Abstract
Centrioles are remarkably stable organelles that play a main role in the assembly of centrosomes and ciliary structures. However, there are several differentiated tissues that must eliminate their centrioles to avoid centrosome formation and improper cell proliferation. Therefore, centriole elimination represents an important process in many organisms to ensure successful cell differentiation and maintenance of tissue homeostasis. In this review, we analyzed centriole inactivation and elimination in various Drosophila cell types in relation to the dynamics of the pericentriolar material.
1. Introduction
Centrioles are widely conserved barrel-shaped organelles composed of triplet, doublet, or single microtubules, usually arranged in a beautiful ninefold symmetry [1,2,3,4,5]. Centrioles are essential for proper cell function: they are found at the focus of the centrosome, the nucleating center for the microtubule network in interphase and cell division, and represent the basis of the ciliary structures involved in cell movement and cell signaling [6,7,8,9]. Therefore, centriole dynamics are crucial for cell homeostasis [10]. Since centrioles represent the core components of centrosomal proteins such as centrosomin (Cnn), spindle defective 2 (Spd2), and γ-tubulin, which are directly involved in the nucleation of the microtubule network, their duplication must be strictly regulated during the cell cycle to avoid too many centrosomes [11,12,13]. Centriole assembly and replication in cycling cells occur during interphase and rely on a set of core proteins forming the so-called Plk4-Stil-Sas6 module [14,15,16,17,18].
Despite the need for centrioles to organize a functional centrosome able to nucleate the network of cytoplasmic microtubules in interphase and during mitosis [19], there are several examples of differentiating cells that eliminate or inactivate their centrioles [20]. The most remarkable example is the Drosophila Spindle assembly abnormal 4 (Sas4) mutant in which adults eclose in the absence of centrioles [21], suggesting that centrioles can be dispensable in some cases for development. However, the centrioles are needed in Sas4 embryos to sustain early development until the beginning of gastrulation when the maternal supplied factors rapidly decrease [22].
Centriole elimination is important to warrant the correct homeostasis of some differentiated tissues. There are several instances in which the loss of centriole integrity or defective centriole/centrosome biogenesis critically contributes to specific diseases, including cancer, ciliopathies, and developmental disorders [23,24,25,26,27,28]. Furthermore, the failure to eliminate centrioles does not prevent the generation and does not alter the fate of supernumerary centrioles in dividing cells [29]. Aneuploidy, which is a hallmark of cancer cells, correlates with the presence of extra centrosomes, which originate from centriole overduplication [13,30,31].
The elimination of the centrioles is a remarkable phenomenon, considering the high stability of this organelle and its persistence within the conditions that lead to microtubule depolymerization. However, despite extensive knowledge of the molecular players involved in centriole assembly, it is still unclear how centriole stability is maintained, and the mechanisms leading to centriole elimination remain an important challenge.
Various Drosophila cell types lack functional centrosomes and organize specialized microtubule networks by non-centrosomal microtubule organizing centers (ncMTOCs) [32]. Non-centrosomal microtubule arrays have been described in Drosophila during oocyte development [33], in muscle cells [34], within dendrite branches [35], along the mitochondrial derivatives during spermatid elongation [36,37], and around the nuclear envelope of larval fat body cells [38]. The lack of functional centrosomes is typical of postmitotic polyploid cells such as salivary glands, midgut, and Malpighian tubule cells [39], although it is still unclear the relationship between the multiple rounds of chromosome replication and the absence of true centrosomes.
Replacement of functional centrosomes with ncMTOCs in Drosophila occurs through an unusual centriole dynamic. Early differentiating ovarian follicle cells [40], salivary gland cells [41], tracheal cells [42], wing epithelial cells [43,44], and ommatidial cells [45,46,47] first assemble a microtubule network by centrosome-dependent mechanisms. Later, however, the centrosome is inactivated, and its core centrioles no longer localize near the new microtubule nucleating foci. Centriole reduction or defects in their structure have been reported in Drosophila after mutations in genes encoding proteins that regulate or build the centriole architecture [48]. These findings suggest that Drosophila could represent a powerful system in which to examine the reduction in centrioles during development and differentiation, and it may be a helpful model to uncover the structural changes leading to centrioles elimination.
The individual examples described below illustrate that there is a subtle diversity in how Drosophila cells ensure centriole elimination or inactivation, suggesting that the mechanisms driving these processes could be slightly different through various tissues (Figure 1).
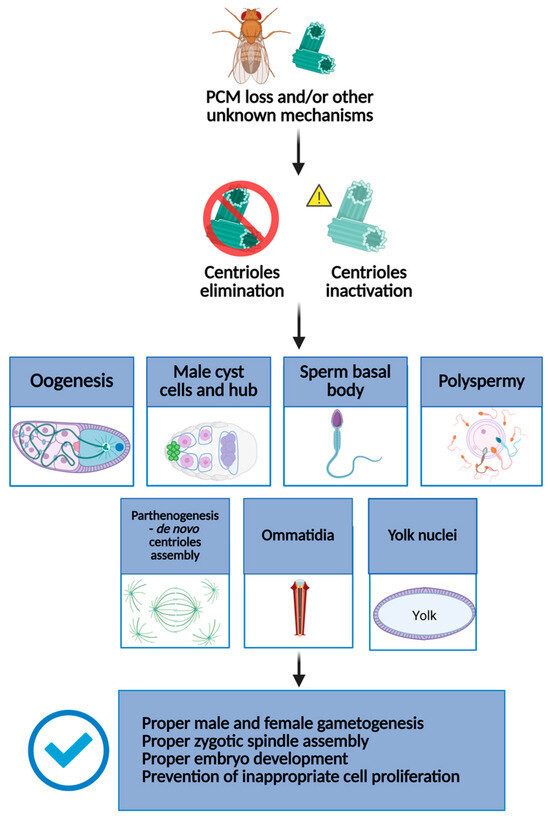
Figure 1.
Schematic representation of how, in Drosophila, centrioles are eliminated or inactivated at key stages of development and cellular differentiation. In particular, centrioles are eliminated or inactivated during oogenesis, spermiogenesis, and parthenogenesis during the early stages of embryo development. Centrioles are also inactivated in differentiated cells, such as ommatidia. These processes are essential to prevent aberrant cell divisions, ensure proper gametogenesis, and support normal embryonic development. Created in BioRender, https://BioRender.com/37eu8sg (accessed on 21 May 2025).
2. The Stem Cell Niche of the Drosophila Testis: Somatic and Germline Centrioles with Different Fates
The apical region of the Drosophila testis contains a niche with a cluster of small somatic cells, the hub cells, surrounded by 8–10 germline stem cells (GSCs) that will be the progenitors of the male gametes. During spermatogenesis and spermiogenesis, each GSC is surrounded by a pair of somatic cyst stem cells (CySCs) that are progenitors of the thin cyst cells [49]. The asymmetric division of each GSC leads to a new stem cell and a gonioblast daughter, which progress through spermatogenesis to form the haploid spermatids that will differentiate in mature sperms. The CySCs also undergo asymmetric divisions to generate new CySCs and cyst cell daughters that withdraw from the cell cycle and will modify their size and shape, becoming very flat and large.
Centrioles in germ cells and cyst cells differ in their functional abilities and timing of activity in accordance with the behavior of their respective cell types. Centrioles of germ cells recruit pericentriolar material (PCM) to assemble functional centrosomes needed to warrant spermatogonial mitosis and spermatocyte meiosis. In addition, the centrioles act as basal bodies to nucleate the axoneme of the cilium-like regions (CLRs) of the spermatocytes and the flagellum of the differentiating sperm cells. Conversely, the cyst cells become quiescent, and their centrioles gradually lose the ability to recruit PCM, including γ-tubulin, but keep the cartwheel component Sas6. This suggests that centrioles are present but inactivated. Indeed, the cyst cells have centriole pairs, at least during the first spermatogonial mitosis, but the daughter centriole is very short and does not elongate further [50], pointing to a correlation between centriole growth and availability of centrosomal material. Similarly, the daughter centriole that remains in the ooplasm at the end of meiosis in the starfish Patiria miniata sheds the surrounding PCM and then disappears [51]. Moreover, centriole duplication does not occur in cyst cells, possibly due to the downregulation of Sas6, a main regulator of centriole architecture [50]. The failure of centriole duplication represents an important limitation since cyst cell division could compromise the integrity of the entire germ cell, impairing the correct progression of gametogenesis.
