Microbial Metabolite Effects on Vasculogenic Mimicry in Metastatic Cancers
Abstract
1. Introduction
2. Definition and Importance of Vasculogenic Mimicry in Metastatic Cancers
3. Vasculogenic Mimicry: Role in the Advancement of Tumors
3.1. Epithelial–Mesenchymal Transition and Vasculogenic Mimicry
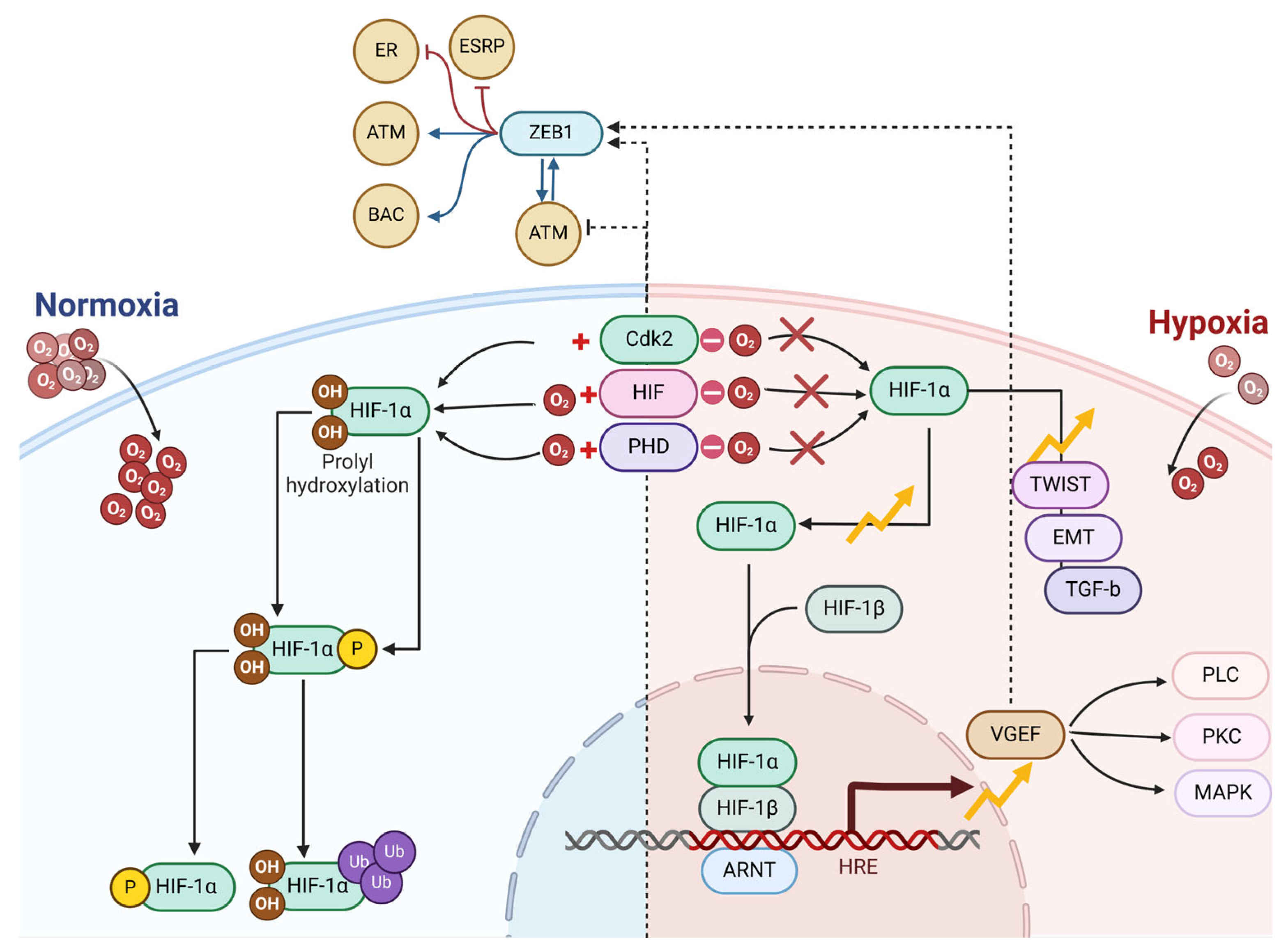
3.2. Hypoxia and Vasculogenic Mimicry
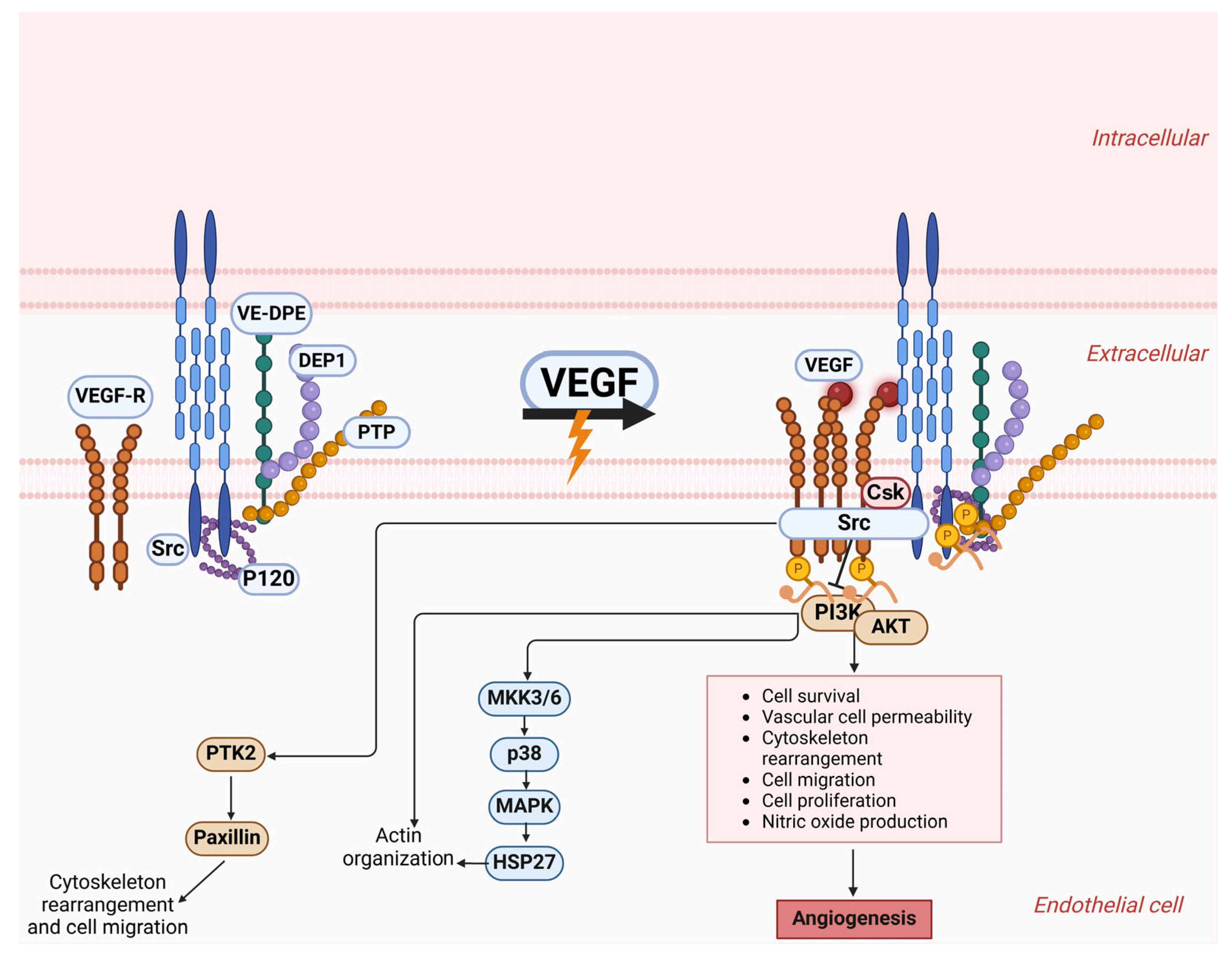
4. Role of Epithelial–Mesenchymal Transition and the Gut Microbiome in Vascular Mimicry
5. Role of Hypoxia and the Gut Microbiome in Vascular Mimicry
5.1. Intestinal Hypoxia and Host Microbiota
5.2. Trimethylamine N-Oxide
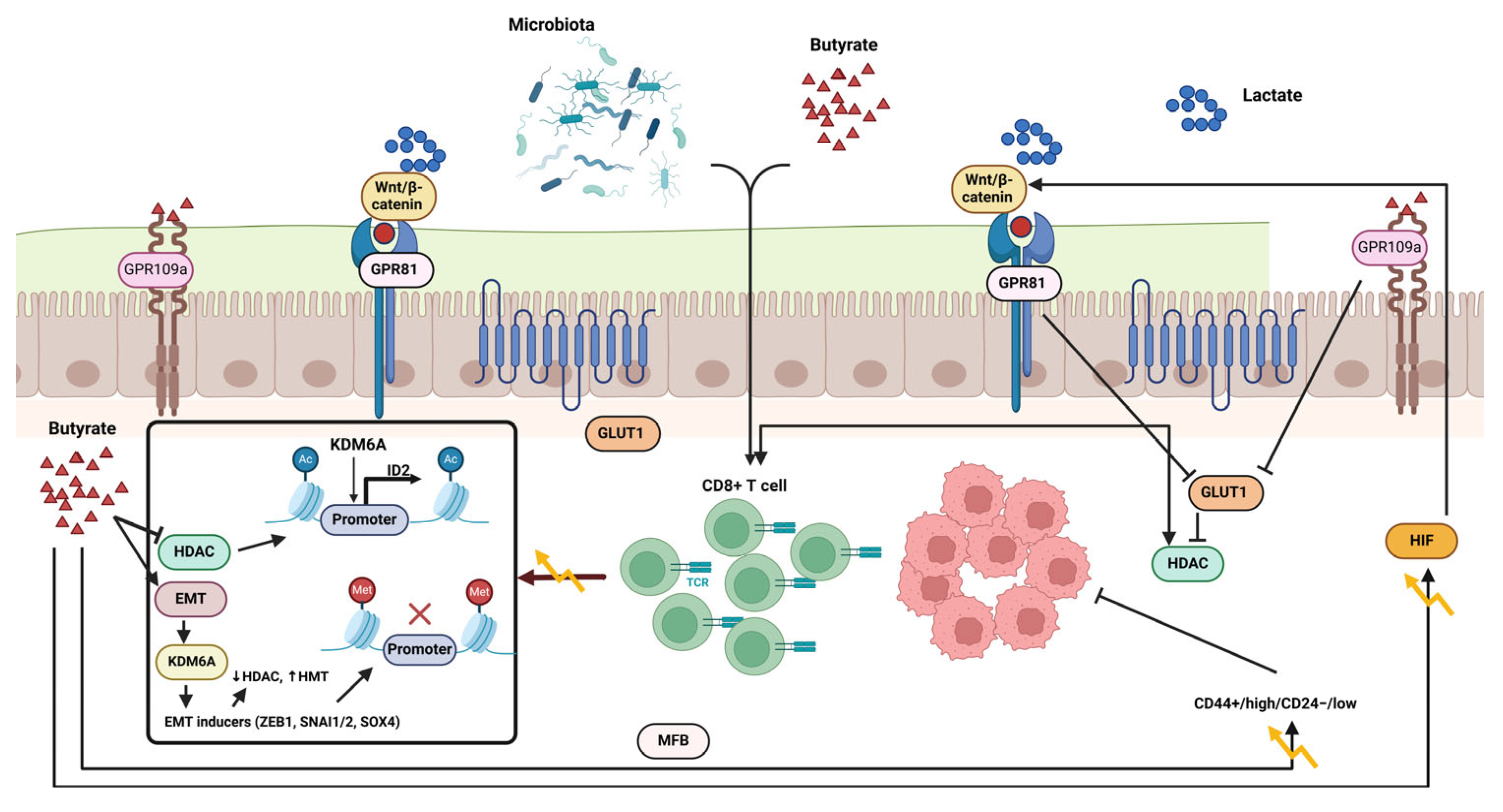
5.3. Butyrate
5.4. Secondary Biliary Acids
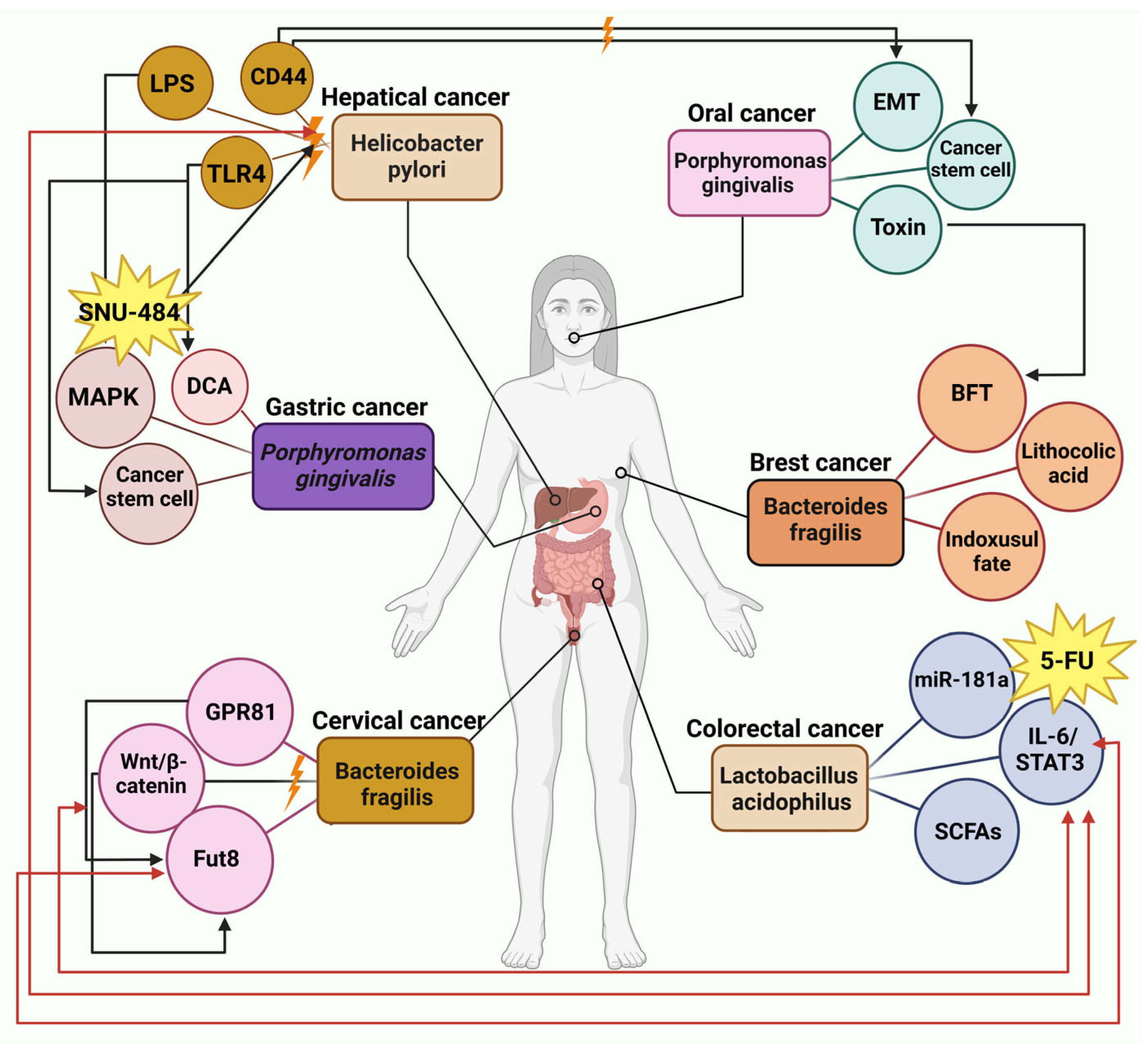
6. Gut Microbiota Metabolites and Cancer Therapy
6.1. Chemotherapy Efficacy
6.2. Immunotherapy Response
6.3. Radiotherapy Efficiency
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ASA | 5-amino salicylic acid |
| EGFR | Epidermal growth factor receptor |
| FexD | Fexaramine D |
| OCA | Obeticholic acid |
| T-βMCA | Tauro-β-muricholic acid |
| FXR | Farnesoid X receptor |
| M3R | Muscarinic acetylcholine receptor M3 |
| LCA | Lithocholic acid |
| BAs | Bile acids |
| MFB | Metformin-butyrate |
| ROS | Reactive oxygen species |
| MAMPs | Microorganism-associated molecular patterns |
| TLRs | Toll-like receptors |
| TLRs | Through Toll-like receptors |
| EMT | Epithelial–mesenchymal transition |
| CRC | Colon cancer |
| LPS | Lipopolysaccharide |
| DCA | Deoxycholic acid |
| CSCs | Cancer stem-like cells |
| ERK | Extracellular-regulated protein kinase |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphatidylinositol 3 kinase |
| AKT | Protein kinase B |
| VE-PTP | Vascular endothelial protein tyrosine phosphatase |
| AJs | Adhesion junctions |
| (VE)-cadherin | Vascular endothelial |
| TWIST1 | Twist-related protein 1 |
| EPHA2 | Ephrin type-A receptor 2 |
| VEGF | Vascular endothelial growth factor |
| MMPs | Matrix metalloproteinases |
| TGFB | Growth factor beta 1 |
| FMO1 | Flavin-containing monooxygenase |
| TMA | Trimethylamine |
| cutC/D | Choline TMA-lyase |
| CntA/B | L-carnitine oxygenase |
| SCFAs | Short-chain fatty acids |
| TMAO | Trimethylamine N-oxide |
| GM | Gut microbiota |
| VM | Vasculogenic mimicry |
References
- Zong, G.; Deng, R.; Pan, Y.; Liu, M.; Zhu, H.; Tao, R.; Shan, Y.; Wei, Z.; Lu, Y. Ginseng polysaccharides ameliorate colorectal tumorigenesis through Lachnospiraceae-mediated immune modulation. Int. J. Biol. Macromol. 2025, 307 Pt 2, 142015. [Google Scholar] [CrossRef] [PubMed]
- Zhra, M.; Elahi, M.A.; Tariq, A.; Abu-Zaid, A.; Yaqinuddin, A. Sirtuins and Gut Microbiota: Dynamics in Health and a Journey from Metabolic Dysfunction to Hepatocellular Carcinoma. Cells 2025, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, Z.; Da, Y.; Liu, X.; Wang, C.; Zhang, T.; Zhang, J.; Wu, T.; Liang, J. Causal Relationships Between Iron Deficiency Anemia, Gut Microbiota, and Metabolites: Insights from Mendelian Randomization and In Vivo Data. Biomedicines 2025, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, T.; Qin, Y.; Xie, J.; Zhang, S.; Xie, Y.; Lao, J.; He, W.; Zeng, H.; Tang, X.; et al. Polygonatum cyrtonema Hua polysaccharides alleviate muscle atrophy and fat lipolysis by regulating the gut microenvironment in chemotherapy-induced cachexia. Front. Pharmacol. 2025, 16, 1503785. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, C.; Li, Z.; Jin, X.; Zou, Y.; Bai, S.; Zheng, H.; Ling, W.; Zhao, Y.; Wang, Y.; et al. Alcaligenes faecalis promotes colitis to colorectal cancer transition through IgA+ B cell suppression and vinculin acetylation. Gut Microbes 2025, 17, 2473511. [Google Scholar] [CrossRef]
- Zhang, B.; Mohd Sahardi, N.F.N.; Di, W.; Long, X.; Shafiee, M.N. The Gut-Endometrium Axis: Exploring the Role of Microbiome in the Pathogenesis and Treatment of Endometrial Cancer—A Narrative Review. Cancers 2025, 17, 1044. [Google Scholar] [CrossRef]
- Pandiar, D.; Smitha, T.; Krishnan, R.P. Vasculogenic mimicry. J. Oral Maxillofac. Pathol. 2023, 27, 228–229. [Google Scholar] [CrossRef]
- Yoshida, A.; Murakami, K.; Tsukamoto, H.; Hotta, Y.; Nakayama, Y.; Saito, R.; Shirakashi, M.; Tsuji, H.; Hiwa, R.; Akizuki, S.; et al. Anti-arthritic effects of polyunsaturated fatty acid rich supplementation combined with selective soluble epoxide hydrolase inhibitors in a collagen-induced arthritis mouse model. Mod. Rheumatol. 2025, roaf019. [Google Scholar] [CrossRef]
- Jahantigh, M.; Tahmasebi, H. Correlation between efflux pumps activity and frequency of pslABD genes in clinical isolates of Pseudomonas aeruginosa collected from diabetic foot infections. J. Knowl. Health Basic Med. Sci. 2024, 19, 22–40. [Google Scholar] [CrossRef]
- Yao, J.; Ning, B.; Ding, J. The gut microbiota: An emerging modulator of drug resistance in hepatocellular carcinoma. Gut Microbes 2025, 17, 2473504. [Google Scholar] [CrossRef]
