A Novel Primary Cell Line Model of Localized Prostate Cancer and Radioresistance—A Role for Nicotinamide N-Methyltransferase
Abstract
1. Introduction
2. Materials and Methods
2.1. Derivation of CaB34
2.2. Cell Culture
2.3. STR Analysis
2.4. Oncomine v3 Assay
2.5. Generation of Radioresistant Cells
2.6. Clonogenic Survival
2.7. Proliferation Assay
2.8. Senescence Assay
2.9. Cell Cycle Analysis
2.10. Soft Agar Assay
2.11. Western Blot
2.12. qRT-PCR
2.13. mRNA-Seq
2.14. Proteomic Sample Preparation and Shotgun Proteomics
2.15. siRNA Knockdown
2.16. Mito Stress Assay
2.17. Organoid 3D Droplets
2.18. Organoid Immunofluorescence Imaging
2.19. Statistical Analysis
3. Results
3.1. Cell Line Derivation
3.2. STR Profiling
3.3. Genomic Alterations
3.4. Generation of Radioresistant Cell Line
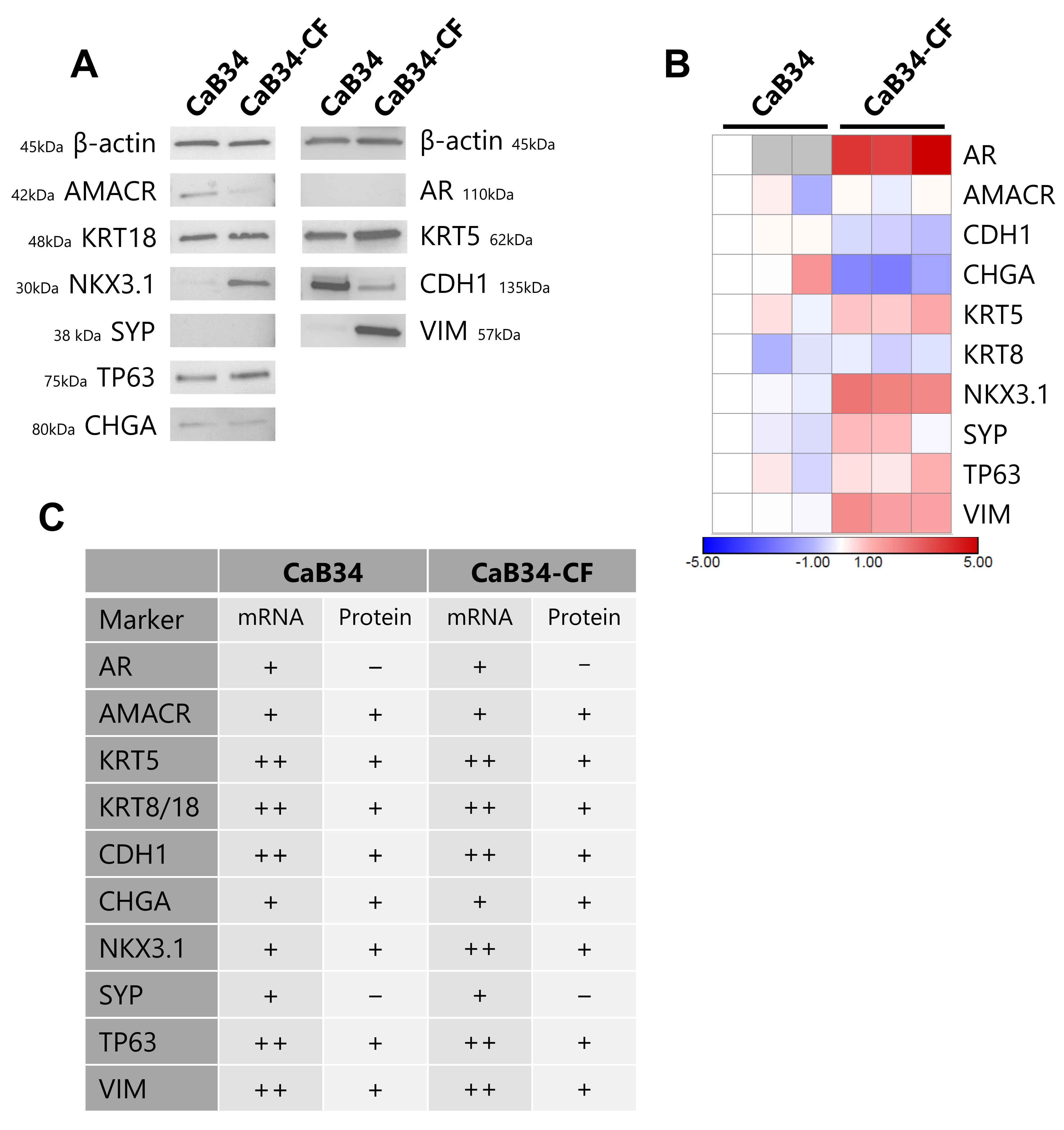
3.5. Molecular Characterization of Cell Lines
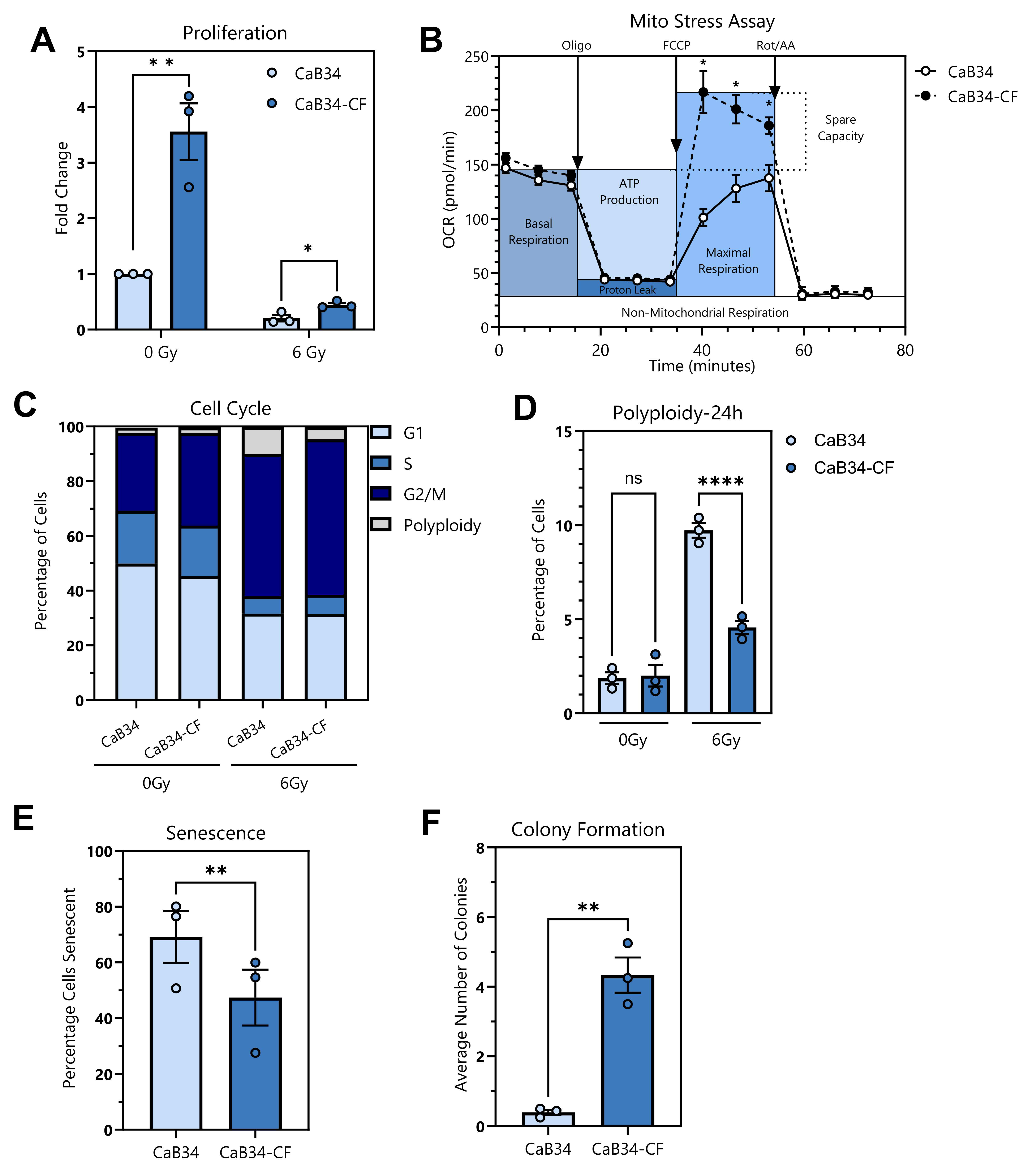
3.6. Phenotypic Characterization of Cell Lines
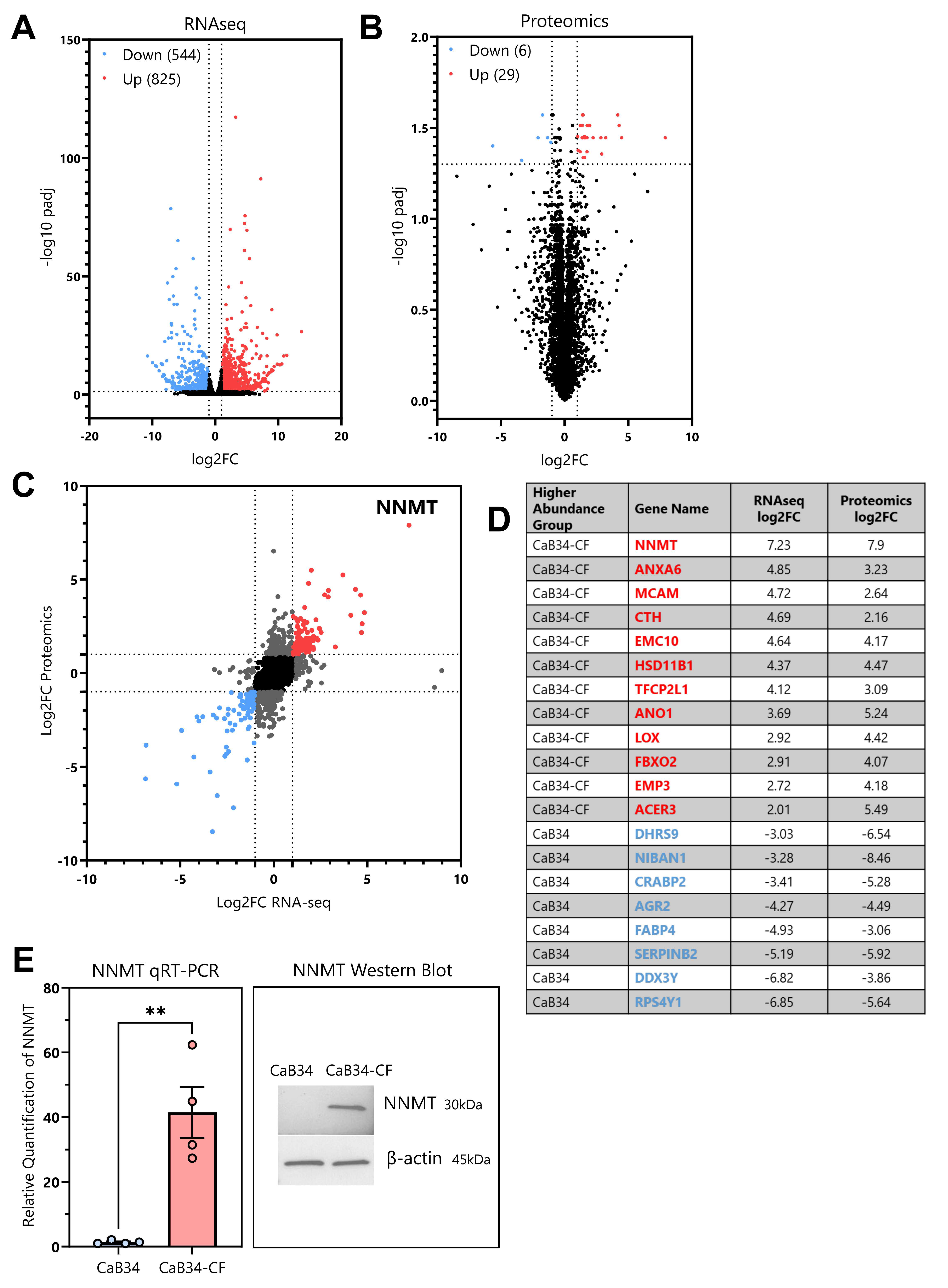
3.7. RNA Seq and Proteomic Analyses
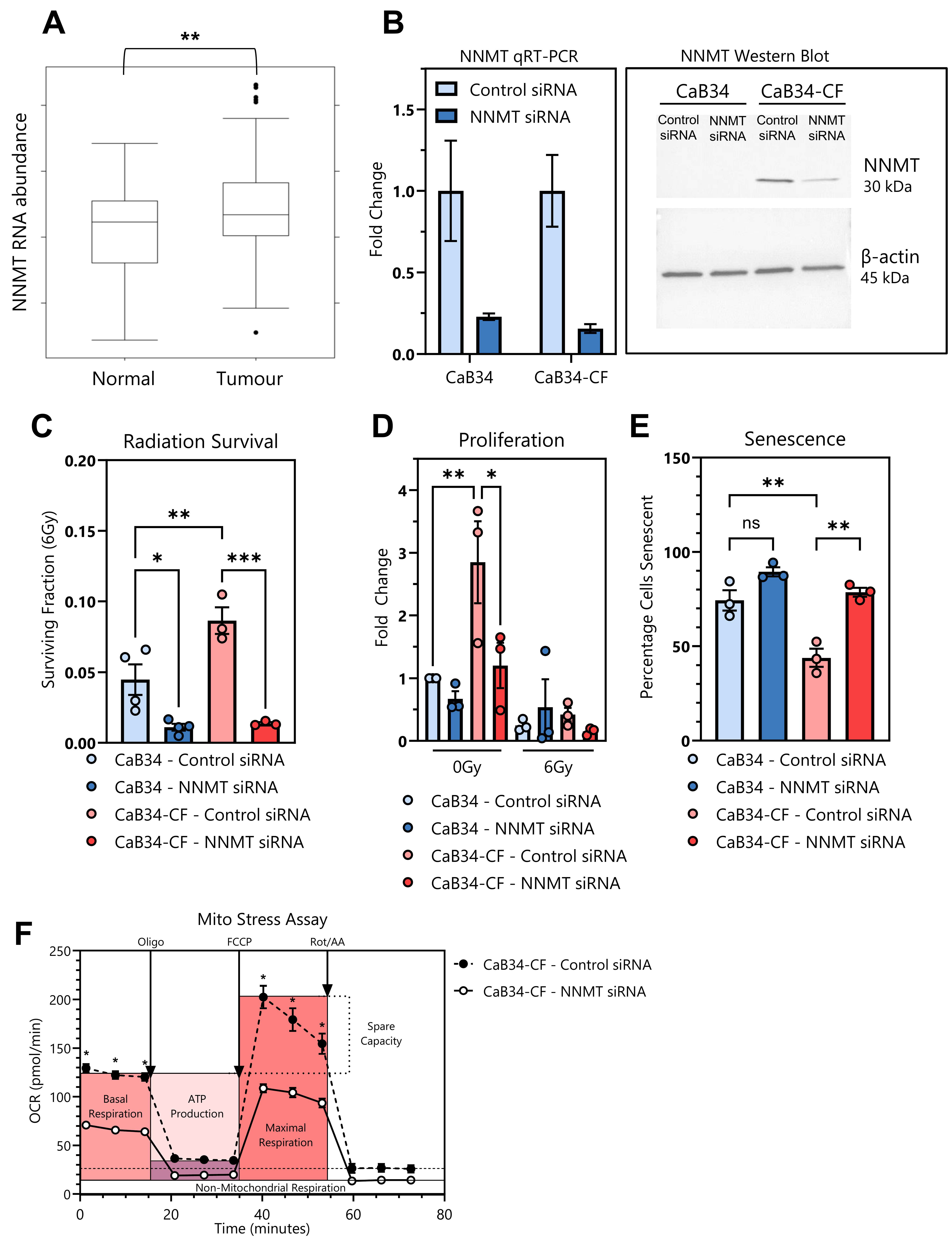
3.8. NNMT
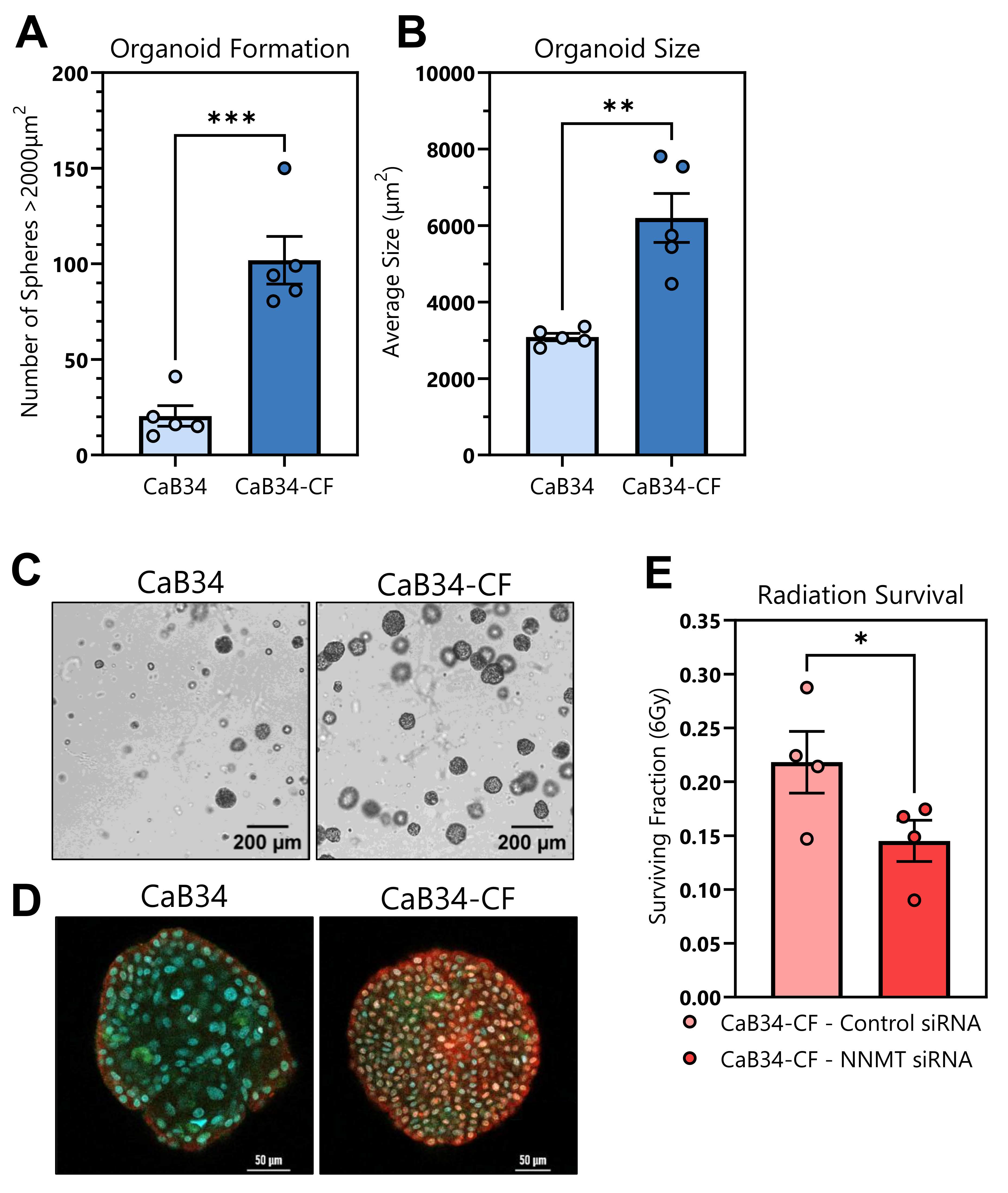
3.9. Organoid Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, Z.S.; Spratt, D.E.; Romesser, P.B.; Pei, X.; Zhang, Z.; Polkinghorn, W.; McBride, S.; Kollmeier, M.; Yamada, Y.; Zelefsky, M.J. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur. Urol. 2015, 67, 1009–1016. [Google Scholar] [CrossRef]
