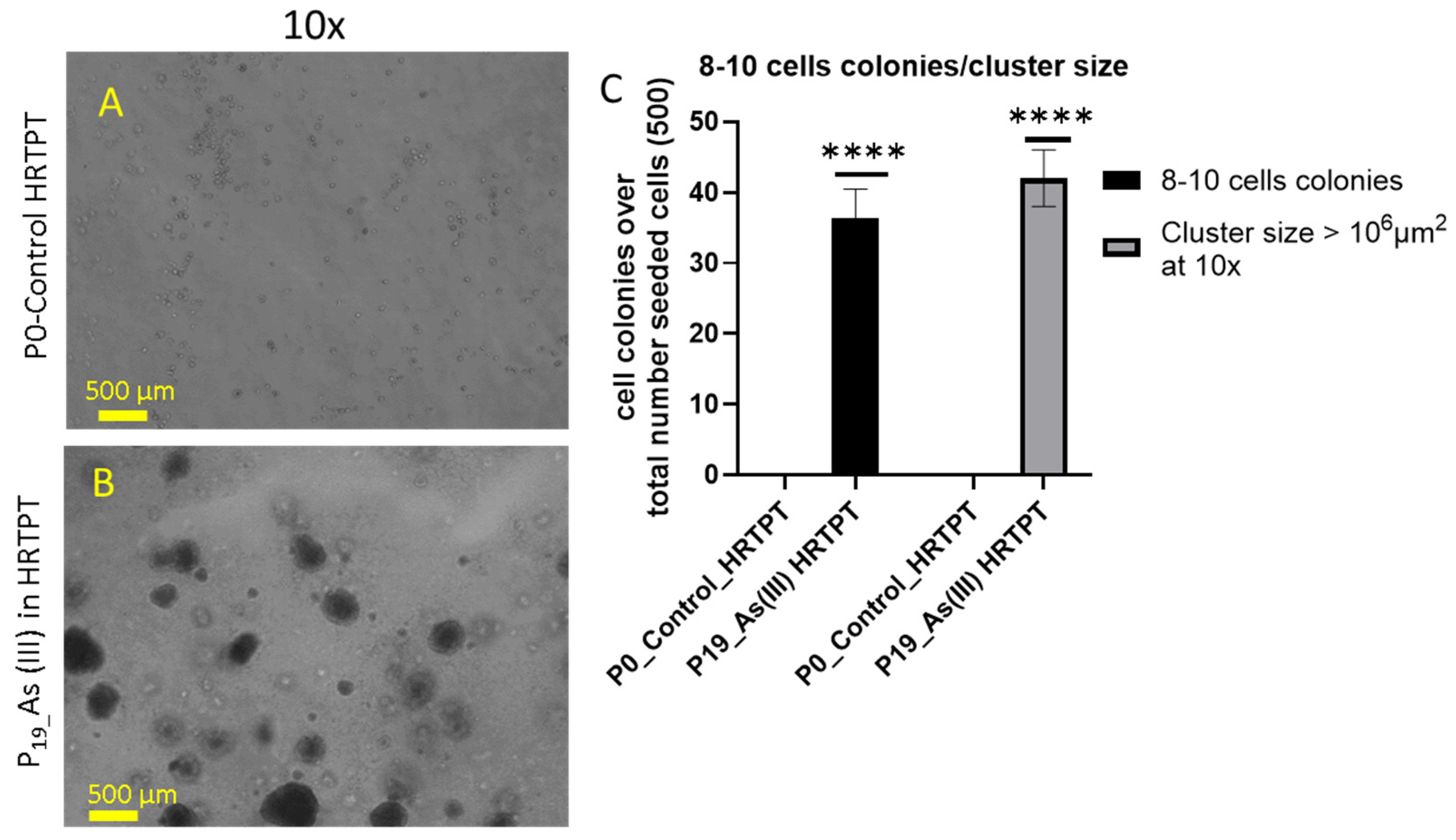
2.8. Soft Agar Assay
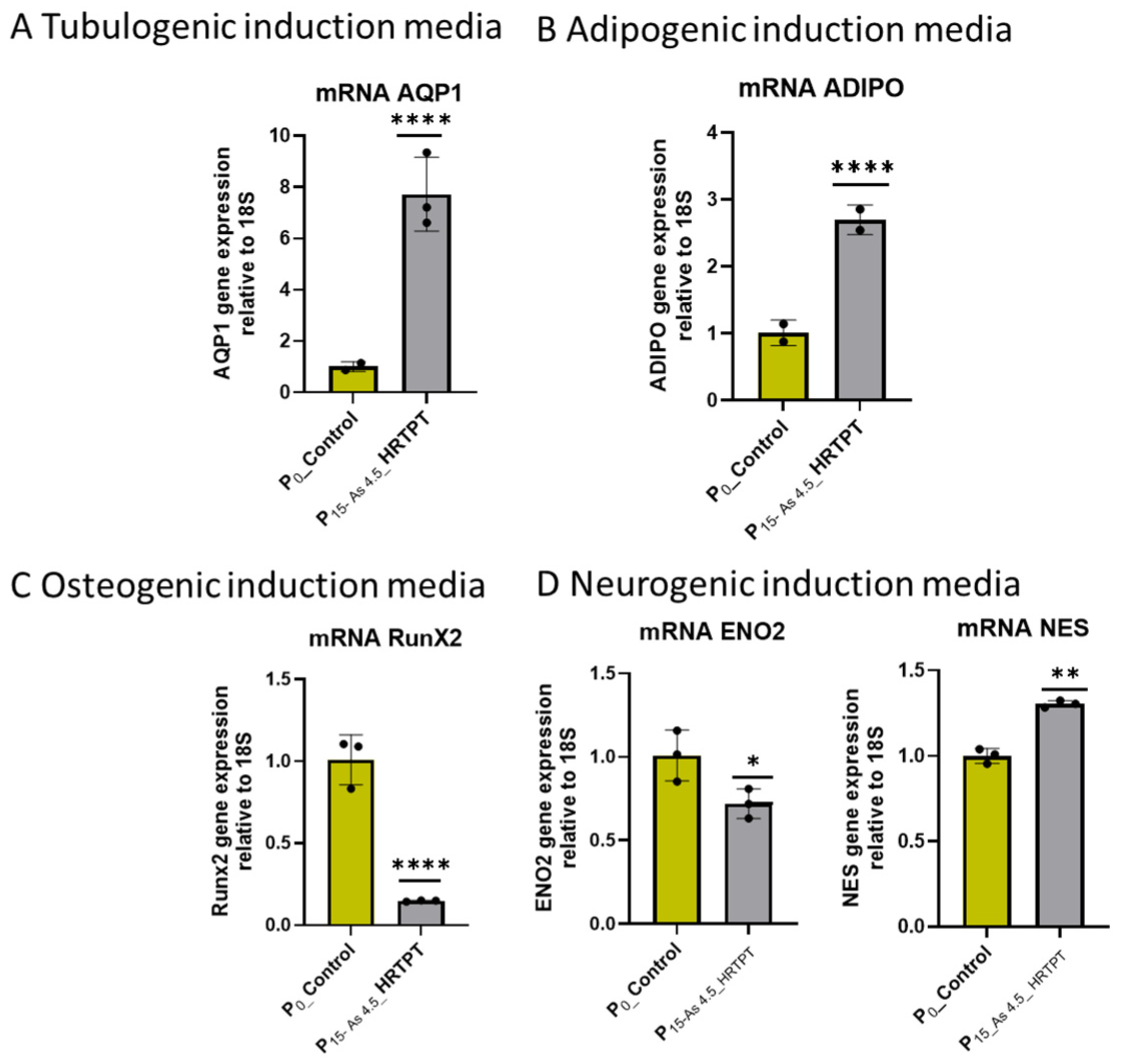
2.9. In Vitro Cell Differentiation Assay
2.10. Strategies for Arsenite Removal from Freeze-Down Chronic Arsenite-Exposed Cells
2.11. Tumor Formation in Immunocompromised Mice
2.12. Statistical Analysis
3. Results
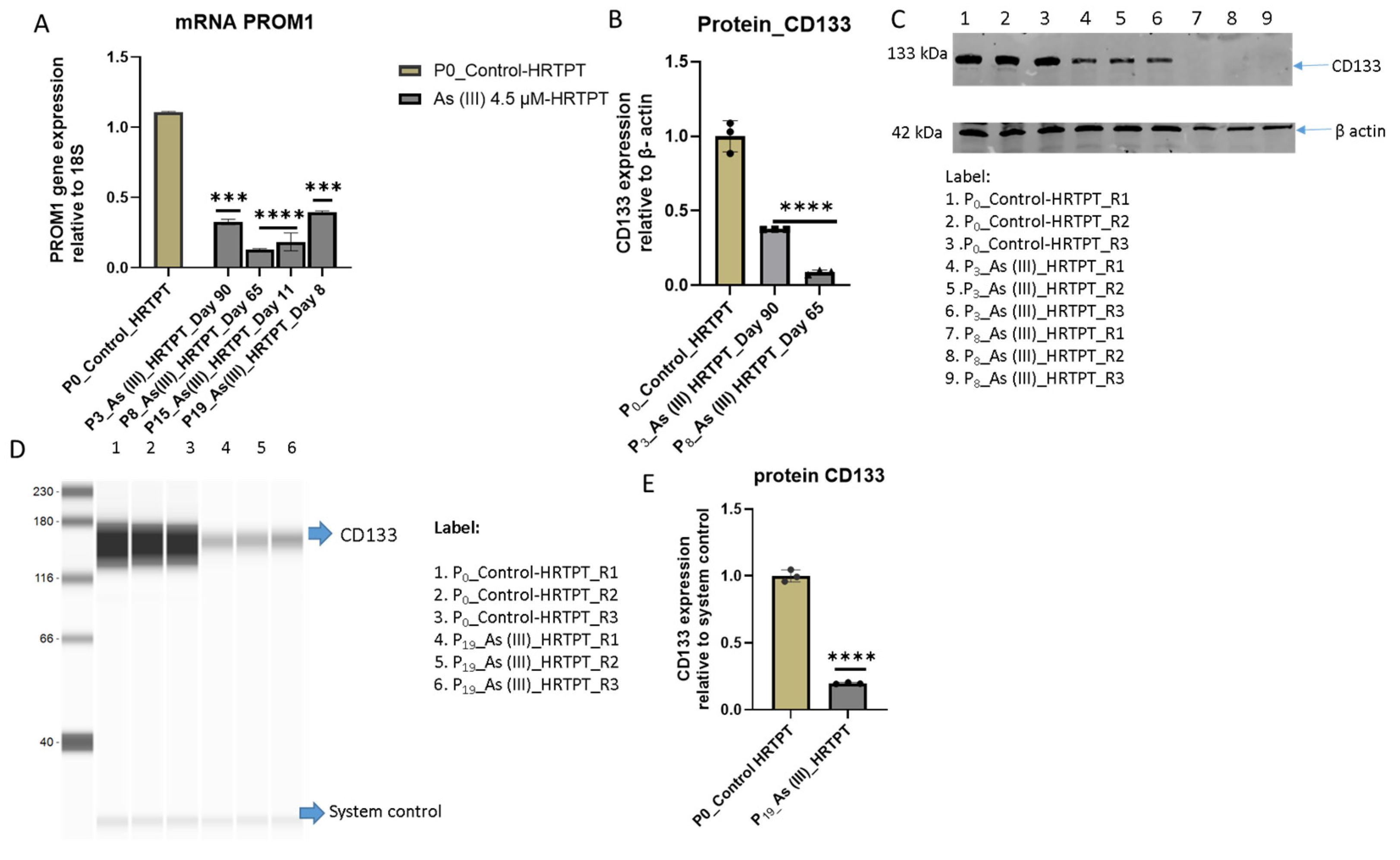
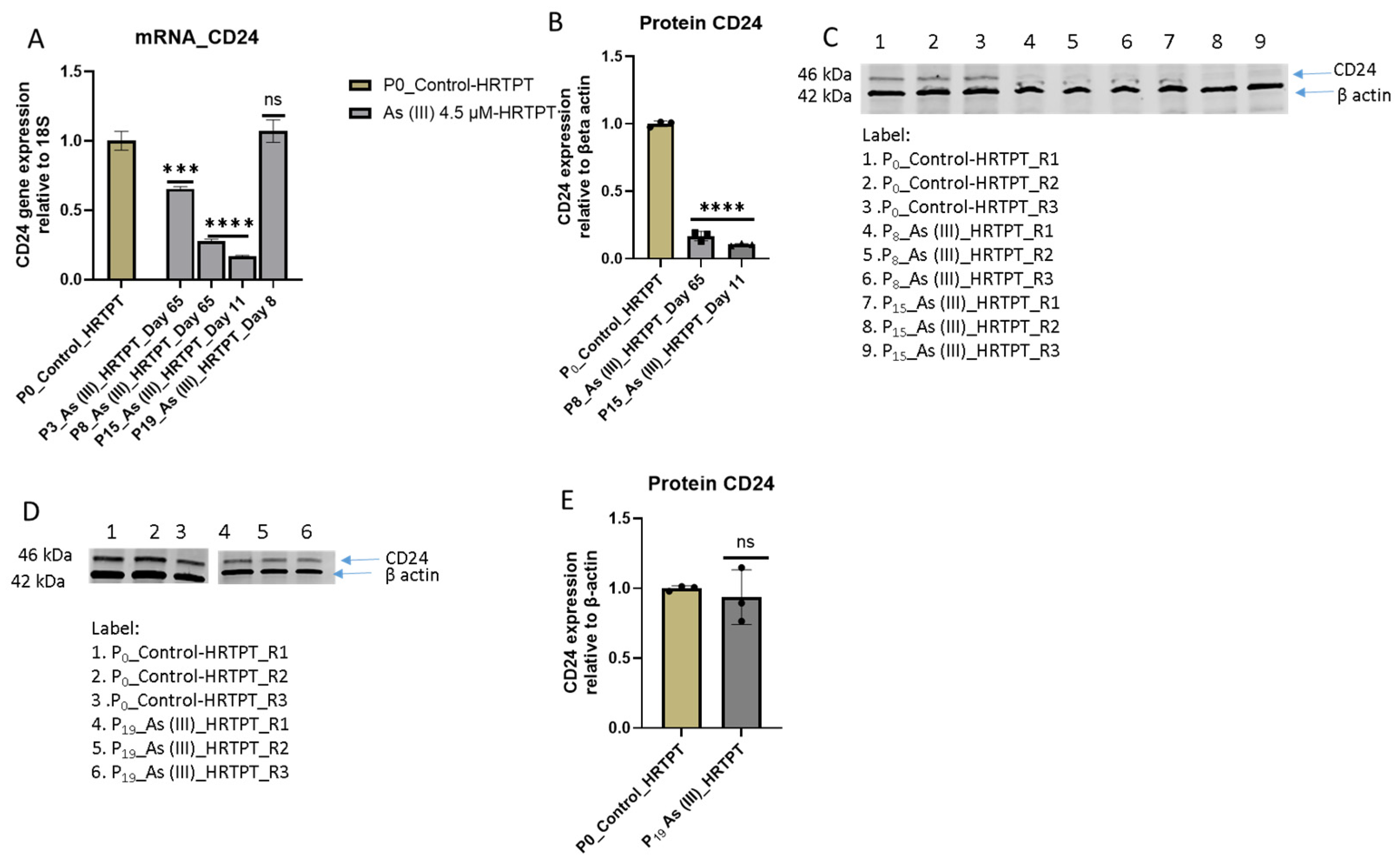
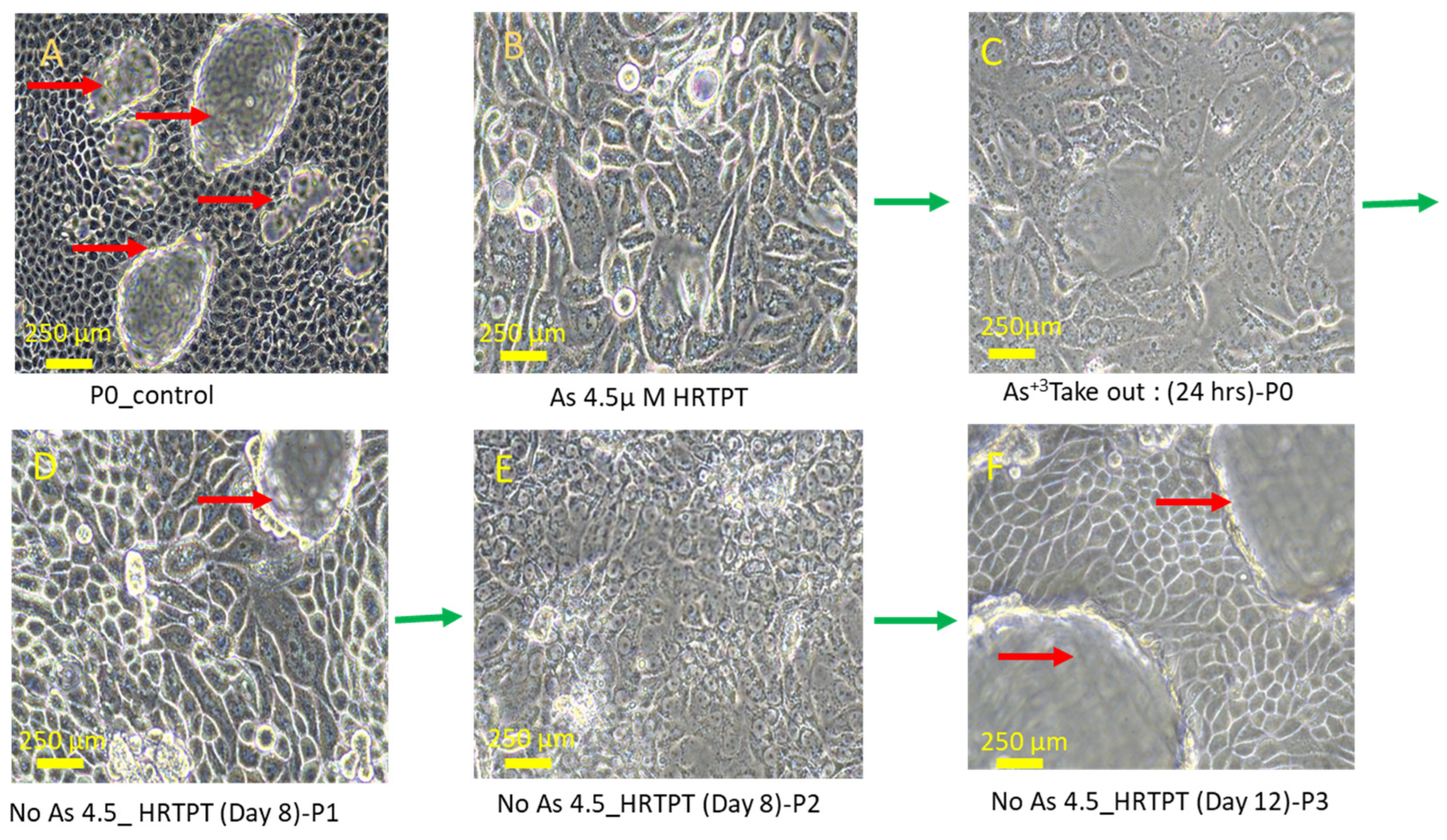
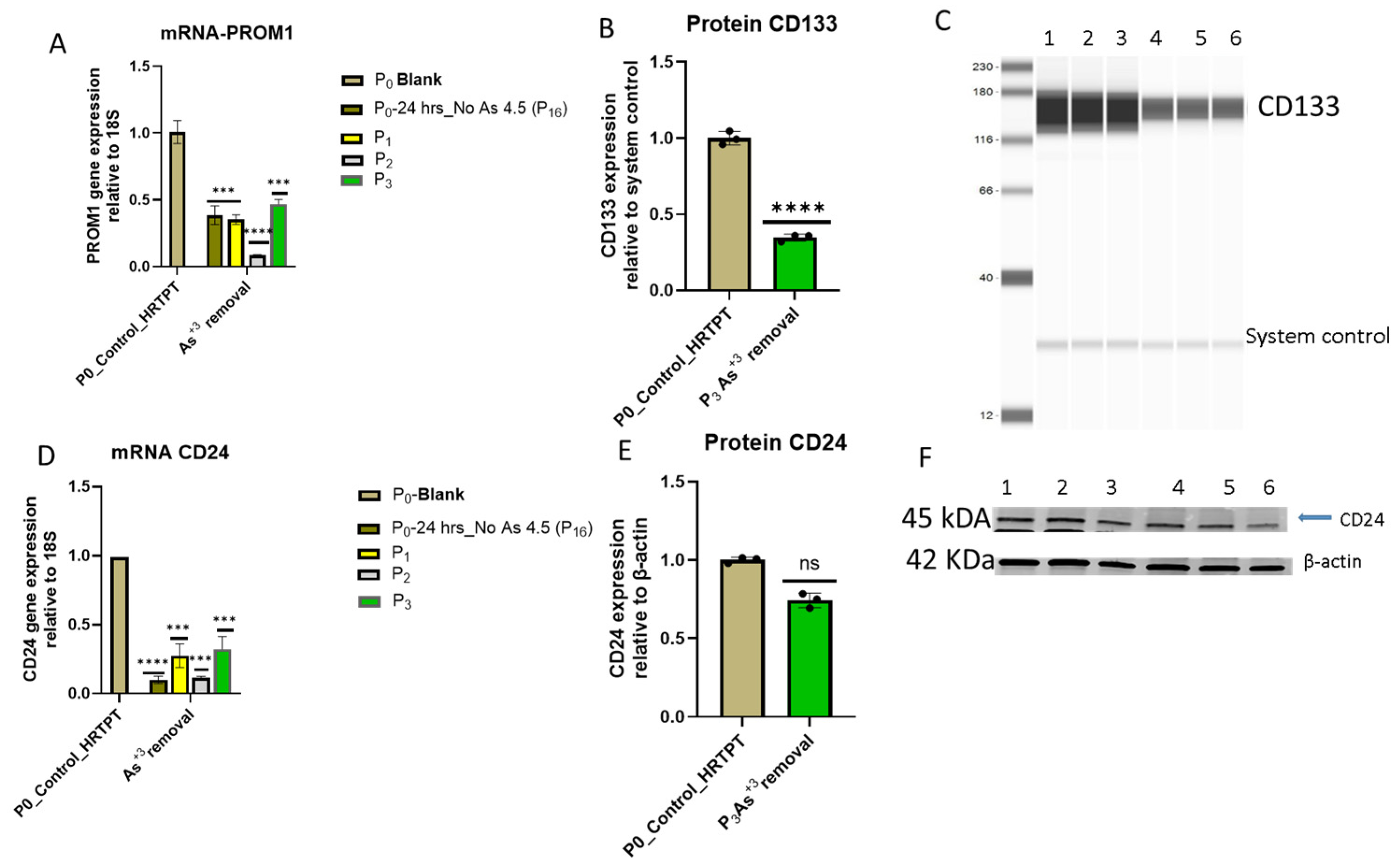
3.1. Growth, Morphology, and PROM1 and CD24 Expression of HRTPT Cells Exposed to 4.5 µM of i-As (III) for 30 Population Doublings
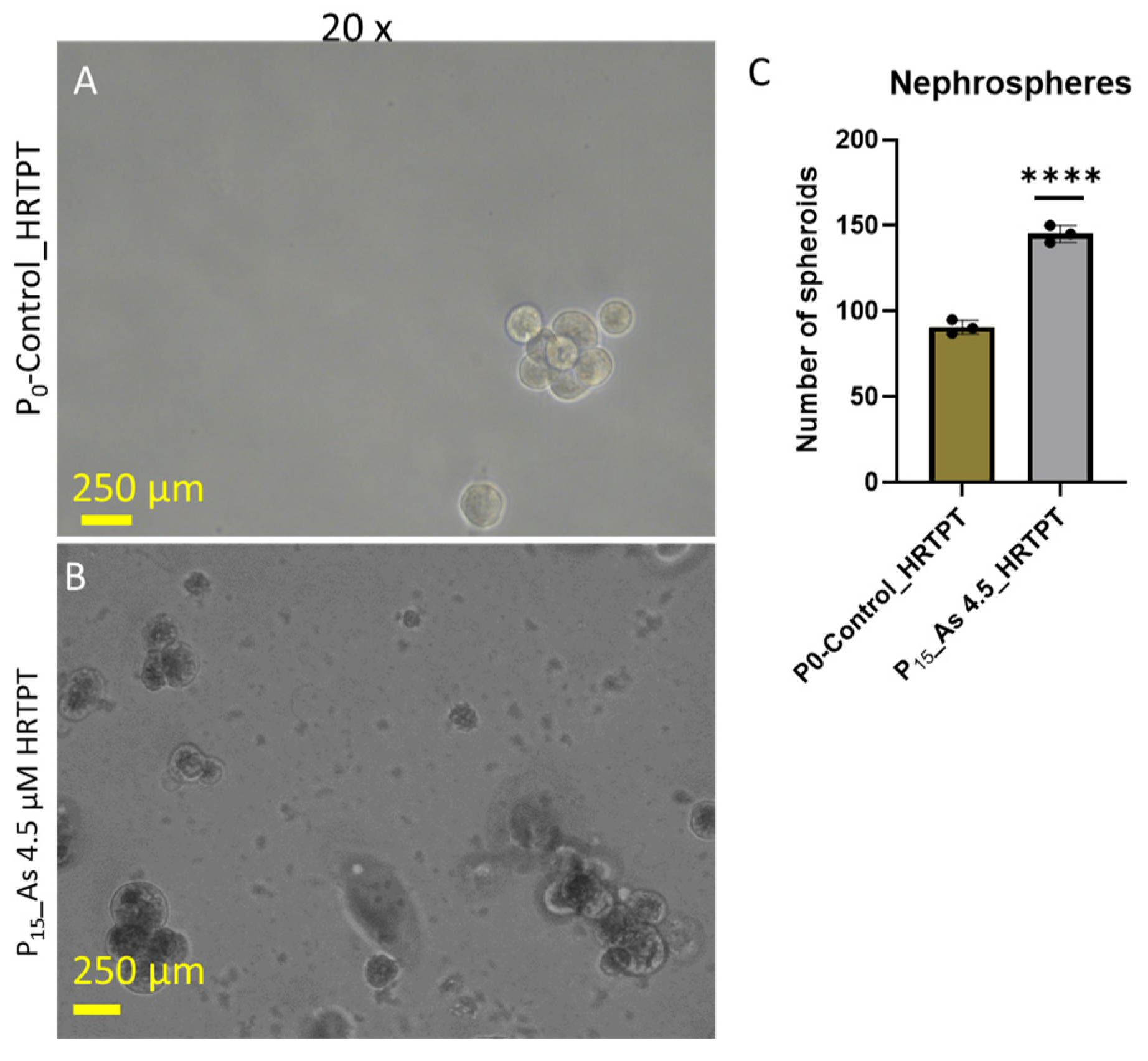
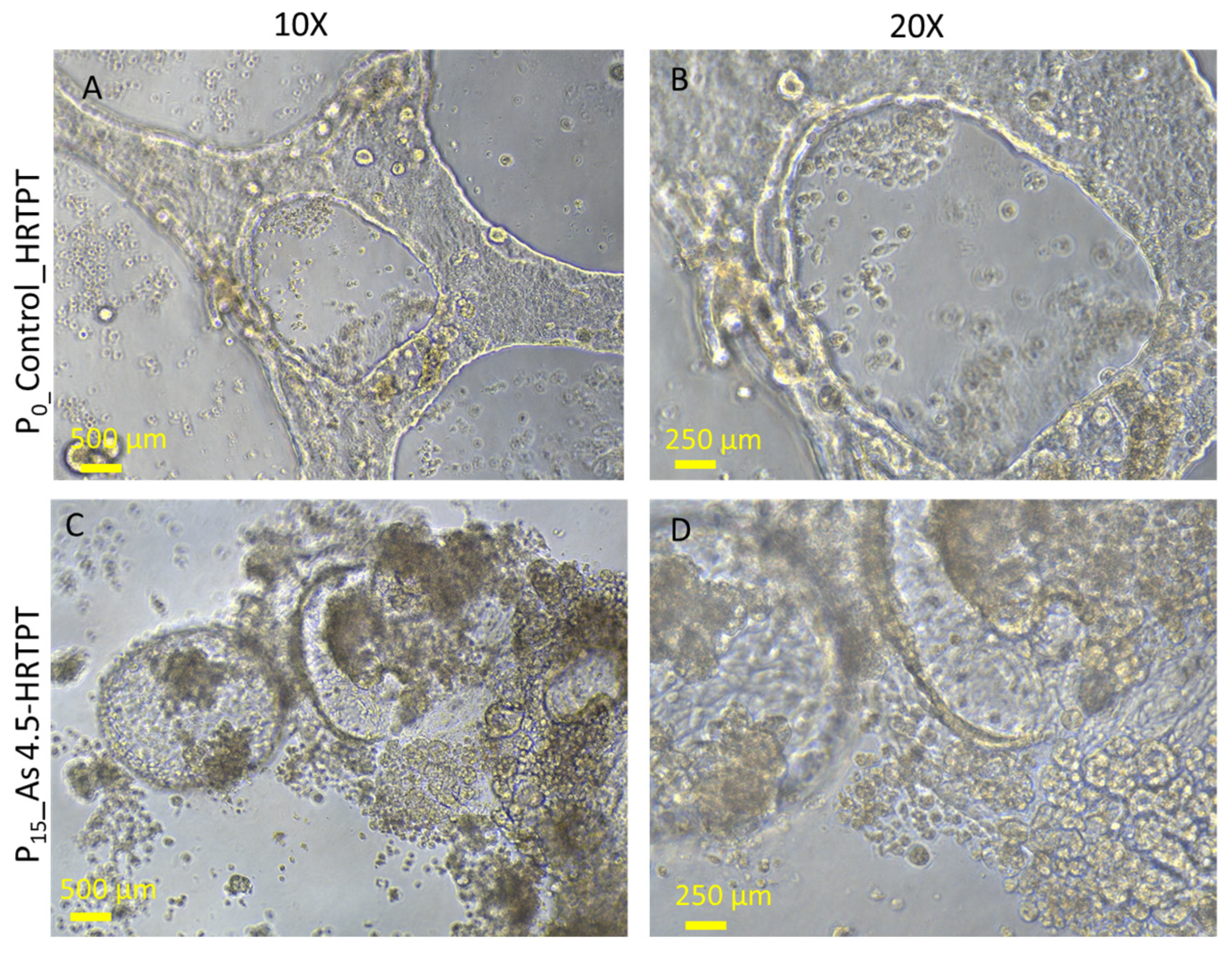
3.2. Progenitor Properties of HRTPT Cells Exposed to 4.5 µM of i-As (III) for 15 Serial Passages/Population Doublings
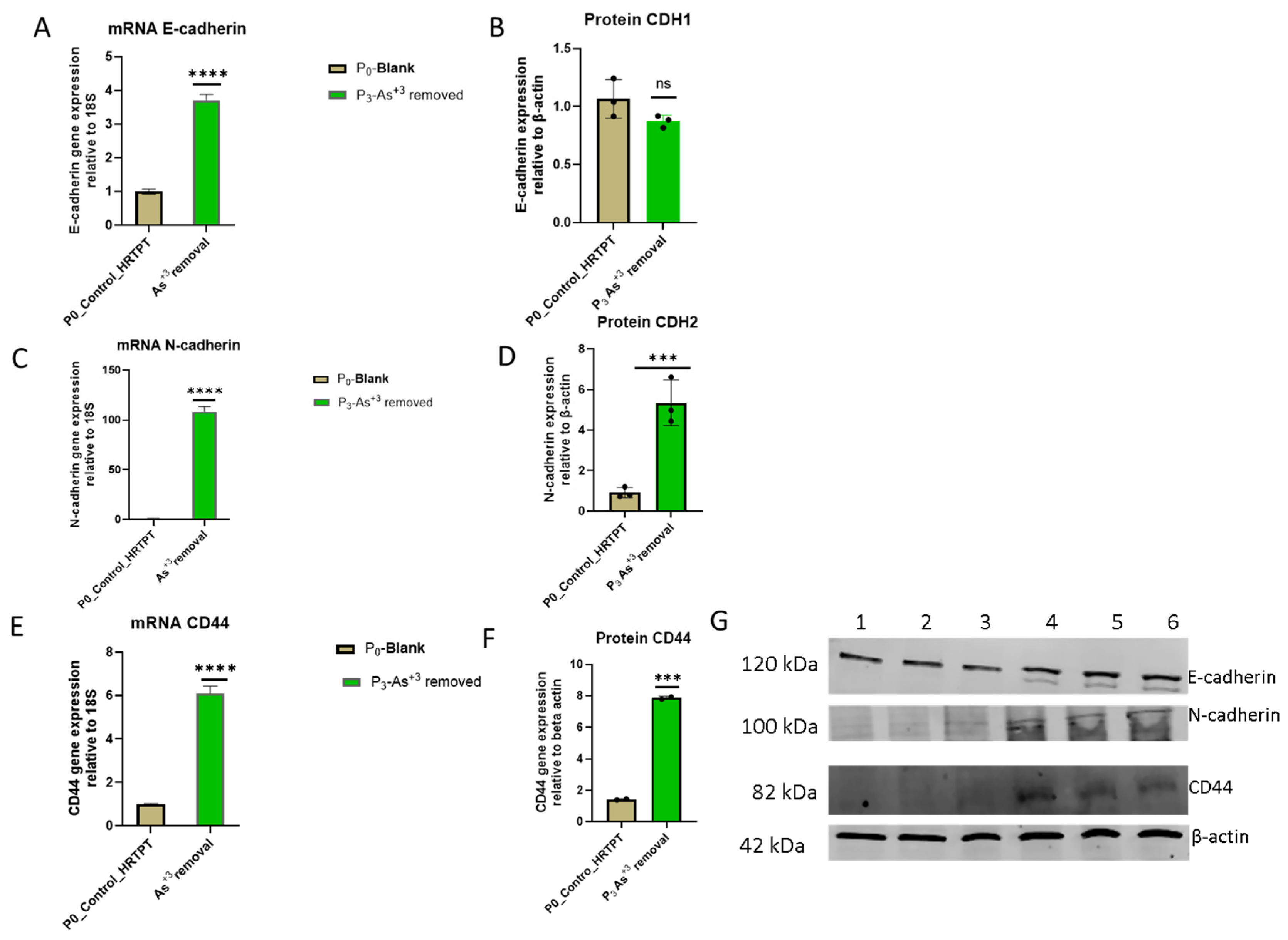
3.3. Epithelial–Mesenchymal Transition (EMT) in HRTPT Cells Exposed to i-As (III) for 19 Passages (30 Population Doublings)
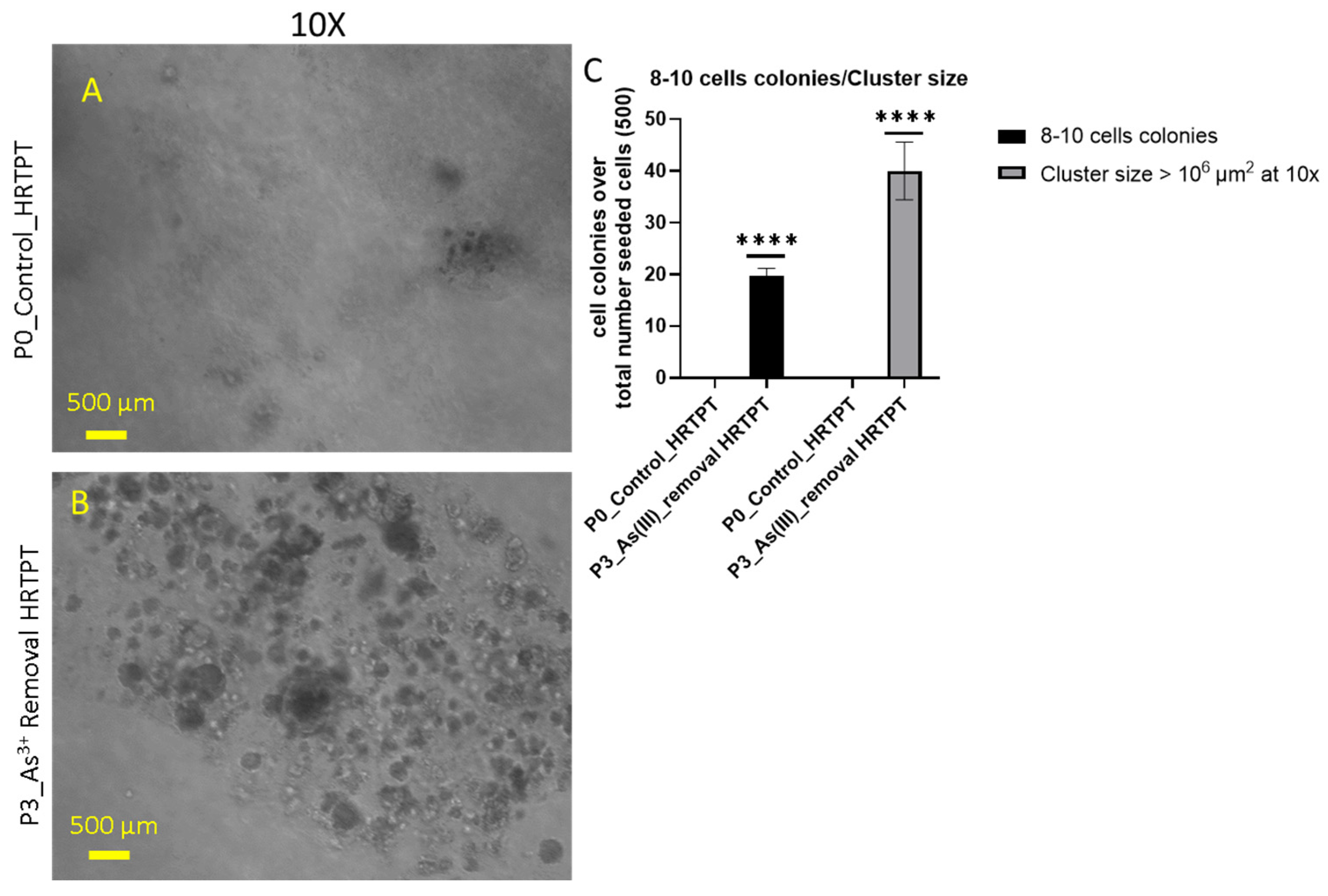
3.4. As3+ Anchorage-Independent Growth and CD44 Expression of HRTPT Cells Exposed to i-As (III) for 19 Passages (30 Population Doublings)
3.5. Effect of iAs (III) Removal from HRTPT Cells Exposed to iAs (III) for 19 Passages
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Ayotte, J.D.; Medalie, L.; Qi, S.L.; Backer, L.C.; Nolan, B.T. Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environ. Sci. Technol. 2017, 51, 12443–12454. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K. An Overview of Arsenic Mass-Poisoning in Bangladesh and West Bengal, India; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2000. Available online: https://pubs.usgs.gov/publication/70198882 (accessed on 25 January 2025).
- Cohen, S.M.; Arnold, L.L.; Eldan, M.; Lewis, A.S.; Beck, B.D. Methylated arsenicals: The implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit. Rev. Toxicol. 2006, 36, 99–133. [Google Scholar] [CrossRef]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef]