- Yang, S.; Liu, H.; Liu, Y. Advances in intestinal epithelium and gut microbiota interaction. Front. Microbiol. 2025, 16, 1499202. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Shi, X.; Zhao, Y.; Liu, X.; Jia, X.; Gao, L.; Yuan, J.; Liao, A.; Yasui, H.; Wang, X.; et al. Microbiota-reprogrammed phosphatidylcholine inactivates cytotoxic CD8 T cells through UFMylation via exosomal SerpinB9 in multiple myeloma. Nat. Commun. 2025, 16, 2863. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ning, L.; Si, Y.; Li, X.; Wang, R.; Ren, Q. Metformin-mediated intestinal AMPK activation ameliorates PCOS through gut microbiota modulation and metabolic pathways. Front. Endocrinol. 2025, 16, 1526109. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, X.; Chen, L.; Ma, H.; Wang, Y.; Liu, W.; Liao, A.; Tan, L.; Gao, X.; Xiao, W.; et al. Gut microbiome and plasma metabolome alterations in ileostomy and after closure of ileostomy. Microbiol. Spectr. 2025, 13, e0119124. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, S.; Lv, J.; Cao, Y.; Chen, Y.; Sun, H.; Dai, S.; Zhang, B.; Zhu, M.; Liu, Y.; et al. Machine learning identification of a novel vasculogenic mimicry-related signature and FOXM1’s role in promoting vasculogenic mimicry in clear cell renal cell carcinoma. Transl. Oncol. 2025, 53, 102312. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, Z.; Chai, Y.; Yao, W.; Ma, G. Unveiling the placental bacterial microbiota: Implications for maternal and infant health. Front. Physiol. 2025, 16, 1544216. [Google Scholar] [CrossRef]
- Wu, S.; Qiao, L.; Liu, H.; Li, Y.L.; Wang, R.; Yin, Y.; Li, E.; Wang, L.; Guan, X.; Yin, L.; et al. Age related gut microbiota regulates energy-related metabolism to influence natural aging phenotypes in the heart. Exp. Gerontol. 2025, 203, 112734. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Wang, H.; Li, Y.; Wang, M.; Li, Q. Cinobufagin modulates vasculogenic mimicry and tumor-associated macrophages to inhibit ovarian cancer progression. Eur. J. Pharmacol. 2025, 987, 177157. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Q.; Zhang, Y.; Wang, X.; Zhai, L.; Wang, R.; Zheng, C.; Hong, Z. Sleep health: An unappreciated key player in colorectal cancer. J. Cancer 2025, 16, 1934–1943. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, Z.; Ma, Y.; Xia, R.; Zheng, Y.; Yin, K.; Pang, C.; Yuan, L.; Cheng, X.; Liu, Z.; et al. Multiomics insights into BMI-related intratumoral microbiota in gastric cancer. Front. Cell Infect. Microbiol. 2025, 15, 1511900. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, W.; Huang, H.; Di, C.; Yang, Q. Huaier enhances the antitumor effects of CDK 4/6 inhibitor by remodeling the immune microenvironment and gut microbiota in breast cancer. J. Ethnopharmacol. 2025, 347, 119723. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Cao, K.; Singh, C.O.; Fang, X.; Yang, H.; Wei, D. Advances in gut microbiota-related treatment strategies for managing colorectal cancer in humans. Cancer Biol. Med. 2025, 22, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Ran, P.; Jiang, F.; Pan, L.; Shu, Y.; Hu, F.; Wang, Y.; Zhao, R.; Wang, W.; Mu, H.; Wang, J.; et al. Polysaccharide from Atractylodes macrocephala Koidz. alleviates pyrotinib-induced diarrhea through regulating cAMP/LKB1/AMPK/CFTR pathway and restoring gut microbiota and metabolites. Int. J. Biol. Macromol. 2025, 308 Pt 3, 142512. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, X.; Yang, B.; Liu, Y.; Tang, D. Importance of intestinal microflora: Dried toad skin-radix clematidis plasma component analysis and anti-CRC core target study. J. Pharm. Biomed. Anal. 2025, 260, 116802. [Google Scholar] [CrossRef]
- Onyeisi, J.O.S.; El-Shorafa, H.M.; Greve, B.; Gotte, M. Role of syndecan-4 in angiogenesis and vasculogenic mimicry in triple negative breast cancer cells. Matrix Biol. 2025, 136, 127–133. [Google Scholar] [CrossRef]
- Onali, T.; Slaba, H.; Jian, C.; Koivumaki, T.; Paivarinta, E.; Marttinen, M.; Maattanen, M.; Salonen, A.; Pajari, A.M. Berry supplementation in healthy volunteers modulates gut microbiota, increases fecal polyphenol metabolites and reduces viability of colon cancer cells exposed to fecal water- a randomized controlled trial. J. Nutr. Biochem. 2025, 141, 109906. [Google Scholar] [CrossRef]
- Lv, J.; Jin, S.; Zhou, Y.; Fu, C.; Shen, Y.; Liu, B.; Li, J.; Li, M.; Zhang, Y.; Feng, N. Gut microbiota-derived metabolite phenylacetylglutamine inhibits the progression of prostate cancer by suppressing the Wnt/beta-catenin signaling pathway. Front. Pharmacol. 2025, 16, 1528058. [Google Scholar] [CrossRef]
- Luan, W.W.; Gu, H.W.; Qiu, D.; Ding, X.; Liu, P.M.; Hashimoto, K.; Yang, J.J.; Wang, X.M. Repeated administration of esketamine ameliorates mechanical allodynia in mice with chemotherapy-induced peripheral neuropathy: A role of gut microbiota and metabolites. Neurochem. Int. 2025, 185, 105961. [Google Scholar] [CrossRef]
- Li, D.; Lan, X.; Xu, L.; Zhou, S.; Luo, H.; Zhang, X.; Yu, W.; Yang, Y.; Fang, X. Influence of gut microbial metabolites on tumor immunotherapy: Mechanisms and potential natural products. Front. Immunol. 2025, 16, 1552010. [Google Scholar] [CrossRef]
- Ou, J.; Yin, H.; Shu, F.; Wu, Z.; Liu, S.; Ye, J.; Zhang, S. Vasculogenic mimicry-related gene prognostic index for predicting prognosis, immune microenvironment in clear cell renal cell carcinoma. Heliyon 2024, 10, e36235. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, Q.; Wang, Y.; Wang, H.; Xiang, Z.; Xu, Y.; Wang, X.; Liu, W.; Wang, A. The vasculogenic mimicry related signature predicts the prognosis and immunotherapy response in renal clear cell carcinoma. BMC Cancer 2024, 24, 420. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, A.P.; Sanchez-Sanchez, G.; Carlos-Reyes, A.; Lopez-Camarillo, C. Functional roles of microRNAs in vasculogenic mimicry and resistance to therapy in human cancers: An update. Expert. Rev. Clin. Immunol. 2024, 20, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Lapkina, E.Z.; Esimbekova, A.R.; Ruksha, T.G. Vasculogenic mimicry. Arkh Patol. 2023, 85, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bellido, D.; Zamudio-Martinez, E.; Fernandez-Cortes, M.; Herrera-Campos, A.B.; Olmedo-Pelayo, J.; Perez, C.J.; Exposito, J.; de Alava, E.; Amaral, A.T.; Valle, F.O.; et al. VE-Cadherin modulates beta-catenin/TCF-4 to enhance Vasculogenic Mimicry. Cell Death Dis. 2023, 14, 135. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. The modes of angiogenesis: An updated perspective. Angiogenesis 2023, 26, 477–480. [Google Scholar] [CrossRef]
