Insulin in Myenteric Neurons: Time-Dependent and Regional Changes in Type 1 Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
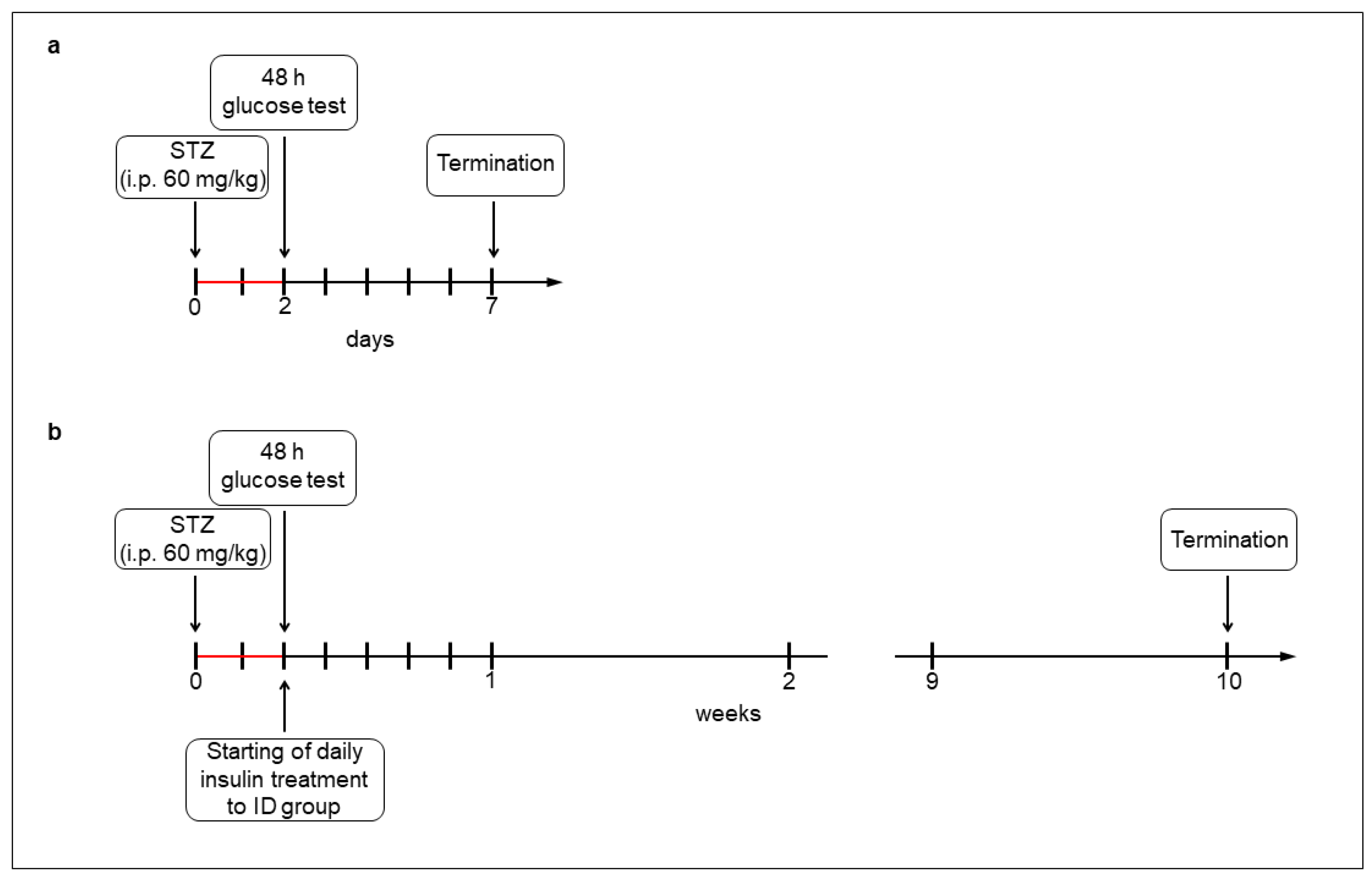
2.1. Acute and Chronic Type 1 Diabetic Animal Models
2.2. Tissue Handling
2.3. Fluorescent Immunohistochemistry
2.4. Post-Embedding Immunogold Electron Microscopy
2.5. Measurement of Tissue Insulin Concentration of Muscle/Myenteric Plexus Homogenates
2.6. RNA Extraction, Reverse Transcription and PCR
2.7. Statistical Analysis
3. Results
3.1. Weight and Glycemic Characteristics of Animals
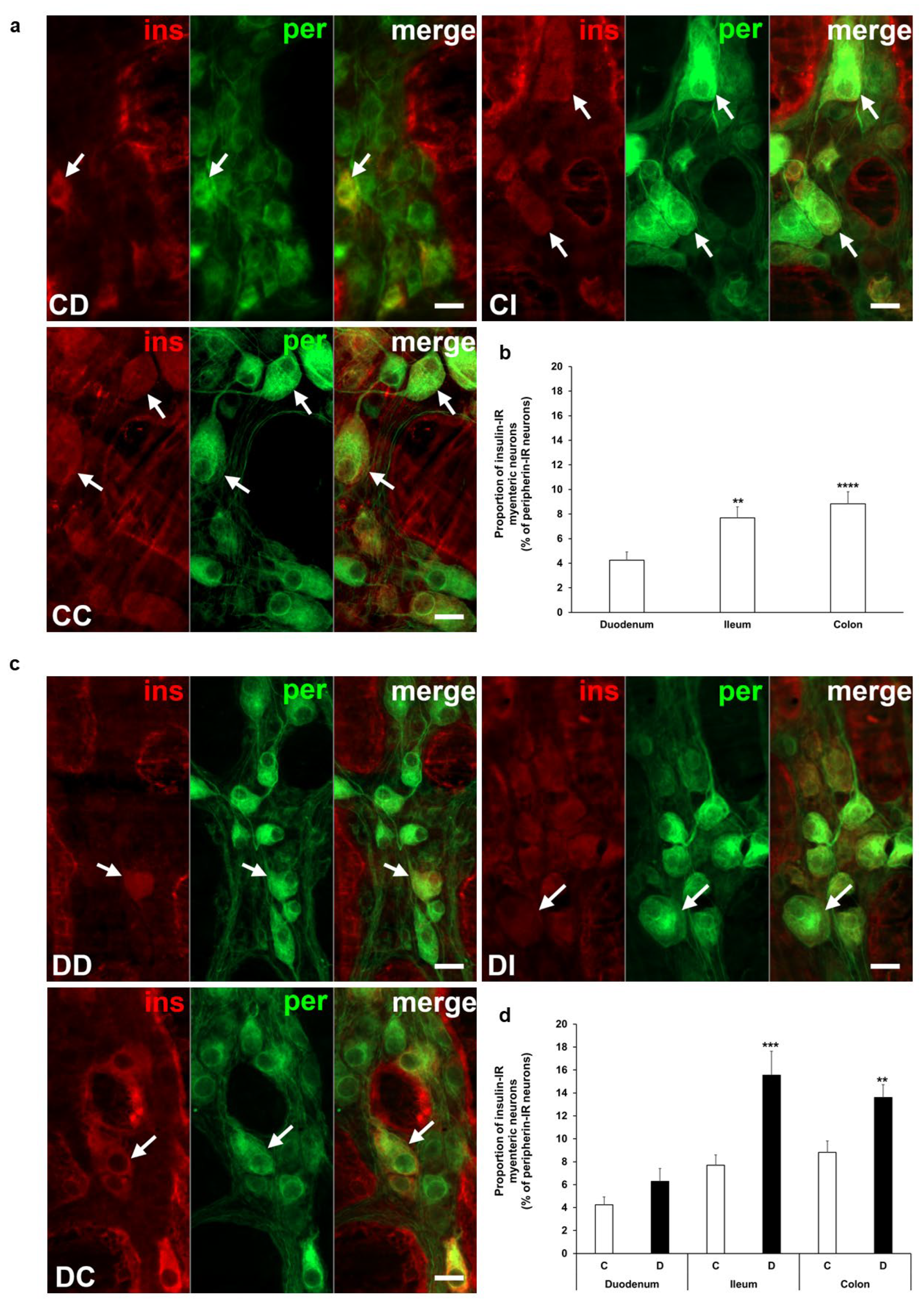
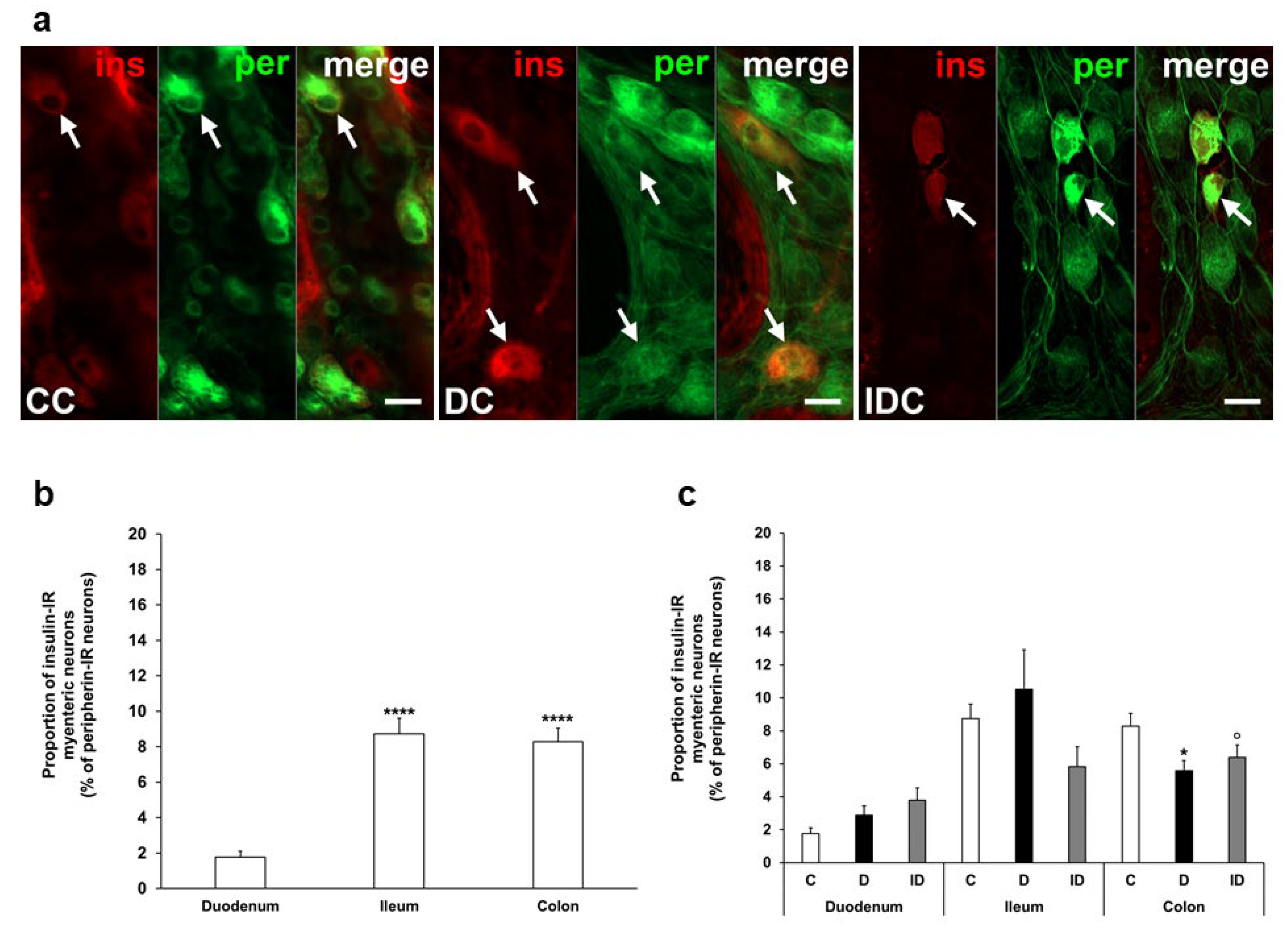
3.2. Proportion of Insulin-Immunoreactive Myenteric Neurons
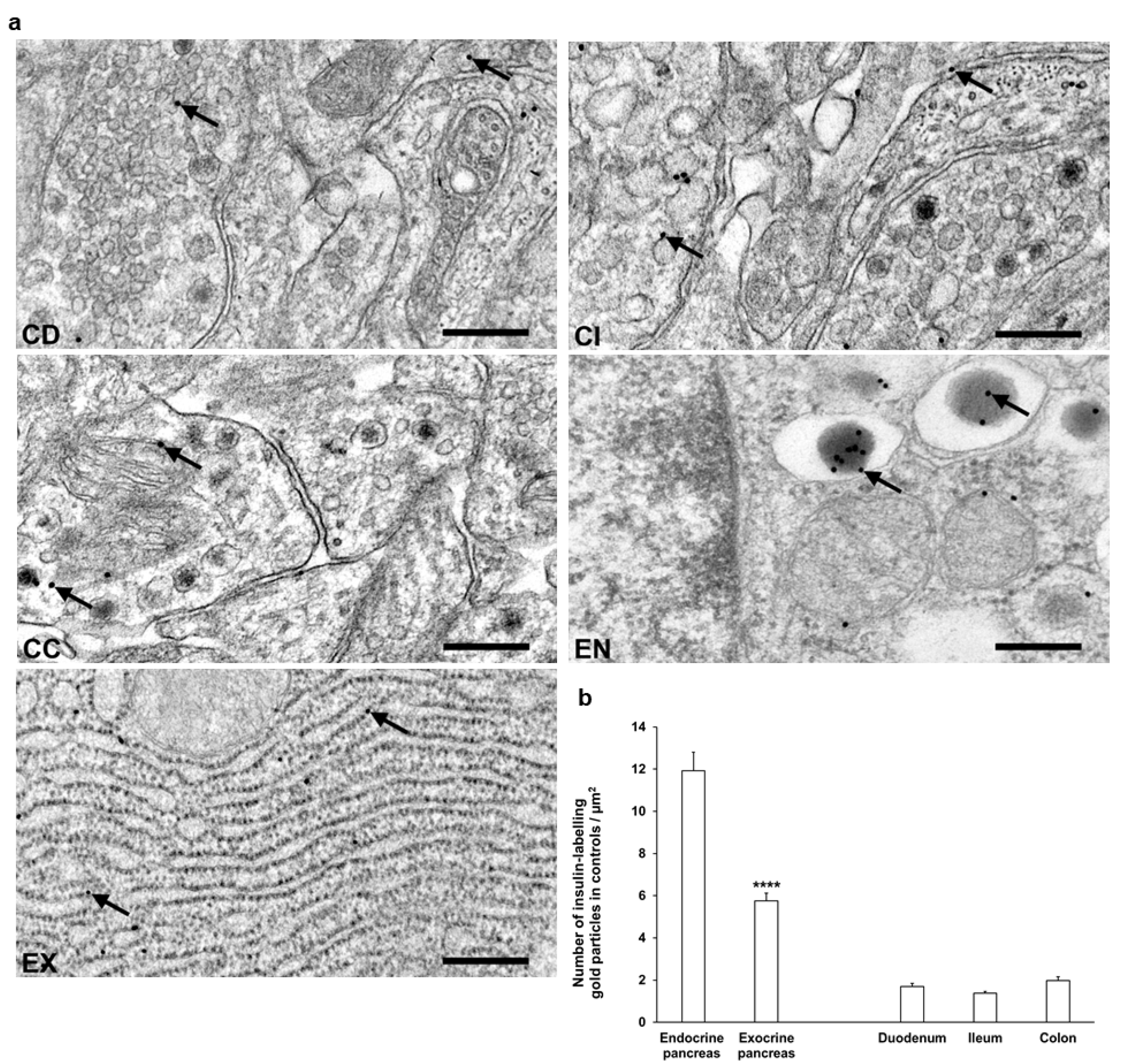
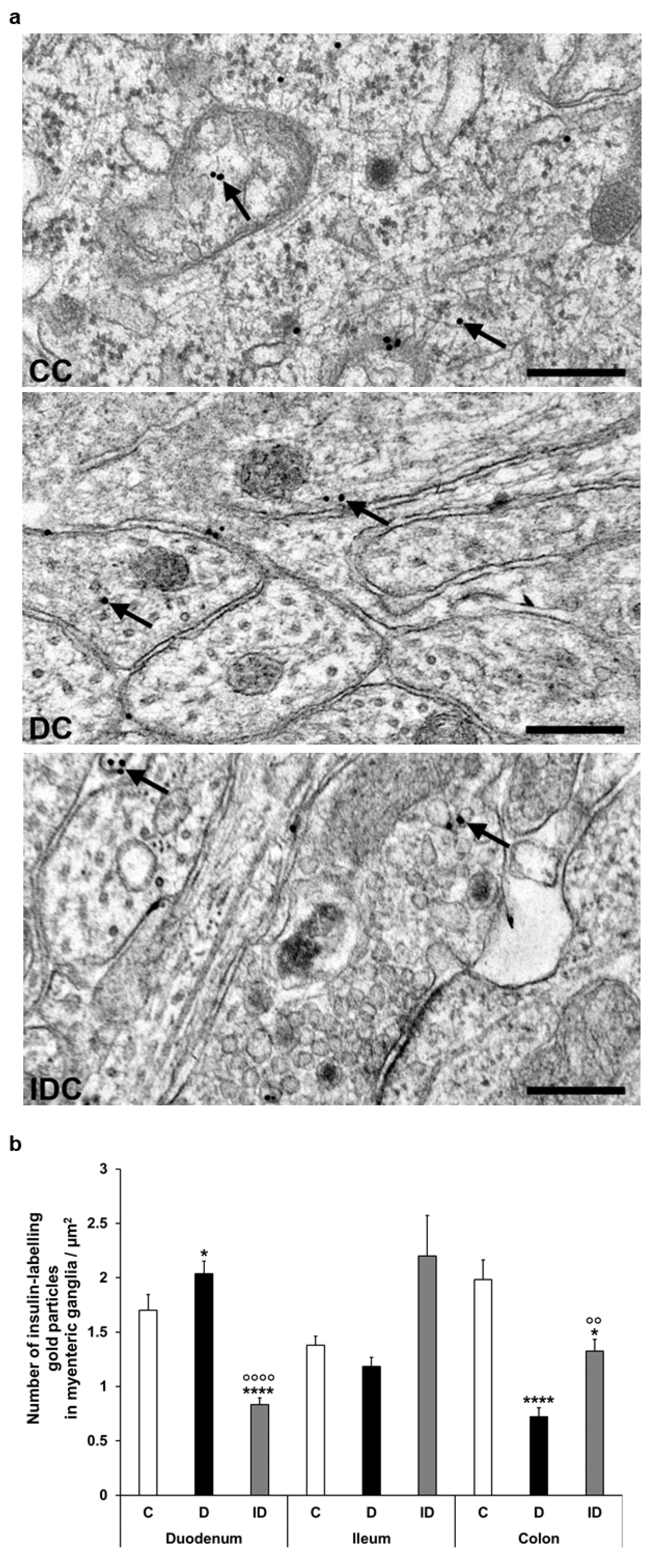
3.3. Subcellular Localization and Distribution of Insulin Within the Ganglia
3.4. Insulin Protein and mRNA Concentration in Intestinal Homogenates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| IR | immunoreactive |
| nNOS | neuronal nitric oxide synthase |
| PB | phosphate buffer |
| STZ | streptozotocin |
References
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.d.A.; Hannan, M.d.A.; Uddin, M.J.; Pang, M.-G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Overview of oxidative stress and inflammation in diabetes. J. Diabetes 2024, 16, e70014. [Google Scholar] [CrossRef] [PubMed]
- Bagyánszki, M.; Bódi, N. Key elements determining the intestinal region-specific environment of enteric neurons in type 1 diabetes. World J. Gastroenterol. 2023, 29, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Barta, B.P.; Onhausz, B.; Egyed-Kolumbán, A.; Al Doghmi, A.; Balázs, J.; Szalai, Z.; Ferencz, Á.; Hermesz, E.; Bagyánszki, M.; Bódi, N. Intestinal Region-Dependent Impact of NFκB-Nrf Crosstalk in Myenteric Neurons and Adjacent Muscle Cells in Type 1 Diabetic Rats. Biomedicines 2024, 12, 2347. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabetes 2014, 5, 89. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Hung, L.-C.; Chen, Y.-C.; Wang, W.-H.; Lin, C.-Y.; Tzeng, H.-H.; Suen, J.-L.; Chen, Y.-H. Insulin Reduces Inflammation by Regulating the Activation of the NLRP3 Inflammasome. Front. Immunol. 2021, 11, 587229. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S. The Neuroprotective Role of Insulin Against MPP+ -Induced Parkinson’s Disease in Differentiated SH-SY5Y Cells. J. Cell Biochem. 2016, 117, 917–926. [Google Scholar] [CrossRef]
- Shaughness, M.; Acs, D.; Brabazon, F.; Hockenbury, N.; Byrnes, K.R. Role of Insulin in Neurotrauma and Neurodegeneration: A Review. Front. Neurosci. 2020, 14, 547175. [Google Scholar] [CrossRef]
- Kojima, H.; Fujimiya, M.; Matsumura, K.; Nakahara, T.; Hara, M.; Chan, L. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc. Natl. Acad. Sci. USA 2004, 101, 2458–2463. [Google Scholar] [CrossRef]
- Chen, X.; Larson, C.S.; West, J.; Zhang, X.; Kaufman, D.B. In vivo detection of extrapancreatic insulin gene expression in diabetic mice by bioluminescence imaging. PLoS ONE 2010, 5, e9397. [Google Scholar] [CrossRef]
- Dakic, T.; Jevdjovic, T.; Lakic, I.; Ruzicic, A.; Jasnic, N.; Djurasevic, S.; Djordjevic, J.; Vujovic, P. The Expression of Insulin in the Central Nervous System: What Have We Learned So Far? Int. J. Mol. Sci. 2023, 24, 6586. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, I.; Berthou, F.; Vicaire, N.; Rouch, C.; Markaki, E.M.; Bailbe, D.; Portha, B.; Taouis, M.; Gerozissis, K. Hypothalamic serotonin-insulin signaling cross-talk and alterations in a type 2 diabetic model. Mol. Cell. Endocrinol. 2012, 350, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Molnár, G.; Faragó, N.; Kocsis, Á.K.; Rózsa, M.; Lovas, S.; Boldog, E.; Báldi, R.; Csajbók, É.; Gardi, J.; Puskás, L.G.; et al. GABAergic Neurogliaform Cells Represent Local Sources of Insulin in the Cerebral Cortex. J. Neurosci. 2014, 34, 1133–1137. [Google Scholar] [CrossRef]