- Hsu, L.I.; Hsieh, F.I.; Wang, Y.H.; Lai, T.S.; Wu, M.M.; Chen, C.J.; Chiou, H.Y.; Hsu, K.H. Arsenic Exposure from Drinking Water and the Incidence of CKD in Low to Moderate Exposed Areas of Taiwan: A 14-Year Prospective Study. Am. J. Kidney Dis. 2017, 70, 787–797. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Gacitúa, T.A.; Gomes-Neto, A.W.; Osté, M.C.J.; Pol, R.A.; Ferreccio, C.; Berger, S.P.; Chong, G.; et al. Circulating Arsenic is Associated with Long-Term Risk of Graft Failure in Kidney Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2020, 9, 417. [Google Scholar] [CrossRef]
- Robles-Osorio, M.L.; Sabath-Silva, E.; Sabath, E. Arsenic-mediated nephrotoxicity. Ren. Fail. 2015, 37, 542–547. [Google Scholar] [CrossRef]
- Peters, B.A.; Hall, M.N.; Liu, X.; Neugut, Y.D.; Pilsner, J.R.; Levy, D.; Ilievski, V.; Slavkovich, V.; Islam, T.; Factor-Litvak, P.; et al. Creatinine, arsenic metabolism, and renal function in an arsenic-exposed population in Bangladesh. PLoS ONE 2014, 9, e113760. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Udensi, U.K.; Pacurari, M.; Stevens, J.J.; Patlolla, A.K.; Noubissi, F.; Kumar, S. State of the science review of the health effects of inorganic arsenic: Perspectives for future research. Environ. Toxicol. 2019, 34, 188–202. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Smeets, B.; Boor, P.; Dijkman, H.; Sharma, S.V.; Jirak, P.; Mooren, F.; Berger, K.; Bornemann, J.; Gelman, I.H.; Floege, J.; et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J. Pathol. 2013, 229, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Remuzzi, G. CD133+ renal stem cells always co-express CD24 in adult human kidney tissue. Stem Cell Res. 2014, 12, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Lasagni, L.; Remuzzi, G. Renal progenitors: An evolutionary conserved strategy for kidney regeneration. Nat. Rev. Nephrol. 2013, 9, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kong, Y.; Xie, C.; Zhou, L. Stem/progenitor cell in kidney: Characteristics, homing, coordination, and maintenance. Stem Cell Res. Ther. 2021, 12, 197. [Google Scholar] [CrossRef]
