Prognostic Significance of Macrophage Phenotypes in Peri-Tumoral Normal Tissue of Early-Stage Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
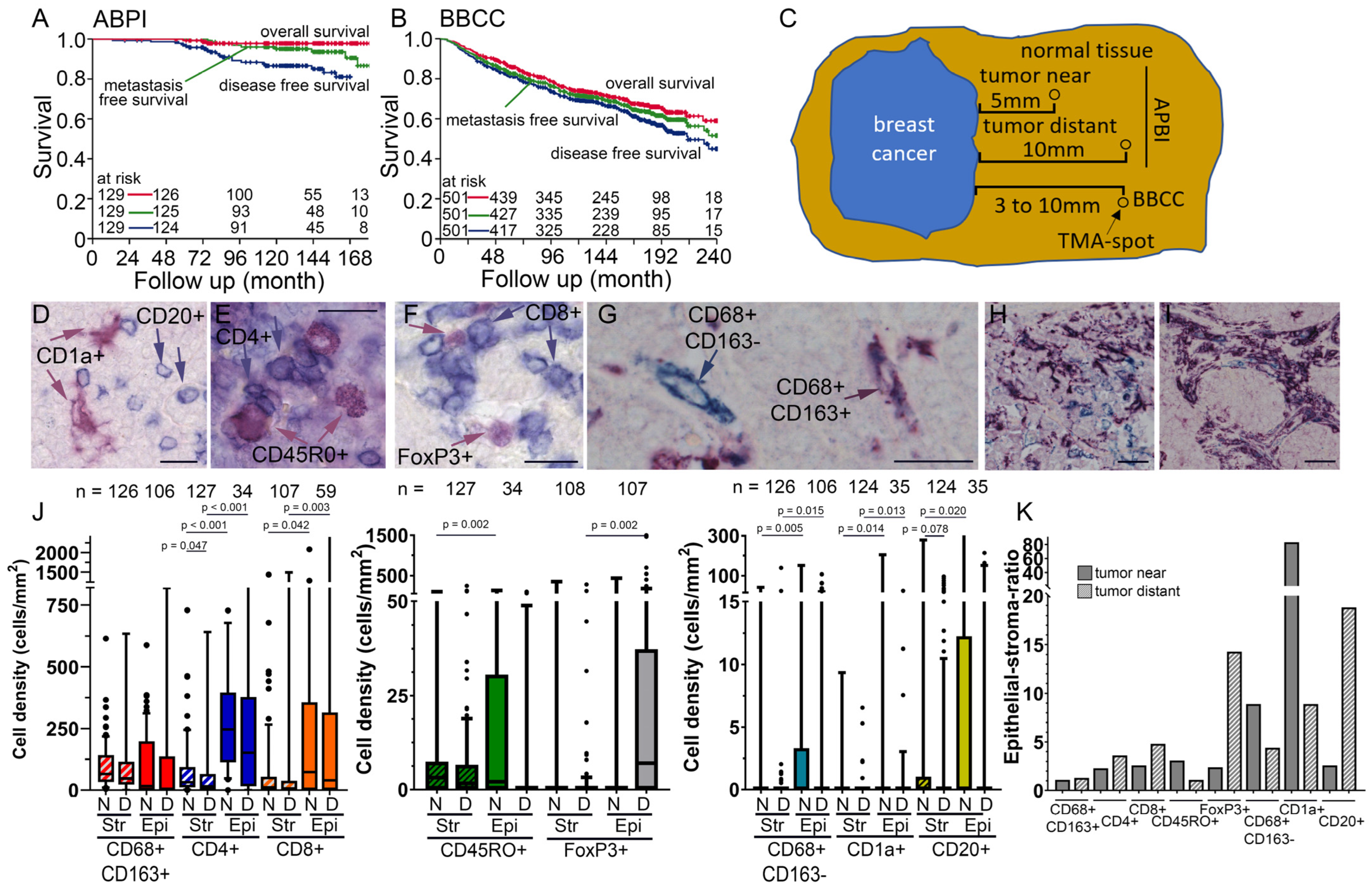
2.1. Clinical Characteristics of the APBI Study
2.2. Clinical Characteristics of the BBCC Study
2.3. Tissue Microarray (TMA) Construction
2.4. Tissue Staining
- ○
- CD68-specific monoclonal mouse antibody (1:1500. PG-M1, Dako GA613, Agilent Technologies, Santa Clara, CA, USA);
- ○
- CD206-specific monoclonal mouse antibody (1:100, Novus biologicals NBP2-52927, Abingdon, UK);
- ○
- MPO-specific polyclonal rabbit antibody (1:200, Abcam ab9535, Cambridge, UK);
- ○
- CD163-specific monoclonal mouse antibody (1:3000, clone 10D6, Novocastra NCL-L-CD163, Leica, Nussloch, Germany);
- ○
- FoxP3-specific monoclonal mouse antibody (1:100, clone 236A/E7, Abcam ab20034, Cambridge, UK);
- ○
- CD8-specific monoclonal mouse antibody (1:50, clone C8/144B, Agilent M7103, Santa Clara, CA, USA) [20];
- ○
- Monoclonal mouse anti-human CD45RO antibody (DAKO Denmark A/S, Glostrup, Denmark);
- ○
- CD1a mouse monoclonal, (clone CD1a007, Abcam ab708, Cambridge, UK);
- ○
- CD20 (1:2000, mouse monoclonal, clone L26, Dako M0755, Agilent Technologies, Santa Clara, CA, USA);
- ○
- Clonal mouse anti-human CD45RO antibody (DAKO Agilent Technologies, Santa Clara, CA, USA);
- ○
- CD4 (Clone C8/144B, Leica NCL-CD4-1F6, Nussloch, Germany) [17].
