Power of Memory: A Natural Killer Cell Perspective
Abstract
1. Introduction
2. Hallmark of NK Cell Memory
2.1. Development and Key Features
2.2. Tissue-Resident Memory NK Cells
3. NK Cell Memory in Viral Infection
3.1. DNA Viruses
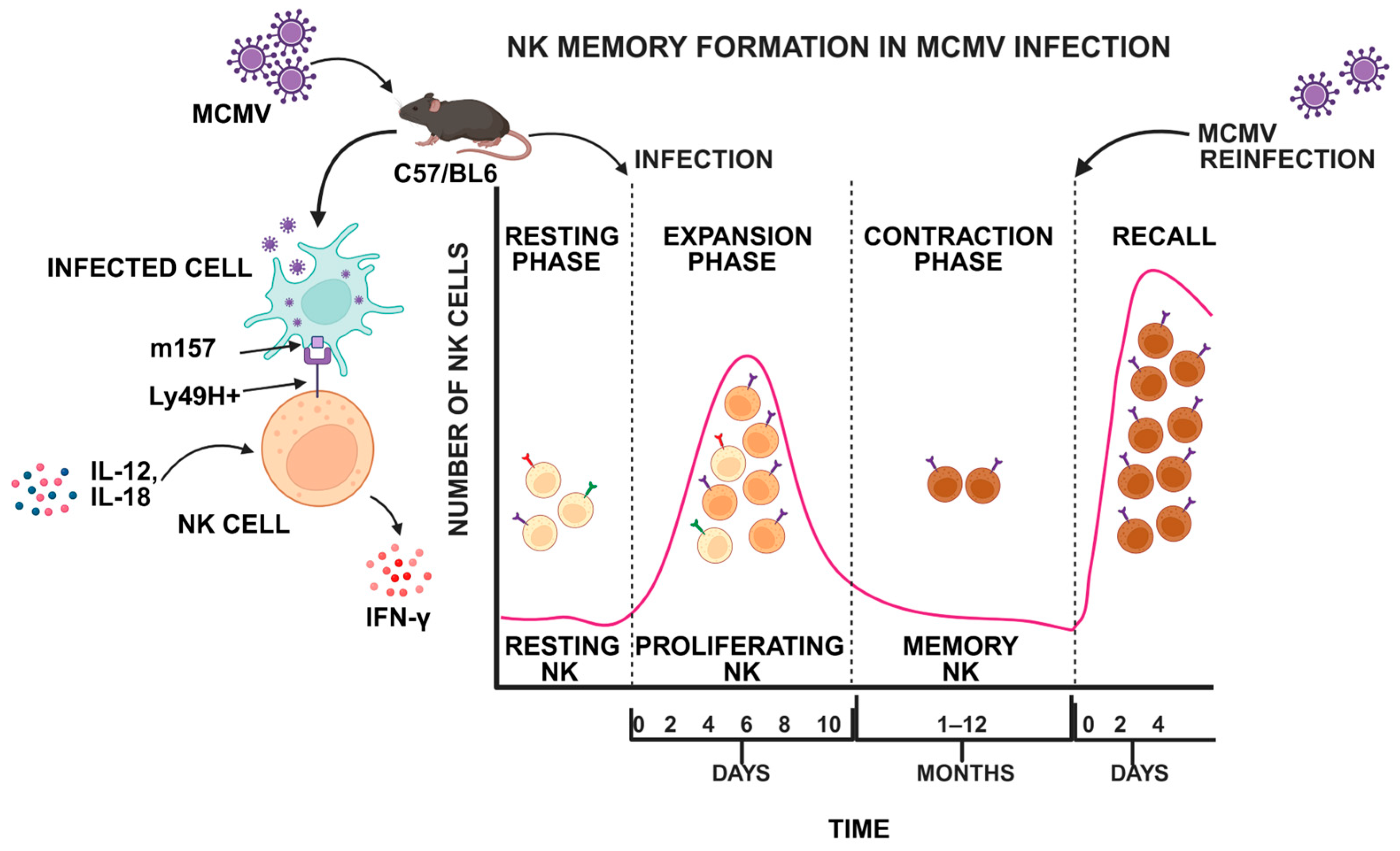
3.1.1. Cytomegalovirus
3.1.2. Epstein–Barr Virus
3.1.3. Herpesvirus
3.2. RNA Viruses
3.2.1. Human Immunodeficiency Virus
3.2.2. Influenza Virus
3.2.3. Severe Acute Respiratory Syndrome Coronavirus 2
3.2.4. Flavivirus
3.2.5. Hantavirus
4. Molecular Mechanisms of Virus-Induced NK Cell Memory Formation
4.1. Receptor Signaling
4.2. Cytokine Signaling
4.3. Epigenetic Reprogramming and Transcriptional Regulation
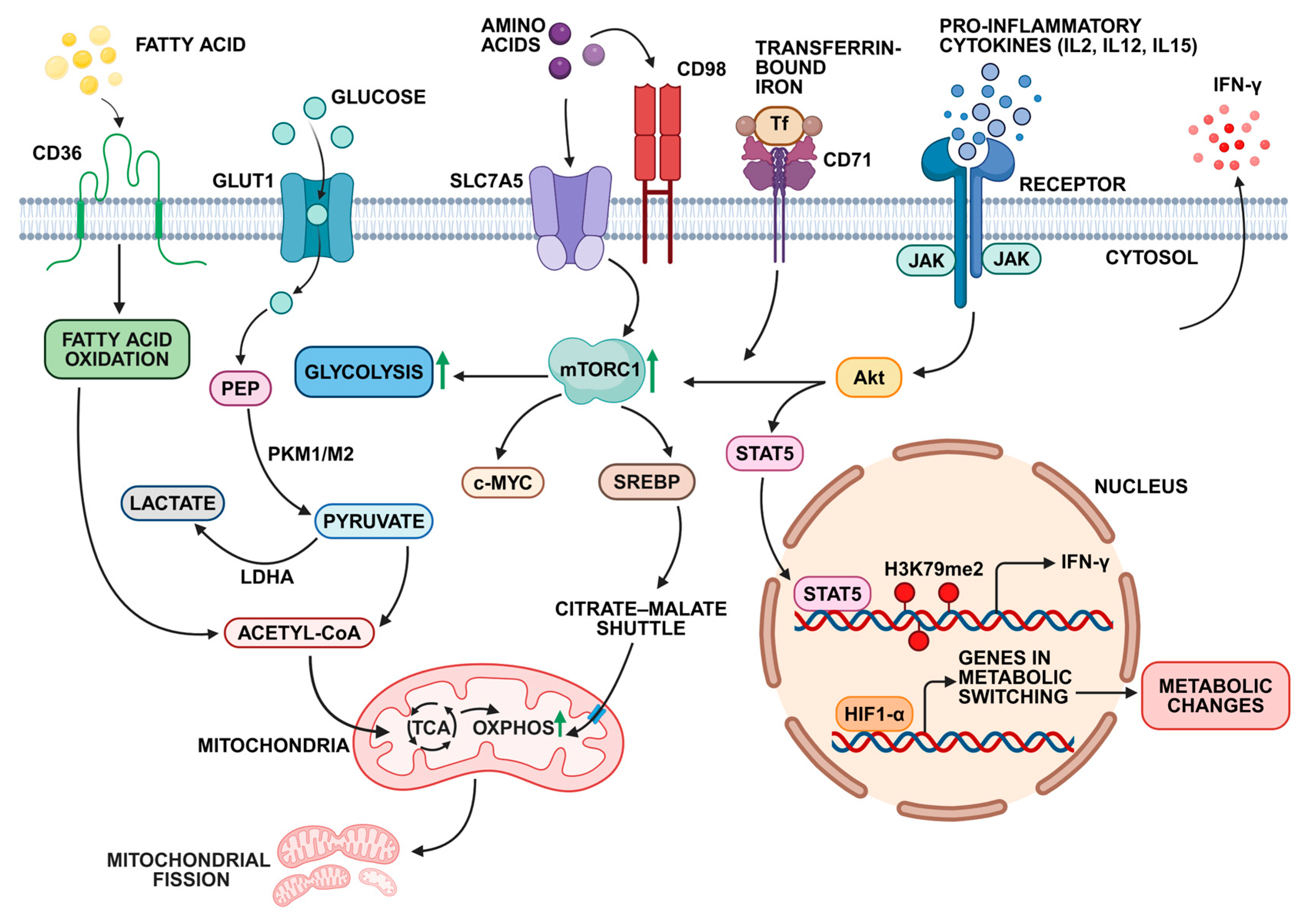
4.4. Metabolic Adaptations for Long-Term NK Cell Survival
5. NK Cell Memory in Bacterial and Parasitic Infection
5.1. Mycobacterium Tuberculosis
5.2. Ehrlichia sp.
5.3. Salmonella Typhi
5.4. Plasmodium sp.
6. NK Cell Memory in Immunological Disorders
7. NK Cell Memory and Cancer
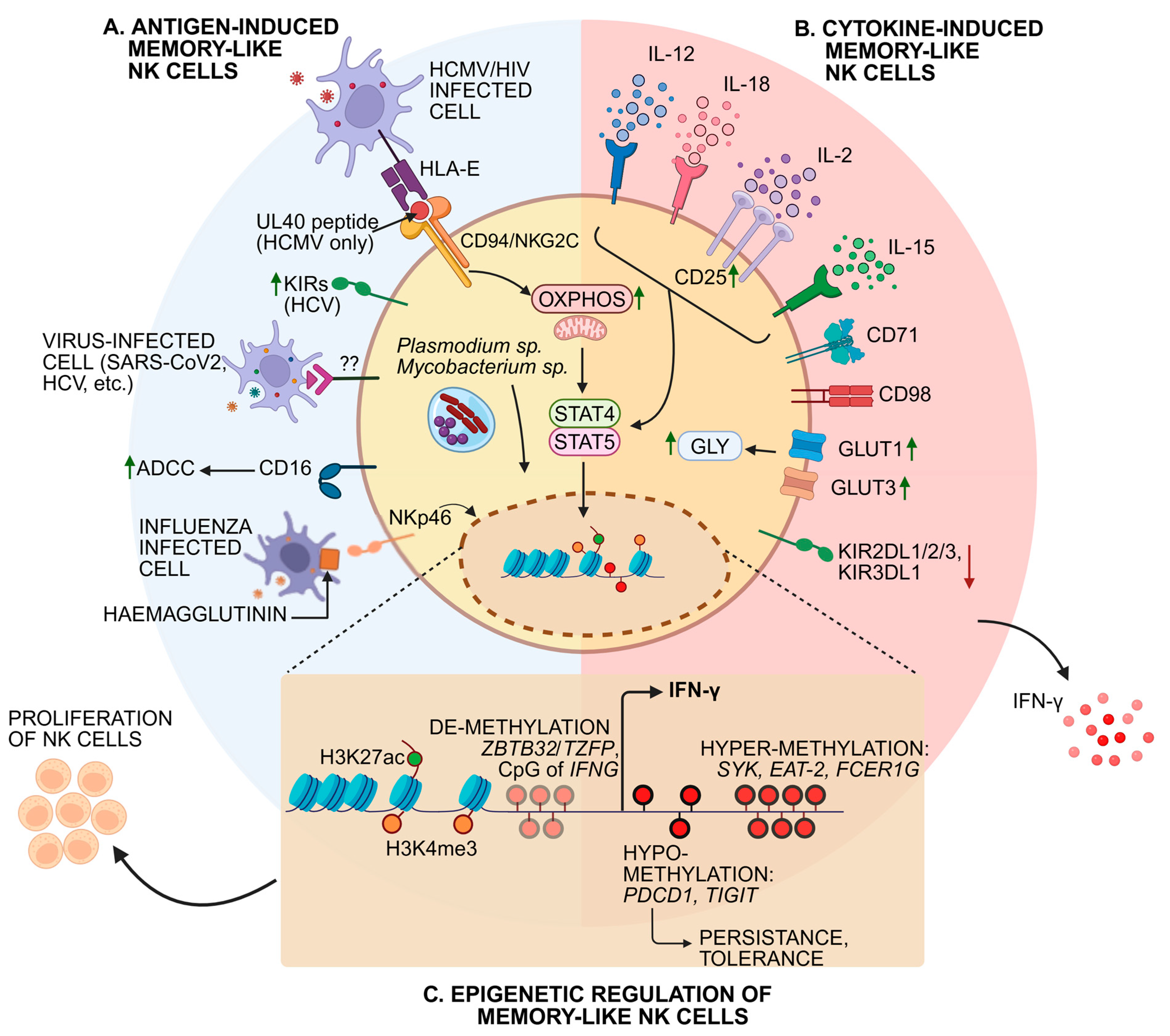
8. Cytokine-Induced Memory NK Cells
9. Molecular Mechanisms of Antigen-Independent NK Cell Memory Formation
10. NK Cell Memory in Immunotherapy Development
11. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prager, I.; Watzl, C. Mechanisms of Natural Killer Cell-Mediated Cellular Cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Zeng, R.Y.; Tan, H.S.W.; et al. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and T-Cell Responses: What We Do and Don’t Know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Quatrini, L.; Della Chiesa, M.; Sivori, S.; Mingari, M.C.; Pende, D.; Moretta, L. Human NK Cells, Their Receptors and Function. Eur. J. Immunol. 2021, 51, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Segerberg, F. Improving NK Cell-Based Immunotherapy of Cancer by Exploration of Migration and Inhibitory Receptor-Ligand Interactions. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2023. [Google Scholar]
- Paust, S.; Von Andrian, U.H. Natural Killer Cell Memory. Nat. Immunol. 2011, 12, 500–508. [Google Scholar] [CrossRef]
- Paust, S.; Senman, B.; Von Andrian, U.H. Adaptive Immune Responses Mediated by Natural Killer Cells. Immunol. Rev. 2010, 235, 286–296. [Google Scholar] [CrossRef]
- Keppel, M.P.; Yang, L.; Cooper, M.A. Murine NK Cell Intrinsic Cytokine-Induced Memory-like Responses Are Maintained Following Homeostatic Proliferation. J. Immunol. 2013, 190, 4754–4762. [Google Scholar] [CrossRef]
- Sun, J.C.; Lopez-Verges, S.; Kim, C.C.; DeRisi, J.L.; Lanier, L.L. NK Cells and Immune “Memory”. J. Immunol. 2011, 186, 1891–1897. [Google Scholar] [CrossRef]
- Beaulieu, A.M. Transcriptional and Epigenetic Regulation of Memory NK Cell Responses. Immunol. Rev. 2021, 300, 125–133. [Google Scholar] [CrossRef]
- O’Sullivan, T.E.; Sun, J.C.; Lanier, L.L. Natural Killer Cell Memory. Immunity 2015, 43, 634–645. [Google Scholar] [CrossRef]
- Freud, A.G.; Yu, J.; Caligiuri, M.A. Human Natural Killer Cell Development in Secondary Lymphoid Tissues. Semin. Immunol. 2014, 26, 132–137. [Google Scholar] [CrossRef]
- Macallan, D.C.; Borghans, J.A.M.; Asquith, B. Human T Cell Memory: A Dynamic View. Vaccines 2017, 5, 5. [Google Scholar] [CrossRef]
- Slamanig, S.A.; Nolte, M.A. The Bone Marrow as Sanctuary for Plasma Cells and Memory T-Cells: Implications for Adaptive Immunity and Vaccinology. Cells 2021, 10, 1508. [Google Scholar] [CrossRef]
- Karo, J.M.; Schatz, D.G.; Sun, J.C. The RAG Recombinase Dictates Functional Heterogeneity and Cellular Fitness in Natural Killer Cells. Cell 2014, 159, 94–107. [Google Scholar] [CrossRef]
- Geary, C.D.; Sun, J.C. Memory Responses of Natural Killer Cells. Semin. Immunol. 2017, 31, 11–19. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-Induced Memory-like Natural Killer Cells Exhibit Enhanced Responses against Myeloid Leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef]
- Miller, J.S.; Verfaillie, C.; Mcglave, P. The Generation of Human Natural Killer Cells from CD34+/DR-Primitive Progenitors in Long-Term Bone Marrow Culture. Blood 1992, 80, 2182–2187. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright Natural Killer (NK) Cells: An Important NK Cell Subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Rebuffet, L.; Melsen, J.E.; Escalière, B.; Basurto-Lozada, D.; Bhandoola, A.; Björkström, N.K.; Bryceson, Y.T.; Castriconi, R.; Cichocki, F.; Colonna, M.; et al. High-Dimensional Single-Cell Analysis of Human Natural Killer Cell Heterogeneity. Nat. Immunol. 2024, 25, 1474–1488. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, Z.; Lin, Y.; Shu, G.; Yin, G.; Zhang, T. Biology and Clinical Relevance of HCMV-Associated Adaptive NK Cells. Front. Immunol. 2022, 13, 830396. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Iwasaki, A. Tissue-Resident Memory T Cells. Immunol. Rev. 2013, 255, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, D.; Scharenberg, M.; Mold, J.E.; Hård, J.; Kekäläinen, E.; Buggert, M.; Nguyen, S.; Wilson, J.N.; Al-Ameri, M.; Ljunggren, H.-G.; et al. Expansions of Adaptive-like NK Cells with a Tissue-Resident Phenotype in Human Lung and Blood. Proc. Natl. Acad. Sci. USA 2021, 118, e2016580118. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.E.; Ostridge, K.; Khakoo, S.I.; Wilkinson, T.M.A.; Staples, K.J. Human CD49a+ Lung Natural Killer Cell Cytotoxicity in Response to Influenza A Virus. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Wang, J.M.; Cheng, Y.Q.; Shi, L.; Ying, R.S.; Wu, X.Y.; Li, G.Y.; Moorman, J.P.; Yao, Z.Q. KLRG1 Negatively Regulates Natural Killer Cell Functions through the Akt Pathway in Individuals with Chronic Hepatitis C Virus Infection. J. Virol. 2013, 87, 11626–11636. [Google Scholar] [CrossRef]
