The Loss of Gonadal Hormones Has a Different Impact on Aging Female and Male Mice Submitted to Heart Failure-Inducing Metabolic Hypertensive Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Echocardiography
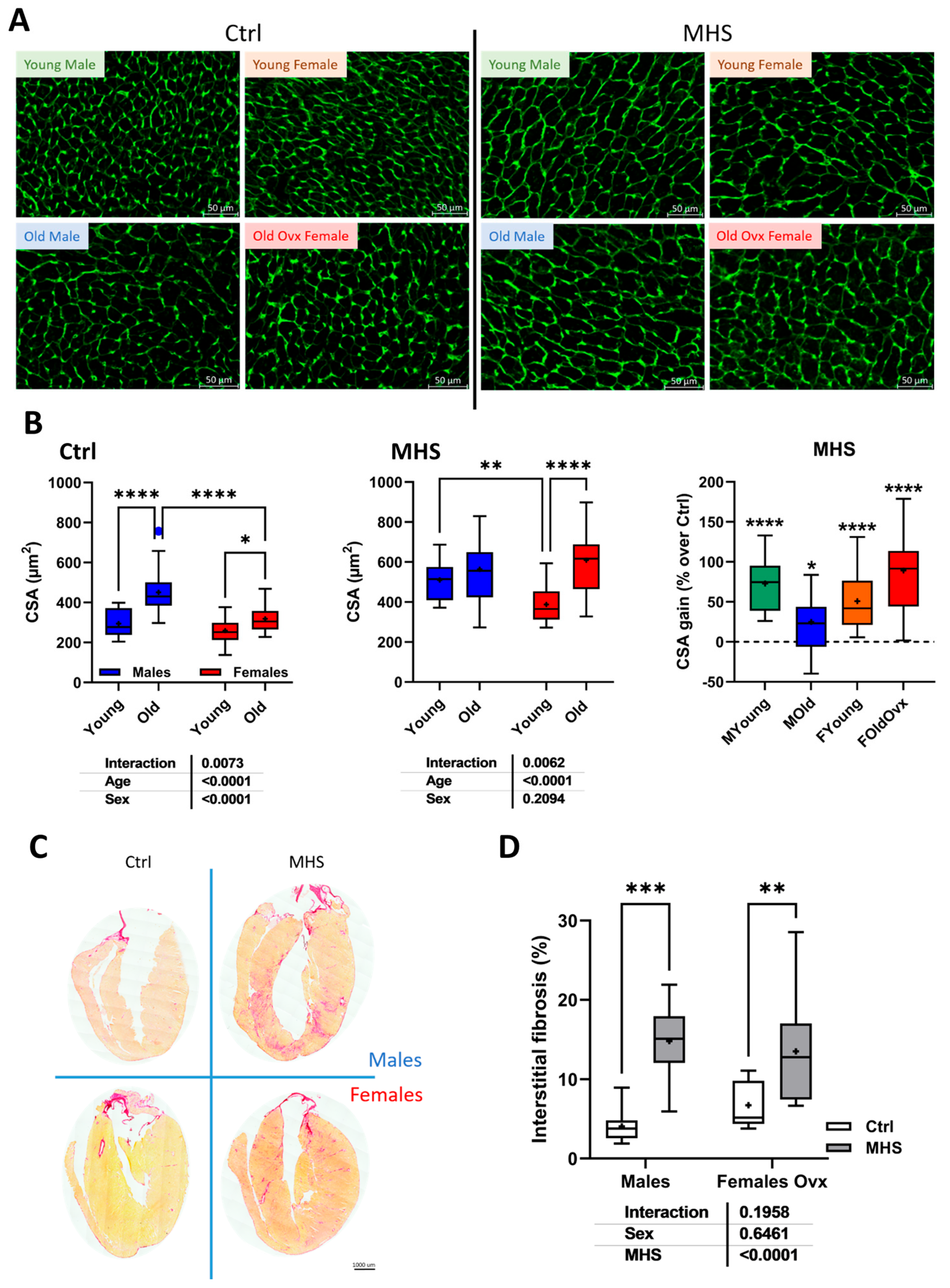
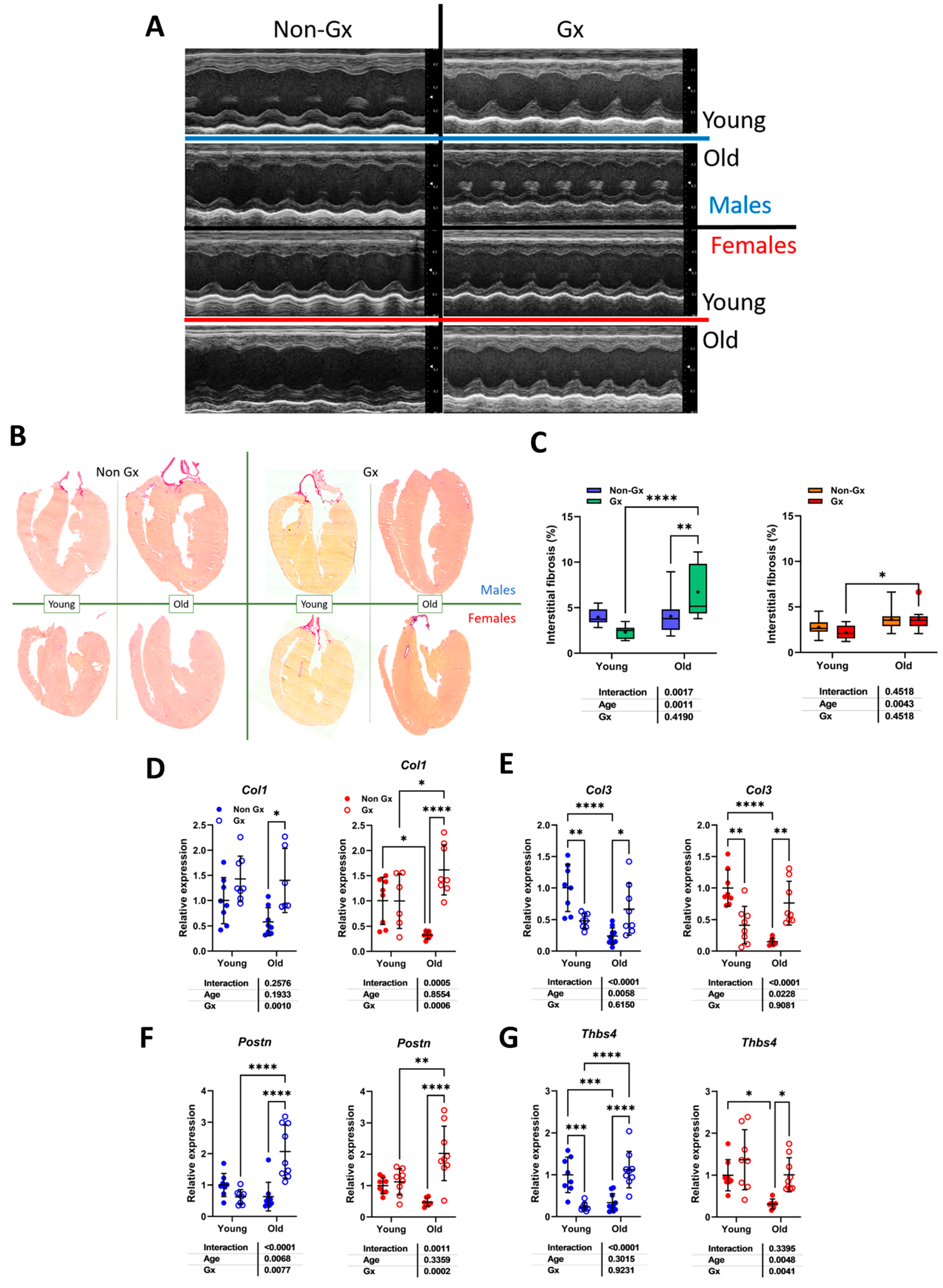
2.3. Myocardial Fibrosis Evaluation
2.4. Cardiomyocyte Cross-Sectional Area
2.5. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
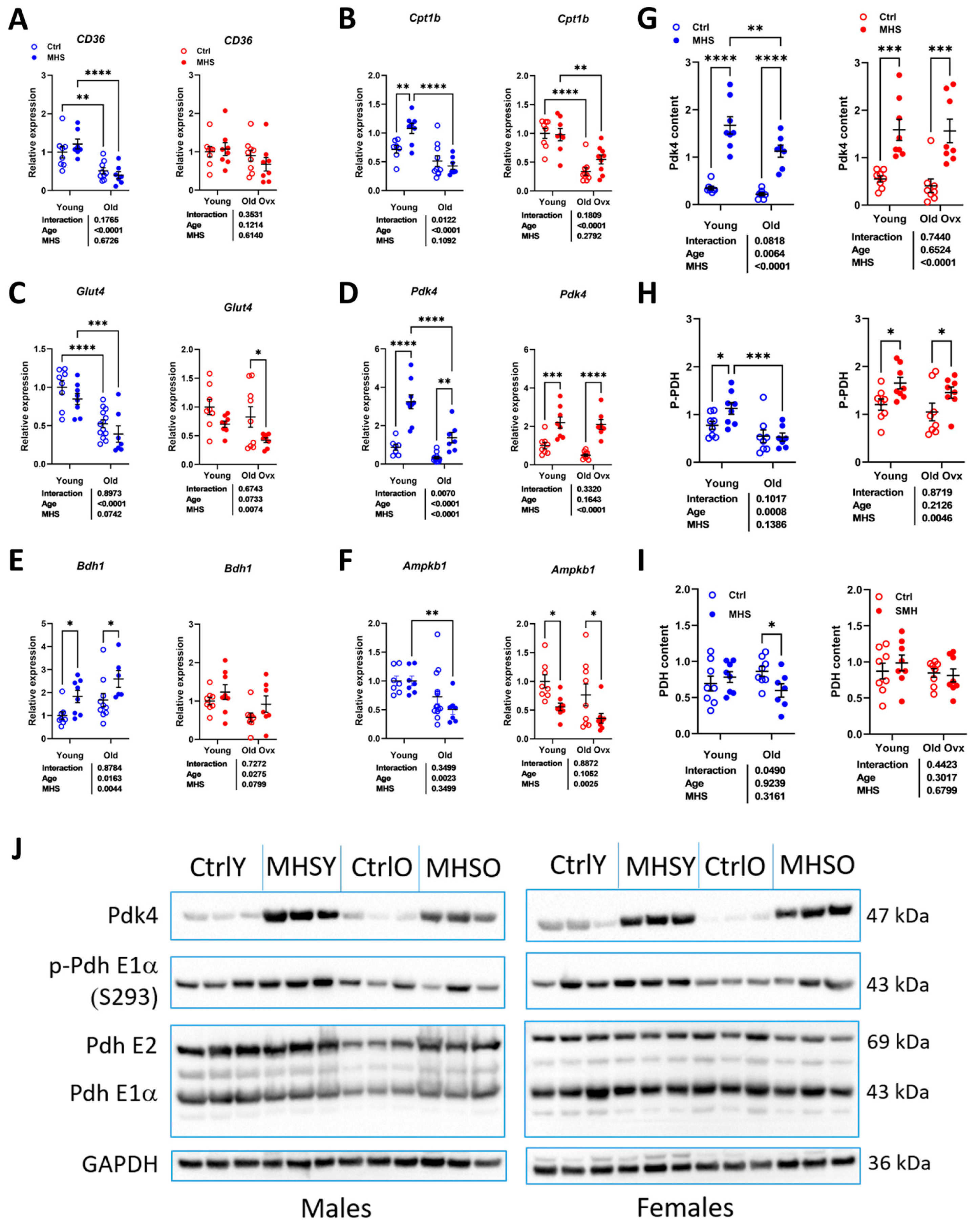
2.6. Western Blots
2.7. Plasma Inflammatory Marker Measurements
2.8. Statistical Analysis
3. Results
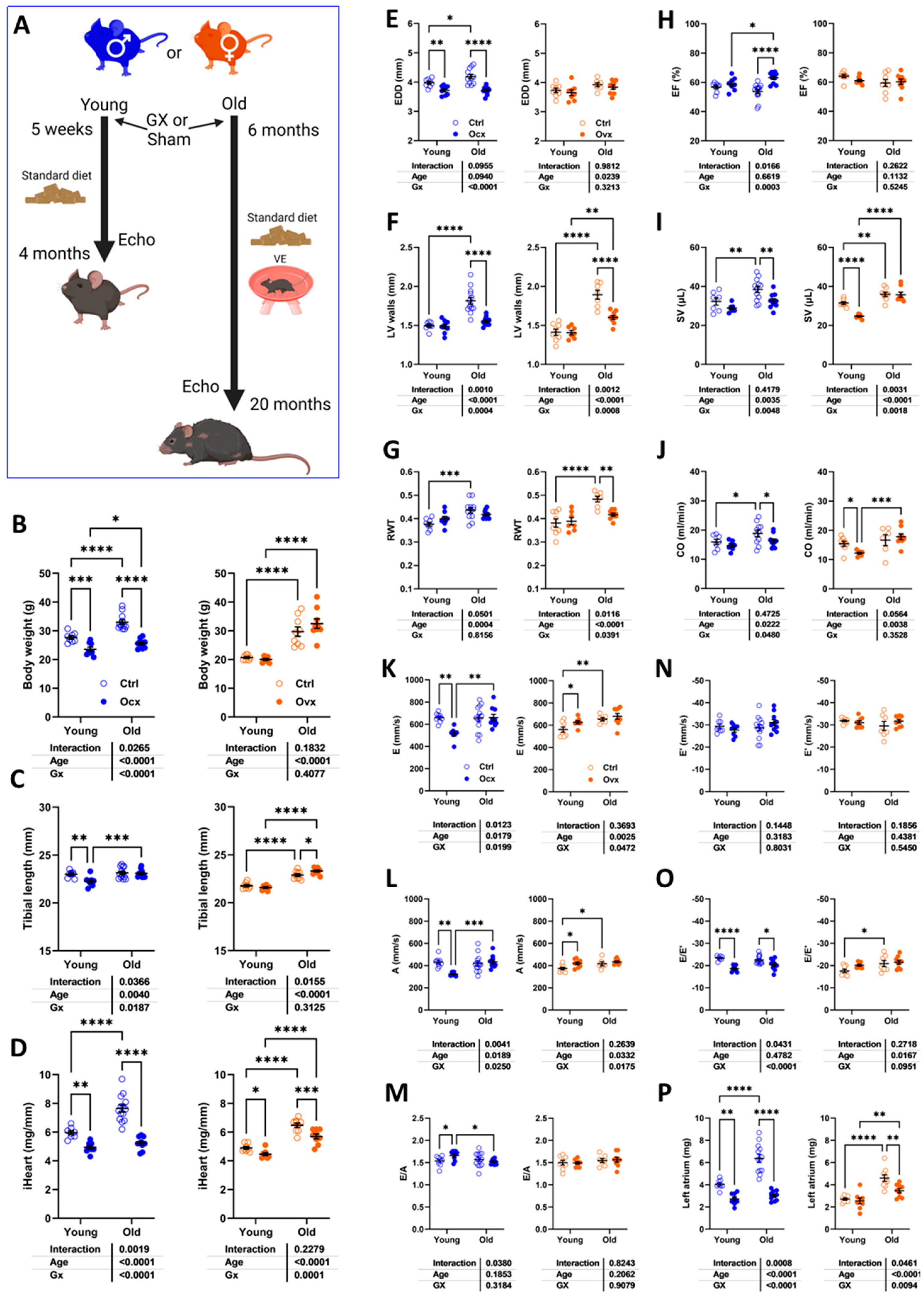
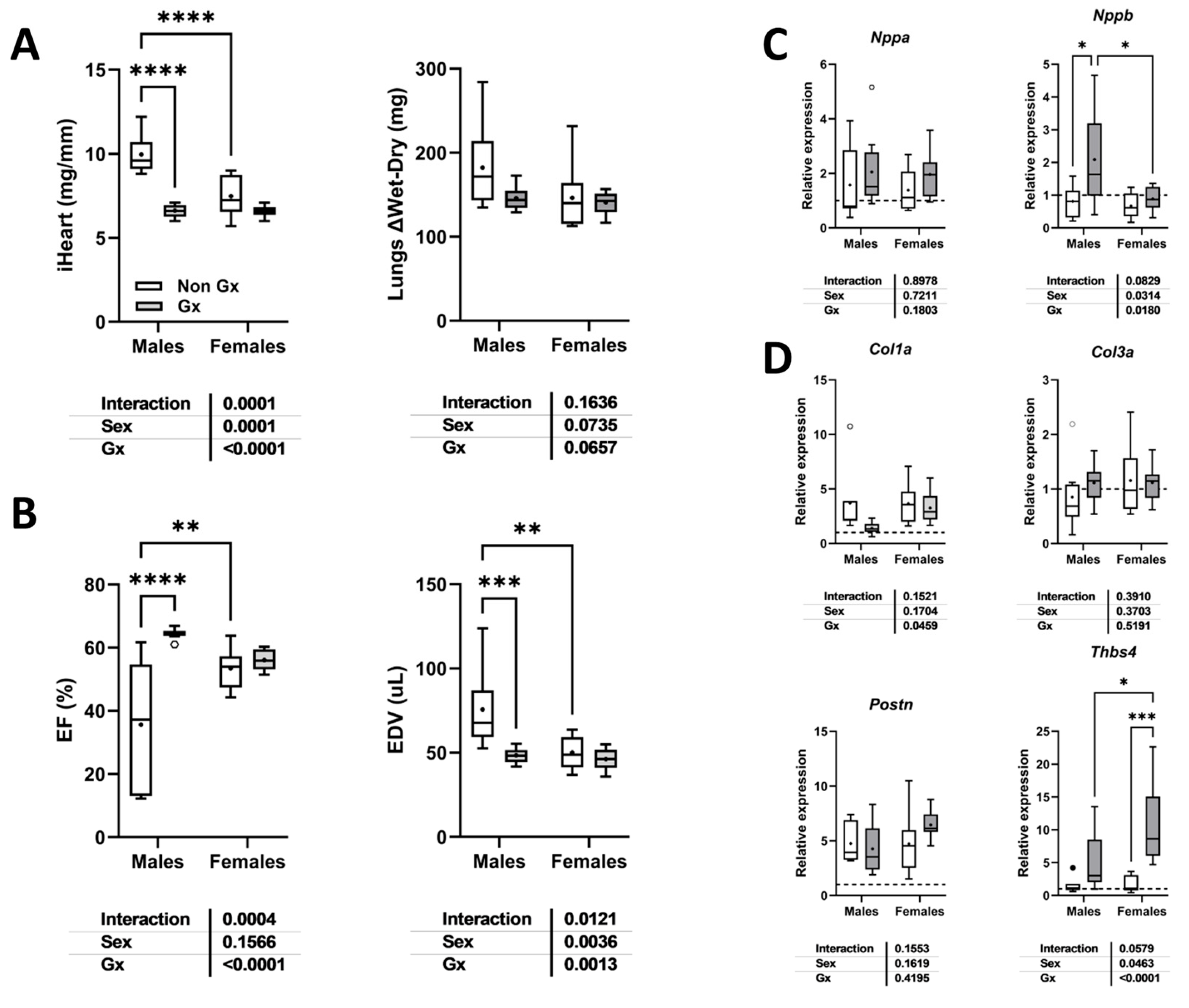
3.1. Short and Long-Term Effects on Cardiac Morphology and Function in Male and Female Mice
3.2. Aging in Female Mice Is Associated with a Circulatory Inflammation Profile
3.3. The Effects of Metabolic Hypertensive Stress (MHS) Are Similar in Mice Regardless of Age, Sex, or Gx
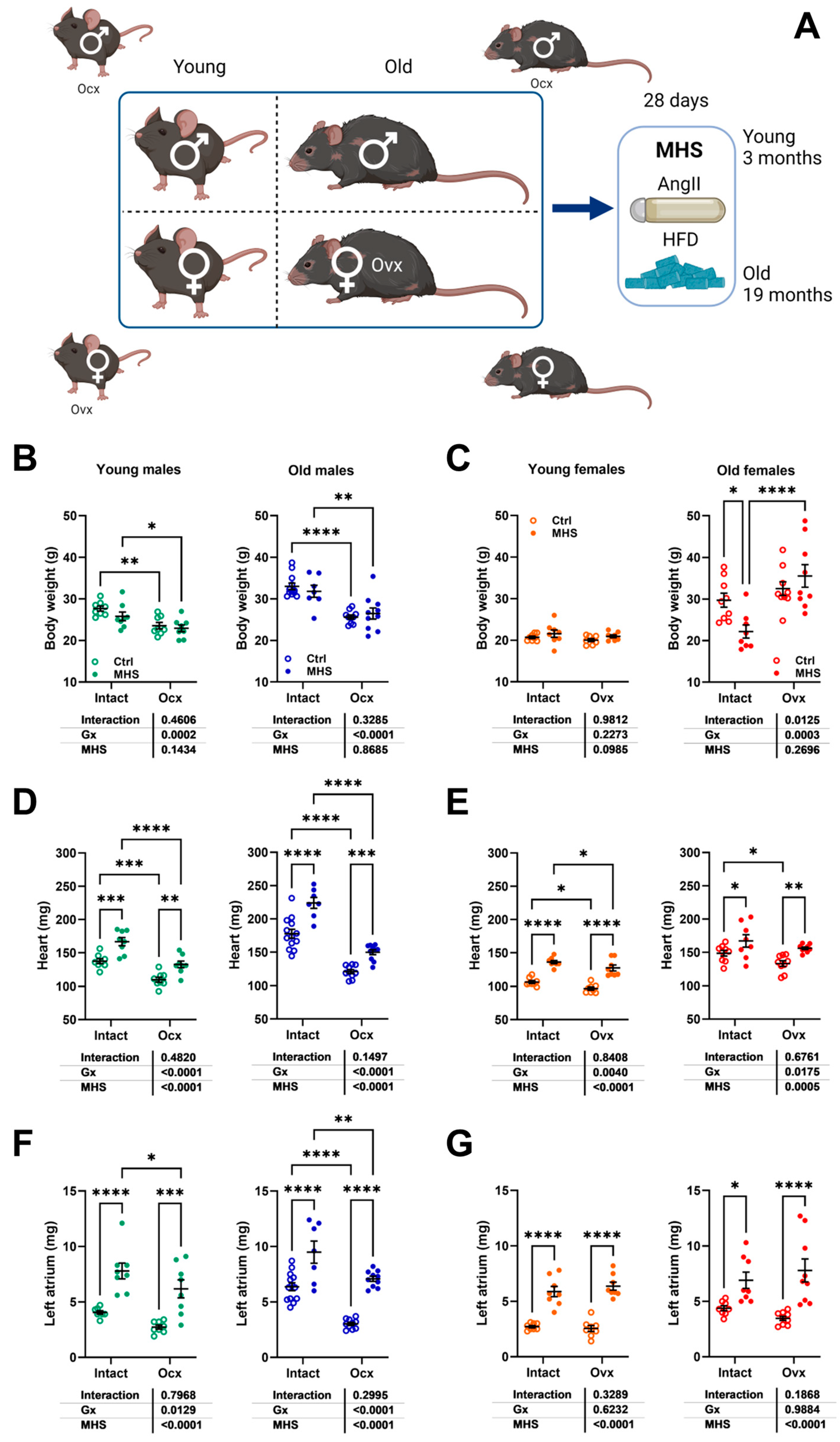
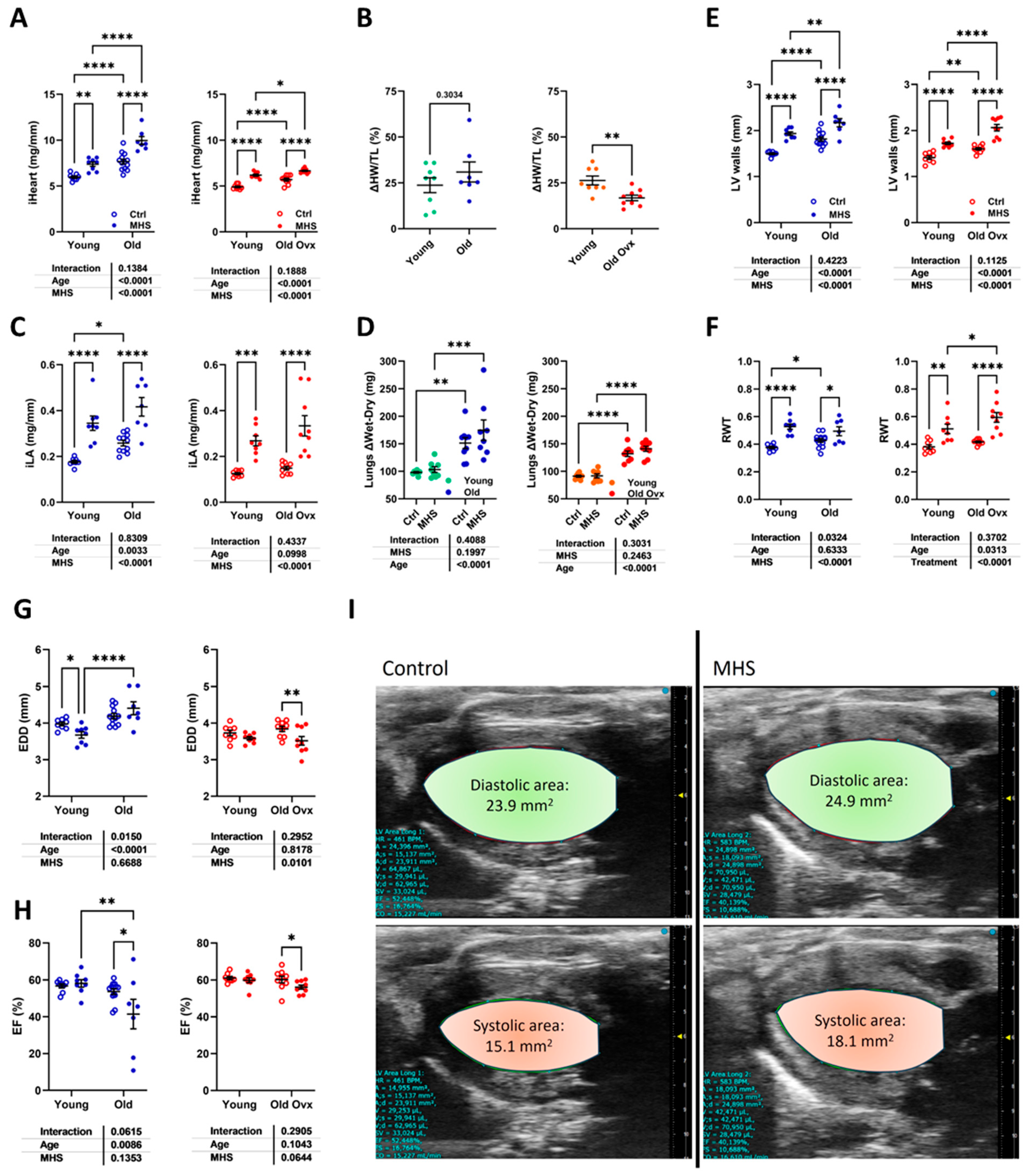
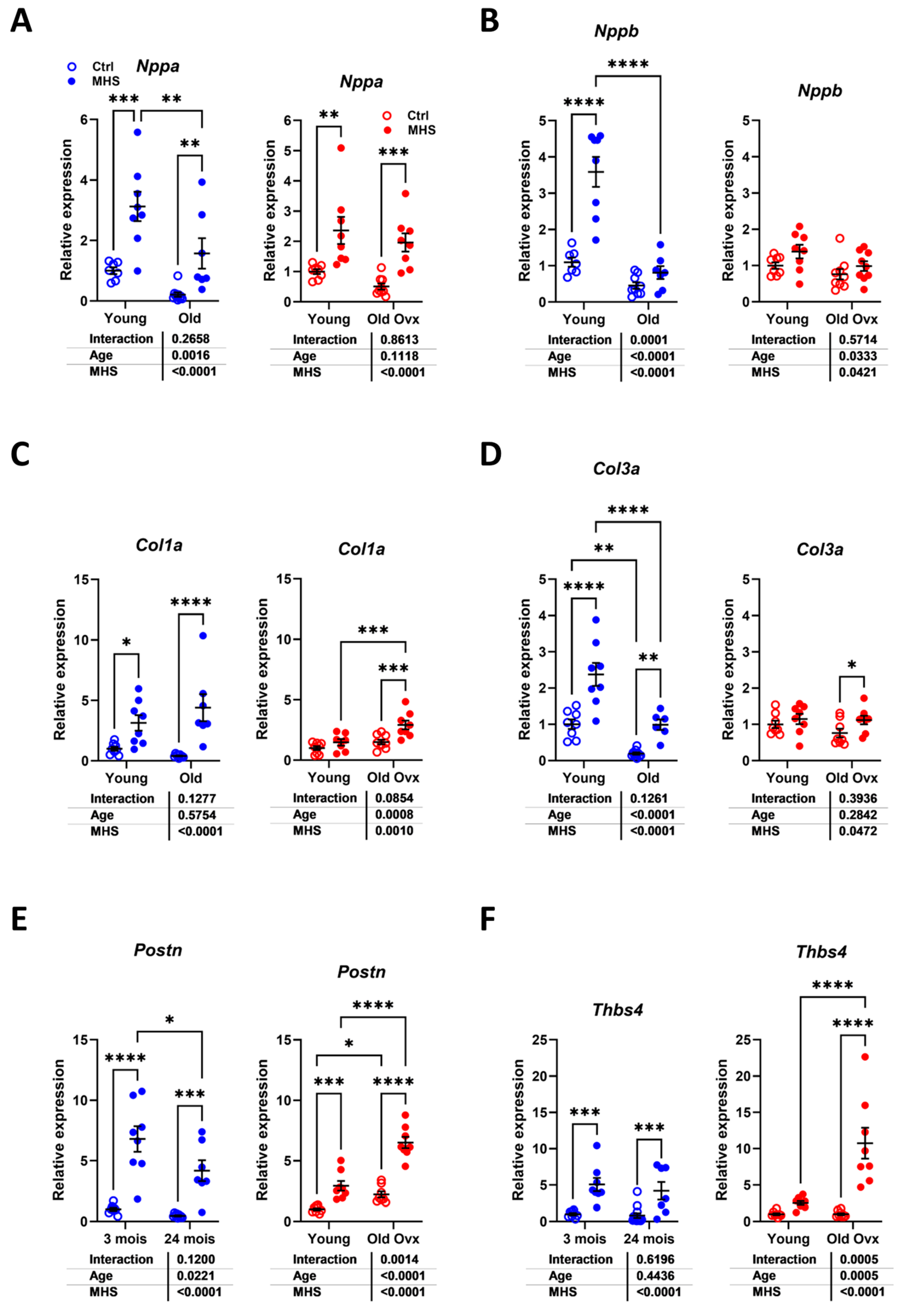
3.4. In Young Female Mice, MHS Induced a Circulatory Inflammation Profile Similar to Aging
3.5. Modulation of Myocardial Hypertrophy and Fibrosis Marker Genes by MHS
3.6. MHS Increases the Pyruvate Dehydrogenase Kinase 4 (PDK4) Protein Content in Young and Old HFpEF Male and Female Mice
3.7. Loss of Testosterone Rescues LV Systolic Function in Old MHS Males
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AngII | Angiotensin II |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| MHS | Metabolic and hypertensive stress (AngII + HFD) |
| HFD | High-fat diet |
| LA | Left atrial or left atrium |
| LV | Left ventricle |
| Gx | Gonadectomized |
| Ovx | Ovariectomized or ovariectomy |
| Ocx | Orchiectomized or orchiectomy |
| CH | Cardiac hypertrophy |
| EF | Ejection fraction |
| FCG | Four core genotypes |
| ECM | Extracellular matrix |
| EDD | End-diastolic diameter |
| ESD | End-systolic diameter |
