Air-Exposure- and Reoxygenation-Stimulated Expressions of Caspase-3 and Induction of Apoptosis in the Central Nervous System of the Crab Erimacrus isenbeckii
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Procedures
2.3. Tissue Collection for Immunohistochemistry
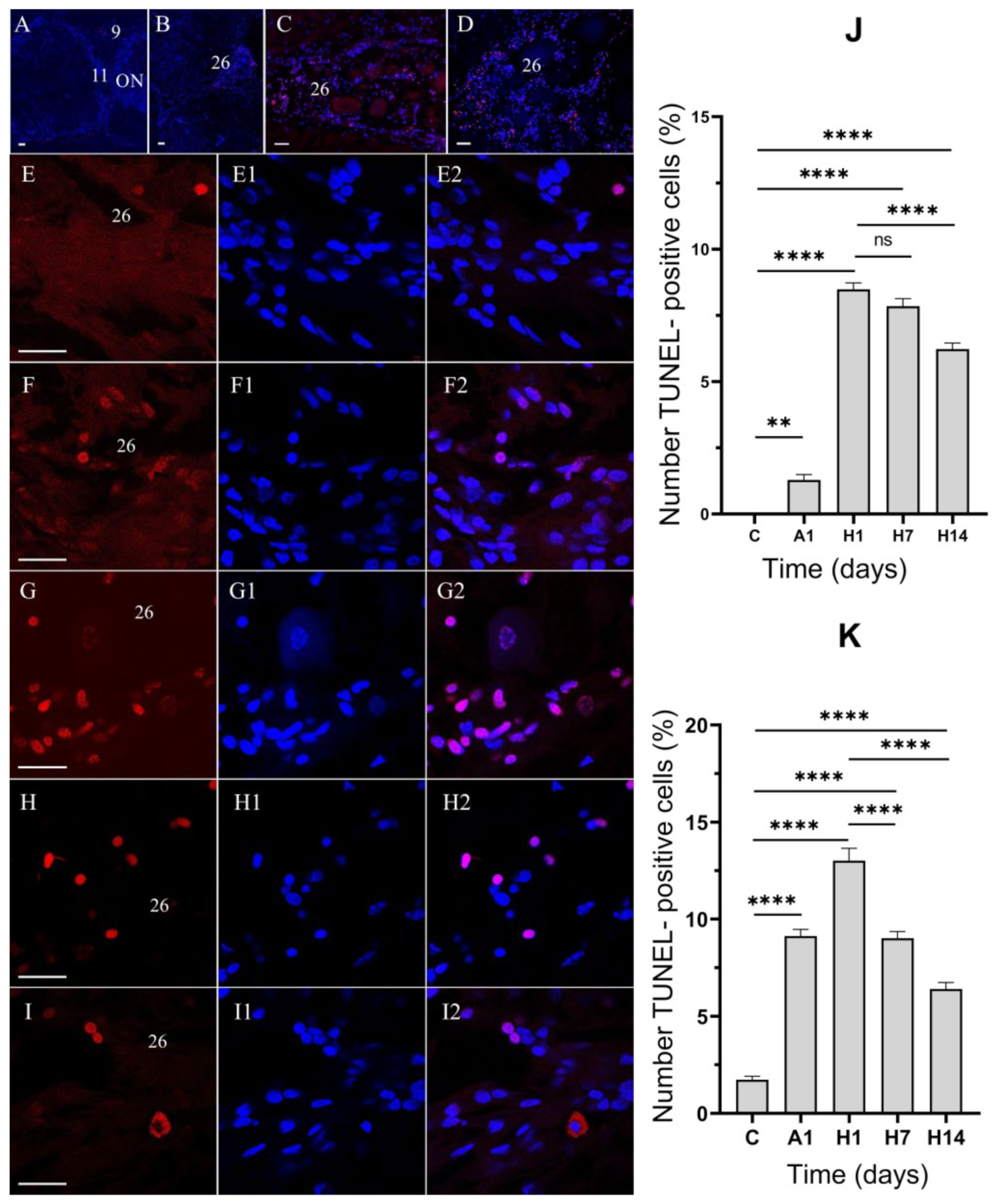
2.4. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL) Staining
2.5. Microscopy and Imaging
2.6. Quantification and Statistical Analysis
2.7. Neuroanatomical Nomenclature
3. Results
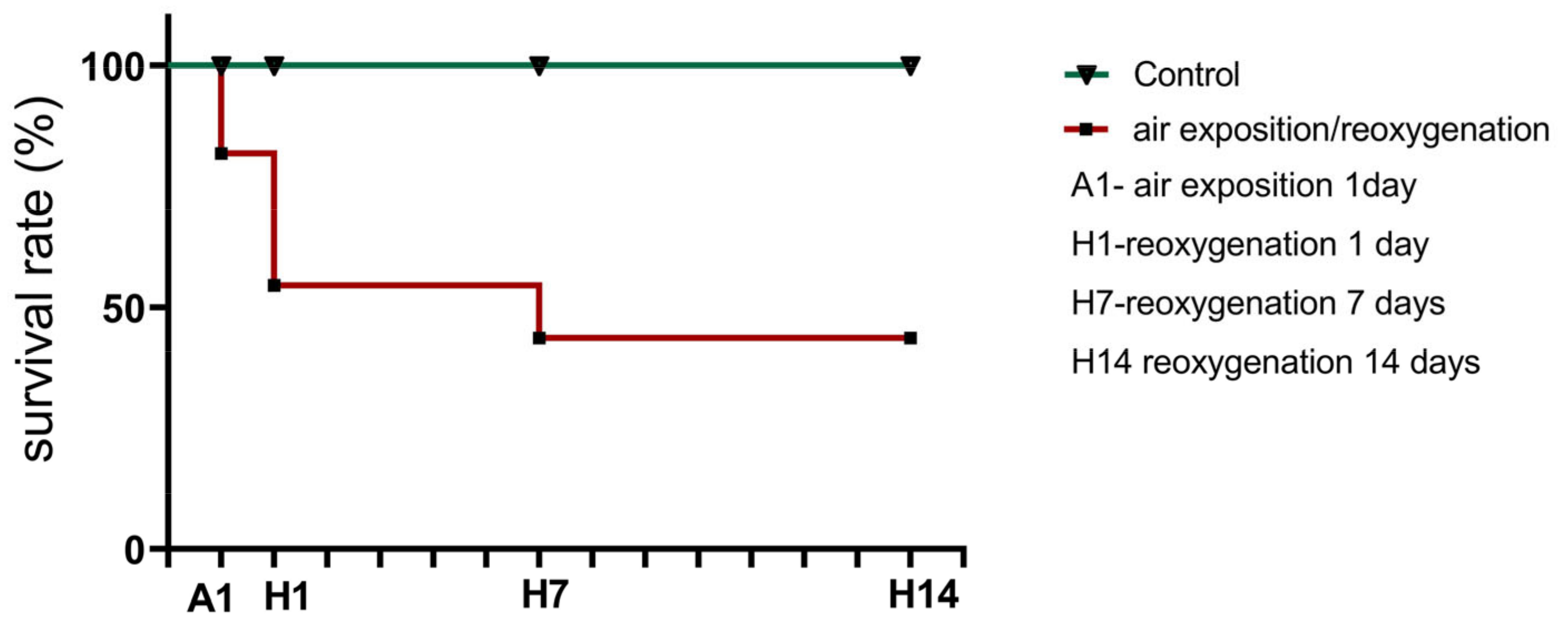
3.1. Effects of Air Exposure and Reoxygenation on Survival Rate
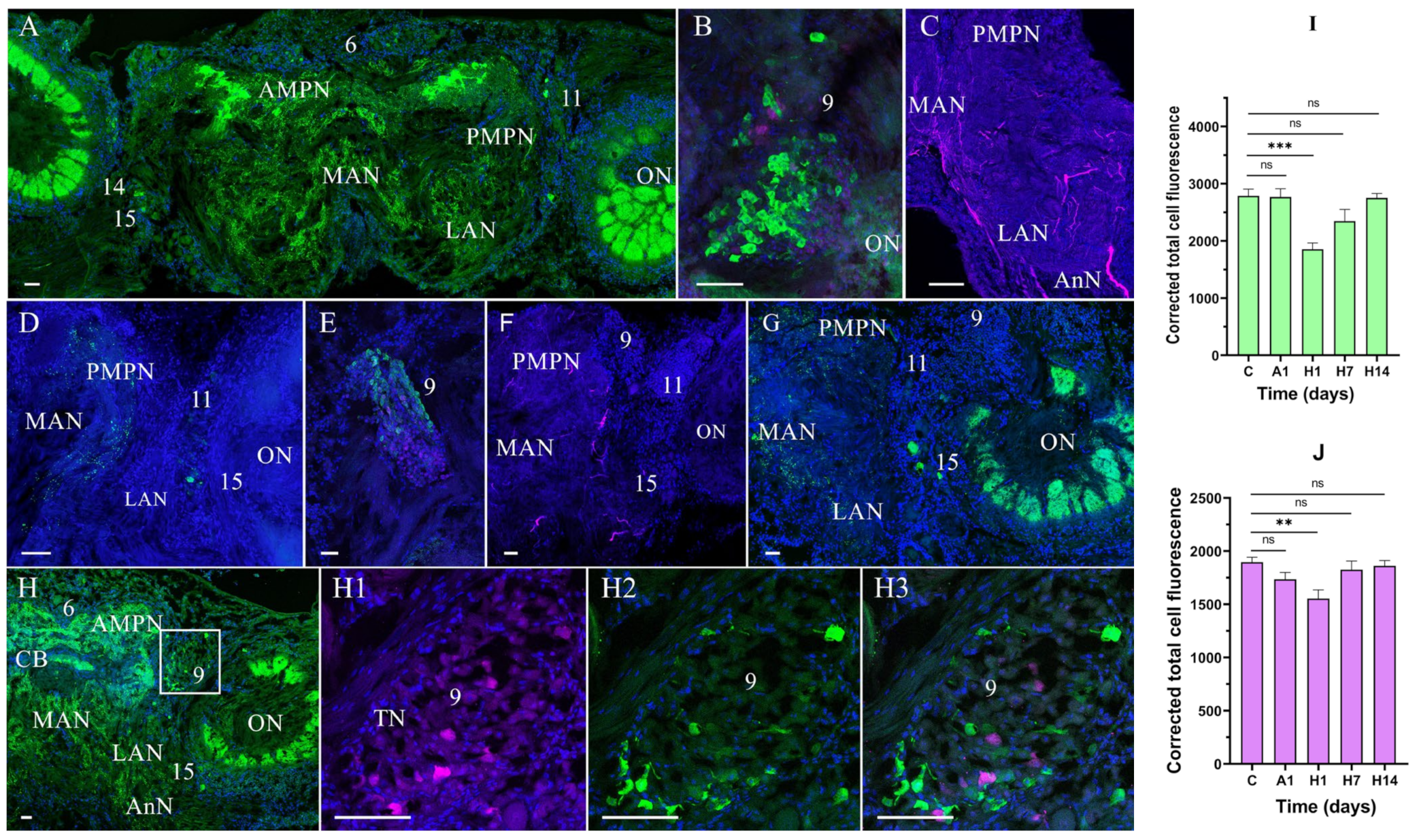
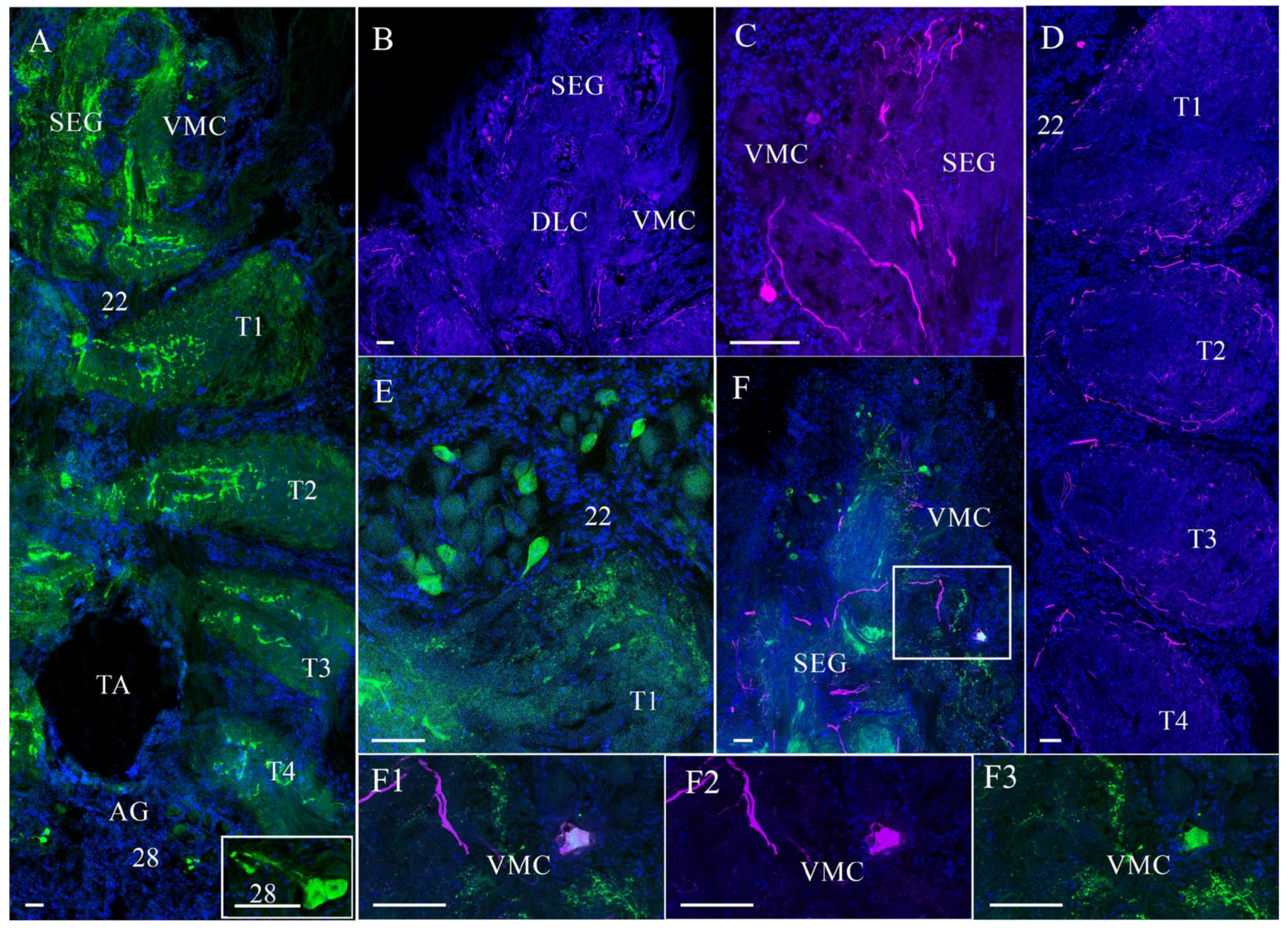
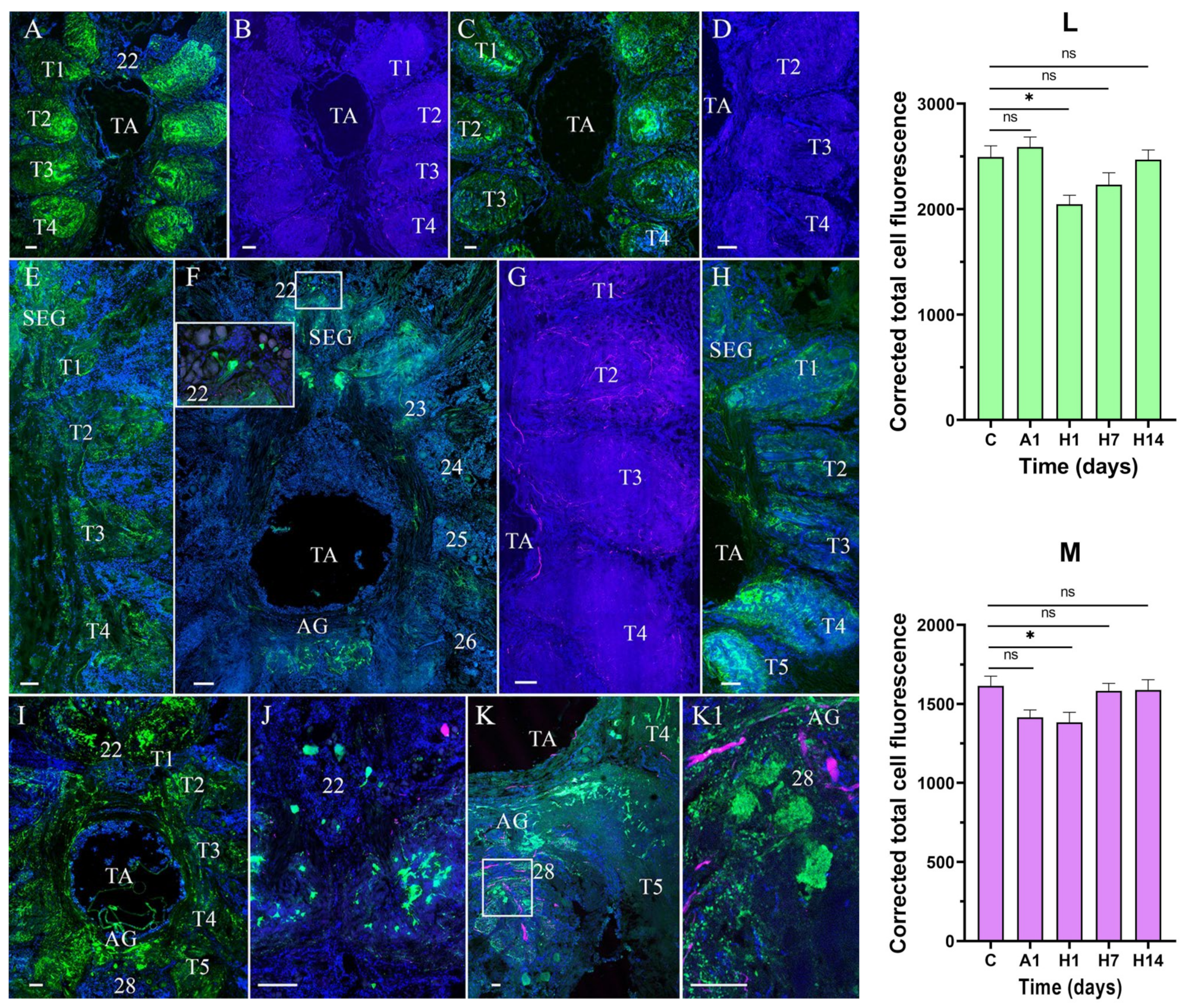
3.2. Effects of Air Exposure and Reoxygenation on Tyrosine Hydroxylase-like and Neuropeptide Y-like Immunoreactivity
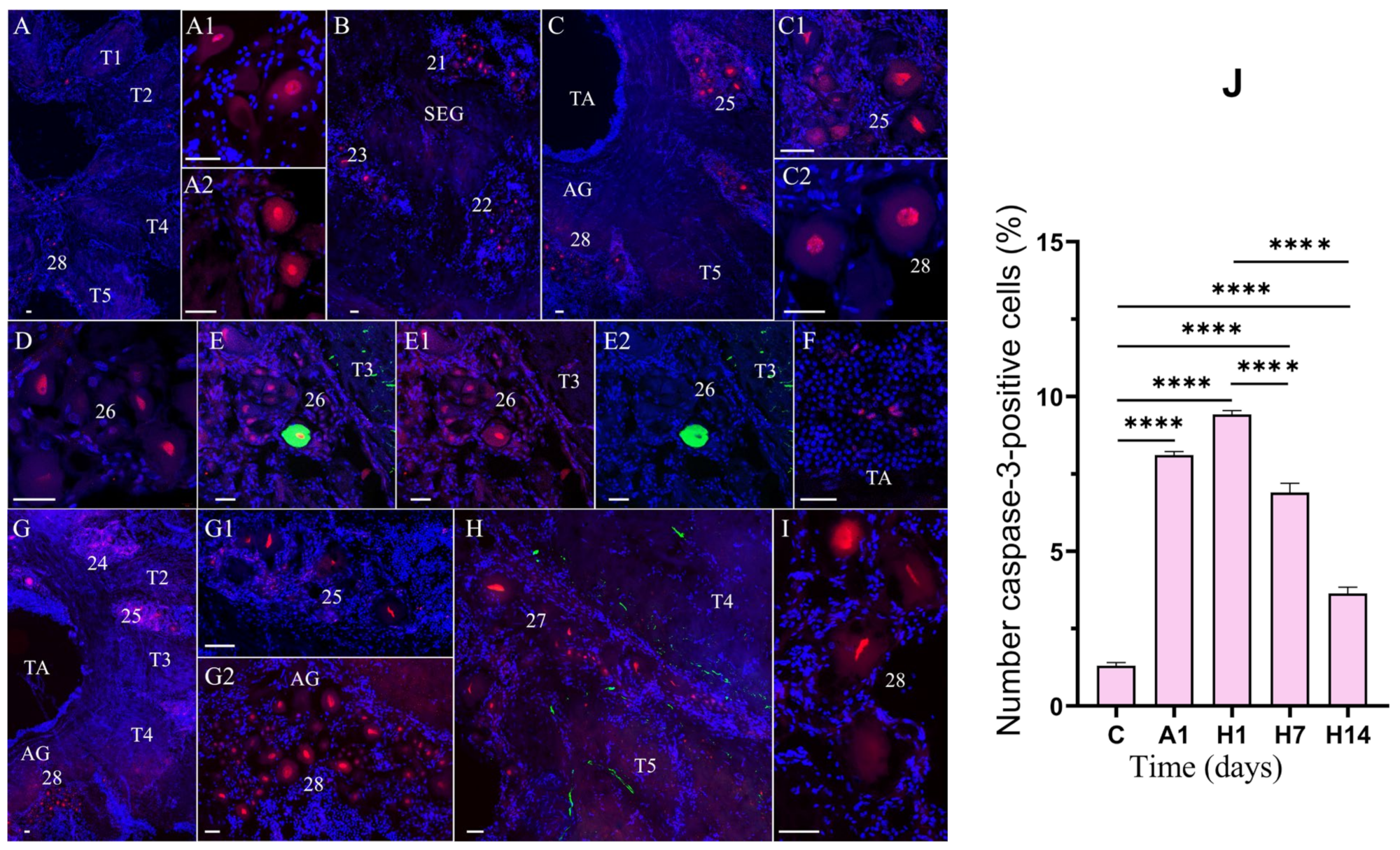
3.3. Effects of Air Exposure and Reoxygenation on Cell Death
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mota, V.C.; Siikavuopio, S.I.; James, P. Physiological responses to live air transport of red king crab (Paralithodes camtschaticus). Fish. Res. 2021, 237, 105882. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.H.; Kuo, C.M. Effects of dissolved oxygen on hemolymph parameters of freshwater giant prawn, Macrobrachium rosenbergii (de Man). Aquaculture 2003, 220, 843–856. [Google Scholar] [CrossRef]
- Romero, M.C.; Ansaldo, M.; Lovrich, G.A. Effect of aerial exposure on the antioxidant status in the subantarctic stone crab Paralomis granulosa (Decapoda: Anomura). Compar. Biochem. Physiol. Part C 2007, 146, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef]
- Linh, N.V.; Khongcharoen, N.; Nguyen, D.H.; Dien, L.T.; Rungrueng, N.; Jhunkeaw, C.; Sangpo, P.; Senapin, S.; Uttarotai, T.; Panphut, W.; et al. Effects of hyperoxia during oxygen nanobubble treatment on innate immunity, growth performance, gill histology, and gut microbiome in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2023, 143, 109191. [Google Scholar] [CrossRef]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef]
