Cistanoside F Ameliorates Lipid Accumulation and Enhances Myogenic Differentiation via AMPK-Dependent Signaling in C2C12 Myotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
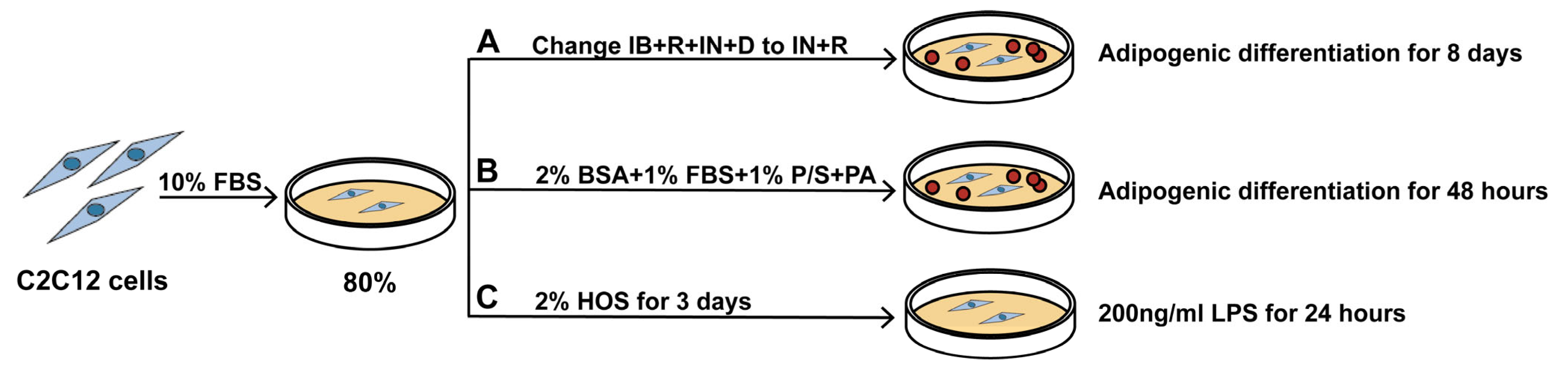
2.2. Cell Culture and Treatments
2.3. Cell Viability Assay
2.4. Oil Red O Staining and High-Content Analysis
2.5. Lipid Tests
2.6. Reverse Transcription Quantitative Polymerase Chain Reaction
2.7. Western Blotting
2.8. ROS and Mitochondrial Membrane Potential Detection (MMP)
2.9. Immunofluorescence
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Molecular Docking
2.12. Statistical Analysis
3. Results
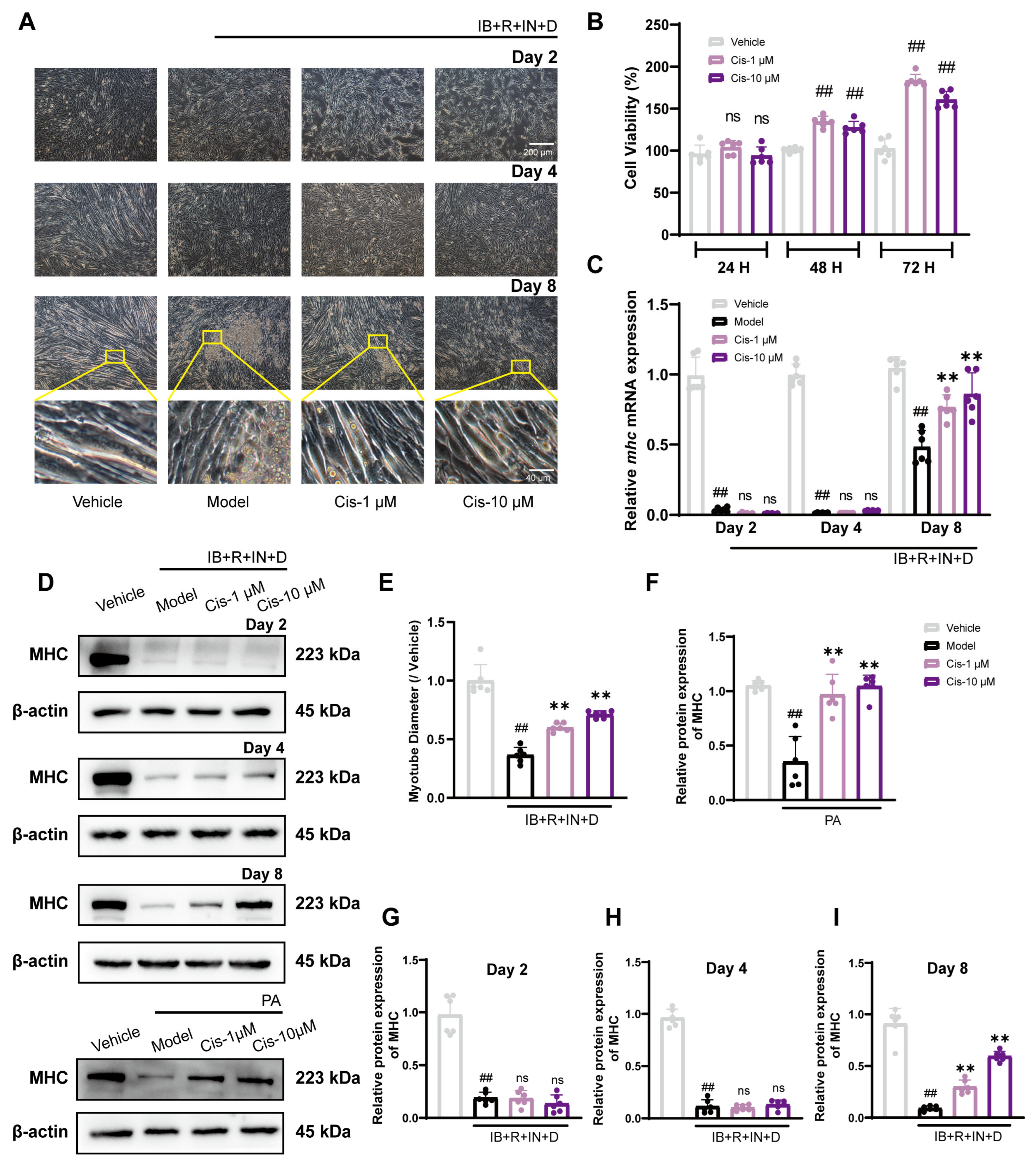
3.1. Cis Enhances C2C12 Myogenic Differentiation in Two Adipogenic Differentiation Models
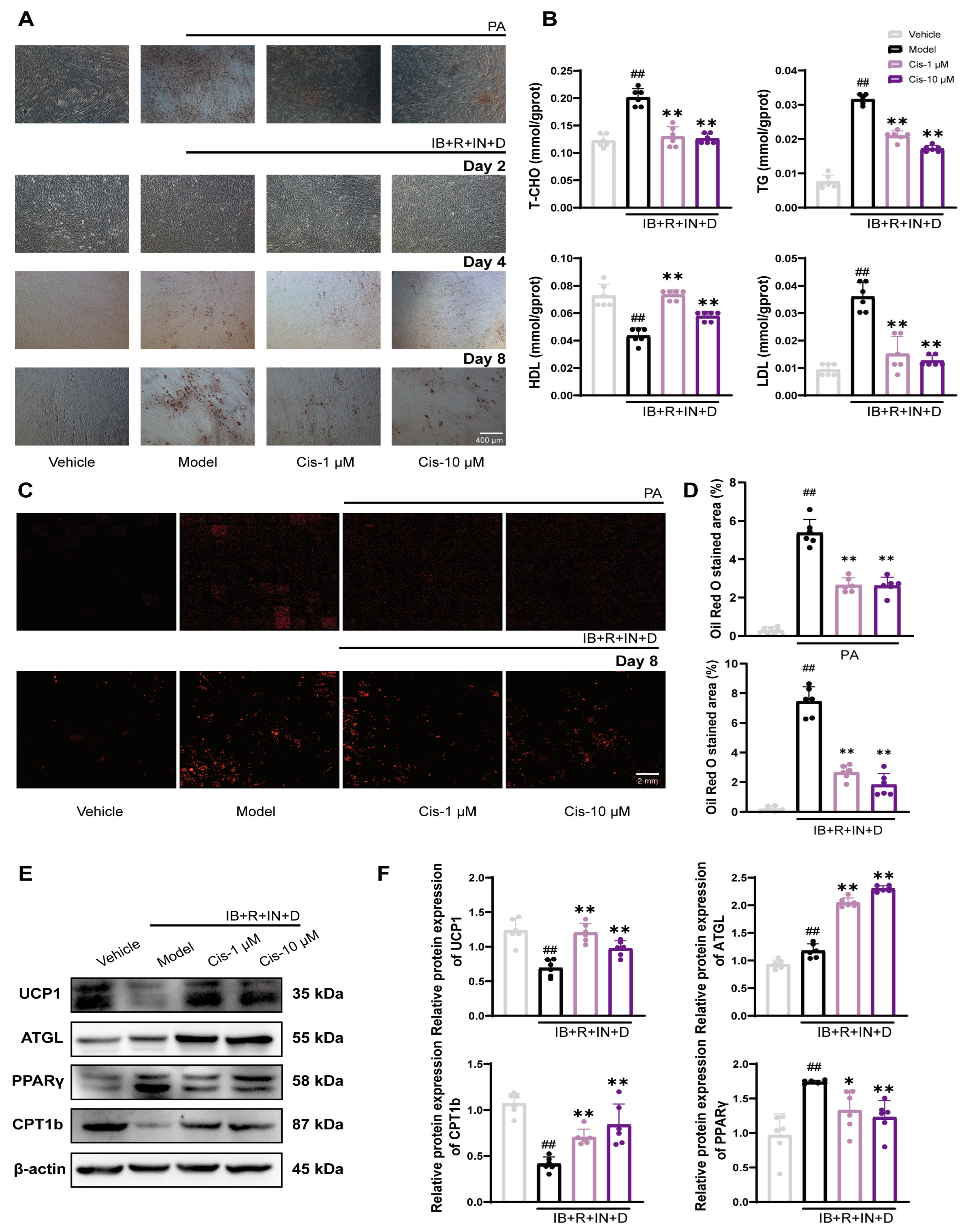
3.2. Cis Reduces Intracellular Lipid Levels in C2C12 Cells
3.3. Cis Effectively Reduces ROS Level and Protects Mitochondrial Function
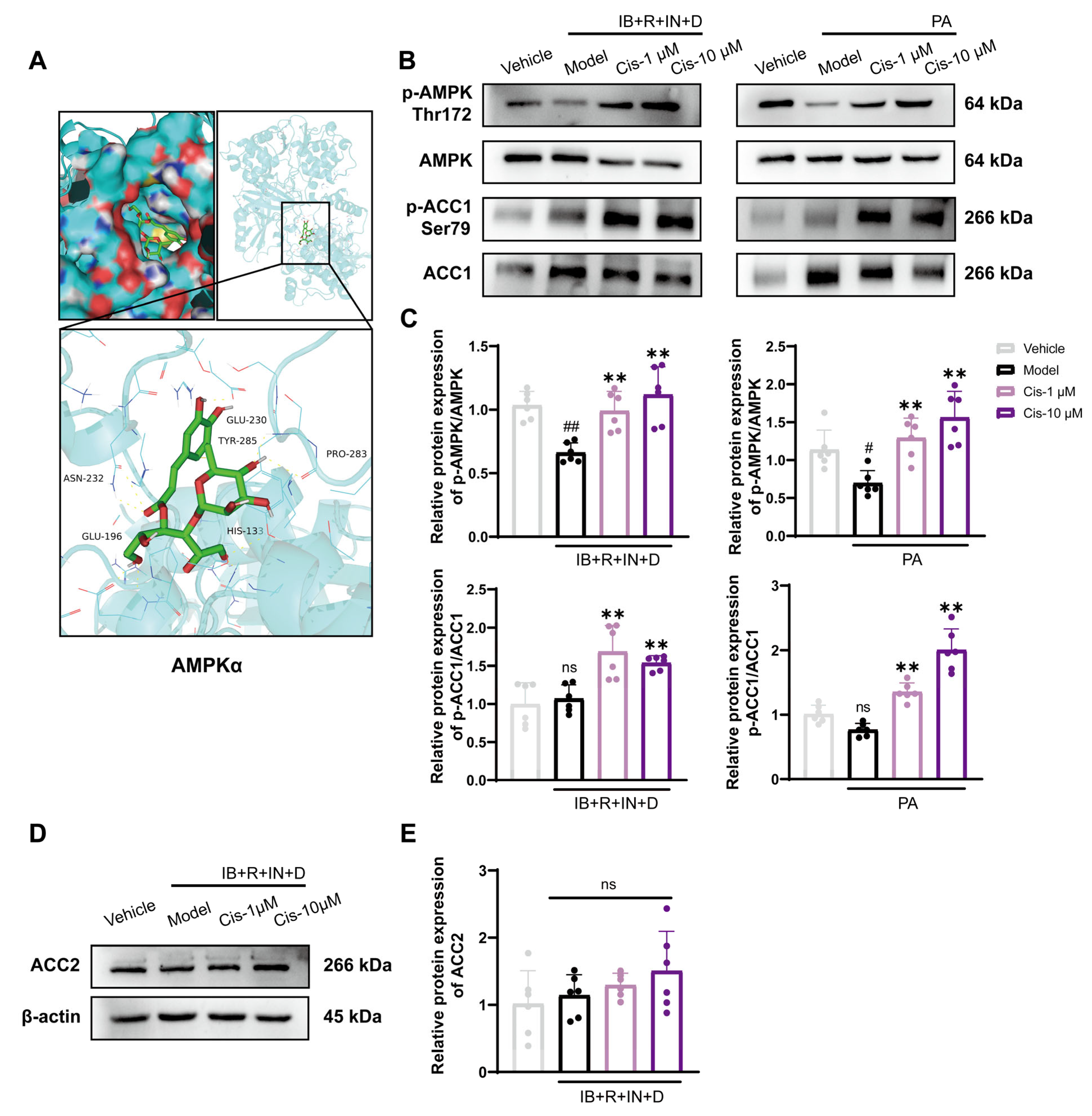
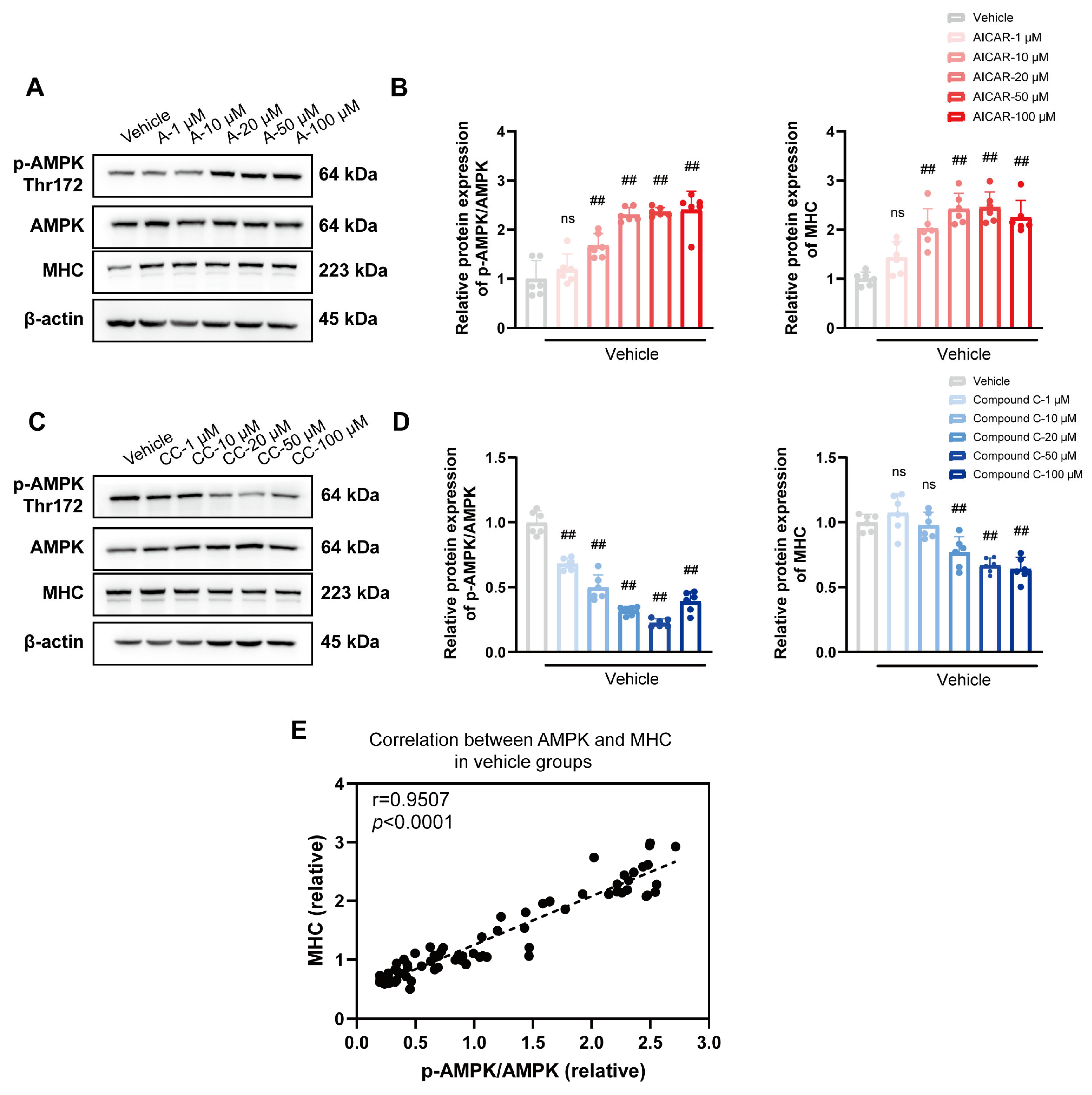
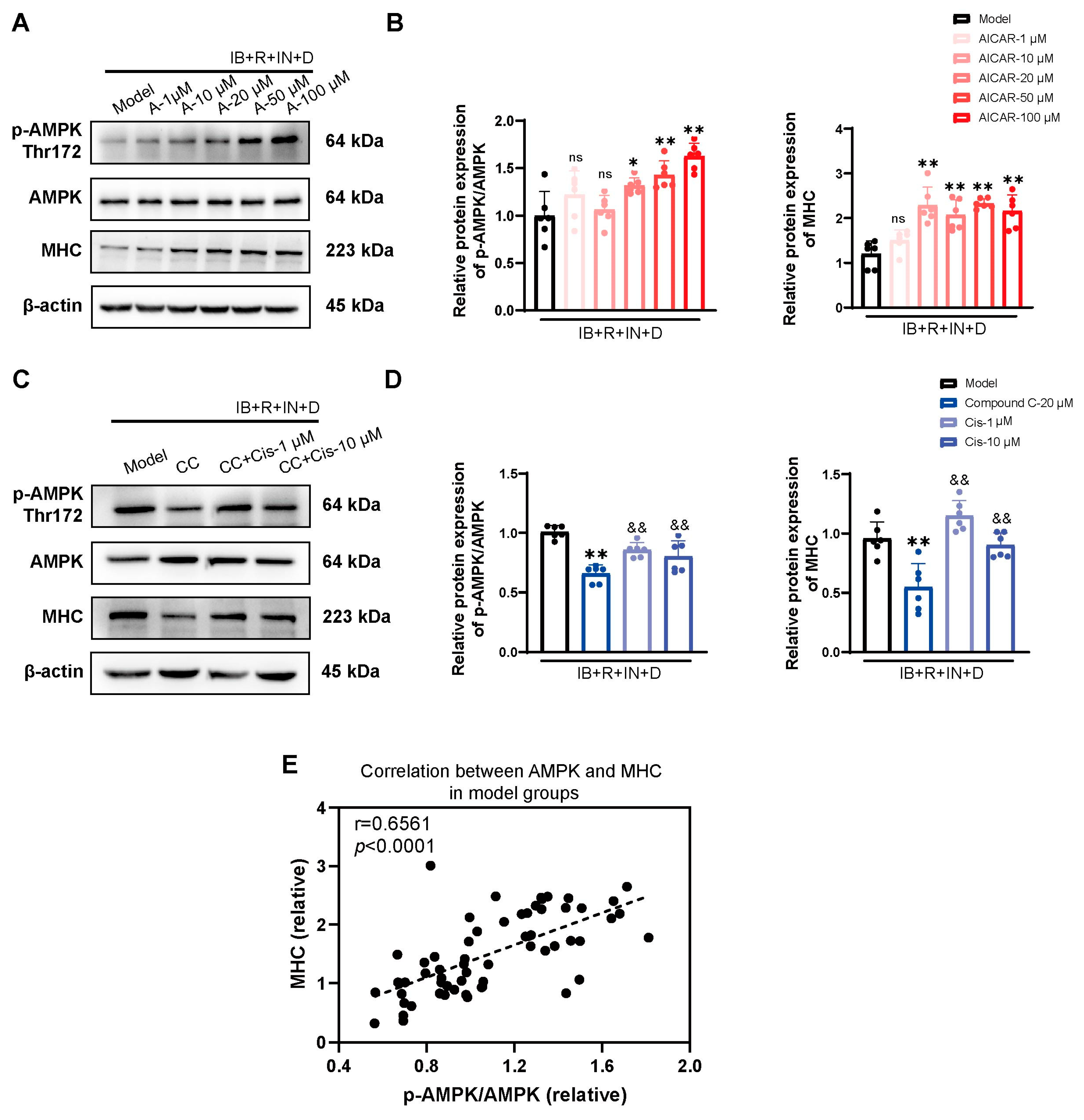
3.4. Role of the Classical AMPK/ACC1 Pathway in Lipid Differentiation
3.5. Cis Effectively Improves the Expression of MHC Associated with AMPK Pathway
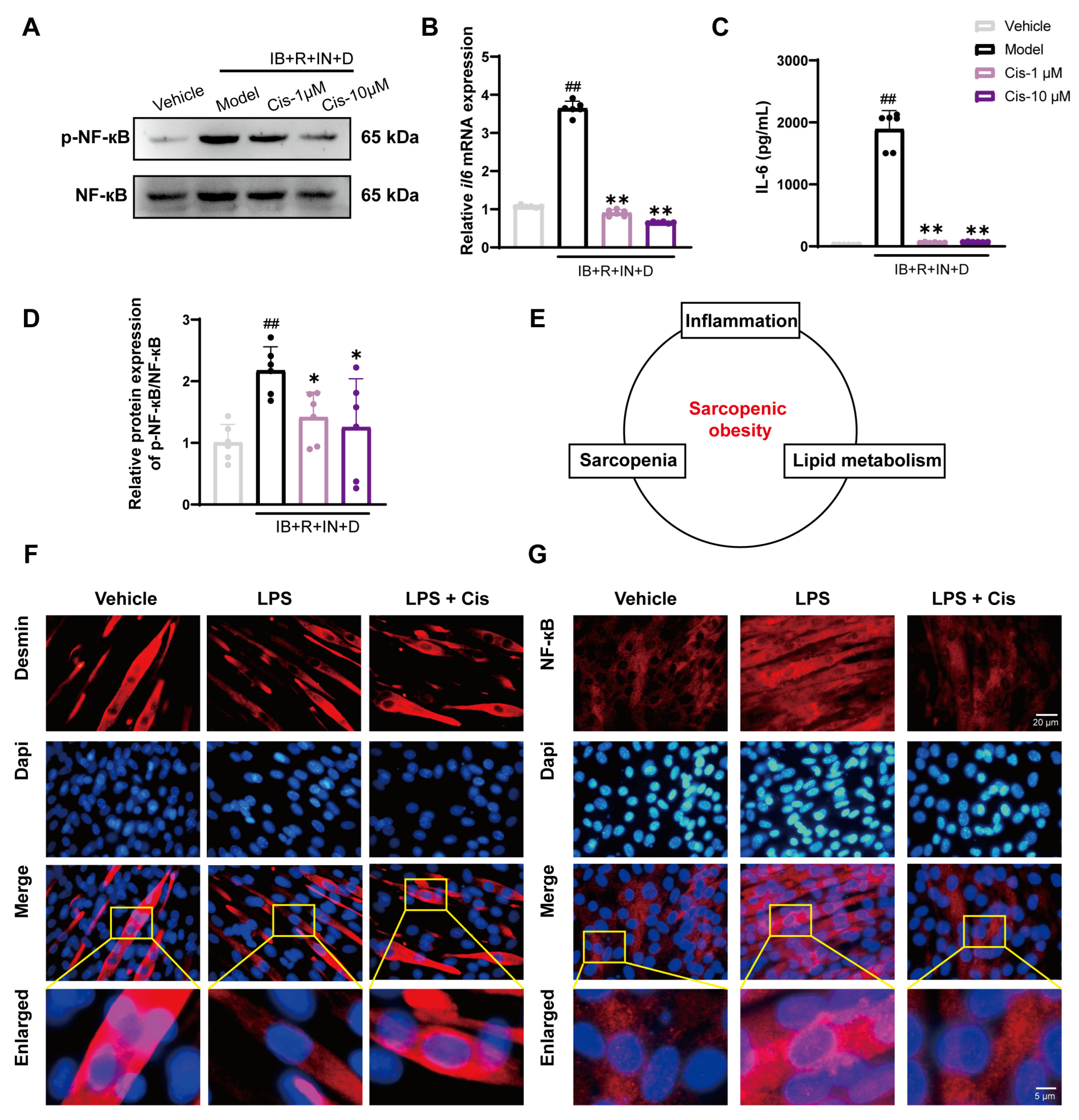
3.6. Cis Effectively Reduces Intracellular Inflammation and Counteracts LPS-Induced Atrophic Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SO | Sarcopenic Obesity |
| Cis | Cistanoside F |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ATGL | Adipose triglyceride lipase |
| CPT1 | Carnitine palmitoyltransferase 1 |
| UCP1 | Uncoupling protein 1 |
| IL | Interleukin |
| NF-κB | Nuclear factor-kappa B |
| AMPK | AMP-activated protein kinase |
| ACC | acetyl-CoA carboxylase |
| MHC | Myosin heavy chain |
| IB+R+IN+D | Isobutylmethylxanthine, dexamethasone, insulin, rosiglitazone (R) |