The other class of somatic cells building the stem cell niche are the small post-mitotic cells of the hub. Centrioles are occasionally found in the hub cells, but they are single and lack procentrioles, suggesting failure to duplicate. Moreover, γ-tubulin, pericentrin-like protein (Plp), and spindle defective 2 (Spd2), usually observed in GSCs and CySCs, are barely detectable or absent in the hub region [52,53]. The failure to recruit these centrosomal proteins confirms that the rare hub centrioles are not functional. The inactivation of the centriole function is an intrinsic property of the cyst cells, and it is unrelated to the germ cells they surround. Indeed, CySCs of the Drosophila mutant nup154 [54] undergo asymmetric division even in the absence of GSCs, and the daughter cyst cells are unable to experience mitosis, and their centrioles do not organize functional centrosomes. However, although hub cells are generally considered terminally differentiated and quiescent, some observations suggest that they can exit quiescence to generate new CySCs [55]. This raises the intriguing question of how these cells regain centrosome function to re-enter the cell cycle and proliferate.
The centrioles of the hub cells have a remarkable architecture and consist of mixed doublet and triplet microtubules. This condition questions the general opinion that Drosophila flies would display two distinct and separate centriole models: centrioles with nine microtubule doublets, only found in somatic cells, and centrioles with nine microtubule triplets typical of male and female germline cells (see [56,57]). The mixed doublet and triplet microtubules of the hub cell centrioles suggest that the acquisition of the C-tubule could sporadically occur in the stem cell niche.
The pole cells, the precursors of the germ cell line, have centrioles with doublet microtubules, like somatic cells, and the GSCs acquire the C-tubule before their asymmetric division [52]. But if it is so, many questions remain open: what kind of information is required to acquire the C-tubule? Why do the hub cells not complete the triplet pattern? Why do the CySCs, located in the stem cell niche, have centrioles with only doublet microtubules?
In addition, the centrioles of the hub cells lack a distinct cartwheel, and the centrioles of the cyst cells show slight signs of degeneration. Remarkably, centriole elimination during C. elegans oogenesis begins with the loss of the central tube, followed by the disassembly of microtubules. [58]. However, the cartwheel, which appears to be a constant structure of Drosophila somatic and germ cell centrioles, lacks both the tandemly aligned centrioles that are at the basis of the olfactory neurons. The distal centriole that nucleates the ciliary axoneme of the olfactory neurons is made up of nine doublets of microtubules, whereas the wall of the proximal centriole consists of both doublet and single microtubules. The single microtubules often display a lateral hook, suggesting that the proximal centrioles grow incompletely or, alternatively, that they are at the beginning of their degradation.
Moreover, the proximal centrioles, despite their incomplete architecture, recruit some typical centrosomal components such as Plp, Anastral spindle 1 (Ana1), Spd2, and γ-tubulin [59] and are exceptionally stable throughout the life of the fly. This suggests that the incomplete wall of the proximal centriole in the olfactory ciliated neurons does not necessarily correlate with decreased function or stability.
A noteworthy structural asymmetry between the centriole pair has been reported in the mature mammalian sperm. However, contrary to the centrioles of the Drosophila olfactory neurons, the distal centriole that templates the flagellar axoneme degenerates during human spermiogenesis, whereas the proximal inactive centriole is structurally unchanged [60,61].
3. Oogenesis: The Main Example of Centriole Elimination
The formation of the zygotic spindle in most organisms usually requires the contribution of the male gamete that provides its basal body at fertilization. Indeed, the basal body functions as a true centriole, recruiting all the components from the oocyte cytoplasm required to organize a functional centrosome capable of nucleating the microtubules that support pronuclear migration. After the pronuclei come into contact, the centrosome duplicates to assemble the bipolar zygotic spindle. Thus, the assembly of the zygotic spindle requires the presence of only a single centrosome at fertilization since multiple centrosomes can nucleate supernumerary astral microtubules that can have deleterious effects on embryonic development. Therefore, the egg must be centriole-free to avoid too many centrosomes at fertilization and ensure that only the sperm basal body can act as the master for the zygotic spindle assembly. The oocyte coming from transit-amplifying oogonial mitotic divisions must eliminate or inactivate any inherited centrioles/centrosomes upon entry into meiosis. Thus, centriole elimination during oogenesis is a widespread phenomenon that occurs throughout metazoan organisms. There are two striking examples of centriole elimination during female meiotic progression: the case of echinoderms and the case of insects and mammals [62]. Echinoderms maintain centrioles, and the meiotic spindles are organized by true centrosomes that progressively reduce their centriole content. Thus, the spindle poles of the first meiotic spindle consist of a centriole pair, whereas during the second meiosis, only one centriole is found at each pole. The extrusion of the last polar body results in the inheritance of the daughter centriole in the egg cytoplasm. However, the daughter centriole is unable to sustain microtubule nucleation and cannot interfere with embryonic development [51]. Conversely, mammalian and insect oocytes progress through meiosis without centrioles, and their spindles are anastral. The fate of centrioles in the Drosophila oocyte is particularly noteworthy [63]. Drosophila oogenesis begins in the anterior region of the ovariole, where two to three GSCs divide asymmetrically to self-renew and give origin to daughter cystoblasts that undergo four incomplete mitotic divisions to form cysts of 16 germ cells interconnected by cytoplasmic bridges, or ring canals [64]. Each of the 16 germ cells inherits a pair of centrioles consisting of nine triplet microtubules. Early in oogenesis, when the oocyte starts to differentiate, the centrioles travel through the ring canals of the sister cells and cluster in the posterior region of the oocyte cytoplasm [65]. The mechanisms driving the movement of each centriole pair from the nurse cells toward the oocyte cytoplasm are still poorly understood. The centrioles are inactive and do not organize functional centrosomes during migration, but a large MTOC appears when the centrioles cluster at the posterior region of the oocyte [66]. Centrioles have been observed at the ultrastructural level in the oocyte cytoplasm during mid-oogenesis [33], and typical centriole markers have also been observed during later stages of oogenesis [67]. Immunofluorescence analysis of the centriolar components Ana1 and Sas6 support the gradual diminution through oogenesis of the centriole number and their loss just before meiotic spindle assembly [67]. The problem here is how the centrioles are eliminated to ensure the correct centriole number upon fertilization. It has been suggested that centriole elimination could be a consequence of the loss of the PCM and that Polo loss could be a critical event in triggering the loss of the PCM, followed by centriole elimination. Indeed, downregulating Polo expression accelerates centriole loss, whereas ectopic tethering of Polo to centrioles prevents the loss of PCM, inhibiting the elimination of the oocyte centrioles [67]. The Polo-induced stability to the oocyte centrioles leads to abnormal meiosis and aborted embryonic development.
Ultrastructural observations of the centriole cluster at the posterior region of the oocyte cytoplasm revealed centrioles with different lengths and centrioles with slight signs of fragmentation.
4. The Sperm Centriole: An Immortal Contribution?
Successful embryonic development in most organisms requires the contribution of the sperm cell that supplies its nucleus and the basal body, with the latter being involved, during spermiogenesis, in the assembly of the axoneme, which gives motility to the male germ cell. The axoneme and the basal body usually separate in the ooplasm of many animals [68,69], presumably to facilitate the role of the inherited centriole as organizer of the functional zygotic centrosome. The elimination of the sperm tail is, indeed, vital to early development in mammals [70], and the persistence of the tail near the zygotic spindle results in developmental failures of the human embryo [71].
The whole sperm cell enters the Drosophila oocyte at fertilization [72,73,74], and the sperm tail, about 2 mm in length, was seen within the yolk region during the blastoderm stage [75] and persisted into the larval midgut after hatching [76]. Remarkably, the sperm tail ends at one mitotic spindle during the nuclear divisions of the early syncytial Drosophila melanogaster [77] and Drosophila simulans [78] embryos. This suggests that the sperm basal body is not excised, but both the structures are intact and remain associated through early development. Indeed, a giant centriole was seen at the apical end of the sperm axoneme [79], confirming a continuity between the sperm axoneme, the centriole, and the mitotic spindle poles. The sperm centriole recruits PCM, and it is found at the core of the true centrosome that organizes the microtubule aster, needed for the apposition of the parental pronuclei, and then it associates with one pole of the zygotic spindle. This process is in apparent contrast with the finding in vertebrate cells that the mother centriole, involved in primary cilium assembly, must shed axonemal microtubules to leave the cell surface and move inward to organize a functional centrosome. However, during insect spermatogenesis, both parental centrioles form microtubule-based CLRs that persist during the assembly of the meiotic spindles [80]. The sperm centriole, despite its link to the axoneme, can duplicate but does not form a mirror image. The daughter centriole is, indeed, smaller than the mother and consists of nine doublets of microtubules, as the ensuing centrioles of somatic cells (and as well all the somatic centrioles that derive from it), while the sperm centriole maintains nine triplets of microtubules.
