Abstract
Physiological oxidative stress plays a pivotal role in supporting proper growth and development. While moderate oxidative stress is essential for activating key metabolic pathways and maintaining normal cellular signaling, excessive production of reactive oxygen species (ROSs) can overwhelm the immature antioxidant systems of newborns, potentially leading to cellular damage and impaired physiological function. This vulnerability is particularly pronounced in the central nervous system, where limited detoxification capacity exacerbates the risk of oxidative damage, following hypoxic–ischemic events. Antioxidants agents—such as melatonin, erythropoietin, allopurinol, N-acetylcisteine, selenium, iminobiotin, taurine, and acetyl-L-carnitine—have demonstrated significant neuroprotective effects in preclinical experimental studies, reducing markers of oxidative injury and improving neurological outcomes. These neuroprotective agents have also been evaluated in clinical trials, demonstrating antioxidant effects. A major issue lies in the complexity of neurological damage, which is not associated with a single pathological pathway. Additionally, the inability of these agents to reach effective concentrations within the central nervous system, along with inconsistencies across clinical trials in terms of dosage and administration methods, hinders the ability to obtain robust results. Future efforts should therefore focus on the development of delivery systems capable of crossing the blood–brain barrier and on establishing standardized clinical trial protocols and study designs. This educational review aims to provide a comprehensive overview of emerging protective strategies, including antioxidant bioactive agents and nutritional interventions. It also explores the underlying mechanisms of oxidative stress and its impact on neonatal brain injury.
1. Introduction
Oxidative stress (OS) refers to the condition in which the production of pro-oxidants overwhelms antioxidant cellular defenses, leading to cellular damage [1]. In biological systems, pro-oxidants are mainly represented by reactive oxygen species (ROS) and reactive nitrogen species (RNS), comprising both free radicals (FRs) and their non-radical intermediates [2]. ROSs consist of free radicals, such as superoxide (●O2−) and hydroxyl radicals (●OH), as well as non-radical molecules like hydrogen peroxide (H2O2) and singlet oxygen (1O2). RNSs comprise nitric oxide (NO●), which is relatively unreactive, and its more reactive derivative, peroxynitrite (ONOO−). These species are produced endogenously during cellular metabolism via both enzymatic and non-enzymatic pathways. Enzymatic sources include the mitochondrial respiratory chain, phagocytic oxidative bursts, prostaglandin synthesis, and the cytochrome P450 enzyme system; non-enzymatic generation occurs through the interaction of molecular oxygen with organic substrates or upon exposure to ionizing radiation [3]. FRs are molecules with a single unpaired electron on the outer shell, which confer them the ability to react rapidly with other molecules [4], such as proteins, lipids, or DNA, altering their structure and function. ROSs and RNSs also play an important role in intracellular signaling cascades regulating vascular tone and cell proliferation, differentiation, and migration [5,6].
Under pathological conditions, NO reacts rapidly with superoxide anions to form peroxynitrite, a highly reactive and damaging species. Peroxynitrite causes nitration of tyrosine residues on proteins and generates hydroxyl radicals, which are potent inducers of lipid peroxidation and DNA damage, amplifying oxidative stress and cellular injury.
Additionally, NO impairs mitochondrial respiration by inhibiting critical components of the electron transport chain, notably cytochrome oxidase (complex IV) and complex I. This disruption leads to energy failure and activates apoptotic pathways, further contributing to neuronal loss [7,8]. Thus, excessive NO levels are not only cytotoxic but also interfere with essential mitochondrial function.
The effects of FR are well controlled by antioxidant cellular defenses, which comprise antioxidant enzymes (catalases, glutathione peroxidases, and superoxide dismutases), small molecules (ascorbate, alfa-tocopherol, glutathione, ubiquinone, etc.), and adaptive mechanisms leading to a higher expression of antioxidant genes. This balance may be altered by an increased production of FRs (toxin exposure, reperfusion after hypoxic–ischemic insult, activation of endogenous enzyme in chronic inflammatory disease) or a deficit in antioxidant defense (nutritional deficit, immaturity of enzymatic activity), leading to OS and damage [1,9]. Newborns—especially those born preterm—are exceptionally vulnerable to oxidative stress. This heightened susceptibility stems from several factors: their antioxidant enzyme systems are underdeveloped, their rapidly increasing energy requirements place a heavy burden on aerobic metabolism, and certain conditions elevate free iron levels, which in turn drive excessive free-radical formation [9,10,11]. OS is a key mediator of organ damage in the distressed fetus and neonate and underlies many prematurity-related complications, including bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, intraventricular hemorrhage, periventricular leukomalacia, and punctate white-matter lesions, which may be part of one entity defined as “the oxygen radical disease of neonatology” [11]. OS is now recognized as an important contributing factor of perinatal disease, including brain injury [9,12].
Oxidative Stress and Neonatal Brain Injury
During the perinatal period, oxidative stress intensifies as a result of inflammation, hyperoxia, hypoxia, ischemia–reperfusion injury, activation of neutrophils and macrophages, and the release of glutamate and free iron [10,11,12,13]. Hypoxia shifts cells toward anaerobic metabolism, causing a rapid buildup of lactic acid and accumulation of reduced intermediates in the mitochondrial electron-transport chain, which in turn drives excessive free-radical generation [11]. Other pathways fueling ROS production and membrane lipid peroxidation include phagocyte activation, arachidonic acid metabolism via cyclooxygenase and lipoxygenase enzymes, reactions catalyzed by elevated intracellular Fe2+, and increased xanthine oxidase activity stemming from accelerated ATP breakdown [14,15]. The combination of high free-iron levels and immature iron-binding/metabolizing systems in newborns further exacerbates their vulnerability to oxidative stress [16,17] (see Figure 1).
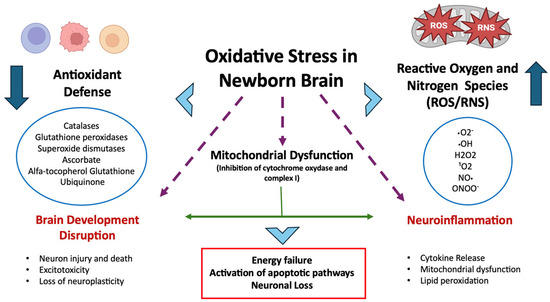
Figure 1.
Oxidative stress in the neonatal brain arises from increased ROS/RNS production (e.g., due to hyperoxia, ischemia–reperfusion, inflammation) and immature antioxidant defenses. This imbalance leads to mitochondrial dysfunction, neuroinflammation, impaired brain development, and brain damage.