| PA | Palmitic acid |
| TG | Triglyceride |
| TCHO | Total Cholesterol |
| LDL | Low Density Lipoprotein |
| HDL | High Density Lipoprotein |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1α |
References
- Barazzoni, R.; Bischoff, S.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Obes. Facts 2018, 11, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; Hill, C.; James, R.S.; Cox, V.M.; Seebacher, F. The effect of obesity on the contractile performance of isolated mouse soleus, EDL, and diaphragm muscles. J. Appl. Physiol. 2017, 122, 170–181. [Google Scholar] [CrossRef]
- Anton, S.D.; Karabetian, C.; Naugle, K.; Buford, T.W. Obesity and diabetes as accelerators of functional decline: Can lifestyle interventions maintain functional status in high risk older adults? Exp. Gerontol. 2013, 48, 888–897. [Google Scholar] [CrossRef]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef]
- Sizoo, D.; de Heide, L.J.M.; Emous, M.; van Zutphen, T.; Navis, G.; van Beek, A.P. Measuring Muscle Mass and Strength in Obesity: A Review of Various Methods. Obes. Surg. 2021, 31, 384–393. [Google Scholar] [CrossRef]
- Busutil, R.; Espallardo, O.; Torres, A.; Martínez-Galdeano, L.; Zozaya, N.; Hidalgo-Vega, Á. The impact of obesity on health-related quality of life in Spain. Health Qual. Life Outcomes 2017, 15, 197. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yang, B. Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics. Molecules 2016, 21, 991. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Pang, H.; Wong, Y.H. Naturally occurring phenylethanoid glycosides: Potential leads for new therapeutics. Curr. Med. Chem. 2008, 15, 2592–2613. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Xiong, Q.; Kadota, S.; Tani, T.; Namba, T. Antioxidative effects of phenylethanoids from Cistanche deserticola. Biol. Pharm. Bull. 1996, 19, 1580–1585. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Matsuda, H.; Morikawa, T.; Xie, H.; Nakamura, S.; Muraoka, O. Phenylethanoid oligoglycosides and acylated oligosugars with vasorelaxant activity from Cistanche tubulosa. Bioorganic Med. Chem. 2006, 14, 7468–7475. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, C.; Gong, X.; Yang, M.; Ji, M.; Jiang, L.; Leonti, M.; Yao, R.; Li, M. The genus Orobanche as food and medicine: An ethnopharmacological review. J. Ethnopharmacol. 2020, 263, 113154. [Google Scholar] [CrossRef]
- Li, Y.M.; Jiang, S.H.; Gao, W.Y.; Zhu, D.Y. Phenylpropanoid glycosides from Scrophularia ningpoensis. Phytochemistry 2000, 54, 923–925. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.Y.; Shao, J.J.; Wang, G.; Wen, R.; Tian, J.Z.; Hou, L. Callicarpa nudiflora Hook. & Arn.: A comprehensive review of its phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 264, 113123. [Google Scholar]
- Fuji, Y.; Uchida, A.; Fukahori, K.; Chino, M.; Ohtsuki, T.; Matsufuji, H. Chemical characterization and biological activity in young sesame leaves (Sesamum indicum L.) and changes in iridoid and polyphenol content at different growth stages. PLoS ONE 2018, 13, e0194449. [Google Scholar] [CrossRef]
- Pan, N.; Hori, H. Antioxidant action of acteoside and its analogs on lipid peroxidation. Redox Rep. Commun. Free Radic. Res. 1996, 2, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kritchevsky, S.B.; Goodpaster, B.H.; Newman, A.B.; Nevitt, M.; Stamm, E.; Harris, T.B. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J. Am. Geriatr. Soc. 2002, 50, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, X.; Advani, V.; Lu, Y.W.; Malizia, A.P.; Singh, G.B.; Huang, Z.P.; Liu, J.; Wang, C.; Oliveira, E.M.; et al. mt-Ty 5′tiRNA regulates skeletal muscle cell proliferation and differentiation. Cell Prolif. 2023, 56, e13416. [Google Scholar] [CrossRef]