2.5. Quantification of Inflammatory Cells in the APBI and BBCC Study Using Two-Color Immunohistochemistry Staining
2.6. Quantification of Inflammatory Cells in the APBI Study Using Four-Color Fluorescence Staining
2.7. Missing Cell Densities from Patients
2.8. M1/M2 Shift Analysis
2.9. Epithelial–Stromal Match Analysis
2.10. Statistical Analysis
3. Results
3.1. Two Early-Stage Breast Cancer Cohorts
3.2. TIIC Densities in Normal Tissue Samples
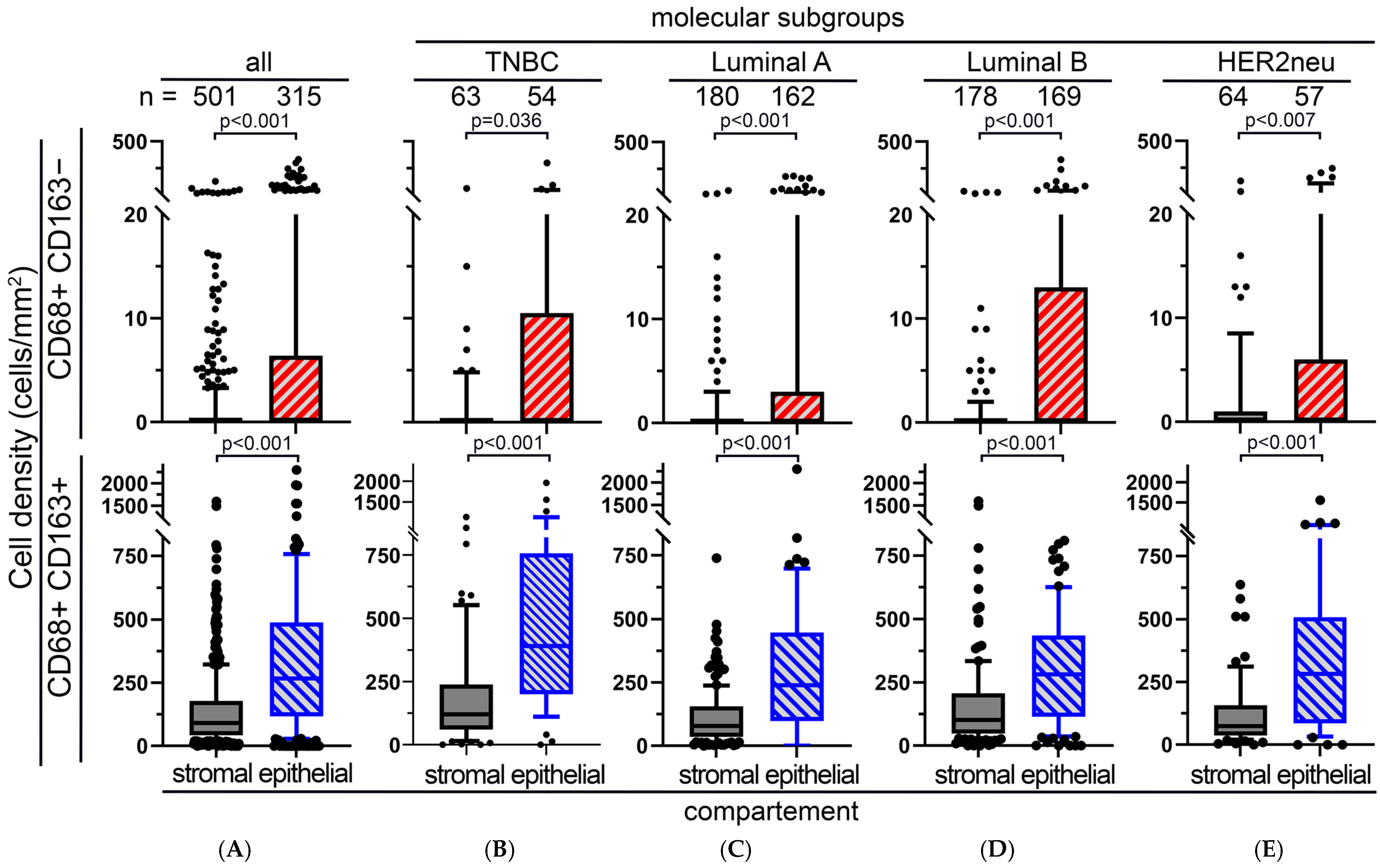
3.3. M1 and M2 Macrophages in the APBI Study
3.4. M1 and M2 Macrophages in the BBCC Study
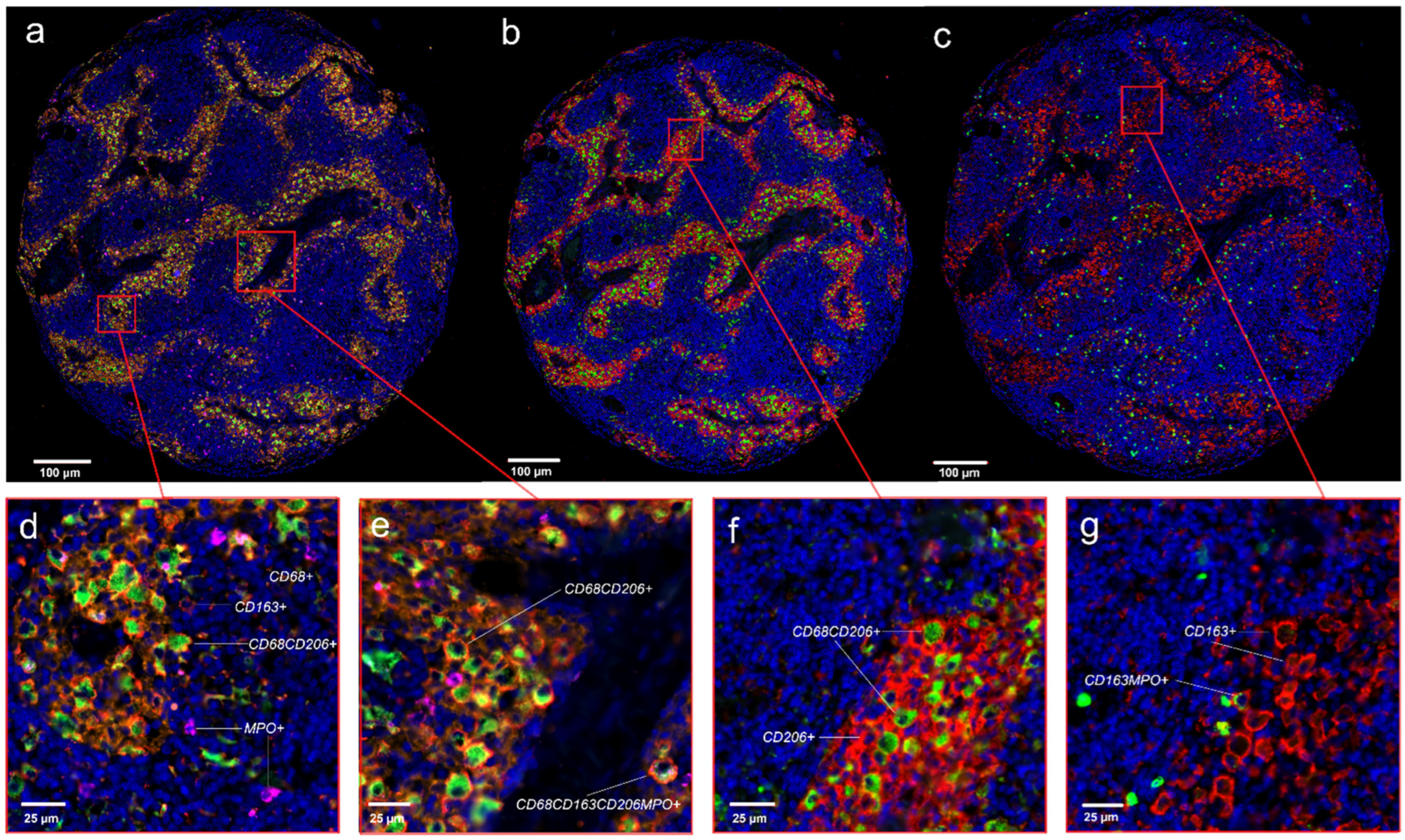
3.5. Four Color Immunostaining of CD68, CD163, MPO and CD206 in the APBI Study
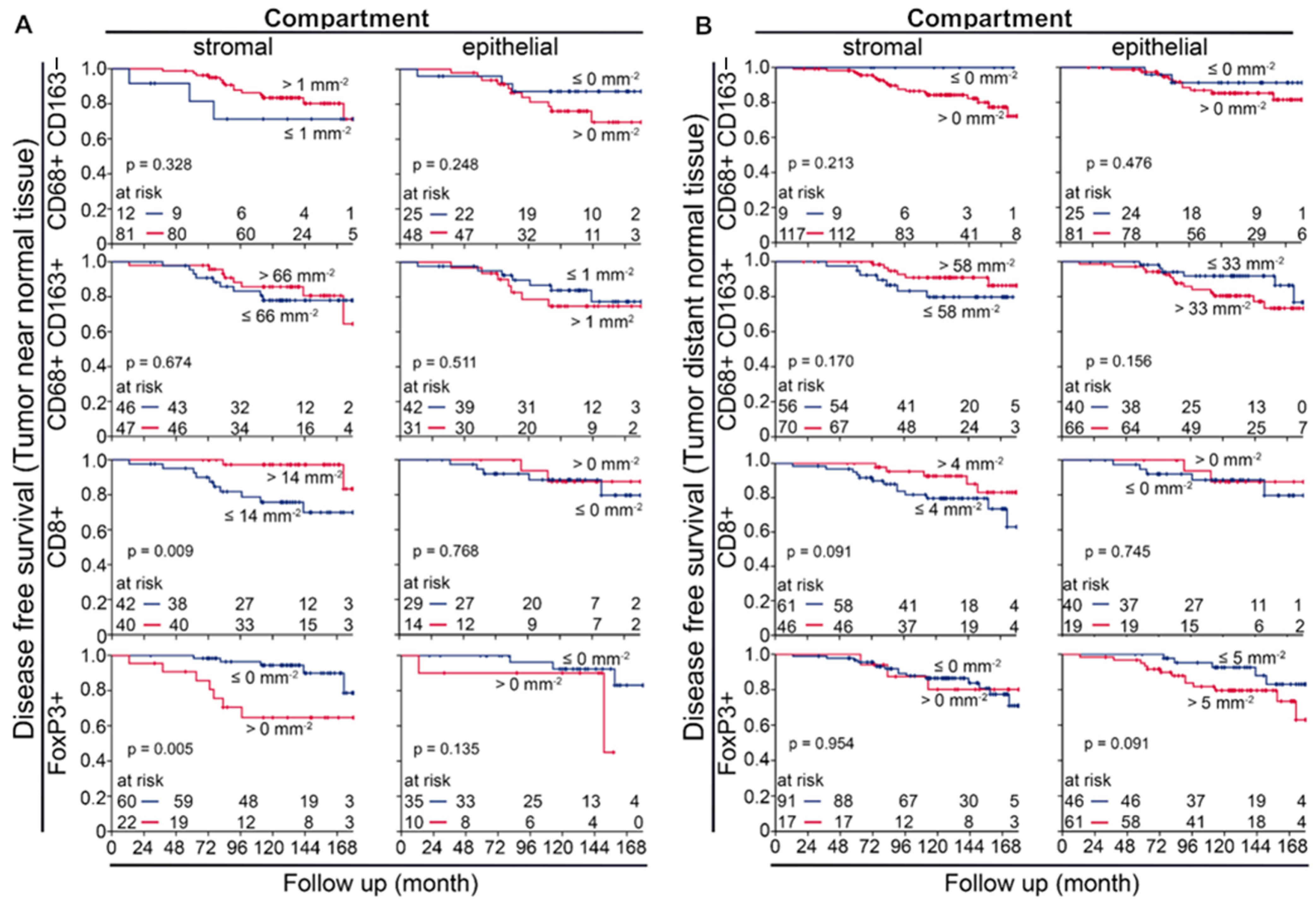
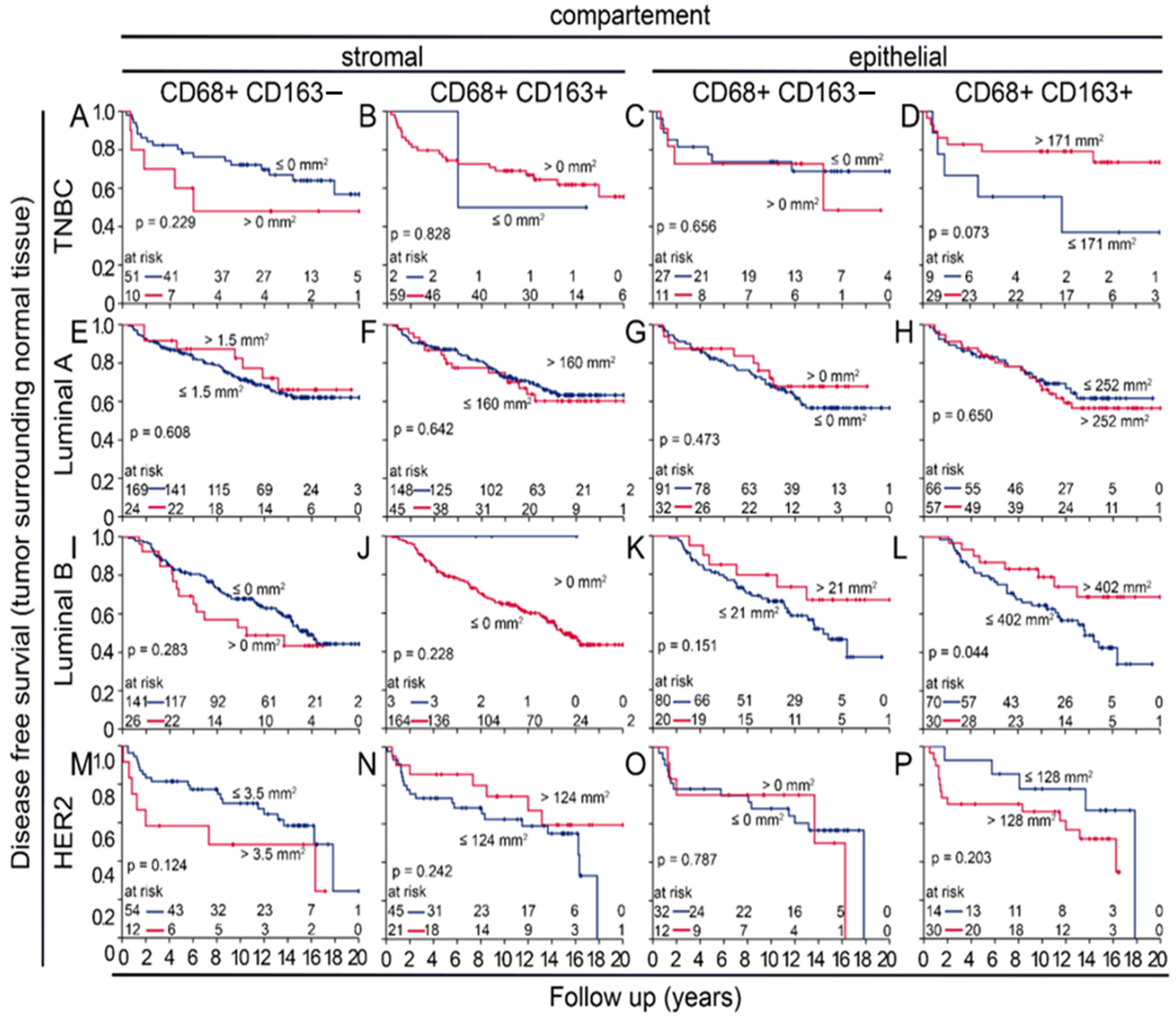
3.6. Prognostic Value of Macrophages in Tumor near and Distant Tissues
3.7. Uni- and Multivariate Analysws of Four-Color Macrophage Staining
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
| HRs | Hormone receptors |
| HER-2 | Human Epidermal Growth Factor Receptor 2 |
| PR | Progesterone Receptor |
| TILs | Tumor-Infiltrating lymphocytes |
| TME | Tumor Microenvironment |
| TAMs | Tumor associated macrophages |
| APBI | Accelerated Partial Breast Irradiation |
| PDR | Pulse-dose-rate |
| HDR | High-dose-rate |
| DFS | Disease-free survival |
| BBCC | Bavarian Breast Cancer Cases and Controls |
| TMA | Tissue Microarray |
| MPO | Myeloperoxidase |
| DAPI | 4′6-diamidino-2-phenylindole |
| TIICs | Tumor-infiltrating inflammatory cells |
| ANOVA | One-way analysis of variance |
| IQR | Interquartile Range |
| Str | Stromal compartment |
| Epi | Epithelial compartment |
| 4C | Four-color |
| IBC | Inflammatory Breast cancer |
| TNBC | Triple-Negative Breast Cancer |
| HE | Hematoxylin and Eosin |
References