- Fishwick, K.J.; Rylett, R.J. Insulin Regulates the Activity of the High-Affinity Choline Transporter CHT. PLoS ONE 2015, 10, e0132934. [Google Scholar] [CrossRef] [PubMed]
- Makhmutova, M.; Caicedo, A. Optical Imaging of Pancreatic Innervation. Front. Endocrinol. 2021, 12, 663022. [Google Scholar] [CrossRef]
- Liu, J.; Meyer, L.; Chung, P.-E.; Tao, Y.; Owen, B.; Murphy, K. Investigating the pancreas-projecting enteric neurons in the regulation of metabolic homeostasis. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2023. [Google Scholar]
- Ryu, G.R.; Lee, E.; Kim, J.J.; Moon, S.-D.; Ko, S.-H.; Ahn, Y.-B.; Song, K.-H. Comparison of enteroendocrine cells and pancreatic β-cells using gene expression profiling and insulin gene methylation. PLoS ONE 2018, 13, e0206401. [Google Scholar] [CrossRef]
- Egozi, A.; Llivichuzhca-Loja, D.; McCourt, B.T.; Bahar Halpern, K.; Farack, L.; An, X.; Wang, F.; Chen, K.; Konnikova, L.; Itzkovitz, S. Insulin is expressed by enteroendocrine cells during human fetal development. Nat. Med. 2021, 27, 2104–2107. [Google Scholar] [CrossRef]
- Izbéki, F.; Wittman, T.; Rosztóczy, A.; Linke, N.; Bódi, N.; Fekete, É.; Bagyánszki, M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res. Clin. Pract. 2008, 80, 192–198. [Google Scholar] [CrossRef]
- Bódi, N.; Szalai, Z.; Bagyánszki, M. Nitrergic Enteric Neurons in Health and Disease-Focus on Animal Models. Int. J. Mol. Sci. 2019, 20, 2003. [Google Scholar] [CrossRef]
