The Role of Serine-Threonine Kinase Receptor-Associated Protein (STRAP) Signaling in Cancer
Abstract
1. Introduction
2. STRAP: Structure, Domain, and Functional Interactions
3. Regulation of Oncogenic Signaling by STRAP: Mechanistic Insights
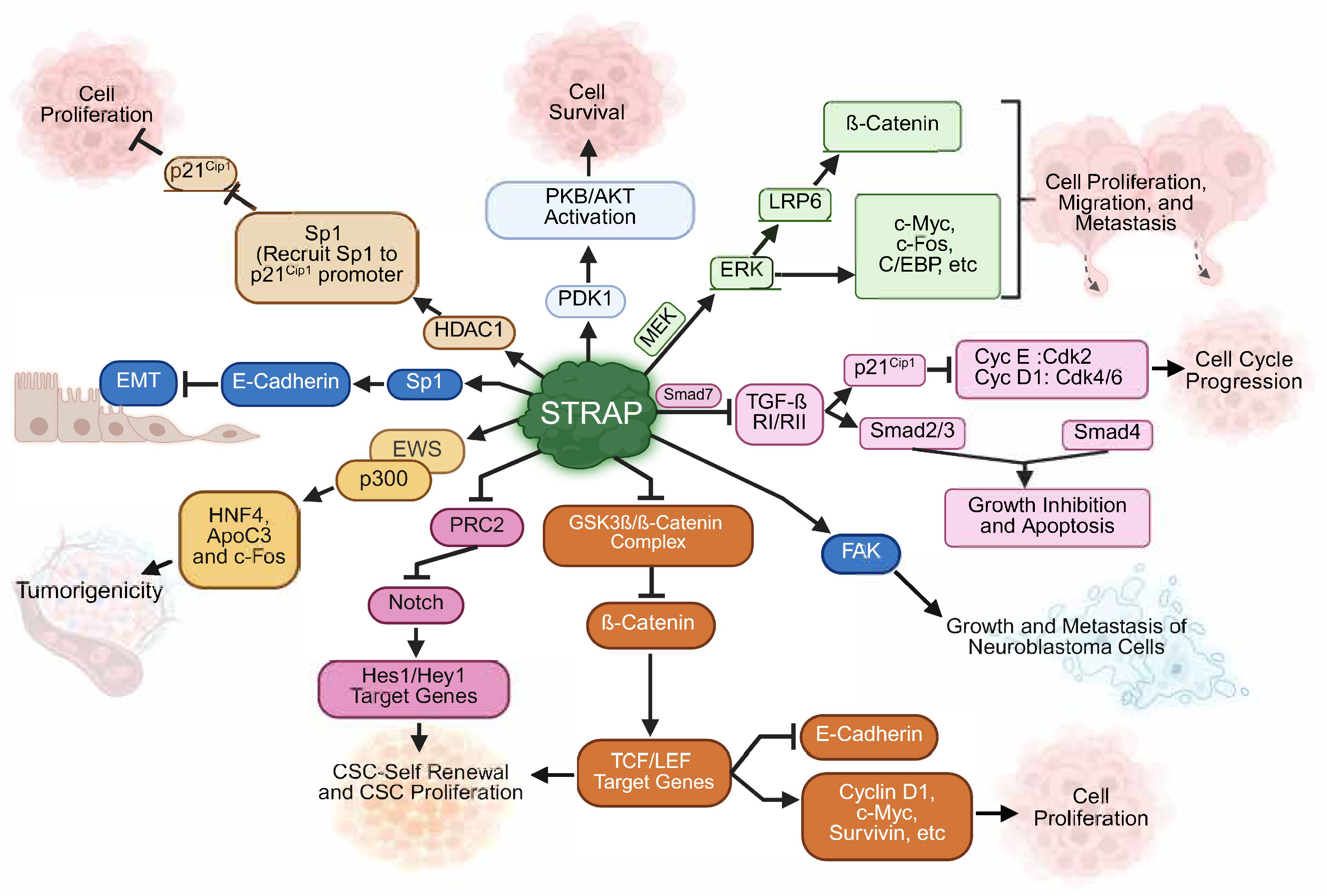
3.1. STRAP Inhibits Smad-Dependent TGF-ß Signaling
3.2. STRAP Promotes Cell Growth by Activating PDK1 Signaling
3.3. STRAP Promotes EMT by Regulating E-Cadherin Expression and Attenuates Sp1/HDAC1-Mediated Transcriptional Regulation
3.4. STRAP Promotes Colon Cancer Cell Stemness by Regulating MEK/ERK, Wnt/β-Catenin, and Notch Signaling
3.5. STRAP Modulates the Function of the Ewing Sarcoma Protein
3.6. STRAP Promotes the Malignant Phenotype in Neuroblastoma and Osteosarcoma
3.7. STRAP in Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma (IHCC)
3.8. Role of STRAP in Non-Small Cell Lung Cancer (NSCLC) Progression
3.9. STRAP Is a Strong Predictor of an Unfavorable Effect of 5-FU-Based Adjuvant Chemotherapy in Colorectal Cancer
4. STRAP Regulates Non-Oncogenic Functions Such as mRNA Splicing, CAP-Independent Translation, and Embryonic Development
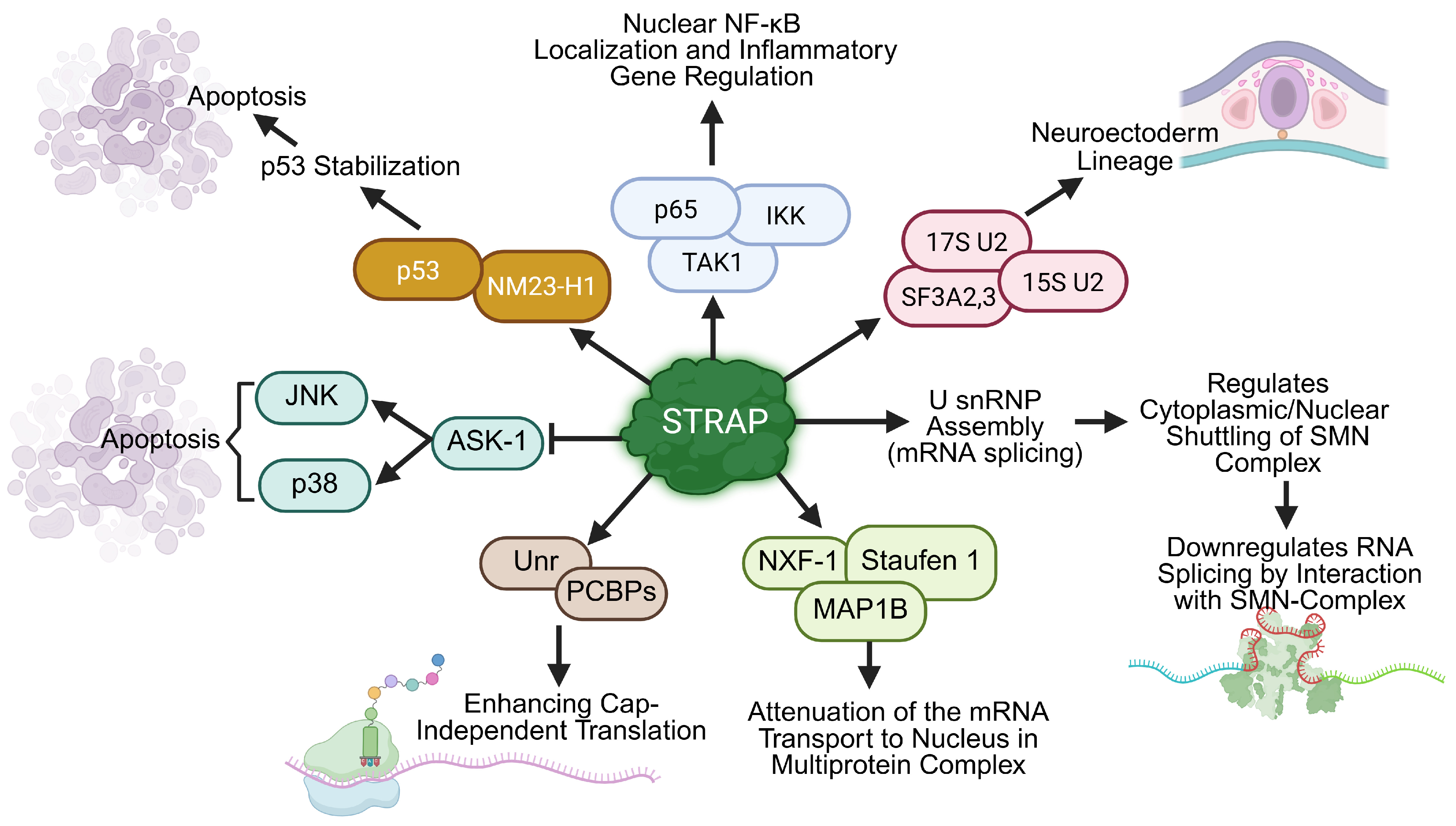
4.1. STRAP Stabilizes NF-κB and Activates Its Signaling by Interacting with TAK1/IKK
4.2. STRAP Activates NM23-H1 Through Scaffold Formation and Inhibits ASK1-Induced Apoptosis
4.3. STRAP Is Involved in mRNA Splicing, Nuclear Transport, and CAP-Independent mRNA Translation
4.4. STRAP Regulates the Splicing Networks of Lineage Commitment That Are Involved in Embryogenesis
4.5. Emerging Role of STRAP in Cellular Stress and Immune Responses
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, P.K.; Chytil, A.; Gorska, A.E.; Moses, H.L. Identification of STRAP, a novel WD domain protein in transforming growth factor-β signaling. J. Biol. Chem. 1998, 273, 34671–34674. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Moses, H.L. STRAP and Smad7 synergize in the inhibition of transforming growth factor β signaling. Mol. Cell. Biol. 2000, 20, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Matera, A.G.; Steiner, R.E.; Mills, C.A.; McMichael, B.D.; Herring, L.E.; Garcia, E.L. Proteomic analysis of the SMN complex reveals conserved and etiologic connections to the proteostasis network. Front. RNA Res. 2024, 2, 1448194. [Google Scholar] [CrossRef] [PubMed]
- Grimmler, M.; Bauer, L.; Nousiainen, M.; Körner, R.; Meister, G.; Fischer, U. Phosphorylation regulates the activity of the SMN complex during assembly of spliceosomal U snRNPs. EMBO Rep. 2005, 6, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Anumanthan, G.; Maddula, R.; Mann, J.; Chytil, A.; Gonzalez, A.L.; Washington, M.K.; Moses, H.L.; Beauchamp, R.D.; Datta, P.K. Oncogenic function of a novel WD-domain protein, STRAP, in human carcinogenesis. Cancer Res. 2006, 66, 6156–6166. [Google Scholar] [CrossRef]
- Matsuda, S.; Katsumata, R.; Okuda, T.; Yamamoto, T.; Miyazaki, K.; Senga, T.; Machida, K.; Thant, A.A.; Nakatsugawa, S.; Hamaguchi, M. Molecular cloning and characterization of human MAWD, a novel protein containing WD-40 repeats frequently overexpressed in breast cancer. Cancer Res. 2000, 60, 13–17. [Google Scholar]