- Cheng, C.H.; Ma, H.L.; Deng, Y.Q.; Feng, J.; Chen, X.L.; Guo, Z.X. Transcriptome analysis and histopathology of the mud crab (Scylla paramamosain) after air exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 228, 108652. [Google Scholar] [CrossRef]
- Kwak, D.J.; Kwak, S.D.; Gauda, E.B. The effect of hyperoxia on reactive oxygen species (ROS) in rat petrosal ganglion neurons during development using organotypic slices. Pediatr. Res. 2006, 60, 371–376. [Google Scholar] [CrossRef]
- Honma, H.; Gross, L.; Windebank, A.J. Hypoxia-induced apoptosis of dorsal root ganglion neurons is associated with DNA damage recognition and cell cycle disruption in rats. Neurosci. Lett. 2004, 354, 95–98. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Su, Y.; Liu, Z.; Mao, Y. Air Exposure Affects Physiological Responses, Innate Immunity, Apoptosis and DNA Methylation of Kuruma Shrimp, Marsupenaeus japonicus. Front. Physiol. 2020, 11, 223. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Qian, X.; Ji, J.; Ning, X.; Zhu, F.; Yin, S.; Zhang, K. Effects of hypoxia and reoxygenation on apoptosis, oxidative stress, immune response and gut microbiota of Chinese mitten crab, Eriocheir sinensis. Aquat. Toxicol. 2023, 260, 106556. [Google Scholar] [CrossRef] [PubMed]
- Menze, M.A.; Fortner, G.; Nag, S.; Hand, S.C. Mechanisms of apoptosis in Crustacea: What conditions induce versus suppress cell death? Apoptosis 2010, 15, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.E.; Fitzgerald, P.; Tait, S.W.G.; Llambi, F.; McStay, G.P.; Tupper, D.O.; Pellettieri, J.; Alvarado, A.S.; Salvesen, G.S.; Green, D.R. Mitochondrial pathway of apoptosis is ancestral in metazoans. Proc. Natl. Acad. Sci. USA 2012, 109, 4904–4909. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lv, C.; Hu, M.; Zhong, G. The Mitochondria-Mediate Apoptosis of Lepidopteran Cells Induced by Azadirachtin. PLoS ONE 2013, 8, e58499. [Google Scholar] [CrossRef]
- Arama, E.; Bader, M.; Srivastava, M.; Bergmann, A.; Steller, H. The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. EMBO J. 2006, 25, 232–243. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Taatjes, D.J.; Sobel, B.E.; Budd, R.C. Morphological and cytochemical determination of cell death by apoptosis. Histochem. Cell Biol. 2008, 129, 33–43. [Google Scholar] [CrossRef]