- Kishan, A.U.; Chu, F.I.; King, C.R.; Seiferheld, W.; Spratt, D.E.; Tran, P.; Wang, X.; Pugh, S.E.; Sandler, K.A.; Bolla, M.; et al. Local Failure and Survival After Definitive Radiotherapy for Aggressive Prostate Cancer: An Individual Patient-level Meta-analysis of Six Randomized Trials. Eur. Urol. 2020, 77, 201–208. [Google Scholar] [CrossRef]
- Haas, R.; Frame, G.; Khan, S.; Neilsen, B.K.; Hong, B.H.; Yeo, C.P.X.; Yamaguchi, T.N.; Ong, E.H.W.; Zhao, W.; Carlin, B.; et al. The Proteogenomics of Prostate Cancer Radioresistance. Cancer Res. Commun. 2024, 4, 2463–2479. [Google Scholar] [CrossRef]
- Lu, X.M.; Long, H. Nicotinamide N-methyltransferase as a potential marker for cancer. Neoplasma 2018, 65, 656–663. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Wang, T.; Deng, H. Complex roles of nicotinamide N-methyltransferase in cancer progression. Cell Death Dis. 2022, 13, 267. [Google Scholar] [CrossRef]
- D’Andrea, F.P.; Safwat, A.; Kassem, M.; Gautier, L.; Overgaard, J.; Horsman, M.R. Cancer stem cell overexpression of nicotinamide N-methyltransferase enhances cellular radiation resistance. Radiother. Oncol. 2011, 99, 373–378. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; You, X.; Xu, Y.; Zhang, C.; Li, Y.; Yang, C.; Wang, Q. Inhibition of NNMT enhances drug sensitivity in lung cancer cells through mediation of autophagy. Front. Pharmacol. 