- Lindgren, D.; Boström, A.K.; Nilsson, K.; Hansson, J.; Sjölund, J.; Möller, C.; Jirström, K.; Nilsson, E.; Landberg, G.; Axelson, H.; et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am. J. Pathol. 2011, 178, 828–837. [Google Scholar] [CrossRef]
- Becherucci, F.; Roperto, R.M.; Materassi, M.; Romagnani, P. Chronic kidney disease in children. Clin. Kidney J. 2016, 9, 583–591. [Google Scholar] [CrossRef]
- Shrestha, S.; Singhal, S.; Kalonick, M.; Guyer, R.; Volkert, A.; Somji, S.; Garrett, S.H.; Sens, D.A.; Singhal, S.K. Role of HRTPT in kidney proximal epithelial cell regeneration: Integrative differential expression and pathway analyses using microarray and scRNA-seq. J. Cell. Mol. Med. 2021, 25, 10466–10479. [Google Scholar] [CrossRef]
- Shrestha, S.; Somji, S.; Sens, D.A.; Slusser-Nore, A.; Patel, D.H.; Savage, E.; Garrett, S.H. Human renal tubular cells contain CD24/CD133 progenitor cell populations: Implications for tubular regeneration after toxicant induced damage using cadmium as a model. Toxicol. Appl. Pharmacol. 2017, 331, 116–129. [Google Scholar] [CrossRef]
- Singhal, S.; Garrett, S.H.; Somji, S.; Schaefer, K.; Bansal, B.; Gill, J.S.; Singhal, S.K.; Sens, D.A. Arsenite Exposure to Human RPCs (HRTPT) Produces a Reversible Epithelial Mesenchymal Transition (EMT): In-Vitro and In-Silico Study. Int. J. Mol. Sci. 2023, 24, 5092. [Google Scholar] [CrossRef]
- Bahrami, M.; Darabi, S.; Roozbahany, N.A.; Abbaszadeh, H.A.; Moghadasali, R. Great potential of renal progenitor cells in kidney: From the development to clinic. Exp. Cell Res. 2024, 434, 113875. [Google Scholar] [CrossRef]
- Shrestha, S.; Garrett, S.H.; Sens, D.A.; Zhou, X.D.; Guyer, R.; Somji, S. Characterization and determination of cadmium resistance of CD133+/CD24+ and CD133−/CD24+ cells isolated from the immortalized human proximal tubule cell line, RPTEC/TERT1. Toxicol. Appl. Pharmacol. 