The brain is particularly vulnerable to free-radical damage because of its very high rate of oxygen consumption, low concentration of antioxidants, water, and high free iron content [13,18]. In addition, neuronal membranes contain a high proportion of polyunsaturated fatty acids (PUFAs), rendering them especially susceptible to oxidative stress, causing devastating brain damage [19]. The newborn brain has large amounts of unsaturated fatty acids, namely adrenic acid, which has its highest level in myelin sheets, and docosahexaenoic acid, which is more present in neuronal membranes as cell bodies and dendrites in gray matter [19,20]. Newborns exhibit low levels of myelination and diminished expression of key ROS-detoxifying enzymes—namely superoxide dismutase, glutathione peroxidase, and catalase—resulting in an underdeveloped antioxidant defense that heightens vulnerability to oxidative injury. In this context, free radicals markedly promote excitotoxicity, neuronal death, and mitochondrial dysfunction [21]. Experimental strategies that boost antioxidant capacity—through overexpression or pharmacological modulation of superoxide dismutases—have demonstrated neuroprotective effects against oxidative insults [13,22]. Additionally, in the immature brain, both nitric-oxide synthase activity and mitochondrial electron leakage are major sources of free radicals [23,24]. OS and inflammation are tightly linked: ROSs can initiate inflammatory pathways, and, conversely, inflammation generates further ROS [25,26]. Finally, oxidative damage undermines blood–brain barrier integrity via multiple mechanisms, including direct injury to endothelial proteins, lipids, and DNA, disruption of tight junctions, activation of matrix metalloproteinases, and cytoskeletal reorganization [15,27].
2. Antioxidant/Neuroprotective Strategies in Experimental and Clinical Studies
Hypothermic treatment is the standard of care for moderate to severe hypoxic–ischemic encephalopathy (HIE). The neuroprotective effects of hypothermia are multifactorial and include also the post-depolarization release of excitatory amino acids and the suppression of OS, inflammation, abnormal receptor activity, and cell death pathways [28,29]. Therapeutic hypothermia has been shown to significantly reduce both mortality and major neurodevelopmental disability, with a number-needed-to-treat of seven [30]. In addition, using room air for the resuscitation of hypoxic neonates has been advocated to minimize reoxygenation-induced injury [31,32], and the latest international newborn resuscitation guidelines now recommend air as the initial gas for neonatal resuscitation [33].
As an adjunct to these measures, antioxidant therapies may provide further neuroprotection after perinatal oxidative stress [34]. The main experimental and clinical studies investigating the use of antioxidant bioactive agents for neuroprotection are reported in Table 1 and Table 2. Such interventions can act at multiple stages of the oxidative injury cascade: by directly scavenging reactive oxygen species, by inhibiting further free-radical generation, by modulating endogenous antiradical defenses, by boosting overall antioxidant levels, and by incorporating lipophilic antioxidants into cell membranes to strengthen their resistance to lipid peroxidation [35,36].

Table 1.
Main experimental studies that have used bioactive antioxidant agents for neuroprotection.

Table 2.
Main clinical studies that have used bioactive antioxidant agents for neuroprotection.
The mechanisms leading to brain injury and the potential protective effects of antioxidant therapies are illustrated in Figure 2.
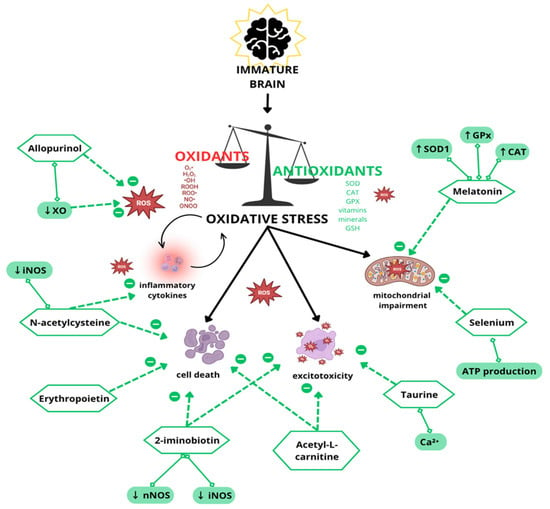
Figure 2.
Schematic representation of mechanisms contributing to oxidative stress and brain injury, including excitotoxicity, mitochondrial impairment, and blood–brain barrier breakdown. This figure also highlights potential antioxidant therapies with protective effects.
2.1. Erythropoietin
Erythropoietin (EPO) is a glycoprotein hormone with erythropoietic and non-erythropoietic effect. EPO may exert a neurotrophic effect through the binding with receptors expressed in neurons, astrocytes, and microglia. In particular, EPO may prevent cell death for its antiapoptotic, anti-inflammatory, and antioxidative actions; moreover, it may promote neuroregeneration and angiogenesis [74,75]. Following cerebral hypoxia, Epo is produced by astrocytes, oligodendrocytes, endothelial cells, neurons, and microglia. A specific receptor named EpoR has been isolated in glial cells, neurons, and brain endothelial cells in the hippocampus, cortex internal capsule, and midbrain regions. When Epo binds to EpoR, cellular survival is promoted, with angiogenesis, oligodendrogenesis, and neurogenesis [75,76].
In a full-term nonhuman primate model of perinatal asphyxia, combining therapeutic hypothermia with erythropoietin led to an increased N-acetylaspartate-to-creatine ratio and a decrease in choline levels during the first 72 h after birth [37].
A nonhuman primate study and small clinical trials provide evidence of the safety and efficacy of a multiple-dose EPO regimen when combined with therapeutic hypothermia (TH), suggesting that EPO has been associated with reduced MRI-detected brain injury, better neuromotor outcomes, and a lower incidence of death or moderate-to-severe cerebral palsy in several studies [37,38,39,77]. However, Sheldon et al. reported that EPO fails to mitigate damage when hydrogen peroxide accumulates excessively and may even worsen injury under conditions of extreme oxidative stress [39]. This suggests that EPO may offer little benefit—or could be harmful—if administered immediately following a severe hypoxic–ischemic insult, possibly because exogenous EPO interferes with the brain’s own early repair mechanisms [57].
Conversely, in settings of milder injury, EPO-based therapies might still favorably alter the course of damage. Yet, a large trial of high-dose EPO given to extremely preterm infants from 24 h after birth until 32 weeks postmenstrual age showed no reduction in overall morbidity or mortality [64].
Eight clinical trials have evaluated the neuroprotective effects of erythropoietin in term infants with hypoxic–ischemic encephalopathy, enrolling 533 neonates at 36–42 weeks’ gestation [56,58,59,60,61,62,63,78]. EPO was initiated within the first postnatal weeks at doses ranging from 200 to 2500 U/kg per administration, given either as a single dose or once daily for three to five days. None of these studies reported increased mortality or disability among EPO-treated infants; the therapy was well tolerated, with no allergic reactions, venous thrombosis, or electrolyte, hepatic, or renal function abnormalities observed [56,61]. As expected from its hematopoietic action, EPO-treated infants also exhibited higher hemoglobin levels and red blood cell counts compared with controls [56].
In two studies, EPO was administered in combination with TH and compared to TH alone [62,78]. Trials comparing EPO to placebo or supportive care alone reported a reduction in both mortality [58,59,61] and disability at 18 months of age [58,61]. Additionally, treatment with EPO alone was associated with reduced brain injury on MRI performed between 10 and 14 days of life [61].
The efficacy of EPO compared to TH alone yielded mixed results. One study found that EPO treatment in moderate HIE led to lower rates of mortality and disability, as well as higher scores on the Bayley Scales of Infant Development-II (BSID-II) at 18 months [56]. However, another study reported a less pronounced effect of EPO on mortality and disability compared to TH alone [59].