| RWT | Relative wall thickness |
| SV | Stroke volume |
| CO | Cardiac output |
| BW | Body weight |
| CSA | Cross-sectional area |
| VE | Voluntary exercise |
| VCD | Vinylcyclohexene dioxide |
| L-NAME | L-NG-Nitroarginine Methyl Ester |
| HRT | Hormonal replacement therapy |
| EDV | End-diastolic volume |
| ESV | End-systolic volume |
| Nppa | Atrial natriuretic peptide |
| Nppb | Brain natriuretic peptide |
| Col1a | Pro-collagen1 alpha |
| Col3a | Pro-collagen3 alpha |
| Postn | Periostin |
| Thbs4 | Thrombospondin 4 |
| CD36/FAT | CD36/fatty acid transporter |
| Cpt1b | Carnitine palmitoyl transferase 1b |
| Glut4 | Glucose transporter 4 |
| Pdk4 | Pyruvate dehydrogenase kinase 4 |
| PDH | Pyruvate dehydrogenase |
| Bdh1 | 3-Hydroxybutyrate Dehydrogenase 1 |
| AMPK1b | Adenosyl monophosphate kinase 1b |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| PW | Posterior wall |
| IVSW | Interventricular septal wall |
| SEM | Standard error of the mean |
References
- Hamo, C.E.; DeJong, C.; Hartshorne-Evans, N.; Lund, L.H.; Shah, S.J.; Solomon, S.; Lam, C.S.P. Heart failure with preserved ejection fraction. Nat. Rev. Dis. Primers 2024, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Radakrishnan, A.; Agrawal, S.; Singh, N.; Barbieri, A.; Shaw, L.J.; Gulati, M.; Lala, A. Underpinnings of Heart Failure With Preserved Ejection Fraction in Women—From Prevention to Improving Function. A Co-publication With the American Journal of Preventive Cardiology and the Journal of Cardiac Failure. J. Card. Fail. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ketema, E.B.; Lopaschuk, G.D. The Impact of Obesity on Cardiac Energy Metabolism and Efficiency in Heart Failure With Preserved Ejection Fraction. Can. J. Cardiol. 2025, in press. [CrossRef] [PubMed]
- Triposkiadis, F.; Giamouzis, G.; Parissis, J.; Starling, R.C.; Boudoulas, H.; Skoularigis, J.; Butler, J.; Filippatos, G. Reframing the association and significance of co-morbidities in heart failure. Eur. J. Heart Fail. 2016, 18, 744–758. [Google Scholar] [CrossRef]