- Smith, T.F.; Gaitatzes, C.; Saxena, K.; Neer, E.J. The WD repeat: A common architecture for diverse functions. Trends Biochem. Sci. 1999, 24, 181–185. [Google Scholar] [CrossRef]
- Li, D.; Roberts, R. Human Genome and Diseases: WD-repeat proteins: Structure characteristics, biological function, and their involvement in human diseases. Cell. Mol. Life Sci. CMLS 2001, 58, 2085–2097. [Google Scholar] [CrossRef]
- Stirnimann, C.U.; Petsalaki, E.; Russell, R.B.; Müller, C.W. WD40 proteins propel cellular networks. Trends Biochem. Sci. 2010, 35, 565–574. [Google Scholar] [CrossRef]
- Janda, L.; Tichý, P.A.; Spízek, J.; Petricek, M. A deduced Thermomonospora curvata protein containing serine/threonine protein kinase and WD-repeat domains. J. Bacteriol. 1996, 178, 1487–1489. [Google Scholar] [CrossRef]
- Grigorieva, G.; Shestakov, S. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS Microbiol. Lett. 1982, 13, 367–370. [Google Scholar] [CrossRef]
- Halder, S.K.; Beauchamp, R.D.; Datta, P.K. A specific inhibitor of TGF-β receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia 2005, 7, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Kashikar, N.D.; Zhang, W.; Massion, P.P.; Gonzalez, A.L.; Datta, P.K. Role of STRAP in regulating GSK3β function and Notch3 stabilization. Cell Cycle 2011, 10, 1639–1654. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Datta, A.; Banister, C.; Jin, L.; Yuan, G.; Samuel, T.; Bae, S.; Eltoum, I.E.; Manne, U.; Zhang, B.; et al. Serine-threonine Kinase Receptor-Associated Protein Is a Critical Mediator of APC Mutation–Induced Intestinal Tumorigenesis Through a Feed-Forward Mechanism. Gastroenterology 2022, 162, 193–208. [Google Scholar] [CrossRef]
- Kashikar, N.D.; Reiner, J.; Datta, A.; Datta, P.K. Serine threonine receptor-associated protein (STRAP) plays a role in the maintenance of mesenchymal morphology. Cell. Signal. 2010, 22, 138–149. [Google Scholar] [CrossRef]
- Jin, L.; Chen, Y.; Crossman, D.K.; Datta, A.; Vu, T.; Mobley, J.A.; Basu, M.K.; Scarduzio, M.; Wang, H.; Chang, C.; et al. STRAP regulates alternative splicing fidelity during lineage commitment of mouse embryonic stem cells. Nat. Commun. 2020, 11, 5941. [Google Scholar] [CrossRef]
- Chen, W.V.; Delrow, J.; Corrin, P.D.; Frazier, J.P.; Soriano, P. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat. Genet. 2004, 36, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.; Seong, H.A.; Ha, H. Dual Roles of Serine-Threonine Kinase Receptor-Associated Protein (STRAP) in Redox-Sensitive Signaling Pathways Related to Cancer Development. Oxid. Med. Cell. Longev. 2018, 2018, 5241524. [Google Scholar] [CrossRef]
- Kashikar, N.D.; Datta, P.K. Serine-Threonine Kinase Receptor-Associated Protein. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Datta, P.K.; Blake, M.C.; Moses, H.L. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-β-induced physical and functional interactions between Smads and Sp1. J. Biol. Chem. 2000, 275, 40014–40019. [Google Scholar] [CrossRef]