- van den Boorn, J.G.; Jakobs, C.; Hagen, C.; Renn, M.; Luiten, R.M.; Melief, C.J.M.; Tüting, T.; Garbi, N.; Hartmann, G.; Hornung, V. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity 2016, 44, 1406–1421. [Google Scholar] [CrossRef]
- Khalil, M.; Malarkannan, S.; Terhune, S.; Barbieri, J.T.; Rao, S.; Boger, R.; Lau, C. Defining Memory NK Cell Development and Functions Following Cytomegalovirus Infection Dissertation Committee. Ph.D. Thesis, The Medical College of Wisconsin, Milwaukee, WI, USA, 2024. [Google Scholar]
- Zhang, C.; Zhang, Y.; Zhuang, R.; Yang, K.; Chen, L.; Jin, B.; Ma, Y.; Zhang, Y.; Tang, K. Alterations in CX3CL1 Levels and Its Role in Viral Pathogenesis. Int. J. Mol. Sci. 2024, 25, 4451. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, E.; Malarkannan, S. Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance. Cancers 2020, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Valero-Pacheco, N.; Beaulieu, A.M. Transcriptional Regulation of Mouse Tissue-Resident Natural Killer Cell Development. Front. Immunol. 2020, 11, 309. [Google Scholar]
- Ochayon, D.E.; Waggoner, S.N. The Effect of Unconventional Cytokine Combinations on NK-Cell Responses to Viral Infection. Front. Immunol. 2021, 12, 645850. [Google Scholar] [CrossRef]
- Forrest, C.; Gomes, A.; Reeves, M.; Male, V. NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development. Vaccines 2020, 8, 394. [Google Scholar] [CrossRef]
- Caldeira-Dantas, S.; Furmanak, T.; Smith, C.; Quinn, M.; Teos, L.Y.; Ertel, A.; Kurup, D.; Tandon, M.; Alevizos, I.; Snyder, C.M. The Chemokine Receptor CXCR3 Promotes CD8+ T Cell Accumulation in Uninfected Salivary Glands but Is Not Necessary after Murine Cytomegalovirus Infection. J. Immunol. 2018, 200, 1133–1145. [Google Scholar] [CrossRef]
- Mah, A.Y.; Rashidi, A.; Keppel, M.P.; Saucier, N.; Moore, E.K.; Alinger, J.B.; Tripathy, S.K.; Agarwal, S.K.; Jeng, E.K.; Wong, H.C.; et al. Glycolytic Requirement for NK Cell Cytotoxicity and Cytomegalovirus Control. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Rölle, A.; Meyer, M.; Calderazzo, S.; Jäger, D.; Momburg, F. Distinct HLA-E Peptide Complexes Modify Antibody-Driven Effector Functions of Adaptive NK Cells. Cell Rep. 2018, 24, 1967–1976.e4. [Google Scholar] [CrossRef]
- Kared, H.; Martelli, S.; Tan, S.W.; Simoni, Y.; Chong, M.L.; Yap, S.H.; Newell, E.W.; Pender, S.L.F.; Kamarulzaman, A.; Rajasuriar, R.; et al. Adaptive NKG2C+CD57+ Natural Killer Cell and Tim-3 Expression during Viral Infections. Front. Immunol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Costa-García, M.; Ataya, M.; Moraru, M.; Vilches, C.; López-Botet, M.; Muntasell, A. Human Cytomegalovirus Antigen Presentation by HLA-DR+ NKG2C+ Adaptive NK Cells Specifically Activates Polyfunctional Effector Memory CD4+ T Lymphocytes. Front. Immunol. 2019, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Martín Almazán, N.; Sala, B.M.; Sandalova, T.; Sun, Y.; Resink, T.; Cichocki, F.; Söderberg-Nauclér, C.; Miller, J.S.; Achour, A.; Sarhan, D. Non-Classical HLA-E Restricted CMV 15-Mer Peptides Are Recognized by Adaptive NK Cells and Induce Memory Responses. Front. Immunol. 2023, 14, 1230718. [Google Scholar] [CrossRef] [PubMed]
- Desimio, M.G.; Covino, D.A.; Rivalta, B.; Cancrini, C.; Doria, M. The Role of NK Cells in EBV Infection and Related Diseases: Current Understanding and Hints for Novel Therapies. Cancers 2023, 15, 1914. [Google Scholar] [CrossRef]
- Hendricks, D.W.; Balfour, H.H.; Dunmire, S.K.; Schmeling, D.O.; Hogquist, K.A.; Lanier, L.L. Cutting Edge: NKG2ChiCD57+ NK Cells Respond Specifically to Acute Infection with Cytomegalovirus and Not Epstein–Barr Virus. J. Immunol. 2014, 192, 4492–4496. [Google Scholar] [CrossRef]
- Jud, A.; Kotur, M.; Berger, C.; Gysin, C.; Nadal, D.; Lünemann, A. Tonsillar CD56brightNKG2A+ NK cells restrict primary Epstein-Barr virus infection in B cells via IFN-γ. Oncotarget 2017, 8, 6130–6141. [Google Scholar] [CrossRef]
- Png, Y.T.; Yang, A.Z.Y.; Lee, M.Y.; Chua, M.J.M.; Lim, C.M. The Role of Nk Cells in Ebv Infection and Ebv-Associated Npc. Viruses 2021, 13, 300. [Google Scholar] [CrossRef]
- Della Chiesa, M.; De Maria, A.; Muccio, L.; Bozzano, F.; Sivori, S.; Moretta, L. Human NK Cells and Herpesviruses: Mechanisms of Recognition, Response and Adaptation. Front. Microbiol. 2019, 10, 2297. [Google Scholar] [CrossRef]
- Abdul-Careem, M.F.; Lee, A.J.; Pek, E.A.; Gill, N.; Gillgrass, A.E.; Chew, M.V.; Reid, S.; Ashkar, A.A. Genital HSV-2 Infection Induces Short-Term NK Cell Memory. PLoS ONE 2012, 7, e32821. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.; Stoian, A.M.M.; Yu, H.; Rahman, M.J.; Banerjee, S.; Stroup, J.N.; Park, C.; Tazi, L.; Rothenburg, S. Molecular Mechanisms of Poxvirus Evolution. mBio 2023, 14, e0152622. [Google Scholar] [CrossRef]
- Hsu, J.; Kim, S.; Anandasabapathy, N. Vaccinia Virus: Mechanisms Supporting Immune Evasion and Successful Long-Term Protective Immunity. Viruses 2024, 16, 870. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.J.; Zhang, X.C.; Wan, L.Y.; Li, Q.Y.; Mu, X.Y.; Guo, A.L.; Zhou, M.J.; Shen, L.L.; Zhang, C.; Fan, X.; et al. Immune Dysfunctions of CD56neg NK Cells Are Associated With HIV-1 Disease Progression. Front. Immunol. 2022, 12, 811091. [Google Scholar] [CrossRef]
- He, L.; Pan, X.; Dou, Z.; Huang, P.; Zhou, X.; Peng, Z.; Zheng, J.; Zhang, J.; Yang, J.; Xu, Y.; et al. The Factors Related to CD4+ T-Cell Recovery and Viral Suppression in Patients Who Have Low CD4+ T Cell Counts at the Initiation of HAART: A Retrospective Study of the National HIV Treatment Sub-Database of Zhejiang Province, China, 2014. PLoS ONE 2016, 11, e0148915. [Google Scholar] [CrossRef] [PubMed]