- Kaur, G.; Lau, E. Sex differences in heart failure with preserved ejection fraction: From traditional risk factors to sex-specific risk factors. Womens Health 2022, 18, 17455057221140209. [Google Scholar] [CrossRef]
- Stolfo, D.; Uijl, A.; Vedin, O.; Strömberg, A.; Faxén, U.L.; Rosano, G.M.C.; Sinagra, G.; Dahlström, U.; Savarese, G. Sex-Based Differences in Heart Failure Across the Ejection Fraction Spectrum: Phenotyping, and Prognostic and Therapeutic Implications. JACC Heart Fail. 2019, 7, 505–515, Erratum in JACC Heart Fail. 2020, 8, 345–346. [Google Scholar] [CrossRef]
- Lam, C.S.; Carson, P.E.; Anand, I.S.; Rector, T.S.; Kuskowski, M.; Komajda, M.; McKelvie, R.S.; McMurray, J.J.; Zile, M.R.; Massie, B.M.; et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Heart Fail. 2012, 5, 571–578. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Garavaglia, G.E.; Messerli, F.H.; Schmieder, R.E.; Nunez, B.D.; Oren, S. Sex differences in cardiac adaptation to essential hypertension. Eur. Heart J. 1989, 10, 1110–1114. [Google Scholar] [CrossRef]
- Sabbatini, A.R.; Kararigas, G. Menopause-Related Estrogen Decrease and the Pathogenesis of HFpEF: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 1074–1082. [Google Scholar] [CrossRef]
- Aidara, M.L.; Walsh-Wilkinson, É.; Thibodeau, S.È.; Labbé, E.A.; Morin-Grandmont, A.; Gagnon, G.; Boudreau, D.K.; Arsenault, M.; Bossé, Y.; Couët, J. Cardiac reverse remodeling in a mouse model with many phenotypical features of heart failure with preserved ejection fraction: Effects of modifying lifestyle. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H1017–H1036. [Google Scholar] [CrossRef] [PubMed]
- Morin-Grandmont, A.; Walsh-Wilkinson, É.; Labbé, E.A.; Thibodeau, S.È.; Dupont, É.; Boudreau, D.K.; Arsenault, M.; Bossé, Y.; Couet, J. Biological sex, sex steroids and sex chromosomes contribute to mouse cardiac aging. Aging 2024, 16, 7553–7577. [Google Scholar] [CrossRef] [PubMed]