- Ueki, N.; Seki, N.; Yano, K.; Masuho, Y.; Saito, T.; Muramatsu, M.A. Isolation and characterization of a novel human gene (HFB30) which encodes a protein with a RING finger motif. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1999, 1445, 232–236. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.E.; Datta, P.K.; Moses, H.L. Signal transduction by transforming growth factor-β: A cooperative paradigm with extensive negative regulation. J. Cell. Biochem. 1998, 72, 111–122. [Google Scholar] [CrossRef]
- Reiner, J.E.; Datta, P.K. TGF-beta-dependent and-independent roles of STRAP in cancer. Front. Biosci. Landmark 2011, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Röijer, E.; Imamura, T.; Souchelnytskyi, S.; Stenman, G.; Heldin, C.H.; ten Dijke, P. Identification of Smad2, a human Mad-related protein in the transforming growth factor β signaling pathway. J. Biol. Chem. 1997, 272, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Lagna, G.; Hata, A.; Hemmati-Brivanlou, A.; Massagué, J. Partnership between DPC4 and SMAD proteins in TGF-β signalling pathways. Nature 1996, 383, 832–836. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.H.; Wu, R.Y.; Derynck, R. Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature 1996, 383, 168–172. [Google Scholar] [CrossRef]
- Yue, J.; Sun, B.; Liu, G.; Mulder, K.M. Requirement of TGF-β receptor-dependent activation of c-Jun N-terminal kinases (JNKs)/stress-activated protein kinases (Sapks) for TGF-β up-regulation of the urokinase-type plasminogen activator receptor. J. Cell. Physiol. 2004, 199, 284–292. [Google Scholar] [CrossRef]
- Łukasik, P.; Załuski, M.; Gutowska, I. Cyclin-dependent kinases (Cdk) and their role in diseases development–review. Int. J. Mol. Sci. 2021, 22, 2935. [Google Scholar] [CrossRef]
- Nakao, A.; Afrakhte, M.; Morn, A.; Nakayama, T.; Christian, J.L.; Heuchel, R.; Itoh, S.; Kawabata, M.; Heldin, N.E.; Heldin, C.H.; et al. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 1997, 389, 631–635. [Google Scholar] [CrossRef]
- Imamura, T.; Takase, M.; Nishihara, A.; Oeda, E.; Hanai, J.I.; Kawabata, M.; Miyazono, K. Smad6 inhibits signalling by the TGF-β superfamily. Nature 1997, 389, 622–626. [Google Scholar] [CrossRef]
- Currie, R.A.; Walker, K.S.; Gray, A.; Deak, M.; Casamayor, A.; Downes, C.P.; Cohen, P.; Alessi, D.R.; Lucocq, J. Role of phosphatidylinositol 3, 4, 5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 1999, 337, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Pullen, N.; Dennis, P.B.; Andjelkovic, M.; Dufner, A.; Kozma, S.C.; Hemmings, B.A.; Thomas, G. Phosphorylation and activation of p70s6k by PDK1. Science 1998, 279, 707–710. [Google Scholar] [CrossRef]
- Cheng, X.; Ma, Y.; Moore, M.; Hemmings, B.A.; Taylor, S.S. Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 9849–9854. [Google Scholar] [CrossRef] [PubMed]
- Le Good, J.A.; Ziegler, W.H.; Parekh, D.B.; Alessi, D.R.; Cohen, P.; Parker, P.J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 1998, 281, 2042–2045. [Google Scholar] [CrossRef]