The two main open questions here are the following: how long is the life of the sperm centriole during development, and how long is its ability to assemble functional centrosomes? The sperm centriole and its tail dissociate from the surface nuclei before cellularization and seek inward. This process is apparently correlated with the reduction in the ability of the sperm centriole to recruit PCM. The amounts of centrosomin (Cnn) and Spd2 slightly reduce when the sperm centriole is at the embryo surface and continues to decrease during early development. A feeble Spd2 labeling is found at the beginning of gastrulation when the sperm centriole is within the yolk region, whereas no more Cnn signal is seen at this stage [79].
Therefore, the first sign of basal body inactivation is the gradual loss of centrosomal material. Presumably, the loss of pericentriolar components could ultimately lead to centriole degradation. This is consistent with the idea that the PCM is needed to warrant centriole duplication and maintenance (integrity, stabilization) in Drosophila melanogaster [67,81,82,83].
5. How Many Centrioles at Fertilization? The PCL Hypothesis
Centriole duplication is accurately monitored during cell division to avoid too many active centrosomes that may have detrimental effects. This process in somatic cells is tightly coupled to DNA replication [84]. However, during male gametogenesis in the majority of animals, mammals included, centriole duplication also occurs during the second meiotic division in the absence of DNA replication. Thus, the haploid spermatids inherit a pair of centrioles that are transferred to the oocyte at fertilization. This is not the rule during insect spermatogenesis in which the centrioles duplicate in primary spermatocytes, according to DNA replication, but fail to duplicate during the second meiosis [56]. Although it appears trivial that the mature sperm carries only its basal body at fertilization, it is still unclear if the sperm cell can also contribute an atypical unstructured procentriole. This conundrum arises from the observation that typical centriolar proteins, namely Ana1, Bld10, Sas6, and Asterless (Asl), are detected as a small dot-like signal in the so-called proximal centriole-like (PCL) structure close to the basal body of early elongating Drosophila spermatids [85,86,87]. These findings point to the presence of a PCL organelle [85]. Ultrastructural observations of early elongating Drosophila spermatids have, indeed, revealed the presence of an unusual procentriole consisting of a distinct cartwheel and an incomplete set of single peripheral tubules, presumably the A-tubules [52]. Remarkably, co-overexpression of the centriolar proteins Sas6 and Poc1B, which are enriched in the PCL [88], induces the formation of ectopic aggregates containing PCL-like structures [89].
The dot-like signals of the centriolar proteins disappear during spermiogenesis [90], and a structured PCL is no longer found in mature sperm [52]. This opens questions on the transient nature and function of the PCL. It has been proposed that the PCL may contribute to the correct anchoring of the basal body to the nuclear envelope during sperm elongation [91] and may represent a focus for the assembly of the second centriole during the formation of the first zygotic spindle [90]. However, it is unclear why the procentriole appears at the onset of spermiogenesis when centriole duplication represents an unexpected and unusual feature for centriole biogenesis.
Distinct procentrioles have also been observed close to the basal body of young spermatids in the coleopteran Adalia [92] and Tribolium [93]. Interestingly, the procentriole of Adalia looks like the one of Drosophila and consists of a cartwheel and peripheral single microtubules, whereas the procentriole of Tribolium lacks microtubules but only displays a dense peripheral ring.
Similar intermediate procentriole-like structures, consisting of a distinct cartwheel and peripheral single microtubules, have also been observed in the lepidopterans Bombix [94], Ephestia [95], and Pieris [96]. These structures, in clusters of three to four, appear before spermiogenesis and are observed in mature primary spermatocytes close to the still-engaged parental centrioles. However, young Pieris spermatids maintain only one procentriole that is no longer detected in mature sperm.
Thus, the male insect gamete could provide at fertilization one true centriole and an unstructured, undisguisable precursor that could act as a scaffold for the rapid assembly of a second zygotic centriole [97]. The problem here is why and how the structured procentriole is eliminated during sperm maturation and what residue, if any, the sperm carries into the egg at fertilization. It is also still unclear how the unstructured precursor can be converted into a functional centriole at fertilization.
6. Polyspermy: Elimination of Redundant Sperm Centrioles
The apposition of male and female pronuclei at fertilization requires the microtubule aster nucleated by the centrosome that is reconstituted around the inherited sperm centriole. Therefore, only one sperm is needed to warrant the successful fusion of the parental pronuclei. Multiple sperm can provide extra centrioles, leading to the formation of supernumerary asters with ensuing developmental defects. Most dioecious animals have developed various strategies to prevent excess sperm from entering the egg and avoid abortion of the first embryonic divisions. However, animals that lay large eggs allow the entry of numerous sperm without detrimental effects on the development of the embryo [98,99]. This process, called physiological polyspermy, which increases the chance that a sperm will meet its female counterpart in a very large cytoplasm, raises intriguing questions about how a single sperm is selected while the others are discarded. It is also unclear how only one centriole organizes the zygotic centrosome, whereas the other will be eliminated.
The entrance of more than one sperm into the egg is a rare event during insect fertilization [100]. Only in a few cases have multiple sperm been observed in the egg cytoplasm of Drosophila melanogaster [63], and supernumerary paternal nuclei have only occasionally been found [101]. Remarkably, Drosophila obscura embryos complete gastrulation with multiple sperm [75]. Although monospermy is the rule in Drosophila melanogaster [102], the few cases in which more than one sperm is present at fertilization raise the question of how supernumerary sperm do not affect embryo development.
Some models have been proposed during urodele amphibian fertilization to explain the selection of one sperm nucleus and the elimination of the supernumerary sperm [99]. Among these models, the failure to enter M-phase could explain the degeneration of the accessory sperm nuclei [99]. Although appealing, this hypothesis does not seem to be applicable in the few cases of Drosophila polyspermy, in which the sperm nuclei are arrested in an anaphase-like configuration [101]. Here, the haploid chromosomes are congressed at the metaphase plate, and the sister chromatids separate at the onset of anaphase but do not migrate to the opposite poles, pointing to defects in the mitotic spindle architecture and dynamics. Each pole of the anaphase-like spindles displays a pair of centrosomes nucleating short astral microtubules, whereas the anaphase zygotic spindles that hold both male and female complements had only one centrosome for each pole. It is unclear whether each centrosome of the pair contains a typical pair of centrioles. If this was the case, we can hypothesize that centriole duplication occurs despite the fact that the chromosomes do not duplicate. Presumably, the centrosomes detach from the abnormal spindles and subsequently degenerate.
7. Parthenogenesis: Too Many Centrioles Do Not Affect the Embryonic Development
Although the centrioles appear to be useless organelles during oogenesis, they must be eliminated because their retention is deleterious for embryonic development. Centriole elimination during oogenesis is needed to ensure the correct centriole number upon fertilization; thus, the mature oocyte usually lacks centrosomes. Some parthenogenetic insects that develop without male contribution, such as the collembolan Folsomia candida [103], the viviparous pea aphid Acyrthosiphon pisum [104], the parasitic hymenopterans Nasonia vitripennis [105] and Muscidifurax uniraptor [106], and the dipterans Drosophila mercatorum [107,108] and Drosophila ananassae [109] also eliminate the maternal centrioles during oogenesis, and the meiotic spindles are anastral. However, a variable number of microtubule asters appeared in the oocyte cytoplasm after anaphase of the first meiosis. Ultrastructural observations revealed that the focus of the cytoplasmic asters contains distinct centrioles [110], supporting the de novo assembly of these organelles despite the absence of pre-existing templates. The presence of cytoplasmic asters appeared as a conserved feature shared among all the insect species until now examined and represents the basis for the assembly of the first zygotic spindle.
Although it is still unclear how the haploid female pronucleus resumes diploidy during parthenogenetic development, we noticed that all the collembolan, aphid, and hymenopteran parthenogenetic eggs develop properly, whereas only 8–10% of the eggs laid by the Drosophila parthenogenetic species eclose, pointing to a stochastic phenomenon rather than to the presence of operative mechanisms. Therefore, the parthenogenetic development of Drosophila mercatorum and Drosophila ananassae appears as a recently acquired process.
A common feature of all the parthenogenetic eggs is the persistence of several astral microtubules during embryonic development, suggesting that the supernumerary centrioles can successfully recruit centrosomal proteins. As discussed above, one main problem at fertilization is the control of the centriole number since the zygotic spindle only needs two pairs of centrioles to be assembled. Thus, the simultaneous presence of several asters within the egg cytoplasm raises the question of how the egg can properly organize the first bipolar spindle and undergo further mitotic divisions without the negative interference of the supernumerary centrioles. Consequently, most animal eggs develop different strategies to avoid supernumerary centrioles at the beginning of embryo development. Such controls prevent the assembly of multipolar spindles and the ensuing developmental defects that are seen during polyspermic fertilization in which multiple sperm asters result in aberrant spindles and developmental failure [68,100]. However, parthenogenesis occurs in a condition resembling polyspermy, but the supernumerary asters do not have a deleterious effect on development and are prevented from interacting with the zygotic spindle.