- Withaar, C.; Meems, L.M.G.; Markousis-Mavrogenis, G.; Boogerd, C.J.; Silljé, H.H.W.; Schouten, E.M.; Dokter, M.M.; Voors, A.A.; Westenbrink, B.D.; Lam, C.S.P.; et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc. Res. 2021, 117, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Walsh-Wilkinson, É.; Aidara, M.L.; Morin-Grandmont, A.; Thibodeau, S.È.; Gagnon, J.; Genest, M.; Arsenault, M.; Couet, J. Age and sex hormones modulate left ventricle regional response to angiotensin II in male and female mice. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H643–H658. [Google Scholar] [CrossRef]
- Morin-Grandmont, A.; Walsh-Wilkinson, E.; Thibodeau, S.È.; Boudreau, D.K.; Arsenault, M.; Bossé, Y.; Couet, J. A murine model of hypertensive heart disease in older women. PeerJ 2024, 12, e17434. [Google Scholar] [CrossRef]
- Walsh-Wilkinson, E.; Arsenault, M.; Couet, J. Segmental analysis by speckle-tracking echocardiography of the left ventricle response to isoproterenol in male and female mice. PeerJ 2021, 9, e11085. [Google Scholar] [CrossRef]
- Labbé, E.A.; Thibodeau, S.È.; Walsh-Wilkinson, È.; Chalifour, M.; Sirois, P.O.; Leblanc, J.; Morin-Grandmont, A.; Arsenault, M.; Couet, J. Relative contribution of correcting the diet and voluntary exercise to myocardial recovery in a murine model of heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Koenig, H.; Goldstone, A.; Lu, C.Y. Testosterone-mediated sexual dimorphism of the rodent heart. Ventricular lysosomes, mitochondria, and cell growth are modulated by androgens. Circ. Res. 1982, 50, 782–787. [Google Scholar] [CrossRef]
- Hooper, A.C.; Brien, T.G.; Lawlor, P.G. The effects of orchidectomy and the role of testosterone in determining the growth of male mice selected for increased body weight. Andrologia 1986, 18, 509–515. [Google Scholar] [CrossRef]
- Dai, D.F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef]