- Fischer, U.; Jänicke, R.; Schulze-Osthoff, K. Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 2003, 10, 76–100. [Google Scholar] [CrossRef]
- Phongdara, A.; Wanna, W.; Chotigeat, W. Molecular cloning and expression of caspase from white shrimp Penaeus merguiensis. Aquaculture 2006, 252, 114–120. [Google Scholar] [CrossRef]
- Leu, J.; Wang, H.; Kou, G.; Lo, C. Penaeus monodon caspase is targeted by a white spot syndrome virus anti-apoptosis protein. Dev. Comp. Immunol. 2008, 32, 476–486. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yang, N.; He, C.; Gao, Y.; Chang, L.-R.; Li, T.; Si, L.-J.; Yan, D.-C. The immune response of Caspase-3 gene in infectious hypodermal and hematopoietic necrosis virus (IHHNV) infection of Litopenaeus vannamei. Aquaculture 2024, 581, 740472. [Google Scholar] [CrossRef]
- Wu, M.H.; Jin, X.K.; Yu, A.Q.; Zhu, Y.T.; Li, D.; Li, W.W.; Wang, Q. Caspase-mediated apoptosis in crustaceans: Cloning and functional characterization of EsCaspase-3-like protein from Eriocheir sinensis. Fish Shellfish Immunol. 2014, 41, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Liu, X.Z.; Ma, H.L.; Liu, G.X.; Deng, Y.Q.; Feng, J.; Jie, Y.K.; Guo, Z.X. The role of caspase 3 in the mud crab (Scylla paramamosain) after Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2021, 118, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhi, B.; Wu, W.; Zhang, X. Requirement for shrimp caspase in apoptosis against virus infection. Dev. Comp. Immunol. 2008, 32, 706–715. [Google Scholar] [CrossRef]
- Kumar, G.K. Hypoxia. 3. Hypoxia and neurotransmitter synthesis. Am. J. Physiol. Cell Physiol. 2011, 300, C743–C751. [Google Scholar] [CrossRef]
- Luo, Y.; Umegaki, H.; Wang, X.; Abe, R.; Roth, G.S. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J. Biol. Chem. 1998, 273, 3756–3764. [Google Scholar] [CrossRef]
- Christie, A.E.; Stemmler, E.A.; Dickinson, P.S. Crustacean neuropeptides. Cell Mol. Life Sci. 2010, 67, 4135–4169. [Google Scholar] [CrossRef]
- Christie, A.E.; Chapline, M.C.; Jackson, J.M.; Dowda, J.K.; Hartline, N.; Malecha, S.R.; Lenz, P.H. Identification, tissue distribution and orexigenic activity of neuropeptide F (NPF) in penaeid shrimp. J. Exp. Biol. 2011, 214, 1386–1396. [Google Scholar] [CrossRef]