Indeed, in Aphids and Hymenopterans, only one or two asters contact the female pronucleus, whereas, in the Drosophila species with occasional parthenogenesis, multiple asters may interact with the chromatin, leading to abnormal spindles and developmental failures [107,108,109]. This indicates that a mechanism limiting aster interaction with the female pronucleus does not work in Drosophila. Moreover, the few Drosophila embryos that develop parthenogenetically have many free cytoplasmic asters, mainly localized in the anterior region of the embryo.
Remarkably, the multiple centrosomes in the Drosophila parthenogenetic eggs seem to duplicate in a cell cycle-dependent manner: single asters have been observed during interphase, whereas distinct pairs are found in prometaphase. Close aster pairs are also visible during late anaphase/telophase, the time the centrioles disengage at the spindle poles. These observations suggest that centriole duplication is unrelated to DNA replication and involves an unknown cytoplasmic clock. Interestingly, it has been demonstrated in Drosophila eggs that centrioles form de novo and duplicate at an elevated Polo-like kinase 4 (Plk4) concentration [111]. This suggests that Plk4 concentration determines the temporal onset of centriole assembly.
Unfortunately, the molecular mechanism triggering centriole de novo assembly at anaphase of the first meiosis remains largely unknown. It is known that overexpression of typical centriolar proteins, such as Plk4, Sas4, Sas6, and Asl, leads to de novo assembly of centrioles in Drosophila unfertilized eggs [112,113,114]. However, a correlation between the overexpression of these proteins and parthenogenetic development needs to be demonstrated.
The asters disappear at different times in different parthenogenetic species. Muscidifurax uniraptor embryos lose their asters during the third mitosis. By contrast, Drosophila mercatorum, Drosophila ananassae, and Acyrthosiphon pisum embryos retain some asters until later cleavage divisions. This suggests that different insect species exploit different mechanisms to inactivate and eliminate supernumerary centrosomes from the cytoplasm of the developing embryo. The mechanisms underlying centriole elimination at different times are still unclear and need further investigation and careful ultrastructural analysis.
8. Yolk Centrosomes: Different Fates in the Same Cytoplasm
The development of the early Drosophila embryo consists of 13 nuclear divisions occurring in a common syncytium that later cellularizes to form a surface blastoderm of about 6000 cells. The first nuclear divisions occur in the central region of the egg, but during telophase of the eighth and ninth mitoses, the majority of the nuclei migrate towards the cortical region of the embryo, whereas other nuclei remain stationary in the inner cytoplasm.
Thus, two distinct nuclear lineages with different fates segregate at the end of the ninth mitotic nuclear division: the peripheral blastodermic nuclei that will be the precursors of the somatic tissues and the inner nuclei that are involved in yolk digestion [115] and midgut development [116]. An unusual and remarkable feature is that each of the nuclear lineages has its own division program despite all residing in a common cytoplasm. The peripheral nuclei undergo four additional mitotic divisions to give rise to a syncytial blastoderm that later cellularizes [117]. By contrast, the yolk nuclei undergo two abnormal divisions followed by two rounds of DNA replication without karyokinesis [77].
The centrosomes associated with the peripheral nuclei organize functional mitotic spindles that support chromosome congression at the metaphase plate and successful chromatid migration to opposite spindle poles. By contrast, the centrosomes in the inner cytoplasm, hereafter called yolk centrosomes, display unusual dynamics. The yolk centrosomes organize normal bipolar spindles during the nuclear cycle 10, but starting from nuclear cycle 11, the majority of the yolk centrosomes duplicate but do not separate properly, and most of the spindles are monopolar. During nuclear cycles 12 and 13, most of the centrosomes detach from the spindle poles and are found free in the inner cytoplasm far from the abnormal chromatin clusters [118]. Remarkably, the free yolk centrosomes can duplicate further, and clusters of four or more centrosomes have been observed during the cellularization of the syncytial blastoderm and at the beginning of gastrulation. This suggests that the yolk centrosomes can duplicate regardless of their incomplete separation and in the absence of nuclear divisions, whereas the centrosome cycle in the cortical region of the embryo is strictly coupled to the nuclear divisions. It is, therefore, intriguing to speculate that centrosomes in the inner cytoplasm and in the cortical region respond to different requirements, although during the early nuclear division cycles, all centrosomes duplicate in synchrony.
The isolated yolk centrosomes recruit γ-tubulin after the nuclear cycle 12 but do not nucleate distinct microtubules, as they do during the previous nuclear cycles, although a small pool of free tubulin is associated with them. This implies a progressive inactivation of the yolk centrosomes that gradually lose their microtubule nucleating properties before disappearing during late gastrulation. However, the molecular mechanisms leading to the inactivation of the yolk centrosome and the elimination of the core centrioles are still unclear.
9. Ommatidial Differentiation: A Tale of Useless Centrioles
The Drosophila eye is formed by ∼750 ommatidial units derived from undifferentiated proliferating cells that form the eye-antennal disc [119,120,121]. The patterning of the ommatidia requires dramatic cell transformations that begin in early third-instar larvae when the eye disc is crossed by the morphogenetic furrow [122]. This structure, in the form of a deep indentation of the epithelium, represents the boundary between the anterior region of the disc where the undifferentiated cells proliferate and the posterior region in which the rhabdomeric cells differentiate to organize the ommatidial units [123,124,125].
The cells of the anterior region of the imaginal disc hold distinct centrosomes that assemble functional mitotic spindles. Such centrosomes, enriched in Cnn and Spd-2, actively recruit γ-tubulin [45,126]. Conversely, the cells of the posterior region of the imaginal disc do not proliferate, and their centrioles are unable to recruit the typical centrosomal proteins. Thus, in the absence of γ-tubulin, the centrioles of the post-mitotic differentiating rhabdomeric cells are not involved in the nucleation of the longitudinal bundles of microtubules that fill the whole cytoplasm [45,47]. Rather, γ-tubulin foci are found at the apical cell membranes, suggesting that they may represent non-conventional MTOC like those described in the cone cells of the Drosophila ommatidia [46]. In addition to the earlier failure of γ-tubulin recruitment, the centrioles of the rhabdomeric cells did not accumulate Cnn and Spd2 proteins during the late larval stages.
Remarkably, the centrioles of the pupal eyes gradually lost the scaffold protein Plp, whereas the core proteins Asl, Ana1, and Sas4 disappeared later, pointing to the progressive disappearance of the centrioles. Although the centrosomal proteins γ-tubulin, Cnn, Spd2, and Plp are lost during late larval and early pupal stages, the structure of the centrioles remains unchanged and displays the usual model of nine doublets of microtubules with a distinct cartwheel, suggesting that the depletion of the PMC does not affect the centriole architecture. However, during later pupal stages, the centriole wall displays doublet and single microtubules. Moreover, the A-tubule holds lateral short hooks, suggesting the progressive deletion of the B-tubule. A distinct cartwheel is no longer observed at this stage of development.
10. Conclusions
Centrioles are very stable organelles that maintain intact their structure during cell cycle progression and embryo development. However, some differentiating tissues eliminate or inactivate their centrioles to avoid the formation of unnecessary functional centrosomes and then prevent inappropriate cell proliferation. Therefore, an important aspect to clarify is to understand the mechanisms that enable centriole stability and the process leading to centriole disassembly. PCM removal plays a critical role in centriole stabilization in some Drosophila cells. Indeed, the continuous expression of cartwheel, centriole wall, and PCM components play a main role in ensuring centriole integrity. However, the relationship between the reduction in PCM components and centriole deconstruction is not a widespread phenomenon, as there are animal cells in which centriole stability is apparently unrelated to PCM persistence [127,128,129].
Although some data on the role of centriolar proteins and centrosomal components involved in managing centriole stability have led to suggestions of hypothetical mechanisms underlying stepwise centriole elimination [20], the structural changes leading to centriole disassembly are still poorly understood.
The few ultrastructural observations concerning the dynamics of centriole disassembly come from analysis of C. elegans oocytes [58] and embryos [20], Naegleria gruberi ameboid form [130], Drosophila mid-oogenesis, ommatidial differentiation and male stem cell niche [45]. These observations show that centriole elimination occurs according to different stereotypical manners in various model systems, suggesting different elimination programs.