The combination of EPO and TH was shown to be safe, with no evidence of increased brain injury on MRI between days 4 and 13, and no adverse impact on disability at 22 months [60]. Furthermore, the combined therapy was associated with lower mortality [78], reduced brain injury scores on early MRI (days 4–7) [62,78], and improved neurodevelopmental outcomes at 12 months compared to TH alone [78].
The findings across these studies suggest that EPO is a safe and potentially effective adjunct to TH in managing newborns with HIE. Although results indicate a reduction in mortality, brain injury, and better neurodevelopmental outcomes, some variability in the effects, particularly when compared to TH alone, highlights the need for further research. Standardized protocols and larger randomized controlled trials are necessary to conclusively determine the optimal dosing, administration schedules, and long-term benefits of EPO therapy in this population.
2.2. Melatonin
Melatonin, along with several of its metabolites, functions as a potent direct scavenger of free radicals. It also exerts significant indirect antioxidant effects by enhancing mitochondrial electron transport efficiency and stimulating the activity of key antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase. Notably, unlike many other antioxidants, melatonin does not exhibit pro-oxidant properties [66,79].
Numerous preclinical studies have demonstrated melatonin’s neuroprotective potential following perinatal hypoxic–ischemic (HI) injury [42,44,46,80]. Its dual action—directly neutralizing ROS and indirectly modulating antioxidant enzyme systems—contributes to mitochondrial protection, supports ATP production, and inhibits oxidative stress-induced apoptosis [42,44,46]. In a newborn piglet model of HI, the combined use of hypothermia and melatonin significantly improved cerebral metabolism in the deep gray matter during the first 48 h after injury, resulting in reduced cellular damage [47].
In preterm and near-term fetal sheep models subjected to intrauterine asphyxia via umbilical cord occlusion, melatonin treatment reduced oxidative stress [40], attenuated cell death, and lessened the neuroinflammatory response [41]. Furthermore, melatonin was shown to decrease caspase-3 activation and improve cellular survival in in vivo models [43]. In juvenile rats, it also improved long-term outcomes, including cognitive and sensorimotor function [45].
More recently, Albertini et al. explored the impact of melatonin on miRNA expressions in a preclinical HI model. They found that HI significantly increased serum levels of miR-126 and miR-146a while decreasing their expression in the ischemic cerebral cortex. Melatonin administration restored both peripheral and cerebral levels of these miRNAs, suggesting a role in modulating ischemic damage at the molecular level [48].
In human studies, melatonin has also shown promise. Its administration to asphyxiated neonates resulted in decreased serum levels of oxidative markers, such as malondialdehyde (MDA) and nitrite/nitrate within the first six hours of life [81]. Aly et al., in a pilot clinical trial, assessed the effect of melatonin in neonates with HIE undergoing whole-body therapeutic hypothermia. Their findings demonstrated that early enteral melatonin administration reduced oxidative stress, improved survival, and was associated with fewer seizures and less white matter damage as seen on MRI and EEG follow-up [65].
Building on this, Weiss et al. conducted a comparative analysis of human and animal specimens to better understand melatonin’s role in neonatal HI injury. A human neonate receiving melatonin and TH was compared to melatonin-treated and untreated HI rats. An analysis of the microRNA expression in plasma and brain tissue helped identify key KEGG pathways associated with HI injury and melatonin response. Expression of proteins such as PKCα, p-Akt, and p-ERK in a rat brain cortex validated a shared, melatonin-sensitive signaling pathway, enhancing translational insight into melatonin’s therapeutic mechanisms [82].
Overall, melatonin’s pleiotropic neuroprotective effects and favorable safety profile make it a promising candidate for adjunctive therapy in neonatal brain injury. However, further research is needed to define the optimal dose, formulation, and timing of administration [66].
2.3. Allopurinol
One of the major sources of ROS during hypoxic–ischemic events is xanthine oxidase. In the initial hypoxic phase, as cells switch to anaerobic metabolism, hypoxanthine accumulates. Upon reoxygenation, xanthine oxidase catalyzes the oxidation of hypoxanthine to xanthine and subsequently to uric acid, generating FRs in the process [49,67].
Allopurinol inhibits xanthine oxidase, thereby preventing the conversion of hypoxanthine into xanthine and uric acid and effectively limiting the excessive production of ROS and the associated reperfusion injury [68,83]. Although studies on allopurinol in animal models of HI brain injury are limited, it has demonstrated neuroprotective effects. In particular, when administered 15 min after the induction of HI in 7-day-old Wistar rats at a high dose (135 mg/kg), allopurinol significantly reduced acute brain edema and long-term cerebral damage [50].
A clinical study examining the administration of Allopurinol within the first hours of life following moderate asphyxia demonstrated a significantly lower incidence of severe adverse outcomes in infants treated with allopurinol compared to the control group [67].
The pharmacokinetics and pharmacodynamics of allopurinol and its metabolite, oxypurinol, were analyzed in 46 newborns with HIE as part of the ALBINO study [69].
The ongoing ALBINO trial is a randomized, placebo-controlled, double-blind, multinational parallel group study designed to assess the superiority of allopurinol in term and near-term infants with neonatal HIE. The primary endpoint centers on long-term outcomes, specifically defined as survival with neurodevelopmental impairment, death, or survival without impairment at two years of age [84]. The neuroprotective effects of allopurinol appear to be highly time-dependent, with the greatest efficacy observed when administered very shortly after the hypoxic–ischemic insult. This presents a major challenge in clinical practice, where the precise timing of the hypoxic event is often unknown, and diagnosis or initiation of treatment may be delayed. These factors can limit the window of therapeutic opportunity and potentially reduce the effectiveness of early interventions. Therefore, future clinical protocols should consider strategies for quickly identification of high risk patients and timely administration of neuroprotective agents to maximize their potential benefit.
2.4. N-Acetylcysteine
N-acetylcysteine (NAC) is a well-established and powerful thiol-containing antioxidant. It acts both as a direct scavenger of oxygen radicals and as a precursor for glutathione synthesis. NAC contributes to the neutralization of ROS, restoration of intracellular glutathione levels, mitigation of redox imbalance, reduction in apoptotic cell death, and suppression of inflammatory cytokines as well as inducible nitric oxide synthase (iNOS), as demonstrated in an adult rat model of stroke [85,86]. In addition, NAC has been shown to activate the cystine/glutamate antiporter, modulating extracellular glutamate levels. In an animal model of HI, pretreatment with NAC increased levels of HIF-1α protein and its downstream targets, EPO and glucose transporter 3, have been observed in the ipsilateral hemispheres of rodents following hypoxic–ischemic injury. Another study demonstrated that administering NAC 10 min after the onset of reoxygenation, followed by continuous infusion, preserved post-resuscitation amino acid neurochemistry at levels comparable to those in sham-treated piglets. Notably, NAC administration did not disrupt cerebral amino acid balance, thereby maintaining cerebral amino acid homeostasis [87].
A follow-up of a randomized controlled trial involving infants with an extremely low birth weight found that postnatal administration of NAC did not provide neuroprotective benefits [88]. However, when administered to term neonates with HIE on days 5–6 of life, following rewarming after TH treatment, NAC was shown to rapidly restore central nervous system glutathione levels, as measured by magnetic resonance spectroscopy [70]. As with allopurinol, the neuroprotective efficacy of NAC has been shown to depend strongly on the timing of administration. In preclinical studies, early intervention—often within minutes of reoxygenation—is crucial to achieve favorable outcomes. However, translating these findings into clinical settings remains complex due to variability in the timing of birth-related hypoxic–ischemic events. These limitations underscore the importance of developing reliable early biomarkers and streamlined protocols for the prompt initiation of therapy.