2024, 15, 1415310. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Huang, Y.; Li, P.; Yang, M.; Zeng, S.; Chen, D.; Wang, Q.; Liu, H.; Luo, K.; et al. Targeting nicotinamide N-methyltransferase overcomes resistance to EGFR-TKI in non-small cell lung cancer cells. Cell Death Discov. 2022, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, J.; Wu, W.; Xie, S.; Yu, H.; Li, G.; Zhu, T.; Li, F.; Lu, J.; Wang, G.Y.; et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Res. 2019, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ory, V.; Chapman, S.; Yuan, H.; Albanese, C.; Kallakury, B.; Timofeeva, O.A.; Nealon, C.; Dakic, A.; Simic, V.; et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 2012, 180, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- MacDonald, K.M.; Khan, S.; Lin, B.; Hurren, R.; Schimmer, A.D.; Kislinger, T.; Harding, S.M. The proteomic landscape of genotoxic stress-induced micronuclei. Molecular Cell 2024, 84, 1377–1391.e1376. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Lam, W.W.; Oakden, W.; Murray, L.; Klein, J.; Iorio, C.; Screaton, R.A.; Koletar, M.M.; Chu, W.; Liu, S.K.; Stanisz, G.J. Differentiation of Normal and Radioresistant Prostate Cancer Xenografts Using Magnetization Transfer-Prepared MRI. Sci. Rep. 2018, 8, 10447. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef] [PubMed]