In particular, the ultrastructural analysis in Drosophila points to a dismantlement of the centrioles by the gradual disappearance of the C- and B-tubules of the wall, depending on germ or somatic cells. The ultrastructural observations uncovering centrioles with signs of degeneration agree with the gradual reduction in the PCM associated with such centrioles. Remarkably, it has been demonstrated that the recruitment of Ana1 by RCD4 is sufficient to stabilize the microtubules triplets during male Drosophila gametogenesis [131], and the expression of Ana1 in the Drosophila oocyte is sufficient to prevent centriole disassembly during oogenesis [83]. Ana1, RCD4, Polo, and PCM components are indeed considered key factors contributing to centriole/centrosome maintenance [28].
Therefore, at least in some Drosophila cells, the gradual disappearance of the PCM components is associated with the reduced functionality of the centrosomes and then with the subsequent degeneration of the centrioles. However, there are Drosophila differentiated cells in which centrioles lost some of their PCM-associated components but persist longer. This suggests that the relationship between PCM reduction and centriole elimination is far from understood and requires further investigation.
Author Contributions
Conceptualization, D.B., G.C. and M.G.R.; writing—review and editing, D.B., G.C. and M.G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Ministero dell’Università e della Ricerca to G.C. (PRIN 2020CLZ5XW and PRIN 2022TLYYPS_003).
Acknowledgments
We would like to thank the Electron Microscopy facility of the Department of Life Sciences for the use of the transmission electron microscope. We apologize to the authors whose work was not cited or cited only indirectly through reviews.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ana1 | Anastral spindle 1 |
| Asl | Asterless |
| CLRs | Cilium-like regions |
| Cnn | Centrosomin |
| CySCs | Cyst stem cells |
| GSCs | Germline stem cells |
| ncMTOCs | Non-centrosomal microtubule organizing centers |
| PCM | Pericentriolar material |
| Plk4 | Polo-like kinase 4 |
| Plp | Pericentrin-like protein |
| Sas | Spindle assembly abnormal |
| Spd2 | Spindle defective 2 |
References
- Bornens, M.; Azimzadeh, J. Origin and Evolution of the Centrosome. In Eukaryotic Membranes and Cytoskeleton; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; Volume 607, pp. 119–129. ISBN 978-0-387-74020-1. [Google Scholar]
- Ito, D.; Bettencourt-Dias, M. Centrosome Remodelling in Evolution. Cells 2018, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T.; Turner, K. The Evolution of Centriole Structure: Heterochrony, Neoteny, and Hypermorphosis. In The Golgi Apparatus and Centriole; Kloc, M., Ed.; Results and Problems in Cell Differentiation; Springer International Publishing: Cham, Switzerland, 2019; Volume 67, pp. 3–15. ISBN 978-3-030-23172-9. [Google Scholar]
- LeGuennec, M.; Klena, N.; Aeschlimann, G.; Hamel, V.; Guichard, P. Overview of the Centriole Architecture. Curr. Opin. Struct. Biol. 2021, 66, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yan, Y.; Fu, J. Nine-fold Symmetry of Centriole: The Joint Efforts of Its Core Proteins. BioEssays 2022, 44, 2100262. [Google Scholar] [CrossRef] [PubMed]
- Nabais, C.; Peneda, C.; Bettencourt-Dias, M. Evolution of Centriole Assembly. Curr. Biol. 2020, 30, R494–R502. [Google Scholar] [CrossRef]
- Joukov, V.; De Nicolo, A. The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle. Cells 2019, 8, 701. [Google Scholar] [CrossRef]
- Breslow, D.K.; Holland, A.J. Mechanism and Regulation of Centriole and Cilium Biogenesis. Annu. Rev. Biochem. 2019, 88, 691–724. [Google Scholar] [CrossRef]
- Qi, F.; Zhou, J. Multifaceted Roles of Centrosomes in Development, Health, and Disease. J. Mol. Cell Biol. 2021, 13, 611–621. [Google Scholar] [CrossRef]
- Aljiboury, A.; Hehnly, H. The Centrosome—Diverse Functions in Fertilization and Development across Species. J. Cell Sci. 2023, 136, jcs261387. [Google Scholar] [CrossRef]
- Fu, J.; Hagan, I.M.; Glover, D.M. The Centrosome and Its Duplication Cycle. Cold Spring Harb. Perspect. Biol. 2015, 7, a015800. [Google Scholar] [CrossRef]
- Fırat-Karalar, E.N.; Stearns, T. The Centriole Duplication Cycle. Phil. Trans. R. Soc. B 2014, 369, 20130460. [Google Scholar] [CrossRef]
- Nigg, E.A.; Holland, A.J. Once and Only Once: Mechanisms of Centriole Duplication and Their Deregulation in Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Gönczy, P.; Hatzopoulos, G.N. Centriole Assembly at a Glance. J. Cell Sci. 2019, 132, jcs228833. [Google Scholar] [CrossRef]
- Arquint, C.; Nigg, E.A. The PLK4–STIL–SAS-6 Module at the Core of Centriole Duplication. Biochem. Soc. Trans. 2016, 44, 1253–1263. [Google Scholar] [CrossRef]
- Ohta, M.; Watanabe, K.; Ashikawa, T.; Nozaki, Y.; Yoshiba, S.; Kimura, A.; Kitagawa, D. Bimodal Binding of STIL to Plk4 Controls Proper Centriole Copy Number. Cell Rep. 2018, 23, 3160–3169.e4. [Google Scholar] [CrossRef]
- Moyer, T.C.; Holland, A.J. PLK4 Promotes Centriole Duplication by Phosphorylating STIL to Link the Procentriole Cartwheel to the Microtubule Wall. eLife 2019, 8, e46054. [Google Scholar] [CrossRef] [PubMed]
- Banterle, N.; Gönczy, P. Centriole Biogenesis: From Identifying the Characters to Understanding the Plot. Annu. Rev. Cell Dev. Biol. 2017, 33, 23–49. [Google Scholar] [CrossRef]
- Uzbekov, R.E.; Avidor-Reiss, T. Principal Postulates of Centrosomal Biology. Version 2020. Cells 2020, 9, 2156. [Google Scholar] [CrossRef] [PubMed]
- Kalbfuss, N.; Gönczy, P. Towards Understanding Centriole Elimination. Open Biol. 2023, 13, 230222. [Google Scholar] [CrossRef]
- Basto, R.; Lau, J.; Vinogradova, T.; Gardiol, A.; Woods, C.G.; Khodjakov, A.; Raff, J.W. Flies without Centrioles. Cell 2006, 125, 1375–1386. [Google Scholar] [CrossRef]
- Stevens, N.R.; Raposo, A.A.S.F.; Basto, R.; St Johnston, D.; Raff, J.W. From Stem Cell to Embryo without Centrioles. Curr. Biol. 2007, 17, 1498–1503. [Google Scholar] [CrossRef]
- Bettencourt-Dias, M.; Hildebrandt, F.; Pellman, D.; Woods, G.; Godinho, S.A. Centrosomes and Cilia in Human Disease. Trends Genet. 2011, 27, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Hildebrandt, F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028191. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.S.; Holland, A.J. The Impact of Mitotic Errors on Cell Proliferation and Tumorigenesis. Genes. Dev. 2018, 32, 620–638. [Google Scholar] [CrossRef] [PubMed]
- Ryniawec, J.M.; Rogers, G.C. Centrosome Instability: When Good Centrosomes Go Bad. Cell. Mol. Life Sci. 2021, 78, 6775–6795. [Google Scholar] [CrossRef]
- Farcy, S.; Hachour, H.; Bahi-Buisson, N.; Passemard, S. Genetic Primary Microcephalies: When Centrosome Dysfunction Dictates Brain and Body Size. Cells 2023, 12, 1807. [Google Scholar] [CrossRef]
- Fernandes-Mariano, C.; Bugalhão, J.N.; Santos, D.; Bettencourt-Dias, M. Centrosome Biogenesis and Maintenance in Homeostasis and Disease. Curr. Opin. Cell Biol. 2025, 94, 102485. [Google Scholar] [CrossRef]
- Shin, B.; Kim, M.S.; Lee, Y.; Jung, G.I.; Rhee, K. Generation and Fates of Supernumerary Centrioles in Dividing Cells. Mol. Cells 2021, 44, 699–705. [Google Scholar] [CrossRef]