2.5. Acetyl-L-Carnitine
Acetyl-L-carnitine is an acetylated derivative of L-carnitine, a naturally occurring metabolite that facilitates the transport of fatty acids across mitochondrial membranes for energy production. It helps prevent the excessive cytoplasmic accumulation of free fatty acids and provides acetyl-CoA to support mitochondrial energy metabolism [89,90]. Xu et al. investigated metabolic changes following neonatal hypoxic–ischemic injury in rats and evaluated the neuroprotective effects of acetyl-L-carnitine. They observed significant oxidative and osmotic stress, impaired phosphorylation, and a shift toward anaerobic glycolysis in the ipsilateral hippocampus 24 h post-injury. In contrast, the group treated with acetyl-L-carnitine showed lower lactate levels, preserved total creatine levels at 24 h, and reduced lesion size compared to untreated animals. These findings suggest that early administration of acetyl-L-carnitine after HI may exert neuroprotective effects by serving as an energy substrate, enhancing oxidative energy production, and reducing reliance on anaerobic glycolysis [51].
2.6. Selenium
Selenium is a vital component of more than two dozen selenoproteins and plays an essential role in antioxidant defense, particularly in neonates [91]. It is crucial for protecting the central nervous system from oxidative damage and for supporting various physiological processes, including infection response, reproduction, thyroid hormone metabolism, and DNA synthesis [92].
El-Mazary et al. found that neonates with HIE had significantly lower serum selenium levels compared to healthy neonates, with the lowest levels observed in those with severe HIE. Moreover, there were significant negative correlations between serum selenium levels and the severity of HIE [71]. These findings suggest that selenium supplementation may help alleviate hypoxic–ischemic-induced neuronal death both in vitro and in vivo. Selenium appears to protect against glutamate and hypoxia-induced cell death by reducing ROS production and increasing the activity of antioxidant enzymes [93,94]. Additionally, selenium helps regulate ATP production and the activity of mitochondrial respiratory chain complexes [52,95], and it can inhibit mitochondria-initiated cell death pathways and autophagy activation, thus promoting neuronal survival. Therefore, selenium supplementation could potentially serve as a beneficial adjunct to hypothermia therapy in treating neonates with HIE in the future.
2.7. Taurine
Taurine is a ubiquitous sulfur-containing amino acid that has been suggested to exert neuroprotective effects, particularly in the developing brain. Its concentration is notably high in the immature brain and gradually decreases with age, indicating a potential role in neurodevelopment and protection during early life stages [96,97]. Its synthesis is tightly coupled with GSH, as they share the same precursor, cysteine [97]. Lima and coworkers have found to be decreased after HI in ipsilateral hippocampus at 24 h after HI in rats; this may reflect a decrease in precursor cysteine levels and might contribute to OS in the damaged hippocampus [98]. The concentration of taurine has been found to be significantly elevated in the cerebrospinal fluid of asphyxiated infants, with levels correlating with the severity of hypoxic–ischemic encephalopathy (HIE) and with different clinical outcomes [72,99]. This suggests that taurine may serve as a potential biomarker for the extent of brain injury and recovery prognosis.
Taurine has demonstrated protective effects against glutamate-induced excitotoxicity, which is a key mechanism of neuronal damage following hypoxic–ischemic insults. These neuroprotective actions are mediated through several mechanisms, including modulation of calcium (Ca2+) and chloride (Cl−) channels, as well as interaction with N-methyl-D-aspartate (NMDA) receptors [53,100,101,102]. Through these pathways, taurine can help stabilize intracellular ion homeostasis, reduce calcium overload, and limit the downstream effects of excitotoxicity, potentially mitigating neural damage in neonates affected by HIE.
2.8. Iminobiotin
The activation of NOS and the resulting overproduction of NO are key contributors to HI-induced cerebral injury in the perinatal period.
Inhibiting NOS activity has been proposed as a potential neuroprotective strategy. Specifically, iminobiotin—a selective inhibitor of both neuronal NOS (nNOS) and inducible NOS (iNOS)—has shown promise in experimental studies.
Importantly, iminobiotin does not significantly inhibit endothelial NOS (eNOS), which is responsible for the vasodilatory effects of nitric oxide essential for maintaining vascular tone and cerebral perfusion. This selective inhibition profile represents a critical therapeutic advantage, allowing for the mitigation of neurotoxic NO production without impairing vascular homeostasis. Moreover, iNOS inhibition may exert indirect antioxidant effects by reducing the formation of peroxynitrite (ONOO−), a highly reactive and damaging oxidant generated by the reaction between NO and superoxide anion. This contributes to the overall protective effect of iminobiotin against oxidative stress-induced neuronal injury [103,104].
By selectively blocking the excessive production of NO during and after HI, iminobiotin may help to interrupt the deleterious cycle of oxidative damage and mitochondrial dysfunction, preserving neuronal integrity [105].
The pathophysiological link between elevated NO concentrations and increased intracellular Ca2+ underscores the importance of NO in signal transduction and cell fate decisions during cerebral ischemia [106]. While basal NO production has physiological roles, excessive NO becomes neurotoxic, promoting both apoptotic and necrotic cell death pathways [107]. These findings support the rationale for exploring NOS inhibitors like iminobiotin in neonatal neuroprotection.
The constitutive NOS isoforms—nNOS and endothelial NOS (eNOS)—produce low physiological levels of NO, which play critical roles in cell signaling and vascular regulation. In contrast, the iNOS is activated under inflammatory or stress conditions, resulting in high-output NO production. This surge contributes significantly to indirect biological effects, such as protein nitrosylation and oxidative damage, exacerbating cellular injury [108]. The pathological activation of nNOS and iNOS following HI events is closely linked to mitochondrial dysfunction and the initiation of apoptotic pathways [109].
2-Iminobiotin, a selective inhibitor of both nNOS and iNOS, has demonstrated promising neuroprotective properties in preclinical models. It effectively interrupts the cytochrome c-caspase 3 apoptotic cascade, providing both short- and long-term neuroprotection in neonatal female rats following HI insult [54]. In neonatal piglets subjected to moderate-to-severe HI injury, a dose of 0.2 mg/kg was shown to be the most effective. Treated animals exhibited significantly reduced protein tyrosine nitration in vulnerable brain regions, such as the thalamus, parietal cortex, and temporal cortex, alongside improved survival and normalization of EEG amplitude within 48 h [55].
Early clinical evaluation includes a Phase IIa trial conducted in Congo, where neonates with birth asphyxia received six doses of 2-iminobiotin (0.16 mg/kg) within 6 h of life, spaced every 4 h. This trial found the treatment to be safe, with no adverse events directly linked to the drug, although it underscored the necessity of adjusting dosing strategies for future trials [73].