- Dai, H.; Zheng, W.; Luo, J.; Yu, G.; Song, C.; Wu, Y.; Xu, J. Inhibiting uptake of extracellular vesicles derived from senescent bone marrow mesenchymal stem cells by muscle satellite cells attenuates sarcopenia. J. Orthop. Transl. 2022, 35, 23–36. [Google Scholar] [CrossRef]
- Deschenes, M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef]
- Yan, Z.; Choi, S.; Liu, X.; Zhang, M.; Schageman, J.J.; Lee, S.Y.; Hart, R.; Lin, L.; Thurmond, F.A.; Williams, R.S. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J. Biol. Chem. 2003, 278, 8826–8836. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef]
- Zeng, Y.; Shen, J.; Zhou, X.; Ouyang, Z.; Zhong, J.; Qin, Y.; Jin, L.; He, X.; Li, L.; Xie, J.; et al. Osteogenic differentiation of bone mesenchymal stem cells on linearly aligned triangular micropatterns. J. Mater. Chem. B 2024, 12, 8420–8430. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, L.; Liu, F.; Al-Bukhaiti, W.Q.; Huang, X.; Lin, T.; Qiu, S.X. New stilbenes from Cajanus cajan inhibit adipogenesis in 3T3-L1 adipocytes through down-regulation of PPARγ. Bioorg. Chem. 2024, 153, 107851. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Moazzami, Z.; Heck, R.; Hu, P.; Nanda, H.; Ren, K.; Sun, Z.; Bartolomucci, A.; Gao, Y.; et al. Brown adipose tissue involution associated with progressive restriction in progenitor competence. Cell Rep. 2022, 39, 110575. [Google Scholar] [CrossRef] [PubMed]
- Ronnett, G.V.; Kleman, A.M.; Kim, E.K.; Landree, L.E.; Tu, Y. Fatty acid metabolism, the central nervous system, and feeding. Obesity 2006, 14 (Suppl. S5), 201s–207s. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, C.; Olivares-Vicente, M.; Rodríguez-Pérez, C.; Herranz-López, M.