- Kurganovs, N.; Wang, H.; Huang, X.; Ignatchenko, V.; Macklin, A.; Khan, S.; Downes, M.R.; Boutros, P.C.; Liu, S.K.; Kislinger, T. A proteomic investigation of isogenic radiation resistant prostate cancer cell lines. Proteom. Clin. Appl. 2021, 15, e2100037. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi Ghiam, A.; Taeb, S.; Huang, X.; Huang, V.; Ray, J.; Scarcello, S.; Hoey, C.; Jahangiri, S.; Fokas, E.; Loblaw, A.; et al. Long non-coding RNA urothelial carcinoma associated 1 (UCA1) mediates radiation response in prostate cancer. Oncotarget 2017, 8, 4668–4689. [Google Scholar] [CrossRef]
- Huang, X.; Taeb, S.; Jahangiri, S.; Emmenegger, U.; Tran, E.; Bruce, J.; Mesci, A.; Korpela, E.; Vesprini, D.; Wong, C.S.; et al. miRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 2013, 73, 6972–6986. [Google Scholar] [CrossRef]
- Mesci, A.; Ahmadi, E.; Ali, A.; Gouran-Savadkoohi, M.; Evelyn Tsakiridis, E.; Biziotis, O.D.; Chow, T.; Kapoor, A.; Sur, M.; Steinberg, G.R.; et al. 18F-DCFPyL (PSMA) PET as a radiotherapy response assessment tool in metastatic prostate cancer. Clin. Transl. Radiat. Oncol. 2023, 39, 100583. [Google Scholar] [CrossRef]
- Ulanovskaya, O.A.; Zuhl, A.M.; Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013, 9, 300–306. [Google Scholar] [CrossRef]