- Chavali, P.L.; Pütz, M.; Gergely, F. Small Organelle, Big Responsibility: The Role of Centrosomes in Development and Disease. Phil. Trans. R. Soc. B 2014, 369, 20130468. [Google Scholar] [CrossRef]
- Cosenza, M.R.; Cazzola, A.; Rossberg, A.; Schieber, N.L.; Konotop, G.; Bausch, E.; Slynko, A.; Holland-Letz, T.; Raab, M.S.; Dubash, T.; et al. Asymmetric Centriole Numbers at Spindle Poles Cause Chromosome Missegregation in Cancer. Cell Rep. 2017, 20, 1906–1920. [Google Scholar] [CrossRef]
- Tillery, M.M.L.; Blake-Hedges, C.; Zheng, Y.; Buchwalter, R.A.; Megraw, T.L. Centrosomal and Non-Centrosomal Microtubule-Organizing Centers (MTOCs) in Drosophila Melanogaster. Cells 2018, 7, 121. [Google Scholar] [CrossRef]
- Januschke, J.; Gervais, L.; Gillet, L.; Keryer, G.; Bornens, M.; Guichet, A. The Centrosome-Nucleus Complex and Microtubule Organization in the Drosophila Oocyte. Development 2006, 133, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.E.; Jan, L.Y.; Jan, Y.N. Reciprocal Localization of Nod and Kinesin Fusion Proteins Indicates Microtubule Polarity in the Drosophila Oocyte, Epithelium, Neuron and Muscle. Development 1997, 124, 461–470. [Google Scholar] [CrossRef]
- Rolls, M.M.; Satoh, D.; Clyne, P.J.; Henner, A.L.; Uemura, T.; Doe, C.Q. Polarity and Intracellular Compartmentalization of Drosophila Neurons. Neural Dev. 2007, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Koizumi, M.; Hayashi, S. Sustained Elongation of Sperm Tail Promoted by Local Remodeling of Giant Mitochondria in Drosophila. Curr. Biol. 2011, 21, 805–814. [Google Scholar] [CrossRef]
- Chen, J.V.; Buchwalter, R.A.; Kao, L.-R.; Megraw, T.L. A Splice Variant of Centrosomin Converts Mitochondria to Microtubule-Organizing Centers. Curr. Biol. 2017, 27, 1928–1940.e6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Buchwalter, R.A.; Zheng, C.; Wight, E.M.; Chen, J.V.; Megraw, T.L. A Perinuclear Microtubule-Organizing Centre Controls Nuclear Positioning and Basement Membrane Secretion. Nat. Cell Biol. 2020, 22, 297–309. [Google Scholar] [CrossRef]
- Schoenfelder, K.P.; Montague, R.A.; Paramore, S.V.; Lennox, A.L.; Mahowald, A.P.; Fox, D.T. Indispensable Pre-Mitotic Endocycles Promote Aneuploidy in the Drosophila Rectum. Development 2014, 141, 3551–3560. [Google Scholar] [CrossRef]
- Nashchekin, D.; Fernandes, A.R.; St Johnston, D. Patronin/Shot Cortical Foci Assemble the Noncentrosomal Microtubule Array That Specifies the Drosophila Anterior-Posterior Axis. Dev. Cell 2016, 38, 61–72. [Google Scholar] [CrossRef]
- Booth, A.J.R.; Blanchard, G.B.; Adams, R.J.; Röper, K. A Dynamic Microtubule Cytoskeleton Directs Medial Actomyosin Function during Tube Formation. Dev. Cell 2014, 29, 562–576. [Google Scholar] [CrossRef]
- Brodu, V.; Baffet, A.D.; Le Droguen, P.-M.; Casanova, J.; Guichet, A. A Developmentally Regulated Two-Step Process Generates a Noncentrosomal Microtubule Network in Drosophila Tracheal Cells. Dev. Cell 2010, 18, 790–801. [Google Scholar] [CrossRef]
- Tucker, J.B.; Milner, M.J.; Currie, D.A.; Muir, J.W.; Forrest, D.A.; Spencer, M.J. Centrosomal Microtubule-Organizing Centres and a Switch in the Control of Protofilament Number for Cell Surface-Associated Microtubules during Drosophila Wing Morphogenesis. Eur. J. Cell Biol. 1986, 41, 279–289. [Google Scholar]
- Harumoto, T.; Ito, M.; Shimada, Y.; Kobayashi, T.J.; Ueda, H.R.; Lu, B.; Uemura, T. Atypical Cadherins Dachsous and Fat Control Dynamics of Noncentrosomal Microtubules in Planar Cell Polarity. Dev. Cell 2010, 19, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Persico, V.; Gottardo, M.; Callaini, G. The Developing Drosophila Eye—A New Model to Study Centriole Reduction. J. Cell Sci. 2018, 131, jcs211441. [Google Scholar] [CrossRef]
- Mogensen, M.M.; Tucker, J.B.; Baggaley, T.B. Multiple Plasma Membrane-Associated MTOC Systems in the Acentrosomal Cone Cells of Drosophila Ommatidia. Eur. J. Cell Biol. 1993, 60, 67–75. [Google Scholar]
- Kos, P.; Baumann, O. Spatial Arrangement, Polarity, and Posttranslational Modifications of the Microtubule System in the Drosophila Eye. Cell Tissue Res. 2024, 398, 123–137. [Google Scholar] [CrossRef]
- Lattao, R.; Kovács, L.; Glover, D.M. The Centrioles, Centrosomes, Basal Bodies, and Cilia of Drosophila Melanogaster. Genetics 2017, 206, 33–53. [Google Scholar] [CrossRef]
- Zoller, R.; Schulz, C. The Drosophila Cyst Stem Cell Lineage: Partners behind the Scenes? Spermatogenesis 2012, 2, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Colozza, G.; Callaini, G. Procentriole Elongation and Recruitment of Pericentriolar Material Are Downregulated in Cyst Cells as They Enter Quiescence. J. Cell Sci. 2009, 122, 3613–3618. [Google Scholar] [CrossRef]
- Borrego-Pinto, J.; Somogyi, K.; Karreman, M.A.; König, J.; Müller-Reichert, T.; Bettencourt-Dias, M.; Gönczy, P.; Schwab, Y.; Lénárt, P. Distinct Mechanisms Eliminate Mother and Daughter Centrioles in Meiosis of Starfish Oocytes. J. Cell Biol. 2016, 212, 815–827. [Google Scholar] [CrossRef]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Structural Characterization of Procentrioles in Drosophila Spermatids. Cytoskeleton 2015, 72, 576–584. [Google Scholar] [CrossRef]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Klp10A Modulates the Localization of Centriole-Associated Proteins during Drosophila Male Gametogenesis. Cell Cycle 2016, 15, 3432–3441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gigliotti, S.; Callaini, G.; Andone, S.; Riparbelli, M.G.; Pernas-Alonso, R.; Hoffmann, G.; Graziani, F.; Malva, C. Nup154, a New Drosophila Gene Essential for Male and Female Gametogenesis Is Related to the Nup155 Vertebrate Nucleoporin Gene. J. Cell Biol. 1998, 142, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Hétié, P.; de Cuevas, M.; Matunis, E. Conversion of Quiescent Niche Cells to Somatic Stem Cells Causes Ectopic Niche Formation in the Drosophila Testis. Cell Rep. 2014, 7, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Persico, V.; Dallai, R.; Callaini, G. Centrioles and Ciliary Structures during Male Gametogenesis in Hexapoda: Discovery of New Models. Cells 2020, 9, 744. [Google Scholar] [CrossRef]
- Jana, S.C. Centrosome Structure and Biogenesis: Variations on a Theme? Semin. Cell Dev. Biol. 2021, 110, 123–138. [Google Scholar] [CrossRef]
- Pierron, M.; Woglar, A.; Busso, C.; Jha, K.; Mikeladze-Dvali, T.; Croisier, M.; Gönczy, P. Centriole Elimination during Caenorhabditis Elegans Oogenesis Initiates with Loss of the Central Tube Protein SAS-1. EMBO J. 2023, 42, e115076. [Google Scholar] [CrossRef]
- Jana, S.C.; Mendonça, S.; Machado, P.; Werner, S.; Rocha, J.; Pereira, A.; Maiato, H.; Bettencourt-Dias, M. Differential Regulation of Transition Zone and Centriole Proteins Contributes to Ciliary Base Diversity. Nat. Cell Biol. 2018, 20, 928–941. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The Sperm Centrioles. Mol. Cell Endocrinol. 2020, 518, 110987. [Google Scholar] [CrossRef]
- Uzbekov, R.; Singina, G.N.; Shedova, E.N.; Banliat, C.; Avidor-Reiss, T.; Uzbekova, S. Centrosome Formation in the Bovine Early Embryo. Cells 2023, 12, 1335. [Google Scholar] [CrossRef]
- Manandhar, G.; Schatten, H.; Sutovsky, P. Centrosome Reduction During Gametogenesis and Its Significance1. Biol. Reprod. 2005, 72, 2–13. [Google Scholar] [CrossRef]