Further pharmacokinetic studies in neonates undergoing TH after perinatal asphyxia confirmed that 2-iminobiotin is well tolerated when co-administered with TH. Target plasma concentrations were attained with a regimen of eight doses of 0.08 mg/kg given every 6 h. Importantly, the drug did not produce safety concerns in the short term [110]. However, further clinical trials are needed to determine the optimal dosing regimen, treatment duration, and long-term efficacy of 2-iminobiotin as a neuroprotective adjunct to TH.
3. Conclusions
Numerous studies have highlighted the significant therapeutic potential of antioxidants as bioactive agents in mitigating brain damage in newborns. Compounds such as selective inhibitors of neuronal and inducible NOS, allopurinol, melatonin, and EPO have been the subject of extensive investigation in clinical trials. When used as adjunct therapies in combination with hypothermia, these agents have demonstrated beneficial effects against HIE. These agents have shown promising anti-inflammatory, anti-apoptotic, and neuroprotective properties in both preclinical and early clinical studies. This growing body of research underscores the promising role these compounds may play in reshaping the landscape of neonatal neuroprotection.
However, despite encouraging results in animal models, clinical translation remains limited, and several compounds have yet to demonstrate consistent efficacy in clinical trials. A major limitation is the poor bioavailability of these drugs and their restricted ability to cross the blood–brain barrier (BBB), which hinders the achievement of therapeutic concentrations in brain tissue. This barrier represents a critical challenge in neonatal neuroprotection and may partly explain the gap between preclinical promise and clinical outcomes. To overcome this obstacle, various strategies are being explored, including the use of nanocarriers such as dendrimers [111]. These delivery systems remain largely at the preclinical stage and are not yet approved for clinical use in humans. For example, a dendrimer conjugated with N-acetylcysteine (NAC) demonstrated a 10- to 100-fold increase in efficacy compared to free NAC in a rabbit model of cerebral palsy, with notable improvements in neuronal injury, myelination, oxidative stress, and inflammation [111,112]. In conclusion, while antioxidant therapies offer a promising avenue for enhancing neonatal neuroprotection, their clinical application requires further validation. Future research should focus on optimizing drug formulations, improving BBB permeability, and identifying the most effective dosage regimens in combination with therapeutic hypothermia to maximize neuroprotective outcomes in newborns.
Author Contributions
V.B.: methodology, writing—original draft, and writing—review and editing; E.S.: methodology and writing—review and editing; S.C.: supervision and writing—review and editing; C.P.: visualization and supervision; V.D.: writing—review and editing; S.R.: writing—review and editing; S.P.: conceptualization, funding acquisition, and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), Project MNESYS (PE0000006)—A multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [PubMed]
- Buonocore, G.; Perrone, S.; Tataranno, M.L. Oxygen toxicity: Chemistry and biology of reactive oxygen species. Semin. Fetal Neonatal Med. 2010, 15, 186–190. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Ostrakhovitch, E.A.; Semenikhin, O.A. The role of redox environment in neurogenic development. Arch. Biochem. Biophys. 2013, 534, 44–54. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.J.; Lin, Y.; Xu, W.M. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef]
- van den Tweel, E.R.; Nijboer, C.; Kavelaars, A.; Heijnen, C.J.; Groenendaal, F.; van Bel, F. Expression of nitric oxide synthase isoforms and nitrotyrosine formation after hypoxia-ischemia in the neonatal rat brain. J. Neuroimmunol. 2005, 167, 64–71. [Google Scholar] [CrossRef]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal Neonatal Med. 2007, 12, 287–295. [Google Scholar] [CrossRef]
- Perrone, S.; Santacroce, A.; Longini, M.; Proietti, F.; Bazzini, F.; Buonocore, G. The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside. Oxidative Med. Cell. Longev. 2018, 2018, 7483062. [Google Scholar] [CrossRef]
- Tataranno, M.L.; Oei, J.L.; Perrone, S.; Wright, I.M.; Smyth, J.P.; Lui, K.; Tarnow-Mordi, W.O.; Longini, M.; Proietti, F.; Negro, S.; et al. Resuscitating preterm infants with 100% oxygen is associated with higher oxidative stress than room air. Acta Paediatr. 2015, 104, 759–765. [Google Scholar] [CrossRef]
- Dennery, P.A. Role of redox in fetal development and neonatal diseases. Antioxid. Redox Signal 2004, 6, 147–153. [Google Scholar] [CrossRef]
- Abad, C.; Chiarello, D.I.; Rojas, D.; Beretta, V.; Perrone, S.; Marín, R. Oxidative Stress in Preeclampsia and Preterm Newborn. In Biomarkers of Oxidative Stress; Andreescu, S., Henkel, R., Khelfi, A., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Perrone, S.; Tataranno, M.L.; Stazzoni, G.; Ramenghi, L.; Buonocore, G. Brain susceptibility to oxidative stress in the perinatal period. J. Matern. Neonatal Med. 2015, 28 (Suppl. S1), 2291–2295. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Longini, M.; Paffetti, P.; Vezzosi, P.; Gatti, M.G.; Bracci, R. Non protein bound iron as early predictive marker of neonatal brain damage. Brain 2003, 126 Pt 5, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, G.; Zani, S.; Perrone, S.; Caciotti, B.; Bracci, R. Intraerythrocyte nonprotein-bound iron and plasma malondialdehyde in the hypoxic newborn. Free Radic. Biol. Med. 1998, 25, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J. Polyunsaturated Fatty Acid Intake and Brain Health: Balance is the Key. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2022, 30, 774–776. [Google Scholar] [CrossRef]
- Sastry, P.S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar]
- VanRollins, M.; Woltjer, R.L.; Yin, H.; Morrow, J.D.; Montine, T.J. F2-dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lipid Res. 2008, 49, 995–1005. [Google Scholar] [CrossRef]
- Baud, O.; Greene, A.E.; Li, J.; Wang, H.; Volpe, J.J.; Rosenberg, P.A. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J. Neurosci. 2004, 24, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, O.; Revuelta, M.; Urigüen, L.; Álvarez, A.; Montalvo, H.; Hilario, E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE 2015, 10, e0142424. [Google Scholar] [CrossRef] [PubMed]