- Morales-Guadarrama, G.; Mendez-Perez, E.A.; Garcia-Quiroz, J.; Avila, E.; Ibarra-Sanchez, M.J.; Esparza-Lopez, J.; Garcia-Becerra, R.; Larrea, F.; Diaz, L. The Inhibition of the FGFR/PI3K/Akt Axis by AZD4547 Disrupts the Proangiogenic Microenvironment and Vasculogenic Mimicry Arising from the Interplay between Endothelial and Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 13770. [Google Scholar] [CrossRef]
- Shie, W.Y.; Chu, P.H.; Kuo, M.Y.; Chen, H.W.; Lin, M.T.; Su, X.J.; Hong, Y.L.; Chou, H.E. Acidosis promotes the metastatic colonization of lung cancer via remodeling of the extracellular matrix and vasculogenic mimicry. Int. J. Oncol. 2023, 63, 136. [Google Scholar] [CrossRef]
- Contreras-Sanzón, E.; Palma-Flores, C.; Flores-Pérez, A.; Salinas-Vera, Y.M.; Silva-Cázares, M.B.; Marchat, L.A.; Avila-Bonilla, R.G.; Hernández de la Cruz, O.N.; Álvarez-Sánchez, M.E.; Pérez-Plasencia, C.; et al. MicroRNA-204/CREB5 axis regulates vasculogenic mimicry in breast cancer cells. Cancer Biomark. 2022, 35, 47–56. [Google Scholar] [CrossRef]
- Andreucci, E.; Peppicelli, S.; Ruzzolini, J.; Bianchini, F.; Calorini, L. Physicochemical aspects of the tumour microenvironment as drivers of vasculogenic mimicry. Cancer Metastasis Rev. 2022, 41, 935–951. [Google Scholar] [CrossRef]
- Murai, T.; Matsuda, S. Targeting the PI3K-Akt-mTOR signaling pathway involved in vasculogenic mimicry promoted by cancer stem cells. Am. J. Cancer Res. 2023, 13, 5039–5046. [Google Scholar]
- Cohen, L.J.; Esterhazy, D.; Kim, S.H.; Lemetre, C.; Aguilar, R.R.; Gordon, E.A.; Pickard, A.J.; Cross, J.R.; Emiliano, A.B.; Han, S.M.; et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017, 549, 48–53. [Google Scholar] [CrossRef]
- Morales-Guadarrama, G.; Garcia-Becerra, R.; Mendez-Perez, E.A.; Garcia-Quiroz, J.; Avila, E.; Diaz, L. Vasculogenic Mimicry in Breast Cancer: Clinical Relevance and Drivers. Cells 2021, 10, 1758. [Google Scholar] [CrossRef]
- Liang, X.; Ma, X.; Luan, F.; Gong, J.; Zhao, S.; Pan, Y.; Liu, Y.; Liu, L.; Huang, J.; An, Y.; et al. Identification of new subtypes of breast cancer based on vasculogenic mimicry related genes and a new model for predicting the prognosis of breast cancer. Heliyon 2024, 10, e36565. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, R.; Sun, X.; Zhang, Z.; Wang, S.; Gao, X.; Sun, Z.; Xue, H.; Li, G. MicroRNA-588 regulates the invasive, migratory and vasculogenic mimicry-forming abilities of hypoxic glioma cells by targeting ROBO1. Mol. Biol. Rep. 2023, 50, 1333–1347. [Google Scholar] [CrossRef]
- Zhang, Y.; Che, N.; Wang, S.; Meng, J.; Zhao, N.; Han, J.; Dong, X.; Li, Y.; Mo, J.; Zhao, X.; et al. Nrf2/ASPM axis regulated vasculogenic mimicry formation in hepatocellular carcinoma under hypoxia. J. Gastroenterol. 2024, 59, 941–957. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, S.S.; Zheng, M.; Dai, L.L.; Wang, K.; Gao, X.L.; Cao, M.X.; Yu, X.H.; Pang, X.; Zhang, M.; et al. Hypoxia promotes vasculogenic mimicry formation by vascular endothelial growth factor A mediating epithelial-mesenchymal transition in salivary adenoid cystic carcinoma. Cell Prolif. 2019, 52, e12600. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, Y.; Li, M.; Zhang, W.; Ding, Y.; Wang, X.; Chen, D.; Liu, T.; Wang, B.; Cao, H.; et al. Clostridium butyricum inhibits epithelial-mesenchymal transition of intestinal carcinogenesis through downregulating METTL3. Cancer Sci. 2023, 114, 3114–3127. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zhang, Y.; Xiao, X.; Chu, F.; Zhang, L. Induction of lncRNA NORAD accounts for hypoxia-induced chemoresistance and vasculogenic mimicry in colorectal cancer by sponging the miR-495-3p/hypoxia-inducible factor-1alpha (HIF-1alpha). Bioengineered 2022, 13, 950–962. [Google Scholar] [CrossRef]
- Pang, V.; Bates, D.O.; Leach, L. Regulation of human feto-placental endothelial barrier integrity by vascular endothelial growth factors: Competitive interplay between VEGF-A(165)a, VEGF-A(165)b, PIGF and VE-cadherin. Clin. Sci. 2017, 131, 2763–2775. [Google Scholar] [CrossRef]
- Milovanovic, J.; Vujasinovic, T.; Todorovic-Rakovic, N.; Greenman, J.; Hranisavljevic, J.; Radulovic, M. Vascular endothelial growth factor (VEGF) -A, -C and VE-cadherin as potential biomarkers in early breast cancer patients. Pathol. Res. Pract. 2023, 252, 154923. [Google Scholar] [CrossRef]
- Hsu, J.L.; Leu, W.J.; Hsu, L.C.; Hsieh, C.H.; Guh, J.H. Doxazosin inhibits vasculogenic mimicry in human non-small cell lung cancer through inhibition of the VEGF-A/VE-cadherin/mTOR/MMP pathway. Oncol. Lett. 2024, 27, 170. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, C.; Zhang, J.; Su, W.; Wang, G.; Wang, Z. The Kynurenine Pathway and Indole Pathway in Tryptophan Metabolism Influence Tumor Progression. Cancer Med. 2025, 14, e70703. [Google Scholar] [CrossRef]
- Baffy, G. Gut Microbiota and Cancer of the Host: Colliding Interests. Adv. Exp. Med. Biol. 2020, 1219, 93–107. [Google Scholar] [CrossRef]
- Wang, S.; Yang, D.; Yuan, C.; Wu, Y.; Wang, Q.; Wu, Y.; Zhang, X. Herbal Formula Yi-Fei-Jie-Du-Tang Regulates Epithelial-Mesenchymal Transition and Vasculogenic Mimicry in Lung Cancer via HIF1A-Mediated Ferroptosis. Adv. Biol. 2025, 9, e2400306. [Google Scholar] [CrossRef]
- Ichikawa, M.K.; Endo, K.; Itoh, Y.; Osada, A.H.; Kimura, Y.; Ueki, K.; Yoshizawa, K.; Miyazawa, K.; Saitoh, M. Ets family proteins regulate the EMT transcription factors Snail and ZEB in cancer cells. FEBS Open Bio 2022, 12, 1353–1364. [Google Scholar] [CrossRef]