- Huettner, A.F. Maturation and Fertilization in Drosophila Melanogaster. J. Morphol. 1924, 39, 249–265. [Google Scholar] [CrossRef]
- Spradling, A.C. Developmental Genetics of Oogenesis. In The Development of Drosophila Melanogaster, 1st ed.; Bate, M., Martinez-Arias, A., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1993; Volume 1, pp. 1–70. [Google Scholar]
- Mahowald, A.P.; Strassheim, J.M. Intercellular Migration of Centrioles in the Germarium of Drosophila melanogaster. J. Cell Biol. 1970, 45, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, J.; Huynh, J.R.; López-Schier, H.; González, C.; St Johnston, D.; González-Reyes, A. Centrosome Migration into the Drosophila Oocyte Is Independent of BicD and Egl, and of the Organisation of the Microtubule Cytoskeleton. Development 2001, 128, 1889–1897. [Google Scholar] [CrossRef]
- Pimenta-Marques, A.; Bento, I.; Lopes, C.A.M.; Duarte, P.; Jana, S.C.; Bettencourt-Dias, M. A Mechanism for the Elimination of the Female Gamete Centrosome in Drosophila melanogaster. Science 2016, 353, aaf4866. [Google Scholar] [CrossRef]
- Schatten, G. The Centrosome and Its Mode of Inheritance: The Reduction of the Centrosome during Gametogenesis and Its Restoration during Fertilization. Dev. Biol. 1994, 165, 299–335. [Google Scholar] [CrossRef] [PubMed]
- Fechter, J.; Schöneberg, A.; Schatten, G. Excision and Disassembly of Sperm Tail Microtubules during Sea Urchin Fertilization: Requirements for Microtubule Dynamics. Cell Motil. Cytoskelet. 1996, 35, 281–288. [Google Scholar] [CrossRef]
- Sutovsky, P.; Schatten, G. Paternal Contributions to the Mammalian Zygote: Fertilization after Sperm-Egg Fusion. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 195, pp. 1–65. ISBN 978-0-12-364599-9. [Google Scholar]
- Simerly, C.; Wu, G.-J.; Zoran, S.; Ord, T.; Rawlins, R.; Jones, J.; Navara, C.; Gerrity, M.; Rinehart, J.; Binor, Z.; et al. The Paternal Inheritance of the Centrosome, the Cell’s Microtubule-Organizing Center, in Humans, and the Implications for Infertility. Nat. Med. 1995, 1, 47–52. [Google Scholar] [CrossRef]
- Perotti, M.E. The Mitochondrial Derivative of the Spermatozoon of Drosophila before and after Fertilization. J. Ultrastruct. Res. 1973, 44, 181–198. [Google Scholar] [CrossRef]
- Karr, T.L.; Swanson, W.J.; Snook, R.R. The Evolutionary Significance of Variation in Sperm–Egg Interactions. In Sperm Biology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 305–365. ISBN 978-0-12-372568-4. [Google Scholar]
- Loppin, B.; Dubruille, R.; Horard, B. The Intimate Genetics of Drosophila Fertilization. Open Biol. 2015, 5, 150076. [Google Scholar] [CrossRef]
- Snook, R.R.; Karr, T.L. Only Long Sperm Are Fertilization-Competent in Six Sperm-Heteromorphic Drosophila Species. Curr. Biol. 1998, 8, 291–294. [Google Scholar] [CrossRef]
- Pitnick, S.; Karr, T.L. Paternal Products and By-Products in Drosophila Development. Proc. Biol. Sci. 1998, 265, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Foe, V.E.; Odell, G.M.; Edgar, B.A. Mitosis and Morphogenesis in the Drosophila Embryo: Point and Counterpoint. In The Development of Drosophila Melanogaster; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 1993; pp. 149–300. [Google Scholar]
- Lassy, C.W.; Karr, T.L. Cytological Analysis of Fertilization and Early Embryonic Development in Incompatible Crosses of Drosophila Simulans. Mech. Dev. 1996, 57, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Callaini, G. Detachment of the Basal Body from the Sperm Tail Is Not Required to Organize Functional Centrosomes during Drosophila Embryogenesis. Cytoskeleton 2010, 67, 251–258. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Callaini, G.; Megraw, T.L. Assembly and Persistence of Primary Cilia in Dividing Drosophila Spermatocytes. Dev. Cell 2012, 23, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Varmark, H.; Llamazares, S.; Rebollo, E.; Lange, B.; Reina, J.; Schwarz, H.; Gonzalez, C. Asterless Is a Centriolar Protein Required for Centrosome Function and Embryo Development in Drosophila. Curr. Biol. 2007, 17, 1735–1745. [Google Scholar] [CrossRef]
- Blachon, S.; Gopalakrishnan, J.; Omori, Y.; Polyanovsky, A.; Church, A.; Nicastro, D.; Malicki, J.; Avidor-Reiss, T. Drosophila Asterless and Vertebrate Cep152 Are Orthologs Essential for Centriole Duplication. Genetics 2008, 180, 2081–2094. [Google Scholar] [CrossRef]
- Pimenta-Marques, A.; Perestrelo, T.; Reis-Rodrigues, P.; Duarte, P.; Ferreira-Silva, A.; Lince-Faria, M.; Bettencourt-Dias, M. Ana1/CEP295 Is an Essential Player in the Centrosome Maintenance Program Regulated by Polo Kinase and the PCM. EMBO Rep. 2024, 25, 102–127. [Google Scholar] [CrossRef]
- Prigent, C.; Uzbekov, R. Duplication and Segregation of Centrosomes during Cell Division. Cells 2022, 11, 2445. [Google Scholar] [CrossRef] [PubMed]
- Blachon, S.; Cai, X.; Roberts, K.A.; Yang, K.; Polyanovsky, A.; Church, A.; Avidor-Reiss, T. A Proximal Centriole-like Structure Is Present in Drosophila Spermatids and Can Serve as a Model to Study Centriole Duplication. Genetics 2009, 182, 133–144. [Google Scholar] [CrossRef]
- Mottier-Pavie, V.; Megraw, T.L. Drosophila Bld10 Is a Centriolar Protein That Regulates Centriole, Basal Body, and Motile Cilium Assembly. Mol. Biol. Cell 2009, 20, 2605–2614. [Google Scholar] [CrossRef]
- Khire, A.; Vizuet, A.A.; Davila, E.; Avidor-Reiss, T. Asterless Reduction during Spermiogenesis Is Regulated by Plk4 and Is Essential for Zygote Development in Drosophila. Curr. Biol. 2015, 25, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Khire, A.; Jo, K.H.; Kong, D.; Akhshi, T.; Blachon, S.; Cekic, A.R.; Hynek, S.; Ha, A.; Loncarek, J.; Mennella, V.; et al. Centriole Remodeling during Spermiogenesis in Drosophila. Curr. Biol. 2016, 26, 3183–3189. [Google Scholar] [CrossRef]
- Jo, K.H.; Jaiswal, A.; Khanal, S.; Fishman, E.L.; Curry, A.N.; Avidor-Reiss, T. Poc1B and Sas-6 Function Together during the Atypical Centriole Formation in Drosophila Melanogaster. Cells 2019, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Blachon, S.; Khire, A.; Avidor-Reiss, T. The Origin of the Second Centriole in the Zygote of Drosophila Melanogaster. Genetics 2014, 197, 199–205. [Google Scholar] [CrossRef]
- Buglak, D.B.; Holmes, K.H.M.; Galletta, B.J.; Rusan, N.M. The Proximal Centriole-like Structure Maintains Nucleus-Centriole Architecture in Sperm. J. Cell Sci. 2024, 137, jcs262311. [Google Scholar] [CrossRef]
- Dallai, R.; Mercati, D.; Lino-Neto, J.; Dias, G.; Lupetti, P. Evidence of a Procentriole during Spermiogenesis in the Coccinellid Insect Adalia Decempunctata (L): An Ultrastructural Study. Arthropod Struct. Dev. 2017, 46, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Fishman, E.L.; Jo, K.; Ha, A.; Royfman, R.; Zinn, A.; Krishnamurthy, M.; Avidor-Reiss, T. Atypical Centrioles Are Present in Tribolium Sperm. Open Biol. 2017, 7, 160334. [Google Scholar] [CrossRef]
- Yamashiki, N.; Kawamura, N. Behavior of Centrioles during Meiosis in the Male Silkworm, Bombyx mori (Lepidoptera). Dev. Growth Differ. 1998, 40, 619–630. [Google Scholar] [CrossRef]
- Wolf, K.; Kyburg, J. The Restructuring of the Flagellar Base and the Flagellar Necklace during Spermatogenesis of Ephestia kuehniella Z. (Pyralidae, Lepidoptera). Cell Tissue Res. 1989, 256. [Google Scholar] [CrossRef]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Procentriole Assembly without Centriole Disengagement: A Paradox of Male Gametogenesis. J. Cell Sci. 2014, 127, 3434–3439. [Google Scholar] [CrossRef]