- Lázár, B.A.; Jancsó, G.; Pálvölgyi, L.; Dobos, I.; Nagy, I.; Sántha, P. Insulin Confers Differing Effects on Neurite Outgrowth in Separate Populations of Cultured Dorsal Root Ganglion Neurons: The Role of the Insulin Receptor. Front. Neurosci. 2018, 12, 732. [Google Scholar] [CrossRef]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.P.; Ponomarev, E.D.; Strekalova, T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 2018, 24, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.R.; Ahmed, M.; Bhave, S.; Hotta, R.; Koppes, R.A.; Goldstein, A.M.; Koppes, A.N. Enteroendocrine Cells Sense Sucrose and Alter Enteric Neuron Excitability via Insulin Signaling. Adv. Biol. 2025, 3, e2300566. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Redox biology of the intestine. Free Radic. Res. 2011, 45, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Baskin, D.G.; Porte, D.; Guest, K.; Dorsa, D.M. Regional Concentrations of Insulin in the Rat Brain. Endocrinology 1983, 112, 898–903. [Google Scholar] [CrossRef]
- Altan, V.M.; Öztürk, Y.; Yildizoǧlu-Ari, N.; Nebigil, C.; Lafçi, D.; Özçelikay, A.T. Insulin action on different smooth muscle preparations. Gen. Pharmacol. Vasc. Syst. 1989, 20, 529–535. [Google Scholar] [CrossRef]
- Bergandi, L.; Silvagno, F.; Russo, I.; Riganti, C.; Anfossi, G.; Aldieri, E.; Ghigo, D.; Trovati, M.; Bosia, A. Insulin Stimulates Glucose Transport Via Nitric Oxide/Cyclic GMP Pathway in Human Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2003, 23, 2215–2221. [Google Scholar] [CrossRef]
- Jones, M.A.; Jadeja, R.N.; Flandrin, O.; Abdelrahman, A.A.; Thounojam, M.C.; Thomas, S.; Dai, C.; Xiao, H.; Chen, J.-K.; Smith, S.B.; et al. Autonomous regulation of retinal insulin biosynthesis in diabetes. Neuropeptides 2022, 94, 102258. [Google Scholar] [CrossRef]
- Baeyens, L.; De Breuck, S.; Lardon, J.; Mfopou, J.K.; Rooman, I.; Bouwens, L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 2005, 48, 49–57. [Google Scholar] [CrossRef]