2019, 375, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Mehus, A.A.; Jones, M.; Trahan, M.; Kinnunen, K.; Berwald, K.; Lindner, B.; Al-Marsoummi, S.; Zhou, X.D.; Garrett, S.H.; Sens, D.A.; et al. Pevonedistat Inhibits SOX2 Expression and Sphere Formation but Also Drives the Induction of Terminal Differentiation Markers and Apoptosis within Arsenite-Transformed Urothelial Cells. Int. J. Mol. Sci. 2023, 24, 9149. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhou, X.D.; Sens, M.A.; Garrett, S.H.; Zheng, Y.; Dunlevy, J.R.; Sens, D.A.; Somji, S. Keratin 6 expression correlates to areas of squamous differentiation in multiple independent isolates of As+3-induced bladder cancer. J. Appl. Toxicol. 2010, 30, 416–430. [Google Scholar] [CrossRef]
- Kalyan, G.; Slusser-Nore, A.; Dunlevy, J.R.; Bathula, C.S.; Shabb, J.B.; Muhonen, W.; Somji, S.; Sens, D.A.; Garrett, S.H.; Theilig, F. Protein interactions with metallothionein-3 promote vectorial active transport in human proximal tubular cells. PLoS ONE 2022, 17, e0267599. [Google Scholar] [CrossRef]
- Larson, J.; Yasmin, T.; Sens, D.A.; Zhou, X.D.; Sens, M.A.; Garrett, S.H.; Dunlevy, J.R.; Cao, L.; Somji, S. SPARC gene expression is repressed in human urothelial cells (UROtsa) exposed to or malignantly transformed by cadmium or arsenite. Toxicol. Lett. 2010, 199, 166–172. [Google Scholar] [CrossRef][Green Version]
- Sagrinati, C.; Netti, G.S.; Mazzinghi, B.; Lazzeri, E.; Liotta, F.; Frosali, F.; Ronconi, E.; Meini, C.; Gacci, M.; Squecco, R.; et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006, 17, 2443–2456. [Google Scholar] [CrossRef]
- McLaren, J.; Whiting, P.; Simpson, J.; Hawksworth, G. Isolation and characterisation of human proximal tubular cells derived from kidney cortical segments. Hum. Exp. Toxicol. 1995, 14, 916–922. [Google Scholar] [CrossRef]
- Du, F.; Zhao, X.; Fan, D. Soft Agar Colony Formation Assay as a Hallmark of Carcinogenesis. Bio Protoc. 2017, 7, e2351. [Google Scholar] [CrossRef]