- Benitez Fuentes, J.D.; Morgan, E.; de Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Moqadami, A.; Ghafari, S.; Khalaj-Kondori, M. Non-coding RNAs modulation in breast cancer radioresponse: Mechanisms and therapeutic implications. Strahlenther. Onkol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Van der Vorst, A.; Lambrecht, M.; Van Aelst, L.; Verhoeven, J.; Jacobs, J.; Baten, A.; Weltens, C. Radiation-induced heart disease in breast cancer patients: A narrative review of epidemiology, risk factors, radiotherapy parameters, and prevention. Strahlenther. Onkol. 2025, 201, 368–382. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.S.; Kumar, A.; Vishakha; Behl, T.; Jha, S.K.; Tang, H. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol. Cancer 2023, 22, 105. [Google Scholar] [CrossRef]
- Barzaman, K.; Moradi-Kalbolandi, S.; Hosseinzadeh, A.; Kazemi, M.H.; Khorramdelazad, H.; Safari, E.; Farahmand, L. Breast cancer immunotherapy: Current and novel approaches. Int. Immunopharmacol. 2021, 98, 107886. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Becherini, C.; Caini, S.; Coles, C.E.; Cortes, J.; Curigliano, G.; de Azambuja, E.; Isacke, C.M.; Harbeck, N.; Kaidar-Person, O.; et al. International multidisciplinary consensus on the integration of radiotherapy with new systemic treatments for breast cancer: European Society for Radiotherapy and Oncology (ESTRO)-endorsed recommendations. Lancet Oncol. 2024, 25, e73–e83. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Danforth, D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef]
- Schnellhardt, S.; Erber, R.; Büttner-Herold, M.; Rosahl, M.C.; Ott, O.J.; Strnad, V.; Beckmann, M.W.; King, L.; Hartmann, A.; Fietkau, R.; et al. Tumour-Infiltrating Inflammatory Cells in Early Breast Cancer: An Underrated Prognostic and Predictive Factor? Int. J. Mol. Sci. 2020, 21, 8238. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Weihbrecht, S.; Haeberle, L.; Gasparyan, A.; Villalobos, I.E.; Ma, Y.; Ekici, A.B.; Wachter, D.L.; Hartmann, A.; Beckmann, M.W.; et al. HER2 and TOP2A amplification in a hospital-based cohort of breast cancer patients: Associations with patient and tumor characteristics. Breast Cancer Res. Treat. 