- Tavakolian, S.; Goudarzi, H.; Faghihloo, E. E-cadherin, Snail, ZEB-1, DNMT1, DNMT3A and DNMT3B expression in normal and breast cancer tissues. Acta Biochim. Pol. 2019, 66, 409–414. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Hu, L. The regulatory effects of metformin on the [SNAIL/miR-34]:[ZEB/miR-200] system in the epithelial-mesenchymal transition(EMT) for colorectal cancer(CRC). Eur. J. Pharmacol. 2018, 834, 45–53. [Google Scholar] [CrossRef]
- Akrida, I.; Makrygianni, M.; Nikou, S.; Mulita, F.; Bravou, V.; Papadaki, H. Hippo pathway effectors YAP, TAZ and TEAD are associated with EMT master regulators ZEB, Snail and with aggressive phenotype in phyllodes breast tumors. Pathol. Res. Pract. 2024, 262, 155551. [Google Scholar] [CrossRef]
- Su, N.W.; Wu, S.H.; Chi, C.W.; Liu, C.J.; Tsai, T.H.; Chen, Y.J. Metronomic Cordycepin Therapy Prolongs Survival of Oral Cancer-Bearing Mice and Inhibits Epithelial-Mesenchymal Transition. Molecules 2017, 22, 629. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Gallardo-Rincon, D.; Ruiz-Garcia, E.; Marchat, L.A.; Valdes, J.; Vazquez-Calzada, C.; Lopez-Camarillo, C. A Three-Dimensional Culture-Based Assay to Detect Early Stages of Vasculogenic Mimicry in Ovarian Cancer Cells. Methods Mol. Biol. 2022, 2514, 53–60. [Google Scholar] [CrossRef]
- Tagliamonte, M.; Buonaguro, L. The impact of antigenic molecular mimicry on anti-cancer T-cell immune response. Front. Oncol. 2022, 12, 1009247. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Hussein, S.; Choi, G.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. The Species of Gut Bacteria Associated with Antitumor Immunity in Cancer Therapy. Cells 2022, 11, 3684. [Google Scholar] [CrossRef]
- Myneedu, K.; Deoker, A.; Schmulson, M.J.; Bashashati, M. Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 1033–1041. [Google Scholar] [CrossRef]
- Maroufi, N.F.; Amiri, M.; Dizaji, B.F.; Vahedian, V.; Akbarzadeh, M.; Roshanravan, N.; Haiaty, S.; Nouri, M.; Rashidi, M.R. Inhibitory effect of melatonin on hypoxia-induced vasculogenic mimicry via suppressing epithelial-mesenchymal transition (EMT) in breast cancer stem cells. Eur. J. Pharmacol. 2020, 881, 173282. [Google Scholar] [CrossRef]
- Resendiz-Hernandez, M.; Garcia-Hernandez, A.P.; Silva-Cazares, M.B.; Coronado-Uribe, R.; Hernandez-de la Cruz, O.N.; Arriaga-Pizano, L.A.; Prieto-Chavez, J.L.; Salinas-Vera, Y.M.; Ibarra-Sierra, E.; Ortiz-Martinez, C.; et al. MicroRNA-204 Regulates Angiogenesis and Vasculogenic Mimicry in CD44+/CD24- Breast Cancer Stem-like Cells. Noncoding RNA 2024, 10, 14. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Zhang, R.; Kong, Z. DAB2IP-knocking down resulted in radio-resistance of breast cancer cells is associated with increased hypoxia and vasculogenic mimicry formation. Int. J. Radiat. Biol. 2023, 99, 1595–1606. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Z.; Yu, Y.; Zhang, P. HIF1alpha lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int. J. Biol. Macromol. 2022, 222, 2225–2243. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, X.; Chen, H.; Wang, Z.; Bu, H.; Lin, S. Rg3 inhibits hypoxia-induced tumor exosomes from boosting pancreatic cancer vasculogenic mimicry through the HIF-1alpha/LARS1/mTOR axis. Phytomedicine 2025, 139, 156437. [Google Scholar] [CrossRef]
- Saha, D.; Mitra, D.; Alam, N.; Sen, S.; Mustafi, S.M.; Majumder, P.K.; Majumder, B.; Murmu, N. Lupeol and Paclitaxel cooperate in hindering hypoxia induced vasculogenic mimicry via suppression of HIF-1alpha-EphA2-Laminin-5gamma2 network in human oral cancer. J. Cell Commun. Signal 2023, 17, 591–608. [Google Scholar] [CrossRef]
- Hu, H.; Ma, T.; Liu, N.; Hong, H.; Yu, L.; Lyu, D.; Meng, X.; Wang, B.; Jiang, X. Immunotherapy checkpoints in ovarian cancer vasculogenic mimicry: Tumor immune microenvironments, and drugs. Int. Immunopharmacol. 2022, 111, 109116. [Google Scholar] [CrossRef]
- Liao, Q.; Zhou, X.; Wu, L.; Yang, Y.; Zhu, X.; Liao, H.; Zhang, Y.; Lian, W.; Zhang, F.; Wang, H.; et al. Gut microbial metabolite 4-hydroxybenzeneacetic acid drives colorectal cancer progression via accumulation of immunosuppressive PMN-MDSCs. J. Clin. Investig. 2025; ahead of print. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, W.; Yin, Z.; Zou, Y.; Liang, S.; Ruan, S.; Chen, P.; Li, S.; Shu, Q.; Cheng, B.; et al. Melittin Inhibits Hypoxia-Induced Vasculogenic Mimicry Formation and Epithelial-Mesenchymal Transition through Suppression of HIF-1alpha/Akt Pathway in Liver Cancer. Evid. Based Complement. Altern. Med. 2019, 2019, 9602935. [Google Scholar] [CrossRef]
- Li, W.; Zong, S.; Shi, Q.; Li, H.; Xu, J.; Hou, F. Hypoxia-induced vasculogenic mimicry formation in human colorectal cancer cells: Involvement of HIF-1a, Claudin-4, and E-cadherin and Vimentin. Sci. Rep. 2016, 6, 37534. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Gao, X.; Wang, J.; Xue, H.; Chen, Z.; Zhang, J.; Guo, X.; Qian, M.; Qiu, W.; et al. Macrophage migration inhibitory factor promotes vasculogenic mimicry formation induced by hypoxia via CXCR4/AKT/EMT pathway in human glioblastoma cells. Oncotarget 2017, 8, 80358–80372. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, S.; Wu, W.; Lu, W.; Jiang, M.; Zheng, N.; Huang, J.; Wang, L.; Liu, H.; Zheng, M.; et al. Regorafenib inhibits migration, invasion, and vasculogenic mimicry of hepatocellular carcinoma via targeting ID1-mediated EMT. Mol. Carcinog. 2021, 60, 151–163. [Google Scholar] [CrossRef]
- Kang, X.; Xu, E.; Wang, X.; Qian, L.; Yang, Z.; Yu, H.; Wang, C.; Ren, C.; Wang, Y.; Lu, X.; et al. Tenascin-c knockdown suppresses vasculogenic mimicry of gastric cancer by inhibiting ERK- triggered EMT. Cell Death Dis. 2021, 12, 890. [Google Scholar] [CrossRef]