- Thongrod, S.; Changklungmoa, N.; Chansela, P.; Siangcham, T.; Kruangkum, T.; Suwansa-Ard, S.; Saetan, J.; Sroyraya, M.; Tinikul, Y.; Wanichanon, C.; et al. Characterization and tissue distribution of neuropeptide F in the eyestalk and brain of the male giant freshwater prawn, Macrobrachium rosenbergii. Cell Tissue Res. 2017, 367, 181–195. [Google Scholar] [CrossRef]
- Thongrod, S.; Wanichanon, C.; Sobhon, P. Distribution of neuropeptide F in the ventral nerve cord and its possible role on testicular development and germ cell proliferation in the giant freshwater prawn, Macrobrachium rosenbergii. Cell Tissue Res. 2019, 376, 471–484. [Google Scholar] [CrossRef]
- Aparicio-Simón, B.; Piñón, M.; Racotta, R.; Racotta, I.S. Neuroendocrine and metabolic responses of Pacific whiteleg shrimp Litopenaeus vannamei exposed to acute handling stress. Aquaculture 2010, 298, 308–314. [Google Scholar] [CrossRef]
- Liu, H.Y.; Pan, L.Q.; Zheng, D.B. Injection of biogenic amines modulates osmoregulation of Litopenaeus vannamei: Response of hemolymph osmotic pressure, ion concentration and osmolality effectors. Comp. Biochem. Physiol. A 2008, 151, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zatta, P. Dopamine, noradrenaline and serotonin during hypo-osmotic stress of Carcinus maenas. Mar. Biol. 1987, 96, 479–481. [Google Scholar] [CrossRef]
- Mapanao, R.; Cheng, W. Cloning and characterization of tyrosine hydroxylase (TH) from the pacific white leg shrimp Litopenaeus vannamei, and its expression following pathogen challenge and hypothermal stress. Fish Shellfish Immunol. 2016, 56, 506–516. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, L.; Ren, Q.; Wang, L.; Miao, J. Effect of salinity on Regulation mechanism of neuroendocrine-immunoregulatory network in Litopenaeus vannamei. Fish Shellfish. Immunol. 2016, 49, 396–406. [Google Scholar] [CrossRef]
- Hu, F.; Pan, L.; Jing, F. Effects of hypoxia on dopamine concentration and the immune response of White Shrimp (Litopenaeus vannamei). J. Ocean Univ. China 2009, 8, 77–82. [Google Scholar] [CrossRef]