- Peppa, D.; Pedroza-Pacheco, I.; Pellegrino, P.; Williams, I.; Maini, M.K.; Borrow, P. Adaptive Reconfiguration of Natural Killer Cells in HIV-1 Infection. Front. Immunol. 2018, 9, 474. [Google Scholar] [CrossRef]
- Naidoo, K.K.; Altfeld, M. The Role of Natural Killer Cells and Their Metabolism in HIV-1 Infection. Viruses 2024, 16, 1584. [Google Scholar] [CrossRef]
- Costanzo, M.C.; Kim, D.; Creegan, M.; Lal, K.G.; Ake, J.A.; Currier, J.R.; Streeck, H.; Robb, M.L.; Michael, N.L.; Bolton, D.L.; et al. Transcriptomic Signatures of NK Cells Suggest Impaired Responsiveness in HIV-1 Infection and Increased Activity Post-Vaccination. Nat. Commun. 2018, 9, 1212. [Google Scholar] [CrossRef]
- Jost, S.; Lucar, O.; Lee, E.; Yoder, T.; Kroll, K.; Sugawara, S.; Smith, S.; Jones, R.; Tweet, G.; Werner, A.; et al. Antigen-Specific Memory NK Cell Responses against HIV and Influenza Use the NKG2/HLA-E Axis. Sci. Immunol. 2023, 8, eadi3974. [Google Scholar] [CrossRef]
- Jost, S.; Lucar, O.; Yoder, T.; Kroll, K.; Sugawara, S.; Smith, S.; Jones, R.; Tweet, G.; Werner, A.; Tomezsko, P.J.; et al. Human Antigen-Specific Memory Natural Killer Cell Responses Develop against HIV-1 and Influenza Virus and Are Dependent on MHC-E Restriction. BioRxiv 2020. [Google Scholar] [CrossRef]
- Macedo, A.B.; Levinger, C.; Nguyen, B.N.; Richard, J.; Gupta, M.; Cruz, C.R.Y.; Finzi, A.; Chiappinelli, K.B.; Crandall, K.A.; Bosque, A. The HIV Latency Reversal Agent HODHBt Enhances NK Cell Effector and Memory-Like Functions by Increasing Interleukin-15-Mediated STAT Activation. J. Virol. 2022, 96, e0037222. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.; Paust, S. Dynamic Natural Killer Cell and T Cell Responses to Influenza Infection. Front. Cell Infect. Microbiol. 2020, 10, 425. [Google Scholar] [CrossRef]
- Dou, Y.; Fu, B.; Sun, R.; Li, W.; Hu, W.; Tian, Z.; Wei, H. Influenza Vaccine Induces Intracellular Immune Memory of Human NK Cells. PLoS ONE 2015, 10, e0121258. [Google Scholar] [CrossRef]
- Zheng, J.; Wen, L.; Yen, H.-L.; Liu, M.; Liu, Y.; Teng, O.; Wu, W.-F.; Ni, K.; Lam, K.-T.; Huang, C.; et al. Phenotypic and Functional Characteristics of a Novel Influenza Virus Hemagglutinin-Specific Memory NK Cell. J. Virol. 2021, 95, e00165-21. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.E.; King, M.L.; Kelvin, A.A. Back to the Future for Influenza Preimmunity—Looking Back at Influenza Virus History to Infer the Outcome of Future Infections. Viruses 2019, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Sun, R. Liver-Resident NK Cells and Their Potential Functions. Cell. Mol. Immunol. 2017, 14, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Stary, V.; Stary, G. NK Cell-Mediated Recall Responses: Memory-Like, Adaptive, or Antigen-Specific? Front. Cell Infect. Microbiol. 2020, 10, 208. [Google Scholar] [CrossRef]
- Herrera, L.; Martin-Inaraja, M.; Santos, S.; Inglés-Ferrándiz, M.; Azkarate, A.; Perez-Vaquero, M.A.; Vesga, M.A.; Vicario, J.L.; Soria, B.; Solano, C.; et al. Identifying SARS-CoV-2 ‘Memory’ NK Cells from COVID-19 Convalescent Donors for Adoptive Cell Therapy. Immunology 2022, 165, 234–249. [Google Scholar] [CrossRef]
- Hasan, M.Z.; Claus, M.; Krüger, N.; Reusing, S.; Gall, E.; Bade-Döding, C.; Braun, A.; Watzl, C.; Uhrberg, M.; Walter, L. SARS-CoV-2 Infection Induces Adaptive NK Cell Responses by Spike Protein-Mediated Induction of HLA-E Expression. Emerg. Microbes Infect. 2024, 13, 2361019. [Google Scholar] [CrossRef]
- Okoye, A.A.; Picker, L.J. CD4+ T-Cell Depletion In Hiv Infection: Mechanisms of Immunological Failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef]
- Alrubayyi, A.; Touizer, E.; Hameiri-Bowen, D.; Charlton, B.; Gea-Mallorquí, E.; Hussain, N.; da Costa, K.A.S.; Ford, R.; Rees-Spear, C.; Fox, T.A.; et al. Natural Killer Cell Responses during SARS-CoV-2 Infection and Vaccination in People Living with HIV-1. Sci. Rep. 2023, 13, 18994. [Google Scholar] [CrossRef] [PubMed]
- Royston, L.; Isnard, S.; Lin, J.; Routy, J.P. Cytomegalovirus as an Uninvited Guest in the Response to Vaccines in People Living with Hiv. Viruses 2021, 13, 1266. [Google Scholar] [CrossRef]
- Kujur, W.; Murillo, O.; Adduri, R.S.R.; Vankayalapati, R.; Konduru, N.V.; Mulik, S. Memory like NK Cells Display Stem Cell like Properties after Zika Virus Infection. PLoS Pathog. 2020, 16, e1009132. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.; Drouin, C.; Bédard, N.; Khakoo, S.I.; Bruneau, J.; Shoukry, N.H. Increased Degranulation of Natural Killer Cells during Acute HCV Correlates with the Magnitude of Virus-Specific T Cell Responses. J. Hepatol. 2010, 53, 805–816. [Google Scholar] [CrossRef]
- Björkström, N.K.; Lindgren, T.; Stoltz, M.; Fauriat, C.; Braun, M.; Evander, M.; Michaëlsson, J.; Malmberg, K.J.; Klingström, J.; Ahlm, C.; et al. Rapid Expansion and Long-Term Persistence of Elevated NK Cell Numbers in Humans Infected with Hantavirus. J. Exp. Med. 2011, 208, 13–21. [Google Scholar] [CrossRef]
- Braun, M.; Björkström, N.K.; Gupta, S.; Sundström, K.; Ahlm, C.; Klingström, J.; Ljunggren, H.G. NK Cell Activation in Human Hantavirus Infection Explained by Virus-Induced IL-15/IL15Rα Expression. PLoS Pathog. 2014, 10, e1004521. [Google Scholar] [CrossRef]
- El Weizman, O.; Song, E.; Adams, N.M.; Hildreth, A.D.; Riggan, L.; Krishna, C.; Aguilar, O.A.; Leslie, C.S.; Carlyle, J.R.; Sun, J.C.; et al. Mouse Cytomegalovirus-Experienced ILC1s Acquire a Memory Response Dependent on the Viral Glycoprotein M12. Nat. Immunol. 2019, 20, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.H.W.; Cerwenka, A.; Ni, J. Memory-Like NK Cells: Remembering a Previous Activation by Cytokines and NK Cell Receptors. Front. Immunol. 2018, 9, 2796. [Google Scholar] [CrossRef]