- Li, X.Y.; Pi, Y.N.; Chen, Y.; Zhu, Q.; Xia, B.R. Nicotinamide N-Methyltransferase: A Promising Biomarker and Target for Human Cancer Therapy. Front. Oncol. 2022, 12, 894744. [Google Scholar] [CrossRef]
- Parsons, R.B.; Facey, P.D. Nicotinamide N-Methyltransferase: An Emerging Protagonist in Cancer Macro(r)evolution. Biomolecules 2021, 11, 1418. [Google Scholar] [CrossRef]
- Zhou, W.; Gui, M.; Zhu, M.; Long, Z.; Huang, L.; Zhou, J.; He, L.; Zhong, K. Nicotinamide N-methyltransferase is overexpressed in prostate cancer and correlates with prolonged progression-free and overall survival times. Oncol. Lett. 2014, 8, 1175–1180. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, W.; Zhu, L.; Yao, D.; Wang, C.; Song, Y.; Hu, S.; Liu, H.; Bai, Y.; Pan, Y.; et al. ANXA6 Contributes to Radioresistance by Promoting Autophagy via Inhibiting the PI3K/AKT/mTOR Signaling Pathway in Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2020, 8, 232. [Google Scholar] [CrossRef]
- Liang, Y.; Voshart, D.; Paridaen, J.; Oosterhof, N.; Liang, D.; Thiruvalluvan, A.; Zuhorn, I.S.; den Dunnen, W.F.A.; Zhang, G.; Lin, H.; et al. CD146 increases stemness and aggressiveness in glioblastoma and activates YAP signaling. Cell Mol. Life Sci. 2022, 79, 398. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.Y.; Jung, C.W.; Lee, Y.S.; An, J.Y.; Kim, E.H.; Kim, K.H.; Lee, S.P.; Park, J.Y.; Park, M.J. ANO1 regulates the maintenance of stemness in glioblastoma stem cells by stabilizing EGFRvIII. Oncogene 2021, 40, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Erler, J.T.; Giaccia, A.J. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 2006, 66, 10238–10241. [Google Scholar] [CrossRef]
- Shen, C.J.; Sharma, A.; Vuong, D.V.; Erler, J.T.; Pruschy, M.; Broggini-Tenzer, A. Ionizing radiation induces tumor cell lysyl oxidase secretion. BMC Cancer 2014, 14, 532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, Y.; Xu, M.; Gao, J.; Li, M. Alkaline ceramidase 3 promotes growth of hepatocellular carcinoma cells via regulating S1P/S1PR2/PI3K/AKT signaling. Pathol. Res. Pract. 2018, 214, 1381–1387. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- van Haren, M.J.; Gao, Y.; Buijs, N.; Campagna, R.; Sartini, D.; Emanuelli, M.; Mateuszuk, L.; Kij, A.; Chlopicki, S.; Escudé Martinez de Castilla, P.; et al. Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity. Biomolecules 2021, 11, 1357. [Google Scholar] [CrossRef]
- van Haren, M.J.; Zhang, Y.; Thijssen, V.; Buijs, N.; Gao, Y.; Mateuszuk, L.; Fedak, F.A.; Kij, A.; Campagna, R.; Sartini, D.; et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT). RSC Chem. Biol. 2021, 2, 1546–1555. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Ramaekers, F.C.; Aalders, T.W.; Schaafsma, H.E.; Debruyne, F.M.; Schalken, J.A. Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res. 1992, 52, 6182–6187. [Google Scholar]