- You, X.; Liu, Q.; Wu, J.; Wang, Y.; Dai, J.; Chen, D.; Zhou, Y.; Lian, Y. Galectin-1 Promotes Vasculogenic Mimicry in Gastric Cancer by Upregulating EMT Signaling. J. Cancer 2019, 10, 6286–6297. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zheng, M.; Tang, Y.L.; Liang, X.H. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review). Oncol. Lett. 2013, 6, 1174–1180. [Google Scholar] [CrossRef]
- Jue, C.; Lin, C.; Zhisheng, Z.; Yayun, Q.; Feng, J.; Min, Z.; Haibo, W.; Youyang, S.; Hisamitsu, T.; Shintaro, I.; et al. Notch1 promotes vasculogenic mimicry in hepatocellular carcinoma by inducing EMT signaling. Oncotarget 2017, 8, 2501–2513. [Google Scholar] [CrossRef]
- Pandey, S.; Anshu, T.; Maharana, K.C.; Sinha, S. Molecular insights into diabetic wound healing: Focus on Wnt/β-catenin and MAPK/ERK signaling pathways. Cytokine 2025, 191, 156957. [Google Scholar] [CrossRef]
- Mathpal, S.; Joshi, T.; Sharma, P.; Maiti, P.; Nand, M.; Pande, V.; Chandra, S. In silico screening of chalcone derivatives as promising EGFR-TK inhibitors for the clinical treatment of cancer. 3 Biotech 2024, 14, 18. [Google Scholar] [CrossRef]
- Entezari, M.; Deldar Abad Paskeh, M.; Orouei, S.; Kakavand, A.; Rezaei, S.; Sadat Hejazi, E.; Pashootan, P.; Nazdari, N.; Tavakolpournegari, A.; Hashemi, M.; et al. Wnt/beta-catenin Signaling in Lung Cancer: Association with Proliferation, Metastasis, and Therapy Resistance. Curr. Cancer Drug Targets 2023, 24, 94–113. [Google Scholar] [CrossRef]
- Jiang, X.W.; Zhang, L.; Liu, Z.C.; Zhou, T.; Li, W.Q.; Liu, W.D.; Zhang, L.F.; You, W.C.; Zhang, Y.; Pan, K.F. Integrative metabolomics and microbiomics analysis reveals distinctive microbiota-metabolites interactions in gastric carcinogenesis. Int. J. Cancer 2025, 156, 2389–2400. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Y.; Zhang, M.J.; An, Q.; Tao, J.N.; Wang, X.H. Interaction Between Hypoxia-Inducible Factor 1-alpha Gene Polymorphism and Helicobacter pylori Infection on Gastric Cancer in a Chinese Tibetan Population. Biochem. Genet. 2024; ahead of print. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, Q.; Hu, Y.; Wang, S.; Xu, J.; Ye, J. Galectin from Trichinella spiralis alleviates DSS-induced colitis in mice by regulating the intestinal microbiota. Vet. Res. 2024, 55, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, T.; Li, X.; Zhang, Y.; Zou, X.; Chen, F.; Yue, L. Single-cell atlas of dental pulp stem cells exposed to the oral bacteria Porphyromonas gingivalis and Enterococcus faecalis. Front. Cell Dev. Biol. 2023, 11, 1166934. [Google Scholar] [CrossRef]
- Sun, X.; Cai, W.; Li, H.; Gao, C.; Ma, X.; Guo, Y.; Fu, D.; Xiao, D.; Zhang, Z.; Wang, Y.; et al. Endothelial-like cancer-associated fibroblasts facilitate pancreatic cancer metastasis via vasculogenic mimicry and paracrine signalling. Gut, 2025; ahead of print. [Google Scholar] [CrossRef]
- Herrera-Vargas, A.K.; Garcia-Rodriguez, E.; Olea-Flores, M.; Mendoza-Catalan, M.A.; Flores-Alfaro, E.; Navarro-Tito, N. Pro-angiogenic activity and vasculogenic mimicry in the tumor microenvironment by leptin in cancer. Cytokine Growth Factor. Rev. 2021, 62, 23–41. [Google Scholar] [CrossRef]
- An, T.Y.; Hu, Q.M.; Ni, P.; Hua, Y.Q.; Wang, D.; Duan, G.C.; Chen, S.Y.; Jia, B. N6-methyladenosine modification of hypoxia-inducible factor-1alpha regulates Helicobacter pylori-associated gastric cancer via the PI3K/AKT pathway. World. J. Gastrointest. Oncol. 2024, 16, 3270–3283. [Google Scholar] [CrossRef]
- Pradhan, N.; Parbin, S.; Kar, S.; Das, L.; Kirtana, R.; Suma Seshadri, G.; Sengupta, D.; Deb, M.; Kausar, C.; Patra, S.K. Epigenetic silencing of genes enhanced by collective role of reactive oxygen species and MAPK signaling downstream ERK/Snail axis: Ectopic application of hydrogen peroxide repress CDH1 gene by enhanced DNA methyltransferase activity in human breast cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1651–1665. [Google Scholar] [CrossRef]
- Gao, Y.; Zandieh, K.; Zhao, K.; Khizanishvili, N.; Fazio, P.D.; Yu, X.; Schulte, L.; Aillaud, M.; Chung, H.R.; Ball, Z.; et al. The long non-coding RNA NEAT1 contributes to aberrant STAT3 signaling in pancreatic cancer and is regulated by a metalloprotease-disintegrin ADAM8/miR-181a-5p axis. Cell. Oncol. 2024, 48, 391–409. [Google Scholar] [CrossRef]
- Hsu, P.C.; Cheng, C.F.; Hsieh, P.C.; Chen, Y.H.; Kuo, C.Y.; Sytwu, H.K. Chrysophanol Regulates Cell Death, Metastasis, and Reactive Oxygen Species Production in Oral Cancer Cell Lines. Evid. Based Complement. Altern. Med. 2020, 2020, 5867064. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.Y.; Qu, F.Z.; Su, G.Y.; Zhao, Y.Q. 4-XL-PPD, a novel ginsenoside derivative, as potential therapeutic agents for gastric cancer shows anti-cancer activity via inducing cell apoptosis medicated generation of reactive oxygen species and inhibiting migratory and invasive. Biomed. Pharmacother. 2019, 118, 108589. [Google Scholar] [CrossRef]
- Sun, M.; Peng, Z.; Shen, W.; Guo, X.; Liao, Y.; Huang, Y.; Ye, P.; Hu, M.; Lin, Q.; Liu, R. Synergism of Fusobacterium periodonticum and N-nitrosamines promote the formation of EMT subtypes in ESCC by modulating Wnt3a palmitoylation. Gut Microbes 2024, 16, 2391521. [Google Scholar] [CrossRef]
- Liu, G.; Wang, P.; Zhang, H. MiR-6838-5p suppresses cell metastasis and the EMT process in triple-negative breast cancer by targeting WNT3A to inhibit the Wnt pathway. J. Gene Med. 2019, 21, e3129. [Google Scholar] [CrossRef]
- Nisar, M.A.; Zheng, Q.; Saleem, M.Z.; Ahmmed, B.; Ramzan, M.N.; Ud Din, S.R.; Tahir, N.; Liu, S.; Yan, Q. IL-1beta Promotes Vasculogenic Mimicry of Breast Cancer Cells Through p38/MAPK and PI3K/Akt Signaling Pathways. Front. Oncol. 2021, 11, 618839. [Google Scholar] [CrossRef]