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Encinar, J.A.; Micol, V. AMPK modulatory activity of olive-tree leaves phenolic compounds: Bioassay-guided isolation on adipocyte model and in silico approach. PLoS ONE 2017, 12, e0173074. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Duan, Y.; Wei, H.; Ning, H.; Bi, C.; Zhao, Y.; Qin, Y.; Li, Y. Acetyl-CoA carboxylase (ACC) as a therapeutic target for metabolic syndrome and recent developments in ACC1/2 inhibitors. Expert Opin. Investig. Drugs 2019, 28, 917–930. [Google Scholar] [CrossRef]
- Foretz, M.; Viollet, B. Activation of AMPK for a Break in Hepatic Lipid Accumulation and Circulating Cholesterol. eBioMedicine 2018, 31, 15–16. [Google Scholar] [CrossRef]
- Oktay, A.A.; Lavie, C.J.; Kokkinos, P.F.; Parto, P.; Pandey, A.; Ventura, H.O. The Interaction of Cardiorespiratory Fitness with Obesity and the Obesity Paradox in Cardiovascular Disease. Prog. Cardiovasc. Dis. 2017, 60, 30–44. [Google Scholar] [CrossRef]
- Roh, E.; Choi, K.M. Health Consequences of Sarcopenic Obesity: A Narrative Review. Front. Endocrinol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Tie, F.; Hu, N.; Dong, Q.; Wang, H. Vitisin A-13-O-β-D-glucoside and Vitisin A from Iris lactea inhibit lipogenesis and promote lipolysis via the PKA/HSL pathway during adipogenic transdifferentiation of C2C12 cells. Eur. J. Pharmacol. 2023, 960, 176154. [Google Scholar] [CrossRef]
- Qi, R.; Liu, H.; Wang, Q.; Wang, J.; Yang, F.; Long, D.; Huang, J. Expressions and Regulatory Effects of P38/ERK/JNK Mapks in the Adipogenic Trans-Differentiation of C2C12 Myoblasts. Cell. Physiol. Biochem. 2017, 44, 2467–2475. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakano, S.; Miyoshi, T.; Yamanouchi, K.; Matsuwaki, T.; Nishihara, M. Age-related resistance of skeletal muscle-derived progenitor cells to SPARC may explain a shift from myogenesis to adipogenesis. Aging 2012, 4, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Huang, P. The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function. Stem Cell Res. Ther. 2019, 10, 103. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Scudese, E.; Marshall, A.G.; Vue, Z.; Exil, V.; Rodriguez, B.I.; Demirci, M.; Vang, L.; López, E.G.; Neikirk, K.; Shao, B.; et al. 3D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights into MFN-2-Mediated Changes. Aging Cell 2025, e70054. [Google Scholar] [CrossRef]
- Zhou, S.; Taskintuna, K.; Hum, J.; Gulati, J.; Olaya, S.; Steinman, J.; Golestaneh, N. PGC-1α repression dysregulates lipid metabolism and induces lipid droplet accumulation in retinal pigment epithelium. Cell Death Dis. 2024, 15, 385. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Fibroblast growth factor 19 alleviates palmitic acid-induced mitochondrial dysfunction and oxidative stress via the AMPK/PGC-1α pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2020, 526, 1069–1076. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, N.; Meng, Q.; Zhong, M.; Yang, M.; Xu, F.; Zhang, L.; Jiang, M.; Wu, J.; Ma, Z.; et al. Mechanisms of Inonotus obliquus (Fr.) Pilát Polysaccharides in Ameliorating Lipid-Induced Skeletal Muscle Insulin Resistance via PI3K/AKT and AMPK/ACC1/CPT1 Signaling Pathways. J. Ethnopharmacol. 2025, 349, 119938. [Google Scholar] [CrossRef]
- Munday, M.R. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 2002, 30 Pt 6, 1059–1064. [Google Scholar] [CrossRef]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. CMLS 2005, 62, 1784–1803. [Google Scholar] [CrossRef]
- Harwood, H.J., Jr.; Petras, S.F.; Shelly, L.D.; Zaccaro, L.M.; Perry, D.A.; Makowski, M.R.; Hargrove, D.M.; Martin, K.A.; Tracey, W.R.; Chapman, J.G.; et al. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. J. Biol. Chem. 2003, 278, 37099–37111. [Google Scholar] [CrossRef]