2014, 145, 193–203. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; Gao, Y.; Jones, A.; Ruebner, M.; Beckmann, M.W.; Wachter, D.L.; Fasching, P.A.; Widschwendter, M. DNA methylation outliers in normal breast tissue identify field defects that are enriched in cancer. Nat. Commun. 2016, 7, 10478. [Google Scholar] [CrossRef]
- Schnellhardt, S.; Hirneth, J.; Büttner-Herold, M.; Daniel, C.; Haderlein, M.; Hartmann, A.; Fietkau, R.; Distel, L. The Prognostic Value of FoxP3+ Tumour-Infiltrating Lymphocytes in Rectal Cancer Depends on Immune Phenotypes Defined by CD8+ Cytotoxic T Cell Density. Front. Immunol. 2022, 13, 781222. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Stavrou, M.; Constantinidou, A. Tumor associated macrophages in breast cancer progression: Implications and clinical relevance. Front. Immunol. 2024, 15, 1441820. [Google Scholar] [CrossRef]
- Dai, Q.; Wu, W.; Amei, A.; Yan, X.; Lu, L.; Wang, Z. Regulation and characterization of tumor-infiltrating immune cells in breast cancer. Int. Immunopharmacol. 2021, 90, 107167. [Google Scholar] [CrossRef]
- Ni, C.; Yang, L.; Xu, Q.; Yuan, H.; Wang, W.; Xia, W.; Gong, D.; Zhang, W.; Yu, K. CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: A retrospective study and meta-analysis. J. Cancer 2019, 10, 4463–4472. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Z.; Li, B.; Li, J.; Ou, Y.; Yu, X.; Zhang, Z.; Liu, S.; Fu, X.; Jin, H.; et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology 2022, 11, 2085432. [Google Scholar] [CrossRef]
- Schnellhardt, S.; Erber, R.; Büttner-Herold, M.; Rosahl, M.C.; Ott, O.J.; Strnad, V.; Beckmann, M.W.; King, L.; Hartmann, A.; Fietkau, R.; et al. Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer. Cancers 2020, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.P.; Atkinson, R.L.; Larson, R.; Burks, J.K.; Smith, D.; Debeb, B.G.; Ruffell, B.; Creighton, C.J.; Bambhroliya, A.; Reuben, J.M.; et al. Mammary stem cell and macrophage markers are enriched in normal tissue adjacent to inflammatory breast cancer. Breast Cancer Res. Treat. 2018, 171, 283–293. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Echarti, A.; Hecht, M.; Büttner-Herold, M.; Haderlein, M.; Hartmann, A.; Fietkau, R.; Distel, L. CD8+ and Regulatory T cells Differentiate Tumor Immune Phenotypes and Predict Survival in Locally Advanced Head and Neck Cancer. Cancers 2019, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Gurski, C.J.; Dittel, B.N. Myeloperoxidase as a Marker to Differentiate Mouse Monocyte/Macrophage Subsets. Int. J. Mol. Sci. 2022, 23, 8246. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Barlow, W.E.; Reynolds, W.; Livingston, R.B.; Yeh, I.T.; Choi, J.Y.; Davis, W.; Rae, J.M.; Tang, L.; Hutchins, L.R.; et al. Myeloperoxidase genotypes and enhanced efficacy of chemotherapy for early-stage breast cancer in SWOG-8897. J. Clin. Oncol. 2009, 27, 4973–4979. [Google Scholar] [CrossRef]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef]
- Bobrie, A.; Massol, O.; Ramos, J.; Mollevi, C.; Lopez-Crapez, E.; Bonnefoy, N.; Boissière-Michot, F.; Jacot, W. Association of CD206 Protein Expression with Immune Infiltration and Prognosis in Patients with Triple-Negative Breast Cancer. Cancers 2022, 14, 4829. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, I.; Kang, S.H.; Shin, H.C.; Kwon, S.Y. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J. Breast Cancer 2019, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Allison, E.; Edirimanne, S.; Matthews, J.; Fuller, S.J. Breast Cancer Survival Outcomes and Tumor-Associated Macrophage Markers: A Systematic Review and Meta-Analysis. Oncol. Ther. 2023, 11, 27–48. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Samoilova, D.V.; Shitova, M.S.; Gratchev, A. Tumor Associated Macrophages in Kidney Cancer. Anal. Cell. Pathol. 2016, 2016, 9307549. [Google Scholar] [CrossRef]