- Bigley, A.B.; Spade, S.; Agha, N.H.; Biswas, S.; Tang, S.; Malik, M.H.; Dai, L.; Masoumi, S.; Patiño-Escobar, B.; Hale, M.; et al. FceRIg-Negative NK Cells Persist in Vivo and Enhance Efficacy of Therapeutic Monoclonal Antibodies in Multiple Myeloma. Blood Adv. 2021, 5, 3021–3031. [Google Scholar] [CrossRef]
- Lau, C.M.; Wiedemann, G.M.; Sun, J.C. Epigenetic Regulation of Natural Killer Cell Memory. Immunol. Rev. 2022, 305, 90–110. [Google Scholar] [CrossRef]
- Liu, L.L.; Landskron, J.; Ask, E.H.; Enqvist, M.; Sohlberg, E.; Traherne, J.A.; Hammer, Q.; Goodridge, J.P.; Larsson, S.; Jayaraman, J.; et al. Critical Role of CD2 Co-Stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep. 2016, 15, 1088–1099. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, H.; Ji, X.; Zhang, Y.; Shi, G.; Dai, L.; Cheng, F.; Wang, H.; Luo, J.; Xu, J.; et al. A Novel Membrane-Bound Interleukin-2 Promotes NK-92 Cell Persistence and Anti-Tumor Activity. Oncoimmunology 2022, 11, 2127282. [Google Scholar] [CrossRef]
- Gill, N.; Chenoweth, M.J.; Verdu, E.F.; Ashkar, A.A. NK Cells Require Type I IFN Receptor for Antiviral Responses during Genital HSV-2 Infection. Cell Immunol. 2011, 269, 29–37. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Tian, L.; Chen, Y. Cytokine-Mediated Immunopathogenesis of Hepatitis B Virus Infections. Clin. Rev. Allergy Immunol. 2016, 50, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.E.; Malle, E.K.; Gardam, S.; Silveira, P.A.; Zammit, N.W.; Walters, S.N.; Brink, R.; Grey, S.T. TRAF2 Regulates Peripheral CD8+ T-Cell and NKT-Cell Homeostasis by Modulating Sensitivity to IL-15. Eur. J. Immunol. 2015, 45, 1820–1831. [Google Scholar] [CrossRef]
- Caldwell, B.A.; Li, L. Epigenetic Regulation of Innate Immune Dynamics during Inflammation. J. Leukoc. Biol. 2024, 115, 589–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.J.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic Modification and Antibody-Dependent Expansion of Memory-like NK Cells in Human Cytomegalovirus-Infected Individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, M.; Romee, R. Cytokine-Induced Memory-like Natural Killer Cells for Cancer Immunotherapy. Stem Cell Res. Ther. 2021, 12, 592. [Google Scholar] [CrossRef]
- Tiku, V.; Tan, M.W.; Dikic, I. Mitochondrial Functions in Infection and Immunity. Trends Cell Biol. 2020, 30, 263–275. [Google Scholar] [CrossRef]
- Cimpean, M.; Cooper, M.A. Metabolic Regulation of NK Cell Antiviral Functions during Cytomegalovirus Infection. J. Leukoc. Biol. 2023, 113, 525–534. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Walls, J.F.; Subleski, J.J.; Palmieri, E.M.; Gonzalez-Cotto, M.; Gardiner, C.M.; McVicar, D.W.; Finlay, D.K. Metabolic but Not Transcriptional Regulation by Pkm2 Is Important for Natural Killer Cell Responses. Elife 2020, 9, e59166. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.; Santosa, E.K.; Lau, C.M.; Violante, S.; Giovanelli, P.; Kim, H.; Cross, J.R.; Li, M.O.; Sun, J.C. Lactate Dehydrogenase A-Dependent Aerobic Glycolysis Promotes Natural Killer Cell Anti-Viral and Anti-Tumor Function. Cell Rep. 2021, 35, 109210. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.Y. Transcription Factors Associated With IL-15 Cytokine Signaling During NK Cell Development. Front. Immunol. 2021, 12, 610789. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yin, J.; Liu, X.; Yu, M.; Li, T.; Yan, H.; Wang, X. Chromatin state dynamics during NK cell activation. Oncotarget 2017, 8, 41854–41865. [Google Scholar] [CrossRef]
- Qiu, B.; Yuan, P.; Du, X.; Jin, H.; Du, J.; Huang, Y. Hypoxia Inducible Factor-1α Is an Important Regulator of Macrophage Biology. Heliyon 2023, 9, e17167. [Google Scholar] [CrossRef]
- Sohn, H.; Cooper, M.A. Metabolic Regulation of NK Cell Function: Implications for Immunotherapy. Immunometabolism 2023, 5, E00020. [Google Scholar] [CrossRef]
- Murillo, O.; Dornelas Moreira, J.; Kujur, W.; Velasco-Alzate, K.; Santara, S.S.; Konduru, N.V.; Mulik, S. Costimulatory CD226 Signaling Regulates Proliferation of Memory-like NK Cells in Healthy Individuals with Latent Mycobacterium Tuberculosis Infection. Int. J. Mol. Sci. 2022, 2022, 12838. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, D.; Wang, S.; Chai, L.Y.A.; Xu, S.; Lam, K.P. Glycolysis and Oxidative Phosphorylation Play Critical Roles in Natural Killer Cell Receptor-Mediated Natural Killer Cell Functions. Front. Immunol. 2020, 11, 202. [Google Scholar] [CrossRef]
- Ham, J.; Yang, W.; Kim, H.Y. Tissue-Specific Metabolic Reprogramming in Innate Lymphoid Cells and Its Impact on Disease. Immune Netw. 2025, 25, e3. [Google Scholar] [CrossRef]
- Yue, M.; Jiang, J.; Gao, P.; Liu, H.; Qing, G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017, 21, 3819–3832. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yu, S.; Yang, B.; Lao, S.; Li, B.; Wu, C. Memory-Like Antigen-Specific Human NK Cells from TB Pleural Fluids Produced IL-22 in Response to IL-15 or Mycobacterium Tuberculosis Antigens. PLoS ONE 2016, 11, e0151721. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, S.; Cheekatla, S.; Paidipally, P.; Tripathi, D.; Welch, E.; Tvinnereim, A.R.; Nurieva, R.; Vankayalapati, R. IL-21-Dependent Expansion of Memory-like NK Cells Enhances Protective Immune Responses against Mycobacterium Tuberculosis. Mucosal Immunol. 2017, 10, 1031–1042. [Google Scholar] [CrossRef]