- Manser, E.; Leung, T.; Salihuddin, H.; Zhao, Z.S.; Lim, L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 1994, 367, 40–46. [Google Scholar] [CrossRef]
- King, C.C.; Gardiner, E.M.; Zenke, F.T.; Bohl, B.P.; Newton, A.C.; Hemmings, B.A.; Bokoch, G.M. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J. Biol. Chem. 2000, 275, 41201–41209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Wu, M.; Wan, W.; Sun, R.; Yang, D.; Sun, X.; Ma, D.; Ying, G.; Zhang, N. Down-regulation of 3-phosphoinositide–dependent protein Kinase-1 levels inhibits migration and experimental metastasis of human breast Cancer cells. Mol. Cancer Res. 2009, 7, 944–954. [Google Scholar] [CrossRef]
- Peifer, C.; Alessi, D.R. New anti-cancer role for PDK1 inhibitors: Preventing resistance to tamoxifen. Biochem. J. 2009, 417, e5–e7. [Google Scholar] [CrossRef]
- Westmoreland, J.J.; Wang, Q.; Bouzaffour, M.; Baker, S.J.; Sosa-Pineda, B. Pdk1 activity controls proliferation, survival, and growth of developing pancreatic cells. Dev. Biol. 2009, 334, 285–298. [Google Scholar] [CrossRef]
- Bayascas, J.R.; Leslie, N.R.; Parsons, R.; Fleming, S.; Alessi, D.R. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN+/− mice. Curr. Biol. 2005, 15, 1839–1846. [Google Scholar] [CrossRef]
- Seong, H.A.; Jung, H.; Choi, H.S.; Kim, K.T.; Ha, H. Regulation of transforming growth factor-β signaling and PDK1 kinase activity by physical interaction between PDK1 and serine-threonine kinase receptor-associated protein. J. Biol. Chem. 2005, 280, 42897–42908. [Google Scholar] [CrossRef]
- Jung, H.; Seong, H.A.; Manoharan, R.; Ha, H. Serine-threonine kinase receptor-associated protein inhibits apoptosis signal-regulating kinase 1 function through direct interaction. J. Biol. Chem. 2010, 285, 54–70. [Google Scholar] [CrossRef]
- Safe, S.; Imanirad, P.; Sreevalsan, S.; Nair, V.; Jutooru, I. Transcription factor Sp1, also known as specificity protein 1 as a therapeutic target. Expert Opin. Ther. Targets 2014, 18, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, M.; Gagliano, N. E-cadherin in pancreatic ductal adenocarcinoma: A multifaceted actor during EMT. Cells 2020, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Datta, P.K. Oncogenic STRAP functions as a novel negative regulator of E-cadherin and p21Cip1 by modulating the transcription factor Sp1. Cell Cycle 2014, 13, 3909–3920. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.Y.; Ngo, L.; Xu, W.S.; Richon, V.M.; Marks, P.A. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl. Acad. Sci. USA 2004, 101, 1241–1246. [Google Scholar] [CrossRef]
- Barbosa, R.; Acevedo, L.A.; Marmorstein, R. The MEK/ERK network as a therapeutic target in human cancer. Mol. Cancer Res. 2021, 19, 361–374. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Fearnhead, N.S.; Britton, M.P.; Bodmer, W.F. The abc of apc. Hum. Mol. Genet. 2001, 10, 721–733. [Google Scholar] [CrossRef]
- Mishra, L. STRAP: A bridge between mutant APC and Wnt/ß-Catenin signaling in intestinal cancer. Gastroenterology 2021, 162, 44. [Google Scholar] [CrossRef]