- Bouchi, R.; Foo, K.S.; Hua, H.; Tsuchiya, K.; Ohmura, Y.; Sandoval, P.R.; Ratner, L.E.; Egli, D.; Leibel, R.L.; Accili, D. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat. Commun. 2014, 5, 4242. [Google Scholar] [CrossRef]
- Lee, S.-H.; Rhee, M.; Kim, J.-W.; Yoon, K.-H. Generation of Insulin-Expressing Cells in Mouse Small Intestine by Pdx1, MafA, and BETA2/NeuroD. Diabetes Metab. J. 2017, 41, 405. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Kuo, T.; Wang, L.; McKimpson, W.M.; Son, J.; Watanabe, H.; Kitamoto, T.; Lee, Y.; Creusot, R.J.; et al. Pharmacological conversion of gut epithelial cells into insulin-producing cells lowers glycemia in diabetic animals. J. Clin. Investig. 2022, 132, e162720. [Google Scholar] [CrossRef] [PubMed]
- De Koning, E.J.P.; Carlotti, F. Human stomach tissue as alternative source of insulin-producing cells. Nat. Rev. Endocrinol. 2023, 19, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Yu, H.; Choi, E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp. Mol. Med. 2020, 52, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Park, S.H.; Choi, E. The insulin receptor endocytosis. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–107. [Google Scholar]
- Chen, Y.; Huang, L.; Qi, X.; Chen, C. Insulin Receptor Trafficking: Consequences for Insulin Sensitivity and Diabetes. Int. J. Mol. Sci. 2019, 20, 5007. [Google Scholar] [CrossRef]
- Jaldin-Fincati, J.R.; Pereira, R.V.S.; Bilan, P.J.; Klip, A. Insulin uptake and action in microvascular endothelial cells of lymphatic and blood origin. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E204–E217. [Google Scholar] [CrossRef]
- Pemberton, S.; Galindo, D.C.; Schwartz, M.W.; Banks, W.A.; Rhea, E.M. Endocytosis of insulin at the blood-brain barrier. Front. Drug Deliv. 2022, 2, 1062366. [Google Scholar] [CrossRef]