- Folkerth, R.D.; Haynes, R.L.; Borenstein, N.S.; Belliveau, R.A.; Trachtenberg, F.; Rosenberg, P.A.; Volpe, J.J.; Kinney, H.C. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J. Neuropathol. Exp. Neurol. 2004, 63, 990–999. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice-Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Castellini, L.; Parladori, R.; Paoletti, V.; Aceti, A.; Corvaglia, L. Free Radicals and Neonatal Brain Injury: From Underlying Pathophysiology to Antioxidant Treatment Perspectives. Antioxidants 2021, 10, 2012. [Google Scholar] [CrossRef]
- Drury, P.P.; Bennet, L.; Gunn, A.J. Mechanisms of hypothermic neuroprotection. Semin. Fetal Neonatal Med. 2010, 15, 287–292. [Google Scholar] [CrossRef]
- Perrone, S.; Szabó, M.; Bellieni, C.V.; Longini, M.; Bangó, M.; Kelen, D.; Treszl, A.; Negro, S.; Tataranno, M.L.; Buonocore, G. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr. Neurol. 2010, 43, 236–240. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 2013, CD003311. [Google Scholar] [CrossRef]
- Vento, M. Oxygen supplementation in the neonatal period: Changing the paradigm. Neonatology 2014, 105, 323–331. [Google Scholar] [CrossRef]
- Perrone, S.; Manti, S.; Petrolini, C.; Dell’Orto, V.G.; Boscarino, G.; Ceccotti, C.; Bertini, M.; Buonocore, G.; Esposito, S.M.R.; Gitto, E. Oxygen for the Newborn: Friend or Foe? Children 2023, 10, 579. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support Collaborators Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142 (Suppl. S1), S185–S221. [Google Scholar] [CrossRef]
- Tataranno, M.L.; Perrone, S.; Longini, M.; Buonocore, G. New antioxidant drugs for neonatal brain injury. Oxidative Med. Cell. Longev. 2015, 2015, 108251. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Akar, M.; Uras, N.; Eras, Z.; Erdeve, O.; Oguz, S.S.; Dilmen, U. Total antioxidant capacity and total oxidant status in perinatal asphyxia in relation to neurological outcome. Neuropediatrics 2011, 42, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mittal, R.; Khanna, H.D.; Basu, S. Free radical injury and blood-brain barrier permeability in hypoxic-ischemic encephalopathy. Pediatrics 2008, 122, e722–e727. [Google Scholar] [CrossRef] [PubMed]
- Traudt, C.M.; McPherson, R.J.; Bauer, L.A.; Richards, T.L.; Burbacher, T.M.; McAdams, R.M.; Juul, S.E. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev. Neurosci. 2013, 35, 491–503. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Wang, Y.; Zhang, R.; Chopp, M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004, 35, 1732–1737. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Windsor, C.; Lee, B.S.; Arteaga Cabeza, O.; Ferriero, D.M. Erythropoietin Treatment Exacerbates Moderate Injury after Hypoxia-Ischemia in Neonatal Superoxide Dismutase Transgenic Mice. Dev. Neurosci. 2017, 39, 228–237. [Google Scholar] [CrossRef]
- Miller, S.L.; Yan, E.B.; Castillo-Meléndez, M.; Jenkin, G.; Walker, D.W. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev. Neurosci. 2005, 27, 200–210. [Google Scholar] [CrossRef]
- Welin, A.K.; Svedin, P.; Lapatto, R.; Sultan, B.; Hagberg, H.; Gressens, P.; Kjellmer, I.; Mallard, C. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr. Res. 2007, 61, 153–158. [Google Scholar] [CrossRef]
- Carloni, S.; Perrone, S.; Buonocore, G.; Longini, M.; Proietti, F.; Balduini, W. Melatonin protects from the long-term consequences of a neonatal hypoxic-ischemic brain injury in rats. J. Pineal Res. 2008, 44, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hutton, L.C.; Abbass, M.; Dickinson, H.; Ireland, Z.; Walker, D.W. Neuroprotective properties of melatonin in a model of birth asphyxia in the spiny mouse (Acomys cahirinus). Dev. Neurosci. 2009, 31, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Ciccoli, L.; Leoncini, S.; Carloni, S.; Perrone, S.; Comporti, M.; Balduini, W.; Buonocore, G. Free iron, total F-isoprostanes and total F-neuroprostanes in a model of neonatal hypoxic-ischemic encephalopathy: Neuroprotective effect of melatonin. J. Pineal Res. 2009, 46, 148–154. [Google Scholar] [CrossRef]
- Lekic, T.; Manaenko, A.; Rolland, W.; Virbel, K.; Hartman, R.; Tang, J.; Zhang, J.H. Neuroprotection by melatonin after germinal matrix hemorrhage in neonatal rats. Acta Neurochir. Suppl. 2011, 111, 201–206. [Google Scholar] [PubMed]
- Balduini, W.; Carloni, S.; Perrone, S.; Bertrando, S.; Tataranno, M.L.; Negro, S.; Proietti, F.; Longini, M.; Buonocore, G. The use of melatonin in hypoxic-ischemic brain damage: An experimental study. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. S1), 119–124. [Google Scholar] [CrossRef]
- Robertson, N.J.; Faulkner, S.; Fleiss, B.; Bainbridge, A.; Andorka, C.; Price, D.; Powell, E.; Lecky-Thompson, L.; Thei, L.; Chandrasekaran, M.; et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013, 136, 90–105. [Google Scholar] [CrossRef]
- Albertini, M.C.; Vanzolini, T.; Perrone, S.; Weiss, M.D.; Buonocore, G.; Dell’Orto, V.; Balduini, W.; Carloni, S. MiR-126 and miR-146a as Melatonin-Responsive Biomarkers for Neonatal Brain Ischemia. J. Mol. Neurosci. 2023, 73, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Vannucci, R.C.; Towfighi, J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr. Res. 1990, 27, 332–336. [Google Scholar] [CrossRef]
- Palmer, C.; Towfighi, J.; Roberts, R.L.; Heitjan, D.F. Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr. Res. 1993, 33, 405–411. [Google Scholar]
- Xu, S.; Waddell, J.; Zhu, W.; Shi, D.; Marshall, A.D.; McKenna, M.C.; Gullapalli, R.P. In vivo longitudinal proton magnetic resonance spectroscopy on neonatal hypoxic-ischemic rat brain injury: Neuroprotective effects of acetyl-L-carnitine. Magn. Reson. Med. 2015, 74, 1530–1542. [Google Scholar] [CrossRef]
- Mehta, S.L.; Kumari, S.; Mendelev, N.; Li, P.A. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012, 13, 79. [Google Scholar] [CrossRef]
- Chan, C.Y.; Sun, H.S.; Shah, S.M.; Agovic, M.S.; Friedman, E.; Banerjee, S.P. Modes of direct modulation by taurine of the glutamate NMDA receptor in rat cortex. Eur. J. Pharmacol. 2014, 728, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Nijboer, C.H.; Groenendaal, F.; Kavelaars, A.; Hagberg, H.H.; van Bel, F.; Heijnen, C.J. Gender-specific neuroprotection by 2-iminobiotin after hypoxia-ischemia in the neonatal rat via a nitric oxide independent pathway. J. Cereb. Blood Flow. Metab. 2007, 27, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, S.T.; Ireland, Z.; Fan, X.; van der Wal, W.M.; Roes, K.C.; Colditz, P.B.; Peeters-Scholte, C.M. Short-term dose-response characteristics of 2-iminobiotin immediately postinsult in the neonatal piglet after hypoxia-ischemia. Stroke 2013, 44, 809–811. [Google Scholar] [CrossRef]