- Roberts, A.B.; Anzano, M.A.; Wakefield, L.M.; Roche, N.S.; Stern, D.F.; Sporn, M.B. Type beta transforming growth factor: A bifunctional regulator of cellular growth. Proc. Natl. Acad. Sci. USA 1985, 82, 119–123. [Google Scholar] [CrossRef]
- San, R.H.; Laspia, M.F.; Soiefer, A.I.; Maslansky, C.J.; Rice, J.M.; Williams, G.M. A survey of growth in soft agar and cell surface properties as markers for transformation in adult rat liver epithelial-like cell cultures. Cancer Res. 1979, 39, 1026–1034. [Google Scholar]
- Sens, D.A.; Park, S.; Gurel, V.; Sens, M.A.; Garrett, S.H.; Somji, S. Inorganic Cadmium- and Arsenite-Induced Malignant Transformation of Human Bladder Urothelial Cells. Toxicol. Sci. 2004, 79, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, E.; Crescioli, C.; Ronconi, E.; Mazzinghi, B.; Sagrinati, C.; Netti, G.S.; Angelotti, M.L.; Parente, E.; Ballerini, L.; Cosmi, L.; et al. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J. Am. Soc. Nephrol. 2007, 18, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Sandquist, E.J.; Somji, S.; Dunlevy, J.R.; Garrett, S.H.; Zhou, X.D.; Slusser-Nore, A.; Sens, D.A. Loss of N-Cadherin Expression in Tumor Transplants Produced from As+3- and Cd+2-Transformed Human Urothelial (UROtsa) Cell Lines. PLoS ONE 2016, 11, e0156310. [Google Scholar] [CrossRef] [PubMed]
- Appel, D.; Kershaw, D.B.; Smeets, B.; Yuan, G.; Fuss, A.; Frye, B.; Elger, M.; Kriz, W.; Floege, J.; Moeller, M.J. Recruitment of Podocytes from Glomerular Parietal Epithelial Cells. J. Am. Soc. Nephrol. 2009, 20, 333–343. [Google Scholar] [CrossRef]
- Chrabańska, M.; Rynkiewicz, M.; Kiczmer, P.; Drozdzowska, B. Does the Immunohistochemical Expression of CD44, MMP-2, and MMP-9 in Association with the Histopathological Subtype of Renal Cell Carcinoma Affect the Survival of Patients with Renal Cancer? Cancers 2023, 15, 1202. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121 Pt 6, 727–735. [Google Scholar] [CrossRef]