- Liu, W.; Scott, J.M.; Langguth, E.; Chang, H.; Park, P.H.; Kim, S. FcRγ Gene Editing Reprograms Conventional NK Cells to Display Key Features of Adaptive Human NK Cells. iScience 2020, 23, 101709. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, J.; Yu, C.; Feng, Z.; Cheng, K.; Ma, J.; Wang, Y.; Duan, C.; Zhang, Y.; Jin, B.; et al. CD226 Deficiency Promotes Glutaminolysis and Alleviates Mitochondria Damage in Vascular Endothelial Cells under Hemorrhagic Shock. FASEB J. 2021, 35, e21998. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Li, L.; Li, Q.; Qiao, D.; Wang, H.; Lao, S.; Fan, Y.; Wu, C.Y. Human Natural Killer Cells Expressing the Memory-Associated Marker CD45RO from Tuberculous Pleurisy Respond More Strongly and Rapidly than CD45RO- Natural Killer Cells Following Stimulation with Interleukin-12. Immunology 2011, 134, 41–49. [Google Scholar] [CrossRef]
- Habib, S.; El Andaloussi, A.; Hisham, A.; Ismail, N. NK Cell-Mediated Regulation of Protective Memory Responses against Intracellular Ehrlichial Pathogens. PLoS ONE 2016, 11, e0153223. [Google Scholar] [CrossRef] [PubMed]
- Blohmke, C.J.; Hill, J.; Darton, T.C.; Carvalho-Burger, M.; Eustace, A.; Jones, C.; Schreiber, F.; Goodier, M.R.; Dougan, G.; Nakaya, H.I.; et al. Induction of Cell Cycle and NK Cell Responses by Live-Attenuated Oral Vaccines against Typhoid Fever. Front. Immunol. 2017, 8, 1276. [Google Scholar] [CrossRef]
- Wilhelm, K.; Happel, K.; Eelen, G.; Schoors, S.; Oellerich, M.F.; Lim, R.; Zimmermann, B.; Aspalter, I.M.; Franco, C.A.; Boettger, T.; et al. FOXO1 Couples Metabolic Activity and Growth State in the Vascular Endothelium. Nature 2016, 529, 216–220. [Google Scholar] [CrossRef]
- Loiseau, C.; Doumbo, O.K.; Traore, B.; Brady, J.L.; Proietti, C.; de Sousa, K.P.; Crompton, P.D.; Doolan, D.L. A Novel Population of Memory-Activated Natural Killer Cells Associated with Low Parasitaemia in Plasmodium Falciparum-Exposed Sickle-Cell Trait Children. Clin. Transl. Immunol. 2020, 9, e1125. [Google Scholar] [CrossRef]
- Tukwasibwe, S.; Nakimuli, A.; Traherne, J.; Chazara, O.; Jayaraman, J.; Trowsdale, J.; Moffett, A.; Jagannathan, P.; Rosenthal, P.J.; Cose, S.; et al. Variations in Killer-Cell Immunoglobulin-like Receptor and Human Leukocyte Antigen Genes and Immunity to Malaria. Cell Mol. Immunol. 2020, 17, 799–806. [Google Scholar] [CrossRef] [PubMed]
- La Marca, V.; Gianchecchi, E.; Fierabracci, A. Type 1 Diabetes and Its Multi-Factorial Pathogenesis: The Putative Role of NK Cells. Int. J. Mol. Sci. 2018, 19, 794. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Hsu, C.Y.; Kuo, M.L.; Lee, P.T.; Hsiao, H.S.; Chen, J.Y. Phenotypic and Functional Characterization of Natural Killer Cells in Rheumatoid Arthritis-Regulation with Interleukin-15. Sci. Rep. 2020, 10, 5858. [Google Scholar] [CrossRef]
- Coyle, C.; Ma, M.; Abraham, Y.; Mahony, C.B.; Steel, K.; Simpson, C.; Guerra, N.; Croft, A.P.; Rapecki, S.; Cope, A.; et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. CI Insight 2021, 6, e149217. [Google Scholar]
- Christensen, A.D.; Haase, C. Immunological Mechanisms of Contact Hypersensitivity in Mice. APMIS 2012, 120, 1–27. [Google Scholar] [CrossRef]
- Williams, N.S.; Klem, J.; Puzanov, I.J.; Sivakumar, P.V.; Schatzle, J.D.; Bennett, M.; Kumar, V. Natural Killer Cell Differentiation: Insights from Knockout and Transgenic Mouse Models and in Vitro Systems. Immunol. Rev. 1998, 165, 47–61. [Google Scholar] [CrossRef]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef] [PubMed]
- Schwab, L.; Bühler, S.; Biedritzky, A.; Schmidt, M.; Andre, M.C. Optimized Flow Cytometry Panel for the Detection and Analysis of Human Tumor-Induced Memory-like NK Cells. J. Immunol. Methods 2023, 515, 113439. [Google Scholar] [CrossRef]
- Matsuo, Y.; Drexler, H.G. Establishment and characterization of human B cell precursor-leukemia cell lines. Leuk. Res. 1998, 22, 567–579. [Google Scholar] [CrossRef]
- Mah, A.Y.; Cooper, M.A. Metabolic Regulation of Natural Killer Cell IFN-γ Production. Crit. Rev. Immunol. 2016, 36, 131–147. [Google Scholar] [CrossRef]
- Schmidt, M.; André, M.C. From Bench to Bedside: Exploiting Memory NK Cell Responses to Leukemia. Adv. Cell Gene Ther. 2019, 2, e28. [Google Scholar] [CrossRef]
- Pal, M.; Schwab, L.; Yermakova, A.; Mace, E.M.; Claus, R.; Krahl, A.C.; Woiterski, J.; Hartwig, U.F.; Orange, J.S.; Handgretinger, R.; et al. Tumor-Priming Converts NK Cells to Memory-like NK Cells. Oncoimmunology 2017, 6, e1317411. [Google Scholar] [CrossRef] [PubMed]
- Creelan, B.C.; Antonia, S.J. The NKG2A Immune Checkpoint—A New Direction in Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 277–278. [Google Scholar] [CrossRef]
- Simiczyjew, A.; Dratkiewicz, E.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. The Influence of Tumor Microenvironment on Immune Escape of Melanoma. Int. J. Mol. Sci. 2020, 21, 8359. [Google Scholar] [CrossRef] [PubMed]
- Lusty, E.; Poznanski, S.M.; Kwofie, K.; Mandur, T.S.; Lee, D.A.; Ashkar, A.A. IL-18/IL-15/IL-12 Synergy Induces Elevated and Prolonged IFN-γ Production by Ex Vivo Expanded NK Cells Which Is Not Due to Enhanced STAT4 Activation. Mol. Immunol. 2017, 88, 138–147. [Google Scholar] [CrossRef]
- Carreira-Santos, S.; López-Sejas, N.; González-Sánchez, M.; Sánchez-Hernández, E.; Pera, A.; Hassouneh, F.; Durán, E.; Solana, R.; Casado, J.G.; Tarazona, R. Enhanced Expression of Natural Cytotoxicity Receptors on Cytokine-Induced Memory-like Natural Killer Cells Correlates with Effector Function. Front. Immunol. 2023, 14, 1256404. [Google Scholar] [CrossRef]