- Zhu, C.; Kang, W.; Xu, F.; Cheng, X.; Zhang, Z.; Jia, L.; Ji, L.; Guo, X.; Xiong, H.; Simbruner, G.; et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics 2009, 124, e218–e226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Bauer, L.A.; Ballard, R.A.; Ferriero, D.M.; Glidden, D.V.; Mayock, D.E.; Chang, T.; Durand, D.J.; Song, D.; Bonifacio, S.L.; et al. Erythropoietin for neuroprotection in neonatal encephalopathy: Safety and pharmacokinetics. Pediatrics 2012, 130, 683–691. [Google Scholar] [CrossRef]
- Avasiloaiei, A.; Dimitriu, C.; Moscalu, M.; Paduraru, L.; Stamatin, M. High-dose phenobarbital or erythropoietin for the treatment of perinatal asphyxia in term newborns. Pediatr. Int. 2013, 55, 589–593. [Google Scholar] [CrossRef]
- El Shimi, M.S.; Awad, H.A.; Hassanein, S.M.; Gad, G.I.; Imam, S.S.; Shaaban, H.A.; El Maraghy, M.O. Single dose recombinant erythropoietin versus moderate hypothermia for neonatal hypoxic ischemic encephalopathy in low resource settings. J. Matern. Fetal Neonatal Med. 2014, 27, 1295–1300. [Google Scholar] [CrossRef]
- Rogers, E.E.; Bonifacio, S.L.; Glass, H.C.; Juul, S.E.; Chang, T.; Mayock, D.E.; Durand, D.J.; Song, D.; Barkovich, A.J.; Ballard, R.A.; et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr. Neurol. 2014, 51, 657–662. [Google Scholar] [CrossRef]
- Malla, R.R.; Asimi, R.; Teli, M.A.; Shaheen, F.; Bhat, M.A. Erythropoietin monotherapy in perinatal asphyxia with moderate to severe encephalopathy: A randomized placebo-controlled trial. J. Perinatol. 2017, 37, 596–601. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Ramakrishnaiah, R.H.; McKinstry, R.C.; Chang, T.; Mathur, A.M.; Mayock, D.E.; Van Meurs, K.P.; Schaefer, G.B.; Luo, C.; Bai, S.; et al. Erythropoietin and Brain Magnetic Resonance Imaging Findings in Hypoxic-Ischemic Encephalopathy: Volume of Acute Brain Injury and 1-Year Neurodevelopmental Outcome. J. Pediatr. 2017, 186, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.Y.; Wu, S.J.; Wang, Q.L.; Yang, L.H.; Ren, P.S.; Qiao, B.J.; Wang, Z.Y.; Li, J.H.; Gu, X.L.; Li, L.X. Effect of erythropoietin combined with hypothermia on serum tau protein levels and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Neural Regen. Res. 2017, 12, 1655–1663. [Google Scholar] [PubMed]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.A.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.; Elmahdy, H.; El-Dib, M.; Rowisha, M.; Awny, M.; El-Gohary, T.; Elbatch, M.; Hamisa, M.; El-Mashad, A.R. Melatonin use for neuroprotection in perinatal asphyxia: A randomized controlled pilot study. J. Perinatol. 2015, 35, 186–191. [Google Scholar] [CrossRef]
- Marseglia, L.; Gitto, E.; Laschi, E.; Giordano, M.; Romeo, C.; Cannavò, L.; Toni, A.L.; Buonocore, G.; Perrone, S. Antioxidant Effect of Melatonin in Preterm Newborns. Oxidative Med. Cell. Longev. 2021, 2021, 6308255. [Google Scholar] [CrossRef]
- Russell, G.A.; Cooke, R.W. Randomised controlled trial of allopurinol prophylaxis in very preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1995, 73, F27–F31. [Google Scholar] [CrossRef]
- Kaandorp, J.J.; Benders, M.J.; Schuit, E.; Rademaker, C.M.; Oudijk, M.A.; Porath, M.M.; Oetomo, S.B.; Wouters, M.G.; van Elburg, R.M.; Franssen, M.T.; et al. Maternal allopurinol administration during suspected fetal hypoxia: A novel neuroprotective intervention? A multicentre randomised placebo controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F216–F223. [Google Scholar] [CrossRef]
- Chu, W.Y.; Annink, K.V.; Nijstad, A.L.; Maiwald, C.A.; Schroth, M.; Bakkali, L.E.; van Bel, F.; Benders, M.J.N.L.; van Weissenbruch, M.M.; Hagen, A.; et al. Pharmacokinetic/Pharmacodynamic Modelling of Allopurinol, its Active Metabolite Oxypurinol, and Biomarkers Hypoxanthine, Xanthine and Uric Acid in Hypoxic-Ischemic Encephalopathy Neonates. Clin. Pharmacokinet. 2022, 61, 321–333. [Google Scholar] [CrossRef]
- Wiest, D.B.; Jenkins, D.D. N-Acetylcysteine rapidly replenishes central nervous system glutathione measured via magnetic resonance spectroscopy in human neonates with hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 950–958. [Google Scholar]
- El-Mazary, A.A.; Abdel-Aziz, R.A.; Mahmoud, R.A.; El-Said, M.A.; Mohammed, N.R. Correlations between maternal and neonatal serum selenium levels in full term neonates with hypoxic ischemic encephalopathy. Ital. J. Pediatr. 2015, 41, 83. [Google Scholar] [CrossRef]
- Gücüyener, K.; Atalay, Y.; Aral, Y.Z.; Hasanoğlu, A.; Türkyilmaz, C.; Biberoglu, G. Excitatory amino acids and taurine levels in cerebrospinal fluid of hypoxic ischemic encephalopathy in newborn. Clin. Neurol. Neurosurg. 1999, 101, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Biselele, T.; Bambi, J.; Betukumesu, D.M.; Ndiyo, Y.; Tabu, G.; Kapinga, J.; Bola, V.; Makaya, P.; Tjabbes, H.; Vis, P.; et al. A Phase IIa Clinical Trial of 2-Iminobiotin for the Treatment of Birth Asphyxia in DR Congo, a Low-Income Country. Paediatr. Drugs 2020, 22, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Balsari, A.; Giallongo, T.; Ottolenghi, S.; Di Giulio, A.M.; Samaja, M.; Carelli, S. Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases. ASN Neuro 2019, 11, 1759091419871420. [Google Scholar] [CrossRef]
- Perrone, S.; Lembo, C.; Gironi, F.; Petrolini, C.; Catalucci, T.; Corbo, G.; Buonocore, G.; Gitto, E.; Esposito, S.M.R. Erythropoietin as a Neuroprotective Drug for Newborn Infants: Ten Years after the First Use. Antioxidants 2022, 11, 652. [Google Scholar] [CrossRef]
- Ott, C.; Martens, H.; Hassouna, I.; Oliveira, B.; Erck, C.; Zafeiriou, M.-P.; Peteri, U.-K.; Hesse, D.; Gerhart, S.; Altas, B.; et al. Widespread Expression of Erythropoietin Receptor in Brain and Its Induction by Injury. Mol. Med. 2015, 21, 803–815. [Google Scholar] [CrossRef]
- Berger, H.R.; Brekke, E.; Widerøe, M.; Morken, T.S. Neuroprotective Treatments after Perinatal Hypoxic-Ischemic Brain Injury Evaluated with Magnetic Resonance Spectroscopy. Dev. Neurosci. 2017, 39, 36–48, Erratum in Dev. Neurosci. 2017, 39, 442. [Google Scholar] [CrossRef]