- Aparicio-Simón, B.; Piñón, M.; Racotta, R.; Racotta, I.S. Neuroendocrine and metabolic responses of Pacific whiteleg shrimp Penaeus vannamei exposed to hypoxia stress. Lat. Am. J. Aquat. Res. 2018, 46, 364–376. [Google Scholar] [CrossRef]
- Rajashekhar, K.P.; Wilkens, J.L. Dopamine and nicotine, but not serotonin, modulate the crustacean ventilatory pattern generator. J. Neurobiol. 1992, 23, 680–691. [Google Scholar] [CrossRef]
- Wilkens, J.L.; Kuramoto, T.; McMahon, B.R. The effects of six pericardial hormones and hypoxia on the semi-isolated heart and sternal arterial valve of the lobster Homarus americanus. Comp. Biochem. Physiol. C 1996, 114, 57–65. [Google Scholar] [CrossRef]
- Lüschen, W.; Willig, A.; Jaros, P.P. The role of biogenic amines in the control of blood glucose level in the decapod crustacean, Carcinus maenas (L.). Comp. Biochem. Physiol. C 1993, 105, 291–296. [Google Scholar] [CrossRef]
- Son, J.H.; Gerenza, A.K.; Bingener, G.M.; Bonkowsky, J.L. Hypoplasia of dopaminergic neurons by hypoxia-induced neurotoxicity is associated with disrupted swimming development of larval zebrafish. Front. Cell Neurosci. 2022, 16, 963037. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S.; Stecyk, J.A.W.; Torp, M.-K.; Løvold, L.Y.; Sørensen, C.; Johansen, I.B.; Stensløkken, K.-O.; Couturier, C.S.; Sloman, K.A.; Nilsson, G.E. Re-oxygenation after anoxia induces brain cell death and memory loss in the anoxia-tolerant crucian carp. J. Exp. Biol. 2017, 220, 3883–3895. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Barker, R.A. Neuropeptide Y and its role in CNS disease and repair. Exp. Neurol. 2012, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Thorsell, A. Central neuropeptide Y in anxiety- and stress-related behavior and in ethanol intake. Ann. N. Y. Acad. Sci. 2008, 1148, 136–140. [Google Scholar] [CrossRef]
- Ali, I.; Bhargava, S. Neuropeptide Y in the brain of Euphlyctis cyanophlyctis tadpoles responds to hypoxic stress. Gen. Comp. Endocrinol. 2017, 251, 38–245. [Google Scholar] [CrossRef]
- Poncet, L.; Denoroy, L.; Dalmaz, Y.; Pequignot, J.M.; Jouvet, M. Alteration in central and peripheral substance P- and neuropeptide Y-like immunoreactivity after chronic hypoxia in the rat. Brain Res. 1996, 733, 64–72. [Google Scholar] [CrossRef]
