Porphyromonas gingivalis-Lipopolysaccharide Induced Caspase-4 Dependent Noncanonical Inflammasome Activation Drives Alzheimer’s Disease Pathologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Small Interfering RNA (siRNA) Mediated Knockdown Assay
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Real-Time Quantitative Polymerase Chain Reaction
2.5. Western Blot Analysis
2.6. Flow Cytometry Analysis
2.7. High-Resolution Respiratory Analysis
2.8. Real-Time Metabolic Flux Assays
2.9. Statistical Analysis
3. Results
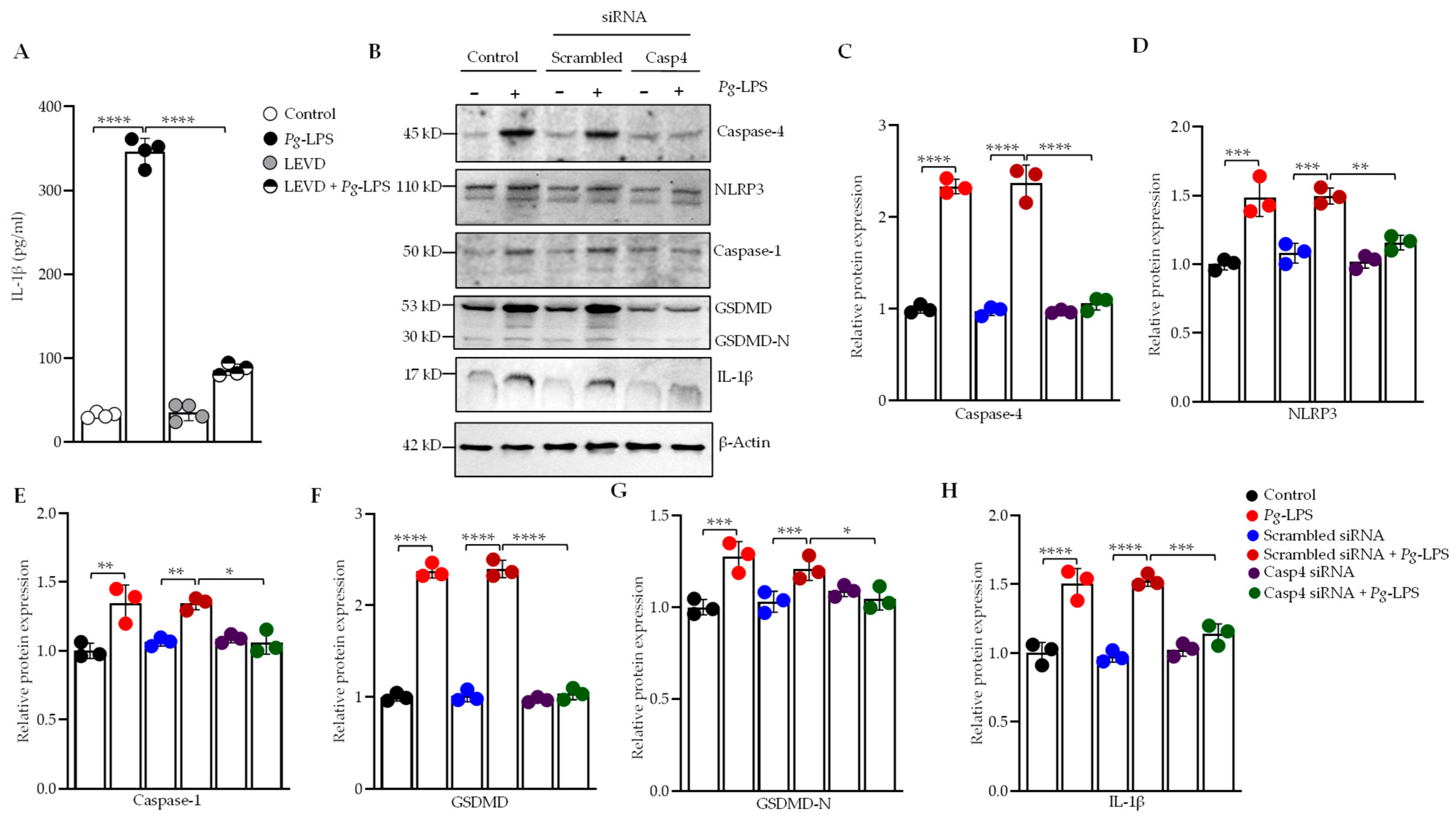