- Gregorieff, A.; Clevers, H. Wnt signaling in the intestinal epithelium: From endoderm to cancer. Genes Dev. 2005, 19, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zhang, B.; Yang, S.; Jin, L.; Datta, A.; Bae, S.; Chen, X.; Datta, P.K. Novel role of STRAP in progression and metastasis of colorectal cancer through Wnt/β-catenin signaling. Oncotarget 2016, 7, 16023. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Inhibition of GSK-3β as a target for cardioprotection: The importance of timing, location, duration and degree of inhibition. Expert Opin. Ther. Targets 2005, 9, 447–456. [Google Scholar] [CrossRef]
- Varnum-Finney, B.; Purton, L.E.; Yu, M.; Brashem-Stein, C.; Flowers, D.; Staats, S.; Moore, K.A.; Le Roux, I.; Mann, R.; Gray, G.; et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood J. Am. Soc. Hematol. 1998, 91, 4084–4091. [Google Scholar]
- Jin, L.; Vu, T.; Yuan, G.; Datta, P.K. STRAP promotes stemness of human colorectal cancer via epigenetic regulation of the NOTCH pathway. Cancer Res. 2017, 77, 5464–5478. [Google Scholar] [CrossRef]
- Nedham, F.N.; Nagaraj, V.; Darwish, A.; Al-Abbasi, T.A. Retroperitoneal blue cell round tumor (Ewing sarcoma in a 35 years old male)-case report. Int. J. Surg. Case Rep. 2022, 94, 107045. [Google Scholar] [CrossRef]
- Janknecht, R. EWS–ETS oncoproteins: The linchpins of Ewing tumors. Gene 2005, 363, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nguyen, P.T.; Shim, H.S.; Hyeon, S.J.; Im, H.; Choi, M.H.; Chung, S.; Kowall, N.W.; Lee, S.B.; Ryu, H. EWSR1, a multifunctional protein, regulates cellular function and aging via genetic and epigenetic pathways. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2019, 1865, 1938–1945. [Google Scholar] [CrossRef]
- Kovar, H.; Aryee, D.; Zoubek, A. The Ewing family of tumors and the search for the Achilles’ heel. Curr. Opin. Oncol. 1999, 11, 275. [Google Scholar] [CrossRef]
- Araya, N.; Hirota, K.; Shimamoto, Y.; Miyagishi, M.; Yoshida, E.; Ishida, J.; Kaneko, S.; Kaneko, M.; Nakajima, T.; Fukamizu, A. Cooperative interaction of EWS with CREB-binding protein selectively activates hepatocyte nuclear factor 4-mediated transcription. J. Biol. Chem. 2003, 278, 5427–5432. [Google Scholar] [CrossRef]
- Anumanthan, G.; Halder, S.K.; Friedman, D.B.; Datta, P.K. Oncogenic serine-threonine kinase receptor-associated protein modulates the function of Ewing sarcoma protein through a novel mechanism. Cancer Res. 2006, 66, 10824–10832. [Google Scholar] [CrossRef]
- Maris, J.M. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr. Opin. Pediatr. 2005, 17, 7–13. [Google Scholar] [CrossRef]
- Bownes, L.V.; Williams, A.P.; Marayati, R.; Quinn, C.H.; Hutchins, S.C.; Stewart, J.E.; Vu, T.; Easlick, J.L.; Mroczek-Musulman, E.; Crossman, D.K.; et al. Serine-threonine kinase receptor-associated protein (STRAP) knockout decreases the malignant phenotype in neuroblastoma cell lines. Cancers 2021, 13, 3201. [Google Scholar] [CrossRef] [PubMed]