- Duszczyk, M.; Ziembowicz, A.; Gadamski, R.; Wieronska, J.M.; Smialowska, M.; Lazarewicz, J.W. Changes in the NPY immunoreactivity in gerbil hippocampus after hypoxic and ischemic preconditioning. Neuropeptides 2009, 43, 31–39. [Google Scholar] [CrossRef]
- Fadda, M.; Hasakiogullari, I.; Temmerman, L.; Beets, I.; Zels, S.; Schoofs, L. Regulation of feeding and metabolism by neuropeptide F and short neuropeptide F in invertebrates. Front. Endocrinol. 2019, 10, 64. [Google Scholar] [CrossRef]
- Stemmler, E.A.; Bruns, E.A.; Gardner, N.P.; Dickinson, P.S.; Christie, A.E. Mass spectrometric identification of pEGFYSQRYamide: A crustacean peptide hormone possessing a vertebrate neuropeptide Y (NPY)-like carboxy-terminus. Gen. Comp. Endocrinol. 2007, 152, 1–7. [Google Scholar] [CrossRef]
- Schoofs, L.; Danger, J.M.; Jegou, S.; Pelletier, G.; Huybrechts, R.; Vaudry, H.; De Loof, A. NPY-like peptides occur in the nervous system and midgut of the migratory locust, Locusta migratoria and in the brain of the grey fleshfly, Sarcophaga bullata. Peptides 1988, 9, 1027–1036. [Google Scholar] [CrossRef]
- Wu, Q.; Wen, T.; Lee, G.; Park, J.H.; Cai, H.N.; Shen, P. Developmental Control of Foraging and Social Behavior by the Drosophila Neuropeptide Y-like System. Neuron 2003, 39, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, M.; Shen, P.A. G-protein-coupled neuropeptide Y-like receptor suppresses behavioral and sensory response to multiple stressful stimuli in Drosophila. J. Neurosci. 2010, 30, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Pan, L.; Wang, H.; Zhang, X. Ammonia-N Exposure Alters Neurohormone Levels in the Hemolymph and mRNA Abundance of Neurohormone Receptors and Associated Downstream Factors in the Gills of Litopenaeus vannamei. J. Exp. Biol. 2019, 222, jeb200204. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, Y.; Jakovlić, I.; Fu, H.; Ge, X.; Qiao, H.; Zhang, W.; Jin, S. Identification of Neuropeptides from Eyestalk Transcriptome Profiling Analysis of Female Oriental River Prawn (Macrobrachium nipponense) under Hypoxia and Reoxygenation Conditions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 241, 110392. [Google Scholar] [CrossRef]