- Wu, Y.W.; Mathur, A.M.; Chang, T.; McKinstry, R.C.; Mulkey, S.B.; Mayock, D.E.; Van Meurs, K.P.; Rogers, E.E.; Gonzalez, F.F.; Comstock, B.A.; et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics 2016, 137, e20160191. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. A review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Furmaga-Jabłońska, W.; Januszewski, S.; Tarkowska, A. Melatonin: A Potential Candidate for the Treatment of Experimental and Clinical Perinatal Asphyxia. Molecules 2023, 28, 1105. [Google Scholar] [CrossRef]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res. 2001, 31, 343–349. [Google Scholar] [CrossRef]
- Weiss, M.D.; Carloni, S.; Vanzolini, T.; Coppari, S.; Balduini, W.; Buonocore, G.; Longini, M.; Perrone, S.; Sura, L.; Mohammadi, A.; et al. Human-rat integrated microRNAs profiling identified a new neonatal cerebral hypoxic-ischemic pathway melatonin-sensitive. J. Pineal Res. 2022, 73, e12818. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, O.; Álvarez, A.; Revuelta, M.; Santaolalla, F.; Urtasun, A.; Hilario, E. Role of Antioxidants in Neonatal Hypoxic-Ischemic Brain Injury: New Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 265. [Google Scholar] [CrossRef]
- Engel, C.; Rüdiger, M.; Benders, M.J.N.L.; van Bel, F.; Allegaert, K.; Naulaers, G.; Bassler, D.; Klebermaß-Schrehof, K.; Vento, M.; Vilan, A.; et al. Detailed statistical analysis plan for ALBINO: Effect of Allopurinol in addition to hypothermia for hypoxic-ischemic Brain Injury on Neurocognitive Outcome—A blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III). Trials 2024, 25, 81. [Google Scholar]
- Liu, X.; Wang, L.; Cai, J.; Liu, K.; Liu, M.; Wang, H.; Zhang, H. N-acetylcysteine alleviates H2O2-induced damage via regulating the redox status of intracellular antioxidants in H9c2 cells. Int. J. Mol. Med. 2019, 43, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, S.A.; Terluk, M.R.; Kartha, R.V. N-acetylcysteine Pharmacology and Applications in Rare Diseases-Repurposing an Old Antioxidant. Antioxidants 2023, 12, 1316. [Google Scholar] [CrossRef]
- Jantzie, L.L.; Cheung, P.Y.; Johnson, S.T.; Bigam, D.L.; Todd, K.G. Cerebral amino acid profiles after hypoxia-reoxygenation and N-acetylcysteine treatment in the newborn piglet. Neonatology 2010, 97, 195–203. [Google Scholar] [CrossRef]
- Kiuru, A.; Ahola, T.; Klenberg, L.; Tommiska, V.; Lano, A.; Kleemola, P.; Haavisto, A.; Fellman, V. Postnatal N-acetylcysteine does not provide neuroprotection in extremely low birth weight infants: A follow-up of a randomized controlled trial. Early Hum. Dev. 2019, 132, 13–17. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef]
- Onofrj, M.; Ciccocioppo, F.; Varanese, S.; di Muzio, A.; Calvani, M.; Chiechio, S.; Osio, M.; Thomas, A. Acetyl-L-carnitine: From a biological curiosity to a drug for the peripheral nervous system and beyond. Expert. Rev. Neurother. 2013, 13, 925–936. [Google Scholar] [CrossRef]
- Tindell, R.; Tipple, T. Selenium: Implications for outcomes in extremely preterm infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2018, 38, 197–202. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Algieri, C.; Oppedisano, F.; Trombetti, F.; Fabbri, M.; Palma, E.; Nesci, S. Selenite ameliorates the ATP hydrolysis of mitochondrial F1FO-ATPase by changing the redox state of thiol groups and impairs the ADP phosphorylation. Free Radic. Biol. Med. 2024, 210, 333–343. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; García-Serrano, A.M.; Duarte, J.M.N. Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes. Nutrients 2022, 14, 1292. [Google Scholar] [CrossRef]
- Lima, L.; Obregón, F.; Roussó, T.; Quintal, M.; Benzo, Z.; Auladell, C. Content and concentration of taurine, hypotaurine, and zinc in the retina, the hippocampus, and the dentate gyrus of the rat at various postnatal days. Neurochem. Res. 2004, 29, 247–255. [Google Scholar] [CrossRef]
- Roldán, A.; Figueras-Aloy, J.; Deulofeu, R.; Jiménez, R. Glycine and other neurotransmitter amino acids in cerebrospinal fluid in perinatal asphyxia and neonatal hypoxic-ischaemic encephalopathy. Acta Paediatr. 1999, 88, 1137–1141. [Google Scholar] [CrossRef]
- Chen, W.Q.; Jin, H.; Nguyen, M.; Carr, J.; Lee, Y.J.; Hsu, C.C.; Faiman, M.D.; Schloss, J.V.; Wu, J.Y. Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J. Neurosci. Res. 2001, 66, 612–619. [Google Scholar] [CrossRef]
- Wu, H.; Jin, Y.; Wei, J.; Jin, H.; Sha, D.; Wu, J.Y. Mode of action of taurine as a neuroprotector. Brain Res. 2005, 1038, 123–131. [Google Scholar] [CrossRef]
- Kulak, A.; Duarte, J.M.; Do, K.Q.; Gruetter, R. Neurochemical profile of the developing mouse cortex determined by in vivo 1H NMR spectroscopy at 14.1 T and the effect of recurrent anaesthesia. J. Neurochem. 2010, 115, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Santacroce, A.; Buonocore, G. 2-Iminobiotin for the treatment of perinatal asphyxia. Expert Opin. Orphan Drugs 2013, 1, 935–945. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Sup, J.; Green, B.G.; Grant, S.K. 2-Iminobiotin is an inhibitor of nitric oxide synthases. Biochem. Biophys. Res. Commun. 1994, 204, 962–968. [Google Scholar] [CrossRef]
- Perrone, S.; Tataranno, M.; Stazzoni, G.; Buonocore, G. Oxidative stress and free radicals related diseases of the newborn. Adv. Biosci. Biotechnol. 2012, 3, 1043–1050. [Google Scholar] [CrossRef]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide 2010, 23, 153–165. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Qiu, L.; Peeters-Scholte, C.; Hagberg, H.; Blomgren, K. Nitrosylation precedes caspase-3 activation and translocation of apoptosis-inducing factor in neonatal rat cerebral hypoxia-ischaemia. J. Neurochem. 2004, 90, 462–471. [Google Scholar] [CrossRef]
- Favié, L.M.A.; Peeters-Scholte, C.M.P.C.D.; Bakker, A.; Tjabbes, H.; Egberts, T.C.G.; van Bel, F.; Rademaker, C.M.A.; Vis, P.; Groenendaal, F. Pharmacokinetics and short-term safety of the selective NOS inhibitor 2-iminobiotin in asphyxiated neonates treated with therapeutic hypothermia. Pediatr. Res. 2020, 87, 689–696. [Google Scholar] [CrossRef]
- Fernandes, L.F.; Bruch, G.E.; Massensini, A.R.; Frézard, F. Recent Advances in the Therapeutic and Diagnostic Use of Liposomes and Carbon Nanomaterials in Ischemic Stroke. Front. Neurosci. 2018, 12, 453. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.A.; Kim, S.Y.; Su, L.; Liu, J.; Kannan, R.M.; Kannan, S. Systemic dendrimer-drug nanomedicines for long-term treatment of mild-moderate cerebral palsy in a rabbit model. J. Neuroinflamm. 2020, 17, 319. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).