- Bownes, L.V.; Marayati, R.; Quinn, C.H.; Hutchins, S.C.; Stewart, J.E.; Anderson, J.C.; Willey, C.D.; Datta, P.K.; Beierle, E.A. Serine-Threonine Kinase Receptor Associate Protein (STRAP) confers an aggressive phenotype in neuroblastoma via regulation of Focal Adhesion Kinase (FAK). J. Pediatr. Surg. 2022, 57, 1026–1032. [Google Scholar] [CrossRef]
- Pruksakorn, D.; Klangjorhor, J.; Lirdprapamongkol, K.; Teeyakasem, P.; Sungngam, P.; Chaiyawat, P.; Phanphaisarn, A.; Settakorn, J.; Srisomsap, C. Oncogenic roles of serine–threonine kinase receptor-associated protein (STRAP) in osteosarcoma. Cancer Chemother. Pharmacol. 2018, 82, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Sherman, M. Epidemiology of hepatocellular carcinoma. Oncology 2010, 78 (Suppl. S1), 7–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, S.; Liu, P.; Sideras, K.; van de Werken, H.J.; van der Heide, M.; Cao, W.; Lavrijsen, M.; Peppelenbosch, M.P.; Bruno, M.; et al. Oncogenic STRAP supports hepatocellular carcinoma growth by enhancing Wnt/β-catenin signaling. Mol. Cancer Res. 2019, 17, 521–531. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, T.; Shi, K.; Fan, S.; Li, C.; Chen, R.; Wang, J.; Jiang, W.; Zhang, Y.; et al. CircPCNXL2 promotes tumor growth and metastasis by interacting with STRAP to regulate ERK signaling in intrahepatic cholangiocarcinoma. Mol. Cancer 2024, 23, 35. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Yang, Y.; Li, L.; Hong, R. STRAP Knockdown Inhibits Migration and Growth of Non-Small Cell Lung Cancer. Bull. Exp. Biol. Med. 2024, 177, 780–786. [Google Scholar] [CrossRef]
- Buess, M.; Terracciano, L.; Reuter, J.; Ballabeni, P.; Boulay, J.L.; Laffer, U.; Metzger, U.; Herrmann, R.; Rochlitz, C. STRAP is a strong predictive marker of adjuvant chemotherapy benefit in colorectal cancer. Neoplasia 2004, 6, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.D.; Ra, E.A.; Lee, T.A.; Kang, S.; Park, A.; Lee, E.; Choi, J.L.; Jang, E.; Lee, J.E.; Lee, S.; et al. STRAP acts as a scaffolding protein in controlling the TLR2/4 signaling pathway. Sci. Rep. 2016, 6, 38849. [Google Scholar] [CrossRef]
- Huh, H.D.; Lee, E.; Shin, J.; Park, B.; Lee, S. STRAP positively regulates TLR3-triggered signaling pathway. Cell. Immunol. 2017, 318, 55–60. [Google Scholar] [CrossRef]
- Kimura, N.; Shimada, N.; Ishijima, Y.; Fukuda, M.; Takagi, Y.; Ishikawa, N. Nucleoside diphosphate kinases in mammalian signal transduction systems: Recent development and perspective. J. Bioenerg. Biomembr. 2003, 35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.A.; Jung, H.; Ha, H. NM23-H1 tumor suppressor physically interacts with serine-threonine kinase receptor-associated protein, a transforming growth factor-β (TGF-β) receptor-interacting protein, and negatively regulates TGF-β signaling. J. Biol. Chem. 2007, 282, 12075–12096. [Google Scholar] [CrossRef]
- Jung, H.; Seong, H.A.; Ha, H. NM23-H1 tumor suppressor and its interacting partner STRAP activate p53 function. J. Biol. Chem. 2007, 282, 35293–35307. [Google Scholar] [CrossRef]