- Andres, S.F.; Simmons, J.G.; Mah, A.T.; Santoro, M.A.; Van Landeghem, L.; Lund, P.K. Insulin receptor isoform switching in intestinal stem cells, progenitors, differentiated lineages and tumors: Evidence that IR-B limits proliferation. J. Cell Sci. 2013, 126, 5645–5656. [Google Scholar] [CrossRef]
- Andres, S.F.; Santoro, M.A.; Mah, A.T.; Keku, J.A.; Bortvedt, A.E.; Blue, R.E.; Lund, P.K. Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 308, G100–G111. [Google Scholar] [CrossRef]
- Gralle, M. The neuronal insulin receptor in its environment. J. Neurochem. 2017, 140, 359–367. [Google Scholar] [CrossRef]
- Frazier, H.N.; Ghoweri, A.O.; Anderson, K.L.; Lin, R.-L.; Popa, G.J.; Mendenhall, M.D.; Reagan, L.P.; Craven, R.J.; Thibault, O. Elevating Insulin Signaling Using a Constitutively Active Insulin Receptor Increases Glucose Metabolism and Expression of GLUT3 in Hippocampal Neurons. Front. Neurosci. 2020, 14, 668. [Google Scholar] [CrossRef]
- Porniece Kumar, M.; Cremer, A.L.; Klemm, P.; Steuernagel, L.; Sundaram, S.; Jais, A.; Hausen, A.C.; Tao, J.; Secher, A.; Pedersen, T.Å.; et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab. 2021, 3, 1662–1679. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Ito, S.; Masuda, T.; Ohtsuki, S. Effect of Insulin Receptor-Knockdown on the Expression Levels of Blood–Brain Barrier Functional Proteins in Human Brain Microvascular Endothelial Cells. Pharm. Res. 2022, 39, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Jarmakiewicz-Czaja, S.; Sokal-Dembowska, A.; Ferenc, K.; Filip, R. Mechanisms of Insulin Signaling as a Potential Therapeutic Method in Intestinal Diseases. Cells 2024, 13, 1879. [Google Scholar] [CrossRef] [PubMed]
- Spielman, L.; Bahniwal, M.; Little, J.; Walker, D.; Klegeris, A. Insulin Modulates In Vitro Secretion of Cytokines and Cytotoxins by Human Glial Cells. Curr. Alzheimer Res. 2015, 12, 684–693. [Google Scholar] [CrossRef]