| Age | Ethnicity | ISUP Grade Group | PSA (ng/mL) | TNM | Risk Group | Hormone Therapy | Radiotherapy | |
|---|---|---|---|---|---|---|---|---|
| CaB34 | 71 | Caribbean | 2 | 31.3 | T1cN0M0 | High | Yes —6 days prior to biopsy | 15 Gy/1 HDR BT then 46 Gy/23 EBRT |
| Component | Concentration in Media | Company | Catalog No. |
|---|---|---|---|
| 3:1 v/v F-12:DMEM | - | Gibco, Waltham, MA, USA | |
| FBS | 5% | Gibco | A3160701 |
| Penicillin–Streptomycin | 1% | Gibco | 15140122 |
| Adenine | 24 µg/mL | Sigma-Aldrich (St. Louis, MO, USA) | A2786 |
| Cholera Toxin | 8.4 ng/mL | Sigma-Aldrich | C8052 |
| EGF | 10 ng/mL | Peprotech (Cranbury, NJ, USA) | AF-100-15 |
| Hydrocortisone | 0.4 µg/mL | Sigma-Aldrich | H0888 |
| Insulin | 5 µg/mL | Sigma-Aldrich | I9278 |
| Y-27632 | 10 µM | StemCell Technologies (Vancouver, BC, Canada) | 72307 |
| D5S818 | D13S317 | D7S820 | D16S539 | vWA | TH01 | AMEL | TPOX | CSF1PO | |
|---|---|---|---|---|---|---|---|---|---|
| CaB34 | 8, 12 | 13, 14 | 8, 10 | 10, 12 | 16, 17 | 5, 7 | X, Y | 6, 11 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, J.A.; White, S.D.; Frame, G.; Bosiljkov, A.; Khan, S.; Haas, R.; Yang, Q.; Sheng, M.; Huang, X.; Higgins, G.S.; et