- Leong, J.W.; Chase, J.M.; Romee, R.; Schneider, S.E.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Preactivation with IL-12, IL-15, and IL-18 Induces Cd25 and a Functional High-Affinity Il-2 Receptor on Human Cytokine-Induced Memory-like Natural Killer Cells. Biol. Blood Marrow Transplant. 2014, 20, 463–473. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Mosteiro, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. Metabolic Changes of Interleu-kin-12/15/18-Stimulated Human NK Cells. Sci. Rep. 2021, 11, 6472. [Google Scholar] [CrossRef]
- Kedia-Mehta, N.; Tobin, L.; Zaiatz-Bittencourt, V.; Pisarska, M.M.; De Barra, C.; Choi, C.; Elamin, E.; O’Shea, D.; Gardiner, C.M.; Finlay, D.K.; et al. Cytokine-Induced Natural Killer Cell Training Is Dependent on Cellular Metabolism and Is Defective in Obesity. Blood Adv. 2021, 5, 4447–4455. [Google Scholar] [CrossRef]
- Becker-Hapak, M.K.; Shrestha, N.; McClain, E.; Dee, M.J.; Chaturvedi, P.; Leclerc, G.M.; Marsala, L.I.; Foster, M.; Schappe, T.; Tran, J.; et al. A Fusion Protein Complex That Combines Il-12, Il-15, and Il-18 Signaling to Induce Memory-like Nk Cells for Cancer Immunotherapy. Cancer Immunol. Res. 2021, 9, 1071–1087. [Google Scholar] [CrossRef]
- Yu, M.; Su, Z.; Huang, X.; Zhou, Y.; Zhang, X.; Wang, B.; Wang, Z.; Liu, Y.; Xing, N.; Xia, M.; et al. Histone Methyltransferase Ezh2 Negatively Regulates NK Cell Terminal Maturation and Function. J. Leukoc. Biol. 2021, 110, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zeng, A.; Cheng, Y.; Li, S. Natural Killer Cell Memory: Challenges and Opportunities for Cancer Immunotherapy. Cancer Biol. Ther. 2024, 25, 2376410. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xu-Monette, Z.Y.; Tzankov, A.; Li, X.; Manyam, G.C.; Murty, V.; Bhagat, G.; Zhang, S.; Pasqualucci, L.; Visco, C.; et al. Loss of PRDM1/BLIMP-1 Function Contributes to Poor Prognosis of Activated B-Cell-like Diffuse Large B-Cell Lymphoma. Leukemia 2017, 31, 625–636. [Google Scholar] [CrossRef]
- Beaulieu, A.M.; Zawislak, C.L.; Nakayama, T.; Sun, J.C. The Transcription Factor Zbtb32 Controls the Proliferative Burst of Virus-Specific Natural Killer Cells Responding to Infection. Nat. Immunol. 2014, 15, 546–553. [Google Scholar] [CrossRef]
- Mansouri, V.; Yazdanpanah, N.; Rezaei, N. The Immunologic Aspects of Cytokine Release Syndrome and Graft versus Host Disease Following CAR T Cell Therapy. Int. Rev. Immunol. 2022, 41, 649–668. [Google Scholar] [CrossRef]
- Gesundheit, B.; Shapira, M.Y.; Resnick, I.B.; Amar, A.; Kristt, D.; Dray, L.; Budowski, E.; Or, R. Successful Cell-Mediated Cytokine-Activated Immunotherapy for Relapsed Acute Myeloid Leukemia after Hematopoietic Stem Cell Transplantation. Am. J. Hematol. 2009, 84, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Haroun-Izquierdo, A.; Vincenti, M.; Netskar, H.; Van Ooijen, H.; Zhang, B.; Bendzick, L.; Kanaya, M.; Momayyezi, P.; Li, S.; Wiiger, M.T.; et al. Adaptive Single-KIR + NKG2C + NK Cells Expanded from Select Superdonors Show Potent Missing-Self Reactivity and Efficiently Control HLA-Mismatched Acute Myeloid Leukemia. J. Immunother. Cancer 2022, 10, e005577. [Google Scholar] [CrossRef]
- Arellano-Ballestero, H.; Zubiak, A.; Dally, C.; Orchard, K.; Alrubayyi, A.; Charalambous, X.; Michael, M.; Torrance, R.; Eales, T.; Das, K.; et al. Proteomic and Phenotypic Characteristics of Memory-like Natural Killer Cells for Cancer Immunotherapy. J. Immunother. Cancer 2024, 12, e008717. [Google Scholar] [CrossRef]
- Rutella, S.; Vadakekolathu, J.; Cashen, A.F.; Mahajan, N.; Ruiz-Heredia, Y.; Martín-Muñoz, A.; Barrio, S.; Berrien-Elliott, M.M.; Davidson-Moncada, J.; Fehniger, T.A. Adoptively Infused Memory-like Natural Killer Cells Impact Adaptive Immune Responses in Patients with Acute Myeloid Leukemia. Blood 2023, 142, 4813. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat4, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Gang, M.; Davila, M.; Lee, H. CAR-Modified Memory-like NK Cells Exhibit Potent Responses to NK-Resistant Lymphomas. Blood J. Am. Soc. Hematol. 2020, 136, 2308–2318. [Google Scholar] [CrossRef]
- Tarannum, M.; Dinh, K.; Vergara, J.; Birch, G.; Abdulhamid, Y.Z.; Kaplan, I.E.; Ay, O.; Maia, A.; Beaver, O.; Sheffer, M.; et al. CAR memory–like NK cells targeting the membrane proximal domain of mesothelin demonstrate promising activity in ovarian cancer. Sci. Adv. 2024, 10, eadn0881. [Google Scholar] [CrossRef] [PubMed]
- Mortara, L.; Balza, E.; Bruno, A.; Poggi, A.; Orecchia, P.; Carnemolla, B. Anti-Cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-Tumor Efficacy. Front. Immunol. 2018, 9, 2905. [Google Scholar] [CrossRef]
- Douka, S.; Papamoschou, V.; Raimo, M.; Mastrobattista, E.; Caiazzo, M. Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy. Pharmaceutics 2024, 16, 1143. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.M.; Birch, G.C.; Hu, G.; Cadavid, J.V.; Nikiforow, S.; Baginska, J.; Ali, A.K.; Tarannum, M.; Sheffer, M.; Abdulhamid, Y.Z.; et al. Expansion, Persistence, and Efficacy of Donor Memory-like NK Cells Infused for Posttransplant Relapse. J. Clin. Investig. 2022, 132, e154334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tu, S.; Wang, C.; Huang, R.; Deng, L.; Song, C.; Yue, C.; He, Y.; Yang, J.; Liang, Z.; et al. Phase I Trial of Fourth-Generation Anti-CD19 Chimeric Antigen Receptor T Cells Against Relapsed or Refractory B Cell Non-Hodgkin Lymphomas. Front. Immunol. 2020, 11, 564099. [Google Scholar] [CrossRef]
- Chiopris, G.; Veronese, P.; Cusenza, F.; Procaccianti, M.; Perrone, S.; Daccò, V.; Colombo, C.; Esposito, S. Congenital Cytomegalovirus Infection: Update on Diagnosis and Treatment. Microorganisms 2020, 8, 1516. [Google Scholar] [CrossRef]