- Zhang, T.; Guan, X.; Choi, U.L.; Dong, Q.; Lam, M.M.T.; Zeng, J.; Xiong, J.; Wang, X.; Poon, T.C.W.; Zhang, H.; et al. Phosphorylation of TET2 by AMPK is indispensable in myogenic differentiation. Epigenet. Chromatin 2019, 12, 32. [Google Scholar] [CrossRef]
- Niesler, C.U.; Myburgh, K.H.; Moore, F. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp. Physiol. 2007, 92, 207–217. [Google Scholar] [CrossRef]
- Hsu, C.G.; Burkholder, T.J. Independent AMP and NAD signaling regulates C2C12 differentiation and metabolic adaptation. J. Physiol. Biochem. 2016, 72, 689–697. [Google Scholar] [CrossRef]
- Mohamed, R.; Jayakumar, C.; Ramesh, G. Chronic administration of EP4-selective agonist exacerbates albuminuria and fibrosis of the kidney in streptozotocin-induced diabetic mice through IL-6. Lab. Investig. 2013, 93, 933–945. [Google Scholar] [CrossRef]
- Cheung, W.W.; Paik, K.H.; Mak, R.H. Inflammation and cachexia in chronic kidney disease. Pediatr. Nephrol. 2010, 25, 711–724. [Google Scholar] [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef]
- Ropelle, E.R.; Pauli, J.R.; Prada, P.O.; de Souza, C.T.; Picardi, P.K.; Faria, M.C.; Cintra, D.E.; Fernandes, M.F.; Flores, M.B.; Velloso, L.A.; et al. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: The role of PTP1B and IRS-1 serine phosphorylation. J. Physiol. 2006, 577 Pt 3, 997–1007. [Google Scholar] [CrossRef]
- Knudsen, S.H.; Hansen, L.S.; Pedersen, M.; Dejgaard, T.; Hansen, J.; Hall, G.V.; Thomsen, C.; Solomon, T.P.; Pedersen, B.K.; Krogh-Madsen, R. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J. Appl. Physiol. 2012, 113, 7–15. [Google Scholar] [CrossRef]