- Roh, J.D.; Houstis, N.; Yu, A.; Chang, B.; Yeri, A.; Li, H.; Hobson, R.; Lerchenmüller, C.; Vujic, A.; Chaudhari, V.; et al. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell 2020, 19, e13159. [Google Scholar] [CrossRef] [PubMed]
- Fares, E.; Pyle, W.G.; Ray, G.; Rose, R.A.; Denovan-Wright, E.M.; Chen, R.P.; Howlett, S.E. The impact of ovariectomy on calcium homeostasis and myofilament calcium sensitivity in the aging mouse heart. PLoS ONE 2013, 8, e74719. [Google Scholar] [CrossRef] [PubMed]
- Banga, S.; Heinze-Milne, S.D.; Godin, J.; Howlett, S.E. Signs of diastolic dysfunction are graded by serum testosterone levels in aging C57BL/6 male mice. Mech. Ageing Dev. 2021, 198, 111523. [Google Scholar] [CrossRef] [PubMed]
- Troy, A.M.; Normukhamedova, D.; Grothe, D.; Momen, A.; Zhou, Y.Q.; McFadden, M.; Hussain, M.; Billia, F.; Cheng, H.M. Impact of ovary-intact menopause in a mouse model of heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H522–H537. [Google Scholar] [CrossRef]
- Methawasin, M.; Strom, J.; Marino, V.A.; Gohlke, J.; Muldoon, J.; Herrick, S.R.; van der Piji, R.; Konhilas, J.P.; Granzier, H. An ovary-intact postmenopausal HFpEF mouse model; menopause is more than just estrogen deficiency. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H719–H733. [Google Scholar] [CrossRef]
- Brenes-Castro, D.; Castillo, E.C.; Vázquez-Garza, E.; Torre-Amione, G.; García-Rivas, G. Temporal Frame of Immune Cell Infiltration during Heart Failure Establishment: Lessons from Animal Models. Int. J. Mol. Sci. 2018, 19, 3719. [Google Scholar] [CrossRef]
- Wang, D.; Weng, X.; Yue, W.; Shang, L.; Wei, Y.; Clemmer, J.S.; Xu, Y.; Chen, Y. CD8 T cells promote heart failure progression in mice with preexisting left ventricular dysfunction. Front. Immunol. 2024, 15, 1472133. [Google Scholar] [CrossRef]