- Grimmler, M.; Otter, S.; Peter, C.; Müller, F.; Chari, A.; Fischer, U. Unrip, a factor implicated in cap-independent translation, associates with the cytosolic SMN complex and influences its intracellular localization. Hum. Mol. Genet. 2005, 14, 3099–3111. [Google Scholar] [CrossRef]
- Carissimi, C.; Baccon, J.; Straccia, M.; Chiarella, P.; Maiolica, A.; Sawyer, A.; Rappsilber, J.; Pellizzoni, L. Unrip is a component of SMN complexes active in snRNP assembly. FEBS Lett. 2005, 579, 2348–2354. [Google Scholar] [CrossRef]
- Tretyakova, I.; Zolotukhin, A.S.; Tan, W.; Bear, J.; Propst, F.; Ruthel, G.; Felber, B.K. Nuclear export factor family protein participates in cytoplasmic mRNA trafficking. J. Biol. Chem. 2005, 280, 31981–31990. [Google Scholar] [CrossRef]
- Hunt, S.L.; Hsuan, J.J.; Totty, N.; Jackson, R.J. unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev. 1999, 13, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Mitchell, S.A.; Spriggs, K.A.; Ostrowski, J.; Bomsztyk, K.; Ostarek, D.; Willis, A.E. Members of the poly (rC) binding protein family stimulate the activity of the c-myc internal ribosome entry segment in vitro and in vivo. Oncogene 2003, 22, 8012–8020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Q.; Pan, M.; Yang, Y.; Dai, X.; You, C. STRAP binds to and promotes the repair of N1-methyldeoxyadenosine in DNA. Chin. Chem. Lett. 2024, 35, 108673. [Google Scholar] [CrossRef]
- He, W.; Chang, H.; Li, C.; Wang, C.; Li, L.; Yang, G.; Chen, J.; Liu, H. STRAP upregulates antiviral innate immunity against PRV by targeting TBK1. Virol. J. 2024, 21, 197. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607. [Google Scholar] [CrossRef]
- Seong, H.A.; Manoharan, R.; Ha, H. B-MYB positively regulates serine-threonine kinase receptor-associated protein (STRAP) activity through direct interaction. J. Biol. Chem. 2011, 286, 7439–7456. [Google Scholar] [CrossRef]
| STRAP Interacting Proteins/Complex | Description | STRAP-Mediated Functional Changes in Relevance to Carcinogenesis | Refs. |
|---|---|---|---|
| TGFß-RI/TGFß-RII | Transmembrane serine/threonine receptor kinases | Formation of a ternary complex that inhibits TGF-ß signaling | [1,2,24] |
| Smad7 | Smad2/3/4 inhibitor (SMAD family member 7) | Inhibits nuclear localization of Smad2/3/4 complex and TGF-ß/Smad signaling | [2,25] |
| MEK1/2 | Dual-specificity protein kinases | Increases MEK/ERK and Wnt/ß-Catenin signaling | [14,35] |
| GSK-3ß | Serine/threonine protein kinase | Stabilization of ß-Catenin and stimulation of Wnt/ß-Catenin signaling in CRC cells | [37] |
| Notch3 | A transmembrane protein that acts as a cell surface receptor | Stabilizes Notch3 by inhibiting its proteasomal degradation and promotes lung tumorigenesis | [13] |
| SUZ12 | A component of the polycomb repressor complex 2 (PRC2) | Regulates Notch signaling gene expression by modulating methylation | [40] |
| Sp1 | A zinc finger transcription factor in the Sp/KLF family | Abrogation of the transcriptional activation of E-cadherin and p21Cip1 and subsequent regulation of EMT and cell proliferation, respectively | [43] |