- Li, J.; Shen, J.; Zhao, Y.; Du, F.; Li, M.; Wu, X.; Chen, Y.; Wang, S.; Xiao, Z.; Wu, Z. Role of miR-181a-5p in cancer (Review). Int. J. Oncol. 2023, 63, 108. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.Z.; Yang, H.; Cao, L.Z.; Wang, X.Y.; Wang, D.L.; Chatterjee, E.; Li, Y.F.; Huang, G. Cardioprotection of voluntary exercise against breast cancer-induced cardiac injury via STAT3. Basic Res. Cardiol. 2025, 120, 113–131. [Google Scholar] [CrossRef]
- Iqbal, M.; Yu, Q.; Tang, J.; Xiang, J. Unraveling the gut microbiota’s role in obesity: Key metabolites, microbial species, and therapeutic insights. J. Bacteriol. 2025, 207, e0047924. [Google Scholar] [CrossRef]
- Han, K.; Luo, D.; Zou, Y.; Dong, S.; Wan, Z.; Yang, X. Modulation of Gut Microbiota by Soybean 7S Globulin Peptide That Involved Lipopolysaccharide-Peptide Interaction. J. Agric. Food Chem. 2019, 67, 2201–2211. [Google Scholar] [CrossRef]
- Porbaran, M.; Tahmasebi, H.; Arabestani, M. A Comprehensive Study of the Relationship between the Production of beta-Lactamase Enzymes and Iron/Siderophore Uptake Regulatory Genes in Clinical Isolates of Acinetobacter baumannii. Int. J. Microbiol. 2021, 2021, 5565537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Han, Y.; Chen, X.; Gong, P.; Zhai, P.; Yao, W.; Ba, Q.; Wang, H. Eucommia bark/leaf extract improves HFD-induced lipid metabolism disorders via targeting gut microbiota to activate the Fiaf-LPL gut-liver axis and SCFAs-GPR43 gut-fat axis. Phytomedicine 2023, 110, 154652. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Deng, C.; Li, Y.; He, S.; Liu, Y.; Pan, S.; Xu, W.; Fang, L.; Zhu, Y.; Wang, Y.; et al. Machine learning-derived diagnostic model of epithelial ovarian cancer based on gut microbiome signatures. J. Transl. Med. 2025, 23, 319. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Bharath, C.; Balakrishnan, S.; Kandasamy, S.; Priyadharshini, I.; Ravi, S. Expression of HIF-1alpha and Nestin in oral squamous cell carcinoma and its association with vasculogenic mimicry. J. Cancer Res. Ther. 2024, 20, 176–180. [Google Scholar] [CrossRef]
- Zhao, B.; Niu, X.; Huang, S.; Yang, J.; Wei, Y.; Wang, X.; Wang, J.; Wang, Y.; Guo, X. TLR4 Agonist and Hypoxia Synergistically Promote the Formation of TLR4/NF-kappaB/HIF-1alpha Loop in Human Epithelial Ovarian Cancer. Anal. Cell. Pathol. 2022, 2022, 4201262. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Gao, R.; Xiu, Z.; Sun, T. Knockdown of cZNF292 suppressed hypoxic human hepatoma SMMC7721 cell proliferation, vasculogenic mimicry, and radioresistance. Cell Signal 2019, 60, 122–135. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Zhu, R.; Wang, S.; Xue, J.; Zhang, D.; Lan, Z.; Zhang, C.; Liang, Y.; Zhang, N.; et al. Gut microbiota and metabolites signatures of clinical response in anti-PD-1/PD-L1 based immunotherapy of biliary tract cancer. Biomark. Res. 2024, 12, 56. [Google Scholar] [CrossRef]
- Tian, J.; Ma, J. The Value of Microbes in Cancer Neoantigen Immunotherapy. Pharmaceutics 2023, 15, 2138. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Ma, K.Y.; Chen, Z.Y. Mangiferin alleviates trimethylamine-N-oxide (TMAO)-induced atherogenesis and modulates gut microbiota in mice. Food Funct. 2023, 14, 9212–9225. [Google Scholar] [CrossRef]
- Tabrizi, E.; Pourteymour Fard Tabrizi, F.; Mahmoud Khaled, G.; Sestito, M.P.; Jamie, S.; Boone, B.A. Unraveling the gut microbiome’s contribution to pancreatic ductal adenocarcinoma: Mechanistic insights and therapeutic perspectives. Front. Immunol. 2024, 15, 1434771. [Google Scholar] [CrossRef]
- Hai, S.; Li, X.; Xie, E.; Wu, W.; Gao, Q.; Yu, B.; Hu, J.; Xu, F.; Zheng, X.; Zhang, B.H.; et al. Intestinal IL-33 promotes microbiota-derived trimethylamine N -oxide synthesis and drives metabolic dysfunction-associated steatotic liver disease progression by exerting dual regulation on HIF-1alpha. Hepatology, 2024; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhen, X.; Liu, Z.; Chen, X.; Liu, Z.; Zhou, M.; Zhou, Z.; Hu, Z.; Zhu, F.; Huang, Q.; et al. Dietary choline, via gut microbe- generated trimethylamine-N- oxide, aggravates chronic kidney disease-induced cardiac dysfunction by inhibiting hypoxia-induced factor 1alpha. Front. Physiol. 2022, 13, 996166. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Cheng, R.; Shen, L.; Sun, Y.; Wang, P.; Jiang, G.; Wen, L.; Li, X.; Peng, X.; Liao, Y.; et al. High-fat diet induces sarcopenic obesity in natural aging rats through the gut-trimethylamine N-oxide-muscle axis. J. Adv. Res. 2025, 70, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, J.; Shan, J.; Hong, Y.; Zhu, W.; Nie, Z.; Zhang, Y.; Ji, N.; Luo, X.; Zhang, T.; et al. Gut-Flora-Dependent Metabolite Trimethylamine-N-Oxide Promotes Atherosclerosis-Associated Inflammation Responses by Indirect ROS Stimulation and Signaling Involving AMPK and SIRT1. Nutrients 2022, 14, 3338. [Google Scholar] [CrossRef]
- Yang, H.; Mo, A.; Yi, L.; Wang, J.; He, X.; Yuan, Y. Selenium attenuated food borne cadmium-induced intestinal inflammation in red swamp crayfish (Procambarus clarkii) via regulating PI3K/Akt/NF-kappaB pathway. Chemosphere 2024, 349, 140814. [Google Scholar] [CrossRef]
- Tokuhira, N.; Kitagishi, Y.; Suzuki, M.; Minami, A.; Nakanishi, A.; Ono, Y.; Kobayashi, K.; Matsuda, S.; Ogura, Y. PI3K/AKT/PTEN pathway as a target for Crohn’s disease therapy (Review). Int. J. Mol. Med. 2015, 35, 10–16. [Google Scholar] [CrossRef]
- Marangon Junior, H.; Rocha, V.N.; Leite, C.F.; de Aguiar, M.C.; Souza, P.E.; Horta, M.C. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2014, 43, 199–204. [Google Scholar] [CrossRef]