- Smolgovsky, S.; Bayer, A.L.; Kaur, K.; Sanders, E.; Aronovitz, M.; Filipp, M.E.; Thorp, E.B.; Schiattarella, G.G.; Hill, J.A.; Blanton, R.M.; et al. Impaired T cell IRE1α/XBP1 signaling directs inflammation in experimental heart failure with preserved ejection fraction. J. Clin. Invest. 2023, 133, e171874. [Google Scholar] [CrossRef]
- Huang, S.; Kang, Y.; Liu, T.; Xiong, Y.; Yang, Z.; Zhang, Q. The role of immune checkpoints PD-1 and CTLA-4 in cardiovascular complications leading to heart failure. Front. Immunol. 2025, 16, 1561968. [Google Scholar] [CrossRef]
- Zhao, Z.; Qi, D.; Zhang, Z.; Du, X.; Zhang, F.; Ma, R.; Liang, Y.; Zhao, Y.; Gao, Y.; Yang, Y. Prognostic Value of Inflammatory Cytokines in Predicting Hospital Readmissions in Heart Failure with Preserved Ejection Fraction. J. Inflamm. Res. 2024, 17, 3003–3012. [Google Scholar] [CrossRef]
- Baumhove, L.; Bomer, N.; Tromp, J.; van Essen, B.J.; Dickstein, K.; Cleland, J.G.; Lang, C.C.; Ng, L.L.; Samani, N.J.; Anker, S.D.; et al. Clinical characteristics and prognosis of patients with heart failure and high concentrations of interleukin-17D. Int. J. Cardiol. 2024, 396, 131384. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. The role of IL-17 family cytokines in cardiac fibrosis. Front. Cardiovasc. Med. 2024, 11, 1470362. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Yan, T.; Liu, J.; Zhang, C.; Yan, J.; Xu, L.; Zhang, N.; Zhang, X. Meta-analysis of the association between interleukin-17 and ischemic cardiovascular disease. BMC Cardiovasc. Disord. 2024, 24, 252. [Google Scholar] [CrossRef]
- Alogna, A.; Koepp, K.E.; Sabbah, M.; Espindola Netto, J.M.; Jensen, M.D.; Kirkland, J.L.; Lam, C.S.P.; Obokata, M.; Petrie, M.C.; Ridker, P.M.; et al. Interleukin-6 in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2023, 11, 1549–1561. [Google Scholar] [CrossRef]
- Albar, Z.; Albakri, M.; Hajjari, J.; Karnib, M.; Janus, S.E.; Al-Kindi, S.G. Inflammatory Markers and Risk of Heart Failure With Reduced to Preserved Ejection Fraction. Am. J. Cardiol. 2022, 167, 68–75. [Google Scholar] [CrossRef]