- Chohan, M.H.; Perry, M.; Laurance-Young, P.; Salih, V.M.; Foey, A.D. Prognostic Role of CD68+ and CD163+ Tumour-Associated Macrophages and PD-L1 Expression in Oral Squamous Cell Carcinoma: A Meta-Analysis. Br. J. Biomed. Sci. 2023, 80, 11065. [Google Scholar] [CrossRef]
- Masuda, S.; Nakazawa, D.; Shida, H.; Miyoshi, A.; Kusunoki, Y.; Tomaru, U.; Ishizu, A. NETosis markers: Quest for specific, objective, and quantitative markers. Clin. Chim. Acta 2016, 459, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Bisheshar, S.K.; van der Kamp, M.F.; de Ruiter, E.J.; Ruiter, L.N.; van der Vegt, B.; Breimer, G.E.; Willems, S.M. The prognostic role of tumor associated macrophages in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oral. Oncol. 2022, 135, 106227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, S. Tumor-Associated CD204-Positive Macrophage Is a Prognostic Marker in Clinical Stage I Lung Adenocarcinoma. Biomed. Res. Int. 2018, 2018, 8459193. [Google Scholar] [CrossRef] [PubMed]
- Yanai, Y.; Kosaka, T.; Mikami, S.; Hongo, H.; Yasumizu, Y.; Takeda, T.; Matsumoto, K.; Miyauchi, J.; Kitano, S.; Oya, M. CD8-positive T cells and CD204-positive M2-like macrophages predict postoperative prognosis of very high-risk prostate cancer. Sci. Rep. 2021, 11, 22495. [Google Scholar] [CrossRef]





| Variables | APBI Cohort (n = 129) | BBCC Cohort (n = 501) |
|---|---|---|
| Age (years) | 59.1; <50: 25 (19.4%); >50: 104 (80.6%); | 58.1; <50: 136 (27.1%); >50: 365 (72.9%); |
| T category | pT1mic: 6 (4.7%); pT1a: 8 (6.2%); pT1b: 31 (24%); pT1c: 75 (58.1%); pT2: 9 (7%); | pT1: 253 (50.5%); pT2: 159 (31.7%); pT3: 18 (3.6%); pT4: 33 (6.6%); n.a.: 38 (7.6%); |
| N category | N0: 126 (97.7%); N1: 3 (2.3%); | pN0: 360 (71.9%); pN1: 117 (23.4%); n.a.: 24 (4.8%); |
| M category | M0: 129 (100%); M1: 0 (0%); | M0: 479 (95.6%); M1: 22 (4.4%); |