- Yu, X.; Ou, J.; Wang, L.; Li, Z.; Ren, Y.; Xie, L.; Chen, Z.; Liang, J.; Shen, G.; Zou, Z.; et al. Gut microbiota modulate CD8+ T cell immunity in gastric cancer through Butyrate/GPR109A/HOPX. Gut Microbes 2024, 16, 2307542. [Google Scholar] [CrossRef]
- Gong, F.; Miller, K.M. Mammalian DNA repair: HATs and HDACs make their mark through histone acetylation. Mutat. Res. 2013, 750, 23–30. [Google Scholar] [CrossRef]
- Littleflower, A.B.; Parambil, S.T.; Antony, G.R.; M, S.A.; Subhadradevi, L. Glut-1 inhibition in breast cancer cells. Vitam. Horm. 2025, 128, 181–211. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zou, Z.; Lin, T.; Guo, C.; Lin, D. Engineered Lactobacillus rhamnosus Producing 3-Hydroxybutyrate: A Dual-Action Therapeutic Strategy for Colon Cancer Cachexia. Biotechnol. Bioeng. 2025, 122, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, K.; Liu, G.; Wu, R.; Zhang, Y.; Wang, S.; Xu, M.; Lu, L.; Li, P. Microbial metabolite butyrate promotes anti-PD-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic CD8 T cell. Gut Microbes 2023, 15, 2249143. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Parsasefat, M.; Bahar, A.; Tahmasebi, H.; Oksenych, V. Behavioral Cooperation or Conflict of Human Intestinal Roundworms and Microbiomes: A Bio-Activity Perspective. Cells 2025, 14, 556. [Google Scholar] [CrossRef]
- Zhang, K.; Ji, X.; Song, Z.; Wu, F.; Qu, Y.; Jin, X.; Xue, X.; Wang, F.; Huang, Y. Butyrate Inhibits Gastric Cancer Cells by Inducing Mitochondriamediated Apoptosis. Comb. Chem. High Throughput Screen. 2023, 26, 630–638. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Liang, G.; Xiao, H.; Zhang, L.; Liu, T. Sonodynamic activated nanoparticles with Glut1 inhibitor and cystine-containing polymer stimulate disulfidptosis for improved immunotherapy in bladder cancer. Biomaterials 2025, 319, 123178. [Google Scholar] [CrossRef]
- Ghotaslou, A.; Azizsoltani, A.; Baghaei, K.; Alizadeh, E. Harnessing HEK293 cell-derived exosomes for hsa-miR-365a-3p delivery: Potential application in hepatocellular carcinoma therapy. Heliyon 2024, 10, e29333. [Google Scholar] [CrossRef]
- Ueno, K.; Ajiki, T.; Tsugawa, D.; Akita, M.; Hashimoto, Y.; Awazu, M.; Mukubo, H.; Komatsu, S.; Kuramitsu, K.; Terai, S.; et al. Effectiveness of omega-3 fatty acid administration on completion rate of adjuvant chemotherapy for biliary tract cancer: Study protocol for a single-centre, open-label, single-arm, historically controlled study. BMJ Open 2019, 9, e029915. [Google Scholar] [CrossRef]
- Goossens, J.F.; Bailly, C. Ursodeoxycholic acid and cancer: From chemoprevention to chemotherapy. Pharmacol. Ther. 2019, 203, 107396. [Google Scholar] [CrossRef]
- Kitamura, T.; Srivastava, J.; DiGiovanni, J.; Kiguchi, K. Bile acid accelerates erbB2-induced pro-tumorigenic activities in biliary tract cancer. Mol. Carcinog. 2015, 54, 459–472. [Google Scholar] [CrossRef]
- Kazmi, I.; Altamimi, A.S.A.; Afzal, M.; Majami, A.A.; AlGhamdi, A.S.; Alkinani, K.B.; Abbasi, F.A.; Almalki, W.H.; Alzera, S.I.; Kukreti, N.; et al. The emerging role of non-coding RNAs in the Wnt/beta-catenin signaling pathway in Prostate Cancer. Pathol. Res. Pract. 2024, 254, 155134. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, U.; Chaudhary, P.; Lim, D.; Dahal, S.; Awasthi, B.P.; Park, H.D.; Kim, J.A.; Jeong, B.S. Synthesis and anticancer evaluation of 6-azacyclonol-2,4,6-trimethylpyridin-3-ol derivatives: M3 muscarinic acetylcholine receptor-mediated anticancer activity of a cyclohexyl derivative in androgen-refractory prostate cancer. Bioorg. Chem. 2021, 110, 104805. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, M.; Larabee, S.M.; Hu, S.; Xie, G.; Raufman, J.P. The Role of M3 Muscarinic Receptor Ligand-Induced Kinase Signaling in Colon Cancer Progression. Cancers 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xia, H.; Tang, Q.; Xu, H.; Wei, G.; Chen, Y.; Dai, X.; Gong, Q.; Bi, F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci. Rep. 2017, 7, 40802. [Google Scholar] [CrossRef]
- Artusa, V.; Calabrone, L.; Mortara, L.; Peri, F.; Bruno, A. Microbiota-Derived Natural Products Targeting Cancer Stem Cells: Inside the Gut Pharma Factory. Int. J. Mol. Sci. 2023, 24, 4997. [Google Scholar] [CrossRef]
- Shi, Q.; Yuan, X.; Zeng, Y.; Wang, J.; Zhang, Y.; Xue, C.; Li, L. Crosstalk between Gut Microbiota and Bile Acids in Cholestatic Liver Disease. Nutrients 2023, 15, 2411. [Google Scholar] [CrossRef]
- Morgan, R.G.; Mortensson, E.; Williams, A.C. Targeting LGR5 in Colorectal Cancer: Therapeutic gold or too plastic? Br. J. Cancer 2018, 118, 1410–1418. [Google Scholar] [CrossRef]
- Wang, W.; Lokman, N.A.; Barry, S.C.; Oehler, M.K.; Ricciardelli, C. LGR5: An emerging therapeutic target for cancer metastasis and chemotherapy resistance. Cancer Metastasis Rev. 2025, 44, 23. [Google Scholar] [CrossRef]
- Ronis, M.J.; Mercer, K.E.; Shankar, K.; Pulliam, C.; Pedersen, K.; Ingelman-Sundberg, M.; Friso, S.; Samuelson, D.; Del Valle, L.; Taylor, C.; et al. Potential role of gut microbiota, the proto-oncogene PIKE (Agap2) and cytochrome P450 CYP2W1 in promotion of liver cancer by alcoholic and nonalcoholic fatty liver disease and protection by dietary soy protein. Chem. Biol. Interact. 2020, 325, 109131. [Google Scholar] [CrossRef]
- Girisa, S.; Henamayee, S.; Parama, D.; Rana, V.; Dutta, U.; Kunnumakkara, A.B. Targeting Farnesoid X receptor (FXR) for developing novel therapeutics against cancer. Mol. Biomed. 2021, 2, 21. [Google Scholar] [CrossRef]