- Xu, H.; Xu, W.H.; Ren, F.; Wang, J.; Wang, H.K.; Cao, D.L.; Shi, G.H.; Qu, Y.Y.; Zhang, H.L.; Ye, D.W. Prognostic value of epithelial-mesenchymal transition markers in clear cell renal cell carcinoma. Aging 2020, 12, 866–883. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Matsushita, K.; Toyoda, T.; Akane, H.; Morikawa, T.; Ogawa, K. Role of CD44 expressed in renal tubules during maladaptive repair in renal fibrogenesis in an allopurinol-induced rat model of chronic kidney disease. J. Appl. Toxicol. 2024, 44, 455–469. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Su, C.T.; Chung, C.J.; Pu, Y.S.; Chu, J.S.; Yang, H.Y.; Wu, C.C.; Hsueh, Y.M. Urinary total arsenic and 8-hydroxydeoxyguanosine are associated with renal cell carcinoma in an area without obvious arsenic exposure. Toxicol. Appl. Pharmacol. 2012, 262, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jacques, N.; Brown, P.; Nauta, L.; Boxall, J.; Parker, L.; Dummer, T.J.B. Estimating the risk of bladder and kidney cancer from exposure to low-levels of arsenic in drinking water, Nova Scotia, Canada. Environ. Int. 2018, 110, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lasorsa, F.; Rutigliano, M.; Milella, M.; Ferro, M.; Pandolfo, S.D.; Crocetto, F.; Autorino, R.; Battaglia, M.; Ditonno, P.; Lucarelli, G. Cancer Stem Cells in Renal Cell Carcinoma: Origins and Biomarkers. Int. J. Mol. Sci. 2023, 24, 13179. [Google Scholar] [CrossRef]
- Corrò, C.; Moch, H. Biomarker discovery for renal cancer stem cells. J. Pathol. Clin. Res. 2018, 4, 3–18. [Google Scholar] [CrossRef]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M.; Saha, K.C. A Review of Arsenic Poisoning and its Effects on Human Health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 213–281. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Chang, Y.-T.; Cheng, H.-L.; Shen, K.-H.; Sung, J.-M.; Guo, H.-R. Associations between arsenic in drinking water and occurrence of end-stage renal disease with modifications by comorbidities: A nationwide population-based study in Taiwan. Sci. Total Environ. 2018, 626, 581–591. [Google Scholar] [CrossRef]