- Vasu, S.; Berg, M.; Davidson-Moncada, J.; Tian, X.; Cullis, H.; Childs, R.W. A Novel Method to Expand Large Numbers of CD56+ Natural Killer Cells from a Minute Fraction of Selectively Accessed Cryopreserved Cord Blood for Immunotherapy after Transplantation. Cytotherapy 2015, 17, 1582–1593. [Google Scholar] [CrossRef]
- Lundqvist, A.; Yokoyama, H.; Smith, A.; Berg, M.; Childs, R. Bortezomib Treatment and Regulatory T-Cell Depletion Enhance the Antitumor Effects of Adoptively Infused NK Cells. Blood 2009, 113, 6120–6127. [Google Scholar] [CrossRef]
- Hamdan, T.A. The Multifaceted Roles of NK Cells in the Context of Murine Cytomegalovirus and Lymphocytic Choriomeningitis Virus Infections. Immune Netw. 2024, 24, e29. [Google Scholar] [CrossRef]
- Parham, P. The Genetic and Evolutionary Balances in Human NK Cell Receptor Diversity. Semin. Immunol. 2008, 20, 311–316. [Google Scholar] [CrossRef]
- Lee, N.I.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; López-Botet, M.; Geraghty, D.E. HLA-E Is a Major Ligand for the Natural Killer Inhibitory Receptor CD94NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Su, B.; Pang, L.; Qiao, L.; Feng, Y.; Ouyang, Y.; Guo, X.; Shi, H.; Wei, F.; Su, X.; et al. High-Dimensional Immune Profiling by Mass Cytometry Revealed Immunosuppression and Dysfunction of Immunity in COVID-19 Patients. Cell Mol. Immunol. 2020, 17, 650–652. [Google Scholar] [CrossRef]
- Widowati, W.; Jasaputra, D.K.; Sumitro, S.B.; Widodo, M.A.; Mozef, T.; Rizal, R.; Kusuma, H.S.W.; Laksmitawati, D.R.; Murti, H.; Bachtiar, I.; et al. Effect of Interleukins (Il-2, Il-15, Il-18) on Receptors Activation and Cytotoxic Activity of Natural Killer Cells in Breast Cancer Cell. Afr. Health Sci. 2020, 20, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Euler, Z.; Alter, G. Exploring the Potential of Monoclonal Antibody Therapeutics for HIV-1 Eradication. AIDS Res. Hum. Retroviruses 2015, 31, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, J.; Yu, H.; Wang, K.; Dai, W.; Zhang, X.; Hu, N.; Yin, L.; Tang, D.; Liu, F.; et al. Single-Cell RNA and ATAC Sequencing Reveal Hemodialysis-Related Immune Dysregulation of Circulating Immune Cell Subpopulations. Front. Immunol. 2022, 13, 878226. [Google Scholar] [CrossRef]
- Ebihara, T.; Song, C.; Ryu, S.H.; Plougastel-Douglas, B.; Yang, L.; Levanon, D.; Groner, Y.; Bern, M.D.; Stappenbeck, T.S.; Colonna, M.; et al. Runx3 Specifies Lineage Commitment of Innate Lymphoid Cells. Nat. Immunol. 2015, 16, 1124–1133. [Google Scholar] [CrossRef]
- MacEk Jilkova, Z.; Decaens, T.; Marlu, A.; Marche, H.; Jouvin-Marche, E.; Marche, P.N. Sex Differences in Spontaneous Degranulation Activity of Intrahepatic Natural Killer Cells during Chronic Hepatitis B: Association with Estradiol Levels. Mediat. Inflamm. 2017, 2017, 3214917. [Google Scholar] [CrossRef]
- Al-Attar, A.; Presnell, S.R.; Peterson, C.A.; Thomas, D.T.; Lutz, C.T. The Effect of Sex on Immune Cells in Healthy Aging: Elderly Women Have More Robust Natural Killer Lymphocytes than Do Elderly Men. Mech. Ageing Dev. 2016, 156, 25–33. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, B.; Liu, X.; Li, H.; Xie, L.; Gao, Y.; Duan, R.; Li, Z.; Zhang, J.; Zheng, Y.; et al. Effects of Sex and Aging on the Immune Cell Landscape as Assessed by Single-Cell Transcriptomic Analysis. Proc. Natl. Acad. Sci. USA 2021, 118, 2023216118. [Google Scholar] [CrossRef] [PubMed]
| Sl. No. | Company Name | Platform/Product Name | Memory NK Cell Type | Cancer Targets | Development Stage | Description | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Nkarta Therapeutics, San Francisco, CA, USA | NKX101 | Engineered memory-like NK cells | AML, B cell malignancies | Phase I | CAR-NK cells with enhanced persistence and cytotoxicity | https://www.nkartatx.com/ |
| 2 | Fate Therapeutics, San Diego, CA, USA | FT536 | iPSC-derived memory NK cells | Solid tumors, NHL | Phase I | Multi-antigen-targeted CAR-NK with IL-15 boost | https://www.fatetherapeutics.com/ |
| 3 | Affimed, Berlin, Germany | AFM13-NK | Cord blood-derived memory NK cells | Hodgkin’s lymphoma | Phase II (ANCHOR) | Innate cell engager (ICE®) combined with preactivated NK cells | https://www.affimed.com/ |
| 4 | GT Biopharma, Brisbane, CA, USA | GTB-3550 | TriKE-activated memory NK cells | AML, MDS | Phase I | Tri-specific NK engager (CD16/IL-15/CD33) to enhance NK memory response | https://www.gtbiopharma.com/ |
| 5 | ImmunityBio, Culver City, CA, USA | haNK | IL-2/IL-15-primed memory NK cells | Solid tumors (pancreatic, NSCLC) | Phase II | Off-the-shelf NK cells with enhanced ADCC | https://immunitybio.com/ |
| 6 | Artiva Biotherapeutics, San Diego, CA, USA | AB-101 | Allogeneic memory-like NK cells | B cell malignancies, solid tumors | Preclinical/Phase I | IL-12/15/18-activated NK cells for improved tumor infiltration | https://www.artivabio.com/ |
| 7 | Wugen, St. Louis, MO, USA | WU-NK-101 | Cytokine-induced memory-like (CIML) phenotype | AML, glioblastoma | Phase I | Cytokine-induced memory-like (CIML) phenotype that supports enhanced antitumor activity, robust trafficking, superior proliferation capacity, and metabolic flexibility | https://wugen.com/ |
| 8 | Glycostem Therapeutics, Oss, The Netherlands | oNKord® | Umbilical cord-derived memory NK cells | AML, ovarian cancer | Phase I/II | Proprietary expansion tech for high-persistence NK cells | https://www.glycostem.com/ |
| 9 | Celularity, Florham Park, NJ, USA | CYNK-101 | Placental-derived memory NK cells | Glioblastoma, AML | Phase I/II | Cryopreserved, off-the-shelf NK cells with cytokine augmentation | https://celularity.com/ |