| Histological typing | ductal: 91 (70.5%); lobular: 19 (14.7%); other: 19 (14.7%); | ductal: 266 (53.1%); lobular: 72 (14.4%); other: 92 (18.4%); n.a.: 71 (14.2%); |
| Molecular class | luminal A: 85 (65.9%); luminal B: 33 (25.6%); Her2: 6 (4.7%); n.a.: 5 (3.9%); | TNBC: 36 (7.2%); luminal A: 113 (22.6%); luminal B: 109 (21.8%); HER2: 66 (13.2%); n.a. (35.3%); |
| Histological grading | G1: 34 (26.4%); G2: 90 (69.8%); G3: 3 (2.3%); n.a.: 2 (1.6%); | G1: 47 (9.4%); G2: 325 (64.9%); G3: 119 (23.8%); n.a.: 10 (2%); |
| Estrogen receptor status | positive: 125 (96.9%); negative: 1 (0.8%); n.a.: 3 (2.3%); | positive: 53 (10.6%); negative:1: 200 (39.9%); n.a.: 248 (49.5%); |
| Progesterone receptor status | positive: 118 (91.5%); negative: 9 (7%); n.a.: 2 (1.6%); | positive: 71 (14.2%); negative:1: 181 (36.1%); n.a.: 249 (49.7%); |
| Her2 status | positive: 7 (5.4%); negative: 119 (92.2%); n.a.: 3 (2.3%); | positive: 209 (41.7%); negative: 94 (18.8%); n.a.: 198 (39.5%); |
| Ki67 | <20%: 102 (79.1%); >20%: 24 (18.6%); n.a.: 3 (2.3%); | <20%: 166 (33.1%); >20%:1: 152 (30.3%); 2: 183 (36.5%); |
| ABPI | BBCC | ||
|---|---|---|---|
| Risk | Near | Distant | |
| low risk | 1000 | ||
| 1001 | 1001 | ||
| 1101 | 1101 | ||
| 1110 | |||
| 1100 | |||
| 0010 | |||
| 0110 | 0110 | ||
| 0111 | |||
| 0101 | |||
| intermediary risk | 0000 | 0000 | |
| 1100 | 1100 | ||
| 0100 | 0100 | ||
| 1111 | 1111 | ||
| 0101 | 0101 | ||
| 1110 | |||
| 1010 | |||
| 0010 | |||
| 0011 | |||
| high risk | 0000 | ||
| 0001 | 0001 | 0001 | |
| 0111 | |||
| 0100 | |||
| 1000 | |||
| 1001 | |||
| 1101 | |||
| 1111 | |||
| early risk | 1110 | ||
| BREAST CANCER | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% Confidence Interval | p-Value | Hazard Ratio | 95% Confidence Interval | p-Value |
| Grade G1 [n = 23] v. G2/3 [n = 64] | 2.985 | 0.368–24.193 | 0.306 | 3.333 | 0.424–26.179 | 0.252 |