- Sans, M.D.; Bruce, J.; Williams, J.A. Regulation of Pancreatic Exocrine Function by Islet Hormones. In Pancreapedia Exocrine Pancreas Knowl Base; American Pancreatic Association: Prairie Village, KS, USA, 2020. [Google Scholar] [CrossRef]
- Cellek, S.; Foxwell, N.A.; Moncada, S. Two Phases of Nitrergic Neuropathy in Streptozotocin-Induced Diabetic Rats. Diabetes 2003, 52, 2353–2362. [Google Scholar] [CrossRef]
- Canabal, D.D.; Song, Z.; Potian, J.G.; Beuve, A.; McArdle, J.J.; Routh, V.H. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R1418–R1428. [Google Scholar] [CrossRef]


| Primary Antibody | Host | Final Dilution | Product Code | Company |
|---|---|---|---|---|
| anti-peripherin polyclonal | rabbit | 1:400 | ab1530 | EMD Millipore Corporation, Burlington, MA, USA |
| anti-nNOS polyclonal | guinea pig | 1:500 | 432 005 | Synaptic Systems GmbH, Goettingen, Germany |
| anti-insulin monoclonal IgG1 | mouse | 1:100 | I2018 | Sigma-Aldrich, Budapest, Hungary |
| anti-insulin monoclonal IgG2a kappa | mouse | 1:200 | IMG-7B2 | ImmunoGenes Ltd., Budakeszi, Hungary |
| anti-insulin polyclonal IgG | rabbit | 1:200 | 15848-1-AP | Proteintech ChromoTek GmbH, Planegg-Martinsried, Germany |
| anti-insulin polyclonal | guinea pig | 1:100 | ab7842 | Abcam, Cambridge, UK |
| Secondary Antibody | Host | Final Dilution | Product Code | Company |
| anti-rabbit Alexa Fluor® 488 | goat | 1:200 | A11008 | Life Technologies Corporation, Molecular Probes, Carlsbad, CA, USA |
| anti-guinea pig Cy5® | goat | 1:200 | ab102372 | Abcam, Cambridge, UK |
| anti-mouse CyTM3 | goat | 1:200 | 115-165-003 | Jackson ImmunoResearch Laboratories, West Grove, PA, USA |
| Target | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| insulin | GCCCTAAGTGACCAGCTACA | TAGAAGAATCCACGCTCCCC |
| GAPDH | GAAGGGCTCATGACCACAGT | GGATGCAGGGATGATGTTCT |