- Fan, K.; Lin, L.; Ai, Q.; Wan, J.; Dai, J.; Liu, G.; Tang, L.; Yang, Y.; Ge, P.; Jiang, R.; et al. Lipopolysaccharide-Induced Dephosphorylation of AMPK-Activated Protein Kinase Potentiates Inflammatory Injury via Repression of ULK1-Dependent Autophagy. Front. Immunol. 2018, 9, 1464. [Google Scholar] [CrossRef]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal muscle energy metabolism in obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′–3′) | Reverse Primer (3′–5′) | Length (bp) |
|---|---|---|---|
| Mhc | CAGACGGAGAGGAGCAGGAAG | CTTGGTGTTGATGAGGCTGGTG | 102 |
| Il6 | CTCCCAACAGACCTGTCTATAC | CCATTGCACAACTCTTTTCTCA | 97 |
| Actb | CTACCTCATGAAGATCCTGACC | CACAGCTTCTCTTTGATGTCAC | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.-L.; Tang, Z.-L.; Chen, L.-P.; Qin, X.-N.; Xiao, K.-F.; Zhu, W.-L.; Zhang, Y.; Gong, Z.-B. Cistanoside F Ameliorates Lipid Accumulation and Enhances Myogenic Differentiation via AMPK-Dependent Signaling in C2C12 Myotubes. Cells 2025, 14, 874. https://doi.org/10.3390/cells14120874
Ma M-L, Tang Z-L, Chen L-P, Qin X-N, Xiao K-F, Zhu W-L, Zhang Y, Gong Z-B. Cistanoside F Ameliorates Lipid Accumulation and Enhances Myogenic Differentiation via AMPK-Dependent Signaling in C2C12 Myotubes. Cells. 2025; 14(12):874. https://doi.org/10.3390/cells14120874
Chicago/Turabian StyleMa, Meng-Ling, Ze-Ling Tang, Li-Ping Chen, Xiang-Nan Qin, Ke-Fei Xiao, Wei-Liang Zhu, Yong Zhang, and Zhang-Bin Gong. 2025. "Cistanoside F Ameliorates Lipid Accumulation and Enhances Myogenic Differentiation via AMPK-Dependent Signaling in C2C12 Myotubes" Cells 14, no. 12: 874. https://doi.org/10.3390/cells14120874
APA StyleMa, M.-L., Tang, Z.-L., Chen, L.-P., Qin, X.-N., Xiao, K.-F., Zhu, W.-L., Zhang, Y., & Gong, Z.-B. (2025). Cistanoside F Ameliorates Lipid Accumulation and Enhances Myogenic Differentiation via AMPK-Dependent Signaling in C2C12 Myotubes. Cells, 14(12), 874. https://doi.org/10.3390/cells14120874