3.1. Silencing of Caspase-4 Reduces P. gingivalis-LPS-Induced Secretion of IL-1β
3.2. P. gingivalis-LPS Activates the AD-Associated Presenilin and Amyloid Secretase Pathway Mediated by Caspase-4
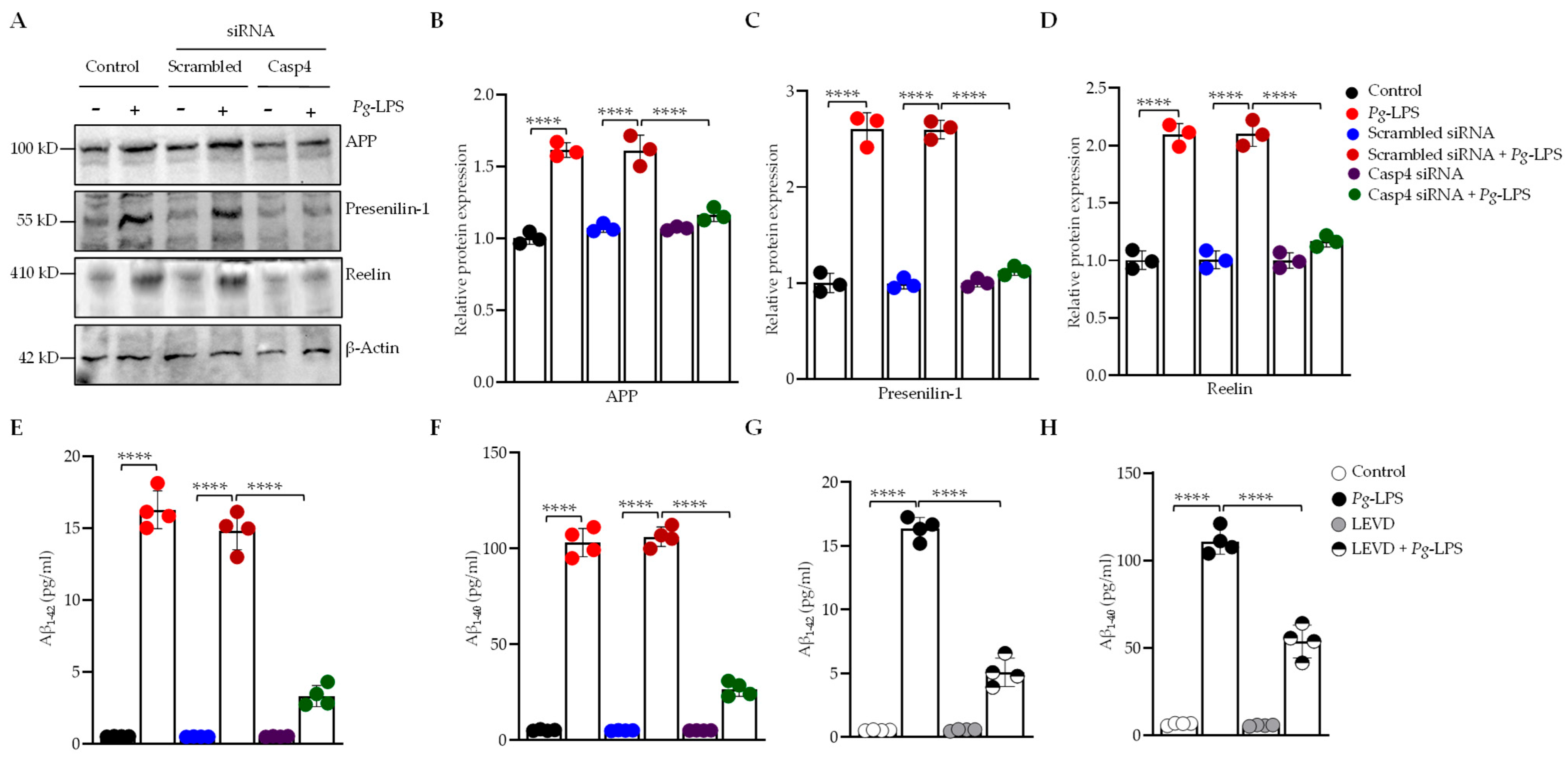
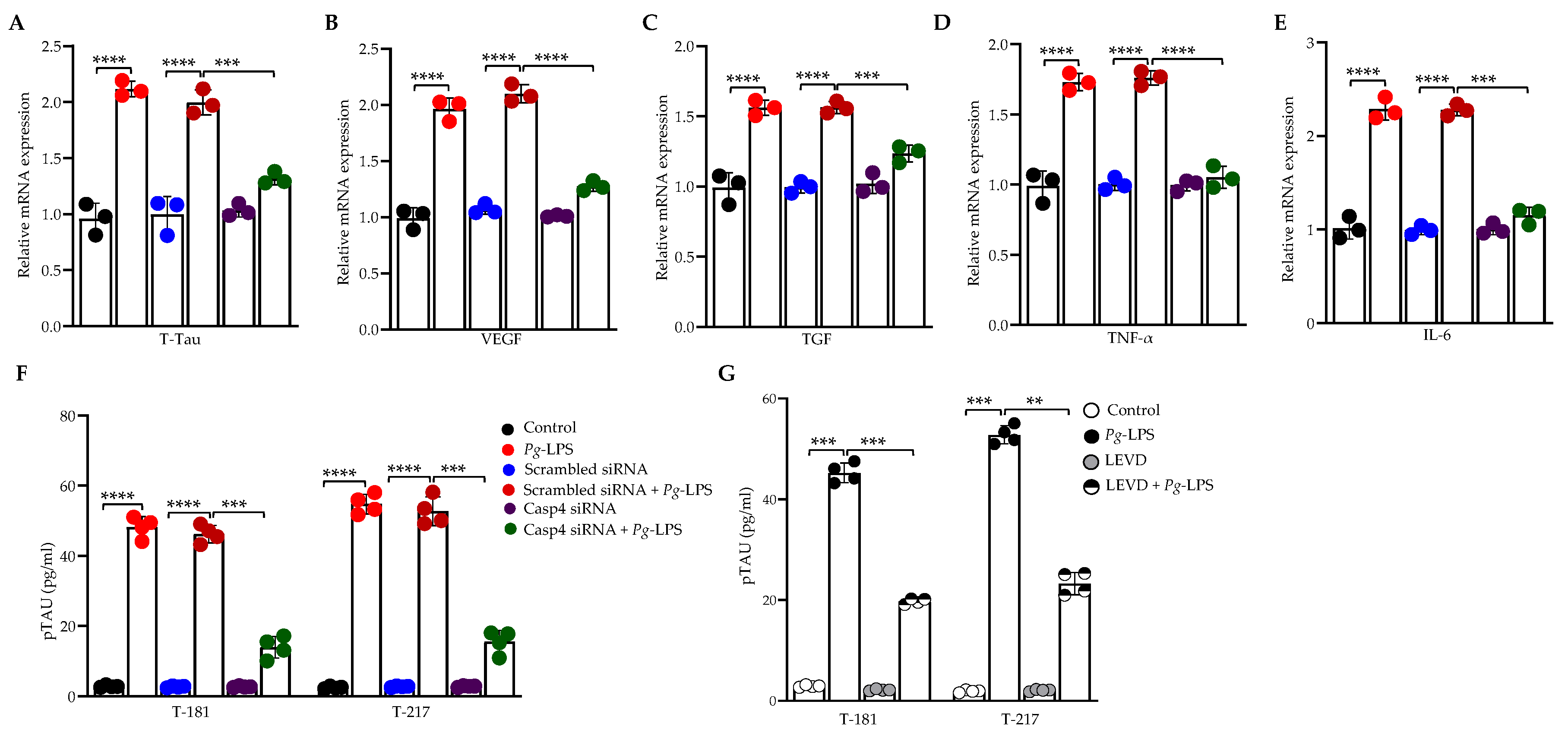
3.3. Caspase-4 Drives P. gingivalis-LPS-Induced Upregulation of Neuroinflammatory Markers