| 10 | Indapta Therapeutics, San Francisco, CA, USA | g-NK | Naturally occurring subset of natural killer cells known as g-NK | Rituximab in NHL, and in combination with daratumumab in multiple myeloma | Preclinical/Phase I | Universal, allogeneic NK cell therapy designed to improve treatment outcomes for cancer and autoimmune diseases, based on subset of NK cells known as g-NK cells | https://indapta.com/ |
| Sl No. | NCT Number | Study Title | Phase | Status | Memory NK Cell type | Conditions | Interventions | Sponsor | Study Type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NCT06321484 | Intraperitoneal Cytokine-Induced Memory Like (CIML) NK Cells in Recurrent Ovarian Cancer | Phase I | Recruiting | Cytokine-Induced Memory-Like NK Cells | Platinum-Resistant Ovarian Cancer, Recurrent Ovary Cancer, Ovarian Cancer, | Interleukin 2 | Dana-Farber Cancer Institute | Interventional |
| 2 | NCT06318871 | Memory-like NK Cell Therapy in Patients with Renal Cell Carcinoma or Urothelial Carcinoma | Phase 1 | Recruiting | Cytokine Induced Memory-Like Natural Killer (CIML NK) Cells | Renal Carcinoma, Renal Cell Carcinoma, Urothelial Carcinoma | Interleukin-2 (IL-2) | Dana-Farber Cancer Institute | Interventional |
| 3 | NCT06158828 | Pilot Study of Memory-like NK (ML NK) Cells After TCRαβ T Cell Depleted Haploidentical Transplant in AML | PhaseI Phase II | Recruiting | Memory-Like NK (ML NK) Cells | AML, ChildhoodAML, Pediatric Acute Myeloid Leukemia | Rabbit Anti-Thymocyte Globulin; Drug: Busulfan; Drug: Fludarabine; 8 More | Washington University School of Medicine | Interventional |
| 4 | NCT06152809 | CIML NK Cells With Venetoclax for AML | Phase I | Recruiting | Cytokine-Induced Memory-Like NK Cells, Interleukin-2 | Acute Myeloid Leukemia, Recurrent Leukemia | Venetoclax | Dana-Farber Cancer Institute | Interventional |
| 5 | NCT06138587 | Preemptive CIML NK Cell Therapy After Hematopoietic Stem Cell Transplantation | Phase I | Recruiting | Cytokine-Induced Memory-Like NK Cells, Interleukin-2 | Acute Myeloid Leukemia, Myeloid Leukemia, 3 More | ----------- | Dana-Farber Cancer Institute | Interventional |
| 6 | NCT05629546 | Memory-Like NK Cells With Nivolumab and Relatlimab in Advanced or Metastatic Melanoma After Progression on Checkpoint Inhibitors | Phase I | Recruiting | Cytokine-Induced Memory-Like NK cells | Advanced Melanoma, Metastatic Melanoma | Relatilmab, Nivolumab | Washington University School of Medicine | Interventional |
| 7 | NCT05580601 | Cytokine-Induced Memory-Like NK Cells (CIML-NK) for Relapsed & Refractory Acute Myeloid Leukemia (AML) | PhaseI Phase II | Recruiting | Cytokine-Induced Memory- Like NK cells | AML | CIML-NK Cells | Children’s Hospital Medical Center, Cincinnati | Interventional |
| 8 | NCT04893915 | Cytokine-induced Memory-like NK Cells in Relapsed/Refractory AML and MDS | Phase II | Withdrawn | Cytokine-Induced Memory- Like NK cells | Relapsed AML, Refractory AML, Myelodysplastic Syndromes | Fludarabine, Cyclophosphamide | Washington University School of Medicine | Interventional |
| 9 | NCT04634435 | Autologous Memory-like NK Cell Therapy With BHV-1100 and Low Dose IL-2 in Multiple Myeloma Patients | Phase I | Completed | Cytokine-Induced Memory-Like NK Cells | Multiple Myeloma | BHV-1100 Plus Cytokine-Induced Memory-Like (CIML) NK Cells plus IVIG and Low-Dose IL-2 | Biohaven Pharmaceuticals, Inc. | Interventional |
| 10 | NCT04354025 | Cytokine-induced Memory-like NK Cells in Combination with Chemotherapy in Pediatric Patents with Refractory or Relapsed AML | Phase II | Withdrawn | Cytokine-Induced Memory- Like NK cells | Refractory AML, Relapsed AML | Fludarabine, Ara-C | Washington University School of Medicine | Interventional |
| 11 | NCT04290546 | CIML NK Cell in Head & Neck Cancer | Phase I | Completed | CIML NK | Squamous Cell Carcinoma of Head and Neck, Recurrent Head and Neck Squamous Cell Carcinoma | Interleukin-15 Superagonist (N-803), CIML NK Cell Infusion, Ipilimumab | Dana-Farber Cancer Institute | Interventional |
| 12 | NCT03068819 | Cytokine Induced Memory-like NK Cell Adoptive Therapy for Relapsed AML After Allogeneic Hematopoietic Cell Transplant | Phase I Phase II | Recruiting | Cytokine-Induced Memory-Like (CIML) NK Cells | AML | CIML NK Cell Infusion, CD3+ T Cell Product Infusion | Washington University School of Medicine | Interventional |
| 13 | NCT02782546 | Cytokine Induced Memory-like NK Cell Adoptive Therapy After Haploidentical Donor Hematopoietic Cell Transplantation | Phase II | Recruiting | Cytokine-Induced Memory-Like NK Cells | AML | Graft Cell Infusion, Tacrolimus, Mycophenolate Mofetil | Washington University School of Medicine | Interventional |
| 14 | NCT01898793 | Cytokine-induced Memory-like NK Cells in Patients AML or Myelodysplastic Syndrome (MDS) | PhaseI Phase II | Terminated | Cytokine-Induced Memory-Like NK Cells | AML | Fludarabine, Cyclophosphamide, Leukapheresis | Washington University School of Medicine | Interventional |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, O.; Abhipsha, S.; Sen Santara, S. Power of Memory: A Natural Killer Cell Perspective. Cells 2025, 14, 846. https://doi.org/10.3390/cells14110846
Sinha O, Abhipsha S, Sen Santara S. Power of Memory: A Natural Killer Cell Perspective. Cells. 2025; 14(11):846. https://doi.org/10.3390/cells14110846
Chicago/Turabian StyleSinha, Oishi, SK Abhipsha, and Sumit Sen Santara. 2025. "Power of Memory: A Natural Killer Cell Perspective" Cells 14, no. 11: 846. https://doi.org/10.3390/cells14110846
APA StyleSinha, O., Abhipsha, S., & Sen Santara, S. (2025). Power of Memory: A Natural Killer Cell Perspective. Cells, 14(11), 846. https://doi.org/10.3390/cells14110846