| Chemotherapy yes [n = 83] v. no [n = 4] | 11.374 | 1.193–108.472 | 0.035 | 12.153 | 2.937–50.294 | 0.001 |
| Ki67 ≤ 20% [n = 67] v. >20 [n = 20] | 1.983 | 0.58–6.779 | 0.275 | 1.930 | 0.583–6.394 | 0.282 |
| Stromal CD68+CD206+ ≤ 17 [n = 24] v. >17 cells/mm2 [n = 63] | 0.121 | 0.034–0.428 | 0.001 | 0.137 | 0.044–0.426 | 0.001 |
| Age ≤ 49 y [n = 15] v. >49 y [n = 72] | 1.469 | 0.24–8.992 | 0.678 | --- | --- | --- |
| Lobular histology [n = 74] v. other [n = 13] | 1.018 | 0.189–5.477 | 0.983 | --- | --- | --- |
| Hormone therapy: yes [n = 5] v. no [n = 82] | 0.337 | 0.039–2.912 | 0.323 | 0.322 | 0.038–2.708 | 0.297 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirschmann, M.; Schnellhardt, S.; Rübner, M.; Segelhorst, S.; Ott, O.; Erber, R.; Daniel, C.; Büttner-Herold, M.; Gass, P.; Fietkau, R.; et al. Prognostic Significance of Macrophage Phenotypes in Peri-Tumoral Normal Tissue of Early-Stage Breast Cancer. Cells 2025, 14, 828. https://doi.org/10.3390/cells14110828
Hirschmann M, Schnellhardt S, Rübner M, Segelhorst S, Ott O, Erber R, Daniel C, Büttner-Herold M, Gass P, Fietkau R, et al. Prognostic Significance of Macrophage Phenotypes in Peri-Tumoral Normal Tissue of Early-Stage Breast Cancer. Cells. 2025; 14(11):828. https://doi.org/10.3390/cells14110828
Chicago/Turabian StyleHirschmann, Marcel, Sören Schnellhardt, Matthias Rübner, Sarah Segelhorst, Oliver Ott, Ramona Erber, Christoph Daniel, Maike Büttner-Herold, Paul Gass, Rainer Fietkau, and et al. 2025. "Prognostic Significance of Macrophage Phenotypes in Peri-Tumoral Normal Tissue of Early-Stage Breast Cancer" Cells 14, no. 11: 828. https://doi.org/10.3390/cells14110828
APA StyleHirschmann, M., Schnellhardt, S., Rübner, M., Segelhorst, S., Ott, O., Erber, R., Daniel, C., Büttner-Herold, M., Gass, P., Fietkau, R., & Distel, L. (2025). Prognostic Significance of Macrophage Phenotypes in Peri-Tumoral Normal Tissue of Early-Stage Breast Cancer. Cells, 14(11), 828. https://doi.org/10.3390/cells14110828