- Buchberger, A.R.; Sauer, C.S.; Vu, N.Q.; DeLaney, K.; Li, L.J. Temporal Study of the Perturbation of Crustacean Neuropeptides Due to Severe Hypoxia Using 4-Plex Reductive Dimethylation. J. Proteome Res. 2020, 19, 1548–1555. [Google Scholar] [CrossRef]
- Buchberger, A.R.; DeLaney, K.; Liu, Y.; Vu, N.Q.; Helfenbein, K.; Li, L. Mass Spectrometric Profiling of Neuropeptides in Callinectes sapidus during Hypoxia Stress. ACS Chem. Neurosci. 2020, 11, 3097–3106. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Saetan, J.; Senarai, T.; Tamtin, M.; Weerachatyanukul, W.; Chavadej, J.; Hanna, P.J.; Parhar, I.; Sobhon, P.; Sretarugsa, P. Histological organization of the central nervous system and distribution of a gonadotropin-releasing hormone-like peptide in the blue crab, Portunus pelagicus. Cell Tissue Res. 2013, 353, 493–510. [Google Scholar] [CrossRef]
- Junn, E.; Mouradian, M.M. Apoptotic signaling in dopamine-induced cell death: The role of oxidative stress, p38 mitogen-activated protein kinase, cytochrome c and caspases. J. Neurochem. 2001, 78, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Thorsell, A.; Michalkiewicz, M.; Dumont, Y.; Quirion, R.; Caberlotto, L.; Rimondini, R.; Mathe, A.A.; Heilig, M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y over expression. Proc. Natl. Acad. Sci. USA 2000, 97, 12852–12857. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yamada, S.; Watanabe, Y. The Role of Neuropeptide Y in the Nucleus Accumbens. Int. J. Mol. Sci. 2021, 22, 7287. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Jonz, M.G.; Gilmour, K.M. Oxygen sensing and the hypoxic ventilatory response. In Fish Physiology; Richards, J.G., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 27, pp. 193–253. [Google Scholar]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef]
- Machado, R.S.; Tenfen, L.; Joaquim, L.; Lanzzarin, E.V.R.; Bernardes, G.C.; Bonfante, S.R.; Mathias, K.; Biehl, E.; Bagio, É.; Stork, S.S.; et al. Hyperoxia by short-term promotes oxidative damage and mitochondrial dysfunction in rat brain. Respir. Physiol. Neurobiol. 2022, 306, 103963. [Google Scholar] [CrossRef]