- Avidor-Reiss, T. Rapid Evolution of Sperm Produces Diverse Centriole Structures That Reveal the Most Rudimentary Structure Needed for Function. Cells 2018, 7, 67. [Google Scholar] [CrossRef]
- Jaffe, L.A.; Gould, M. Polyspermy-Preventing Mechanisms. In Biology of Fertilization; Academic Press: New York, NY, USA, 1985; Volume 3, pp. 223–250. [Google Scholar]
- Iwao, Y.; Kimoto, C.; Fujimoto, A.; Suda, A.; Hara, Y. Physiological Polyspermy: Selection of a Sperm Nucleus for the Development of Diploid Genomes in Amphibians. Mol. Reprod. Devel. 2020, 87, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Snook, R.R.; Hosken, D.J.; Karr, T.L. The Biology and Evolution of Polyspermy: Insights from Cellular and Functional Studies of Sperm and Centrosomal Behavior in the Fertilized Egg. Reproduction 2011, 142, 779–792. [Google Scholar] [CrossRef]
- Callaini, G.; Riparbelli, M.G. Fertilization inDrosophila Melanogaster:Centrosome Inheritance and Organization of the First Mitotic Spindle. Dev. Biol. 1996, 176, 199–208. [Google Scholar] [CrossRef]
- Hildreth, P.E.; Lucchesi, J.C. Fertilization in Drosophila. Dev. Biol. 1963, 6, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Giordano, R.; Callaini, G. Centrosome Inheritance in the Parthenogenetic Egg of the Collembolan Folsomia Candida. Cell Tissue Res. 2006, 326, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Tagu, D.; Bonhomme, J.; Callaini, G. Aster Self-Organization at Meiosis: A Conserved Mechanism in Insect Parthenogenesis? Dev. Biol. 2005, 278, 220–230. [Google Scholar] [CrossRef]
- Tram, U.; Sullivan, W. Reciprocal Inheritance of Centrosomes in the Parthenogenetic Hymenopteran Nasonia Vitripennis. Curr. Biol. 2000, 10, 1413–1419. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Stouthamer, R.; Dallai, R.; Callaini, G. Microtubule Organization during the Early Development of the Parthenogenetic Egg of the HymenopteranMuscidifurax Uniraptor. Dev. Biol. 1998, 195, 89–99. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Callaini, G. Drosophila Parthenogenesis: A Model for de Novo Centrosome Assembly. Dev. Biol. 2003, 260, 298–313. [Google Scholar] [CrossRef]
- Eisman, R.; Kaufman, T.C. Cytological Investigation of the Mechanism of Parthenogenesis in Drosophila Mercatorum. Fly 2007, 1, 317–329. [Google Scholar] [CrossRef]
- Hirai, K.; Inoue, Y.H.; Matsuda, M. Mitotic Progression and Dual Spindle Formation Caused by Spindle Association of de Novo-Formed Microtubule-Organizing Centers in Parthenogenetic Embryos of Drosophila Ananassae. Genetics 2023, 223, iyac178. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Gottardo, M.; Callaini, G. Parthenogenesis in Insects: The Centriole Renaissance. In Oocytes; Kloc, M., Ed.; Results and Problems in Cell Differentiation; Springer International Publishing: Cham, Switzerland, 2017; Volume 63, pp. 435–479. ISBN 978-3-319-60854-9. [Google Scholar]
- Nabais, C.; Pessoa, D.; de-Carvalho, J.; van Zanten, T.; Duarte, P.; Mayor, S.; Carneiro, J.; Telley, I.A.; Bettencourt-Dias, M. Plk4 Triggers Autonomous de Novo Centriole Biogenesis and Maturation. J. Cell Biol. 2021, 220, e202008090. [Google Scholar] [CrossRef]
- Peel, N.; Stevens, N.R.; Basto, R.; Raff, J.W. Overexpressing Centriole-Replication Proteins in Vivo Induces Centriole Overduplication and de Novo Formation. Curr. Biol. 2007, 17, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Martins, A.; Riparbelli, M.; Callaini, G.; Glover, D.M.; Bettencourt-Dias, M. Revisiting the Role of the Mother Centriole in Centriole Biogenesis. Science 2007, 316, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Dzhindzhev, N.S.; Yu, Q.D.; Weiskopf, K.; Tzolovsky, G.; Cunha-Ferreira, I.; Riparbelli, M.; Rodrigues-Martins, A.; Bettencourt-Dias, M.; Callaini, G.; Glover, D.M. Asterless Is a Scaffold for the Onset of Centriole Assembly. Nature 2010, 467, 714–718. [Google Scholar] [CrossRef]
- Bownes, M. Ovarian Yolk-Protein Synthesis in Drosophila Melanogaster. J. Insect Physiol. 1982, 28, 953–960. [Google Scholar] [CrossRef]
- Walker, J.J.; Lee, K.K.; Desai, R.N.; Erickson, J.W. The Drosophila Melanogaster Sex Determination Gene sisA Is Required in Yolk Nuclei for Midgut Formation. Genetics 2000, 155, 191–202. [Google Scholar] [CrossRef]
- Foe, V.E.; Alberts, B.M. Studies of Nuclear and Cytoplasmic Behaviour during the Five Mitotic Cycles That Precede Gastrulation in Drosophila Embryogenesis. J. Cell Sci. 1983, 61, 31–70. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Callaini, G. Assembly of Yolk Spindles in the Early Drosophila Embryo. Mech. Dev. 2003, 120, 441–454. [Google Scholar] [CrossRef]
- Gehring, W. Cell heredity and changes of determination in cultures of imaginal discs in Drosophila melanogaster. J. Embryol. Exp. Morphol. 1966, 15, 77–111. [Google Scholar] [PubMed]
- Haynie, J.L.; Bryant, P.J. Development of the Eye-antenna Imaginal Disc and Morphogenesis of the Adult Head in Drosophila melanogaster. J. Exp. Zool. 1986, 237, 293–308. [Google Scholar] [CrossRef]
- Carthew, R.W. Pattern Formation in the Drosophila Eye. Curr. Opin. Genet. Dev. 2007, 17, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.P. Building an Ommatidium One Cell at a Time. Dev. Dyn. 2012, 241, 136–149. [Google Scholar] [CrossRef]
- Wolff, T.; Ready, D.F. The Beginning of Pattern Formation in the Drosophila Compound Eye: The Morphogenetic Furrow and the Second Mitotic Wave. Development 1991, 113, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Cagan, R. Principles of Drosophila Eye Differentiation. Curr. Top. Dev. Biol. 2009, 89, 115–135. [Google Scholar] [CrossRef]
- Treisman, J.E. Retinal Differentiation in Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 545–557. [Google Scholar] [CrossRef]
- Fernandes, V.M.; McCormack, K.; Lewellyn, L.; Verheyen, E.M. Integrins Regulate Apical Constriction via Microtubule Stabilization in the Drosophila Eye Disc Epithelium. Cell Rep. 2014, 9, 2043–2055. [Google Scholar] [CrossRef]
- Pierron, M.; Kalbfuss, N.; Borrego-Pinto, J.; Lénárt, P.; Gönczy, P. Centriole Foci Persist in Starfish Oocytes despite Polo-like Kinase 1 Inactivation or Loss of Microtubule Nucleation Activity. Mol. Biol. Cell 2020, 31, 873–880. [Google Scholar] [CrossRef]
- Garbrecht, J.; Laos, T.; Holzer, E.; Dillinger, M.; Dammermann, A. An Acentriolar Centrosome at the C. Elegans Ciliary Base. Curr. Biol. 2021, 31, 2418–2428.e8. [Google Scholar] [CrossRef]
- Magescas, J.; Eskinazi, S.; Tran, M.V.; Feldman, J.L. Centriole-Less Pericentriolar Material Serves as a Microtubule Organizing Center at the Base of C. Elegans Sensory Cilia. Curr. Biol. 2021, 31, 2410–2417.e6. [Google Scholar] [CrossRef] [PubMed]
- Woglar, A.; Busso, C.; Garcia-Rodriguez, G.; Douma, F.; Croisier, M.; Knott, G.; Gönczy, P. Mechanisms of Axoneme and Centriole Elimination in Naegleria Gruberi. EMBO Rep. 2025, 26, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Ladinsky, M.S.; Glover, D.M. 9-Fold Symmetry Is Not Essential for Centriole Elongation and Formation of New Centriole-like Structures. Nat. Commun. 2024, 15, 4467. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).