| HDAC1 | A histone deacetylase that regulates physiological processes | Regulates its binding to the Sp1-binding region (C-terminal domain) of p21Cip1 promoter, downregulating its expression | [43,44] |
| PDK-1 | A phospho-inositide-dependent kinase | Positively regulates PDK1 activity, inhibition of TNF-α/TGF-ß mediated cell apoptosis | [55] |
| ASK-1 | A member of MAP kinase family | Phosphorylation of ASK-1(Thr/Ser) leads to inhibition of ASK1-mediated cell death | [55,56] |
| EWS | The Ewing Sarcoma (EWS) is a multifaceted oncogenic RNA-binding protein (RBP) | Attenuates EWS/p300-dependent activation of HNF4, ApoC3, and c-Fos in the nucleus and elevates the oncogenic properties of EWS | [62] |
| TAK1/IKK-α/p65 complex | P65 is a component of the NF-κB transcription factor complex TAK1/IKK-α is a kinase complex in the core element of the NF-κB cascade | Formation of scaffold with TAK1, IKKα, and p65, facilitates NF-κB nuclear translocation and inflammatory genes regulation | [68] |
| Gemin 6/7, SmB, SmD2 and SmD3 components of SMN complex | Nuclear ribonucleoproteins in the RNA–protein complex that participate in the splicing of pre-mRNAs | Downregulates snRNP complex assembly and regulates pre-mRNA splicing | [78,79] |
| NXF1/MAP1B/Stau1 Complex | Nuclear RNA export factor 1, Microtubule-associated protein 1B, and Staufen1 are proteins that interact with each other to facilitate mRNA transport | Forms a multiprotein complex and facilitates nuclear mRNA export in neuronal cells | [80] |
| Unr/PCBPs complex | Members of the cytoplasmic RNA-binding protein family | Increases c-Myc internal ribosomal entry site (IRES) translation and enhances cap-independent translation | [81,82] |
| NM23-H1/P53 | p53 is the guardian of the genome and a tumor suppressor protein. NM23-H1 is a metastasis suppressor | Enhances p53 stabilization and upregulates effector gene expression, may promote apoptosis and cell cycle regulation | [72] |
| B-MYB | G1/S phase transcription factor of the MYB family | Inhibition of nuclear localization of Smad3, increase in p53 nuclear localization, and suppression of TGF-ß mediated growth inhibition and apoptosis | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karfa, S.; Saurav, S.; Feng, B.; Li, S.; Law, B.K.; Datta, P.K. The Role of Serine-Threonine Kinase Receptor-Associated Protein (STRAP) Signaling in Cancer. Cells 2025, 14, 854. https://doi.org/10.3390/cells14120854
Karfa S, Saurav S, Feng B, Li S, Law BK, Datta PK. The Role of Serine-Threonine Kinase Receptor-Associated Protein (STRAP) Signaling in Cancer. Cells. 2025; 14(12):854. https://doi.org/10.3390/cells14120854
Chicago/Turabian StyleKarfa, Sourajeet, Shashank Saurav, Bryan Feng, Song Li, Brian K. Law, and Pran K. Datta. 2025. "The Role of Serine-Threonine Kinase Receptor-Associated Protein (STRAP) Signaling in Cancer" Cells 14, no. 12: 854. https://doi.org/10.3390/cells14120854
APA StyleKarfa, S., Saurav, S., Feng, B., Li, S., Law, B. K., & Datta, P. K. (2025). The Role of Serine-Threonine Kinase Receptor-Associated Protein (STRAP) Signaling in Cancer. Cells, 14(12), 854. https://doi.org/10.3390/cells14120854