- Wang, X.X.; Xie, C.; Libby, A.E.; Ranjit, S.; Levi, J.; Myakala, K.; Bhasin, K.; Jones, B.A.; Orlicky, D.J.; Takahashi, S.; et al. The role of FXR and TGR5 in reversing and preventing progression of Western diet-induced hepatic steatosis, inflammation, and fibrosis in mice. J. Biol. Chem. 2022, 298, 102530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Kang, W.; Liu, S.; Liu, J.; Shi, M.; Wang, Y.; Liu, X.; Chen, X.; Huang, K. Aflatoxin B1 induces liver injury by disturbing gut microbiota-bile acid-FXR axis in mice. Food Chem. Toxicol. 2023, 176, 113751. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lv, X.; Liu, G.; Li, S.; Fan, J.; Chen, L.; Huang, Z.; Lin, G.; Xu, X.; Huang, Z.; et al. Gut microbiota-related bile acid metabolism-FXR/TGR5 axis impacts the response to anti-alpha4beta7-integrin therapy in humanized mice with colitis. Gut Microbes 2023, 15, 2232143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, W.; Wang, Y.; Li, S.; Shan, C.; Dong, J.; Xu, Z.; Zou, H.; Pan, Y.; Chen, X.; et al. Calycosin regulates gut microbiota-bile acid-FXR axis to protect rats from cerebral ischemia-reperfusion injury. Eur. J. Pharmacol. 2025, 1000, 177707. [Google Scholar] [CrossRef]
- Liu, X.; Fang, W.; Pang, S.; Song, G.; Wang, Y.; Qi, W. Total dietary fiber of tartary buckwheat alleviates T2DM through the IRS-1/PI3K/AKT pathway and gut microbiota-bile acids-TGR5/FXR axis in db/db mice. Int. J. Biol. Macromol. 2025, 308 Pt 3, 142145. [Google Scholar] [CrossRef]
- Wang, J.; Zang, J.; Yu, Y.; Liu, Y.; Cao, H.; Guo, R.; Zhang, L.; Liu, M.; Zhang, Z.; Li, X.; et al. Lingguizhugan oral solution alleviates MASLD by regulating bile acids metabolism and the gut microbiota through activating FXR/TGR5 signaling pathways. Front. Pharmacol. 2024, 15, 1426049. [Google Scholar] [CrossRef]
- Sun, H.; Zhai, Q.; Liu, J.; Shi, K.; Fan, W. Interplay between the gut microbiota, its metabolites and carcinogens. Clin. Transl. Oncol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Laaraj, J.; Lachance, G.; Bergeron, A.; Fradet, Y.; Robitaille, K.; Fradet, V. New insights into gut microbiota-prostate cancer crosstalk. Trends Mol. Med. 2025; ahead of print. [Google Scholar] [CrossRef]
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schonlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023, 615, 168–174, Erratum in: Nature 2025, ahead of print. [Google Scholar] [CrossRef]
- Yao, B.; Wei, W.; Zhang, H. Efficacy of probiotics or synbiotics supplementation on chemotherapy-induced complications and gut microbiota dysbiosis in gastrointestinal cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2024; ahead of print. [Google Scholar] [CrossRef]
- Nakamoto, S.; Kajiwara, Y.; Taniguchi, K.; Hida, A.I.; Miyoshi, Y.; Kin, T.; Yamamoto, M.; Takabatake, D.; Kubo, S.; Hikino, H.; et al. Baseline gut microbiota as a predictive marker for the efficacy of neoadjuvant chemotherapy in patients with early breast cancer: A multicenter prospective cohort study in the Setouchi Breast Project-14. Breast Cancer Res. Treat. 2024, 208, 67–77. [Google Scholar] [CrossRef]
- Kwon, B. A metabolite of the gut microbiota: A facilitator of chemotherapy efficacy in cancer. Signal Transduct. Target. Ther. 2023, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhuang, Y.; Liang, Y.; Chen, H.; Qiu, W.; Xu, H.; Zhou, H. Curcumin exerts anti-tumor activity in colorectal cancer via gut microbiota-mediated CD8(+) T Cell tumor infiltration and ferroptosis. Food Funct. 2025, 16, 3671–3693. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, H.; Liu, F.; Chen, Z.S.; Tang, H. Intratumoral microbiota in orchestrating cancer immunotherapy response. J. Transl. Int. Med. 2024, 12, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, L.; Ren, Q.; Zhu, C.; Du, H.; Wang, Z.; Qi, Y.; Xian, X.; Chen, D. Gut microbiota as a biomarker and modulator of anti-tumor immunotherapy outcomes. Front. Immunol. 2024, 15, 1471273. [Google Scholar] [CrossRef]
- Xiao, K.; Li, K.; Xiao, K.; Yang, J.; Zhou, L. Gut Microbiota and Hepatocellular Carcinoma: Metabolic Products and Immunotherapy Modulation. Cancer Med. 2025, 14, e70914. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, W.; Zhu, S.; Lin, T.; Mao, R.; Wu, N.; Zhang, P.; Kang, M. Gut and Intratumoral microbiota: Key to lung Cancer development and immunotherapy. Int. Immunopharmacol. 2025, 156, 114677. [Google Scholar] [CrossRef]
- Vucinic, D.; Redzovic, A.; Hauser, G.; Mikolasevic, I. Microbiota and Radiotherapy: Unlocking the Potential for Improved Gastrointestinal Cancer Treatment. Biomedicines 2025, 13, 526. [Google Scholar] [CrossRef]
- Kwiatkowski, D.; Schuch, L.F.; Klaus, N.M.; Martins, M.D.; Hilgert, J.B.; Hashizume, L.N. Oral microbiota in head and neck cancer patients during radiotherapy: A systematic review. Support. Care Cancer 2025, 33, 127. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamalabadi Farahani, M.; Bahar, A.; Tahmasebi, H.; Oksenych, V.; Jahantigh, M. Microbial Metabolite Effects on Vasculogenic Mimicry in Metastatic Cancers. Cells 2025, 14, 811. https://doi.org/10.3390/cells14110811
Kamalabadi Farahani M, Bahar A, Tahmasebi H, Oksenych V, Jahantigh M. Microbial Metabolite Effects on Vasculogenic Mimicry in Metastatic Cancers. Cells. 2025; 14(11):811. https://doi.org/10.3390/cells14110811
Chicago/Turabian StyleKamalabadi Farahani, Mohammad, Aisa Bahar, Hamed Tahmasebi, Valentyn Oksenych, and Mojdeh Jahantigh. 2025. "Microbial Metabolite Effects on Vasculogenic Mimicry in Metastatic Cancers" Cells 14, no. 11: 811. https://doi.org/10.3390/cells14110811
APA StyleKamalabadi Farahani, M., Bahar, A., Tahmasebi, H., Oksenych, V., & Jahantigh, M. (2025). Microbial Metabolite Effects on Vasculogenic Mimicry in Metastatic Cancers. Cells, 14(11), 811. https://doi.org/10.3390/cells14110811