- Gama, J.M.; Oliveira, R.C. CD44 and Its Role in Solid Cancers—A Review: From Tumor Progression to Prognosis and Targeted Therapy. Front. Biosci. 2025, 30, 24821. [Google Scholar] [CrossRef]
- Weng, X.; Maxwell-Warburton, S.; Hasib, A.; Ma, L.; Kang, L. The membrane receptor CD44: Novel insights into metabolism. Trends Endocrinol. Metab. 2022, 33, 318–332. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Elakad, O.; Häupl, B.; Labitzky, V.; Yao, S.; Küffer, S.; von Hammerstein-Equord, A.; Danner, B.C.; Jücker, M.; Urlaub, H.; Lange, T.; et al. Activation of CD44/PAK1/AKT signaling promotes resistance to FGFR1 inhibition in squamous-cell lung cancer. NPJ Precis. Oncol. 2022, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X.; Chen, L.; Gu, L.; Zhang, Y.; Zhang, F.; Ouyang, Y.; Gao, Y.; Huang, Q.; Zhang, X. Prognostic value of CD44 expression in renal cell carcinoma: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 13157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, W.; Ma, H.; Zhang, J.; Zhu, L.; Wang, C.; Yang, Y. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 2017, 7, 13856. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Komohara, Y.; Ohnishi, K.; Shimoji, T.; Kuwahara, N.; Sakumura, Y.; Matsuishi, K.; Fujiwara, Y.; Motoshima, T.; Takahashi, W.; et al. Infiltration of tumor-associated macrophages is involved in CD44 expression in clear cell renal cell carcinoma. Cancer Sci. 2016, 107, 700–707. [Google Scholar] [CrossRef]
- Rouschop, K.M.; Sewnath, M.E.; Claessen, N.; Roelofs, J.J.; Hoedemaeker, I.; van der Neut, R.; Aten, J.; Pals, S.T.; Weening, J.J.; Florquin, S. CD44 deficiency increases tubular damage but reduces renal fibrosis in obstructive nephropathy. J. Am. Soc. Nephrol. 2004, 15, 674–686. [Google Scholar] [CrossRef]
- Papanastasiou, A.D.; Peroukidis, S.; Sirinian, C.; Arkoumani, E.; Chaniotis, D.; Zizi-Sermpetzoglou, A. CD44 Expression in Clear Cell Renal Cell Carcinoma (ccRCC) Correlates with Tumor Grade and Patient Survival and Is Affected by Gene Methylation. Genes 2024, 15, 537. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Zanjani, L.S.; Madjd, Z.; Abolhasani, M.; Rasti, A.; Fodstad, O.; Andersson, Y.; Asgari, M. Increased expression of CD44 is associated with more aggressive behavior in clear cell renal cell carcinoma. Biomark. Med. 2018, 12, 45–61. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; De Palma, G.; Picerno, A.; Curci, C.; Sebastiano, S.; Campioni, M.; Cicirelli, A.; Rizzo, A.; Di Lorenzo, V.F.; et al. Human Adult Renal Progenitor Cells Prevent Cisplatin-Nephrotoxicity by Inducing CYP1B1 Overexpression and miR-27b-3p Down-Regulation through Extracellular Vesicles. Cells 2023, 12, 1655. [Google Scholar] [CrossRef]



| Number of Passages | Days to Next Passage at 4.5 μM of As+3 |
|---|---|
| P0 | 1 |
| P1 | 7 |
| P2 | 25 |
| P3 | 90 |
| P4 | 13 |
| P5 | 18 |
| P6 | 45 |
| P7 | 40 |
| P8 | 65 |
| P9 | 37 |
| P10 | 6 |
| P11 | 13 |
| P12 | 12 |
| P13 | 20 |
| P14 | 12 |
| P15 | 11 |
| P16 | 12 |
| P17 | 11 |
| P18 | 7 |
| P19 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.E.; Shrestha, S.; Sens, D.A.; Garrett, S.H. Inorganic Arsenite [As (III)] Represses Human Renal Progenitor Cell Characteristics and Induces Neoplastic-like Transformation. Cells 2025, 14, 877. https://doi.org/10.3390/cells14120877
Haque ME, Shrestha S, Sens DA, Garrett SH. Inorganic Arsenite [As (III)] Represses Human Renal Progenitor Cell Characteristics and Induces Neoplastic-like Transformation. Cells. 2025; 14(12):877. https://doi.org/10.3390/cells14120877
Chicago/Turabian StyleHaque, Md Ehsanul, Swojani Shrestha, Donald A. Sens, and Scott H. Garrett. 2025. "Inorganic Arsenite [As (III)] Represses Human Renal Progenitor Cell Characteristics and Induces Neoplastic-like Transformation" Cells 14, no. 12: 877. https://doi.org/10.3390/cells14120877
APA StyleHaque, M. E., Shrestha, S., Sens, D. A., & Garrett, S. H. (2025). Inorganic Arsenite [As (III)] Represses Human Renal Progenitor Cell Characteristics and Induces Neoplastic-like Transformation. Cells, 14(12), 877. https://doi.org/10.3390/cells14120877