al. A Novel Primary Cell Line Model of Localized Prostate Cancer and Radioresistance—A Role for Nicotinamide N-Methyltransferase. Cells 2025, 14, 819. https://doi.org/10.3390/cells14110819
Wright JA, White SD, Frame G, Bosiljkov A, Khan S, Haas R, Yang Q, Sheng M, Huang X, Higgins GS, et al. A Novel Primary Cell Line Model of Localized Prostate Cancer and Radioresistance—A Role for Nicotinamide N-Methyltransferase. Cells. 2025; 14(11):819. https://doi.org/10.3390/cells14110819
Chicago/Turabian StyleWright, Jessica A., Stephanie D. White, Gavin Frame, Ana Bosiljkov, Shahbaz Khan, Roni Haas, Qian Yang, Minzhi Sheng, Xiaoyong Huang, Geoff S. Higgins, and et al. 2025. "A Novel Primary Cell Line Model of Localized Prostate Cancer and Radioresistance—A Role for Nicotinamide N-Methyltransferase" Cells 14, no. 11: 819. https://doi.org/10.3390/cells14110819
APA StyleWright, J. A., White, S. D., Frame, G., Bosiljkov, A., Khan, S., Haas, R., Yang, Q., Sheng, M., Huang, X., Higgins, G. S., Mills, I., Downes, M. R., Vesprini, D., Chung, H. T., Screaton, R. A., Leong, H. S., Boutros, P. C., Kislinger, T., & Liu, S. K. (2025). A Novel Primary Cell Line Model of Localized Prostate Cancer and Radioresistance—A Role for Nicotinamide N-Methyltransferase. Cells, 14(11), 819. https://doi.org/10.3390/cells14110819