| ACUTE EXPERIMENT | Body Weight (g) | Blood Glucose Concentration (mmol/L) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final (Average) | |
| Controls (n = 6) | 219 ± 7.18 | 275 ± 9.21 * | 6.83 ± 0.26 | 6.39 ± 0.12 |
| Diabetics (n = 8) | 216.5 ± 4.98 | 256.3 ± 8.19 * | 5.91 ± 0.19 | 29.1 ± 1.01 ****, ° |
| CHRONIC EXPERIMENT | Body Weight (g) | Blood Glucose Concentration (mmol/L) | ||
| Initial | Final | Initial | Final (Average) | |
| Controls (n = 17) | 203.9 ± 3.17 | 432.2 ± 10.3 **** | 5.09 ± 0.31 | 5.61 ± 0.11 |
| Diabetics (n = 16) | 206.8 ± 4.27 | 318.5 ± 11.94 * | 5.38 ± 0.4 | 27.88 ± 1.2 ****, °°°° |
| Insulin-treated diabetics (n = 14) | 206.5 ± 4.77 | 446.8 ± 13.83 **** | 5.08 ± 0.40 | 13.66 ± 0.91 ***, ° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egyed-Kolumbán, A.; Onhausz, B.; Barta, B.P.; Szalai, Z.; Huliák, I.; Kiricsi, M.; Bagyánszki, M.; Bódi, N. Insulin in Myenteric Neurons: Time-Dependent and Regional Changes in Type 1 Diabetic Rats. Cells 2025, 14, 809. https://doi.org/10.3390/cells14110809
Egyed-Kolumbán A, Onhausz B, Barta BP, Szalai Z, Huliák I, Kiricsi M, Bagyánszki M, Bódi N. Insulin in Myenteric Neurons: Time-Dependent and Regional Changes in Type 1 Diabetic Rats. Cells. 2025; 14(11):809. https://doi.org/10.3390/cells14110809
Chicago/Turabian StyleEgyed-Kolumbán, Abigél, Benita Onhausz, Bence Pál Barta, Zita Szalai, Ildikó Huliák, Mónika Kiricsi, Mária Bagyánszki, and Nikolett Bódi. 2025. "Insulin in Myenteric Neurons: Time-Dependent and Regional Changes in Type 1 Diabetic Rats" Cells 14, no. 11: 809. https://doi.org/10.3390/cells14110809
APA StyleEgyed-Kolumbán, A., Onhausz, B., Barta, B. P., Szalai, Z., Huliák, I., Kiricsi, M., Bagyánszki, M., & Bódi, N. (2025). Insulin in Myenteric Neurons: Time-Dependent and Regional Changes in Type 1 Diabetic Rats. Cells, 14(11), 809. https://doi.org/10.3390/cells14110809