- Ferreira, C.; Trindade, F.; Ferreira, R.; Neves, J.S.; Leite-Moreira, A.; Amado, F.; Santos, M.; Nogueira-Ferreira, R. Sexual dimorphism in cardiac remodeling: The molecular mechanisms ruled by sex hormones in the heart. J. Mol. Med. 2022, 100, 245–267. [Google Scholar] [CrossRef]
- Elagizi, A.; Köhler, T.S.; Lavie, C.J. Testosterone and Cardiovascular Health. Mayo Clin. Proc. 2018, 93, 83–100. [Google Scholar] [CrossRef]
- Withaar, C.; Meems, L.M.G.; Nollet, E.E.; Schouten, E.M.; Schroeder, M.A.; Knudsen, L.B.; Niss, K.; Madsen, C.T.; Hoegl, A.; Mazzoni, G.; et al. The Cardioprotective Effects of Semaglutide Exceed Those of Dietary Weight Loss in Mice with HFpEF. JACC Basic. Transl. Sci. 2023, 8, 1298–1314. [Google Scholar] [CrossRef]
- Fleury, M.A.; Clavel, M.A. Sex and Race Differences in the Pathophysiology, Diagnosis, Treatment, and Outcomes of Valvular Heart Diseases. Can. J. Cardiol. 2021, 37, 980–991. [Google Scholar] [CrossRef]
- Sun, Q.; Güven, B.; Wagg, C.S.; Almeida de Oliveira, A.; Silver, H.; Zhang, L.; Chen, B.; Wei, K.; Ketema, E.B.; Karwi, Q.G.; et al. Mitochondrial fatty acid oxidation is the major source of cardiac adenosine triphosphate production in heart failure with preserved ejection fraction. Cardiovasc. Res. 2024, 120, 360–371. [Google Scholar] [CrossRef]
- Güven, B.; Sun, Q.; Wagg, C.S.; Almeida de Oliveira, A.; Silver, H.; Persad, K.L.; Onay-Besikci, A.; Vu, J.; Oudit, G.Y.; Lopaschuk, G.D. Obesity Is a Major Determinant of Impaired Cardiac Energy Metabolism in Heart Failure with Preserved Ejection Fraction. J. Pharmacol. Exp. Ther. 2024, 388, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Fang, L.; Jiang, Y.; Liang, M.; Wang, J.; Shen, Y.; Wang, Z.; Liang, F.; Huo, H.; Pan, C.; et al. Angiotensin II Promotes White Adipose Tissue Browning and Lipolysis in Mice. Oxid. Med. Cell Longev. 2022, 2022, 6022601. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.; Alluri, K.; Peng, H.; Ortiz, P.A.; Xu, J.; Sabbah, H.N.; Rhaleb, N.E.; Matrougui, K. Unveiling the vulnerability of C57BL/6J female mice to HFpEF and its related complications. J. Mol. Cell Cardiol. Plus. 2024, 7, 100062. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, A.; McMillen, T.S.; Wang, Y.; Zhou, B.; Chen, H.; Banerjee, D.; Herrero, M.; Wang, P.; Muraoka, N.; Wang, W.; et al. Blunted Cardiac Mitophagy in Response to Metabolic Stress Contributes to HFpEF. Circ. Res. 2024, 135, 1004–1017. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teou, D.C.; Labbé, E.-A.; Thibodeau, S.-È.; Walsh-Wilkinson, É.; Morin-Grandmont, A.; Trudeau, A.-S.; Arsenault, M.; Couet, J. The Loss of Gonadal Hormones Has a Different Impact on Aging Female and Male Mice Submitted to Heart Failure-Inducing Metabolic Hypertensive Stress. Cells 2025, 14, 870. https://doi.org/10.3390/cells14120870
Teou DC, Labbé E-A, Thibodeau S-È, Walsh-Wilkinson É, Morin-Grandmont A, Trudeau A-S, Arsenault M, Couet J. The Loss of Gonadal Hormones Has a Different Impact on Aging Female and Male Mice Submitted to Heart Failure-Inducing Metabolic Hypertensive Stress. Cells. 2025; 14(12):870. https://doi.org/10.3390/cells14120870
Chicago/Turabian StyleTeou, Diwaba Carmel, Emylie-Ann Labbé, Sara-Ève Thibodeau, Élisabeth Walsh-Wilkinson, Audrey Morin-Grandmont, Ann-Sarah Trudeau, Marie Arsenault, and Jacques Couet. 2025. "The Loss of Gonadal Hormones Has a Different Impact on Aging Female and Male Mice Submitted to Heart Failure-Inducing Metabolic Hypertensive Stress" Cells 14, no. 12: 870. https://doi.org/10.3390/cells14120870
APA StyleTeou, D. C., Labbé, E.-A., Thibodeau, S.-È., Walsh-Wilkinson, É., Morin-Grandmont, A., Trudeau, A.-S., Arsenault, M., & Couet, J. (2025). The Loss of Gonadal Hormones Has a Different Impact on Aging Female and Male Mice Submitted to Heart Failure-Inducing Metabolic Hypertensive Stress. Cells, 14(12), 870. https://doi.org/10.3390/cells14120870