- Kerr, L.E.; McGregor, A.L.; Amet, E.; Asada, T.; Spratt, C.; Allsopp, T.E.; Harmar, A.J.; Shen, S.; Carlson, G.; Logan, N.; et al. Mice overexpressing human caspase 3 appear phenotypically normal but exhibit increased apoptosis and larger lesion volumes in response to transient focal cerebral ischaemia. Cell Death Differ. 2004, 11, 1102–1111. [Google Scholar] [CrossRef]
- Li, J.; Dong, L.; Zhu, D.; Zhang, M.; Wang, K.; Chen, F. An effector caspase Sp-caspase first identified in mud crab Scylla paramamosain exhibiting immune response and cell apoptosis. Fish Shellfish Immunol. 2020, 103, 442–453. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Östlund-Nilsson, S. Does size matter for hypoxia tolerance in fish? Biol. Rev. 2008, 83, 173–189. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Hollmann, G.; Linden, R.; Giangrande, A.; Allodi, S. Increased p53 and decreased p21 accompany apoptosis induced by ultraviolet radiation in the nervous system of a crustacean. Aquat. Toxicol. 2016, 173, 1–8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsyuba, E.; Dyachuk, V. Air-Exposure- and Reoxygenation-Stimulated Expressions of Caspase-3 and Induction of Apoptosis in the Central Nervous System of the Crab Erimacrus isenbeckii. Cells 2025, 14, 827. https://doi.org/10.3390/cells14110827
Kotsyuba E, Dyachuk V. Air-Exposure- and Reoxygenation-Stimulated Expressions of Caspase-3 and Induction of Apoptosis in the Central Nervous System of the Crab Erimacrus isenbeckii. Cells. 2025; 14(11):827. https://doi.org/10.3390/cells14110827
Chicago/Turabian StyleKotsyuba, Elena, and Vyacheslav Dyachuk. 2025. "Air-Exposure- and Reoxygenation-Stimulated Expressions of Caspase-3 and Induction of Apoptosis in the Central Nervous System of the Crab Erimacrus isenbeckii" Cells 14, no. 11: 827. https://doi.org/10.3390/cells14110827
APA StyleKotsyuba, E., & Dyachuk, V. (2025). Air-Exposure- and Reoxygenation-Stimulated Expressions of Caspase-3 and Induction of Apoptosis in the Central Nervous System of the Crab Erimacrus isenbeckii. Cells, 14(11), 827. https://doi.org/10.3390/cells14110827