3.4. P. gingivalis-LPS Induces Oxidative Stress and Decreases Mitochondrial Membrane Potential Mediated by Caspase-4
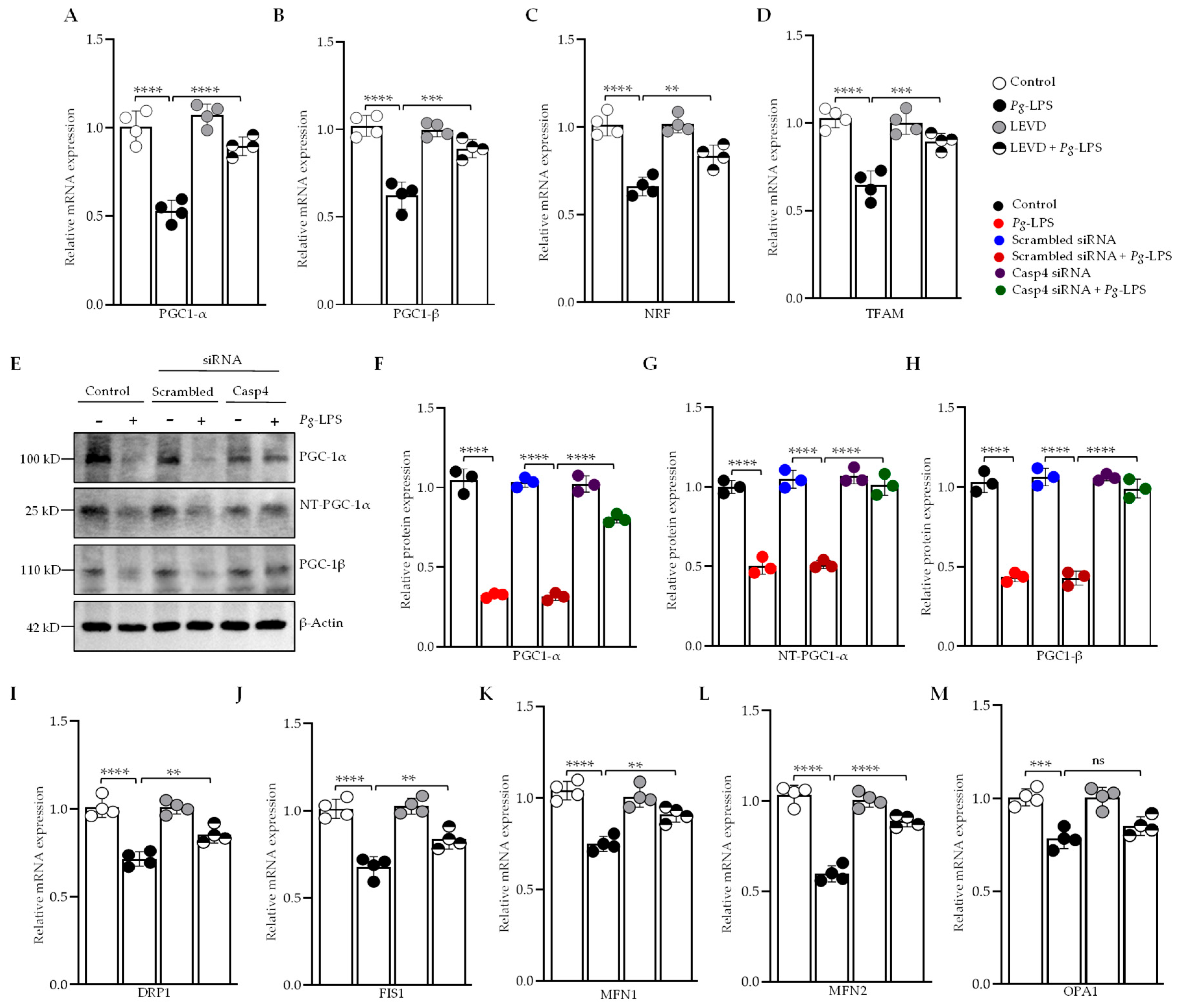
3.5. Caspase-4 Modulates Mitochondrial Biogenesis, Fission, and Fusion in Response to P. gingivalis-LPS
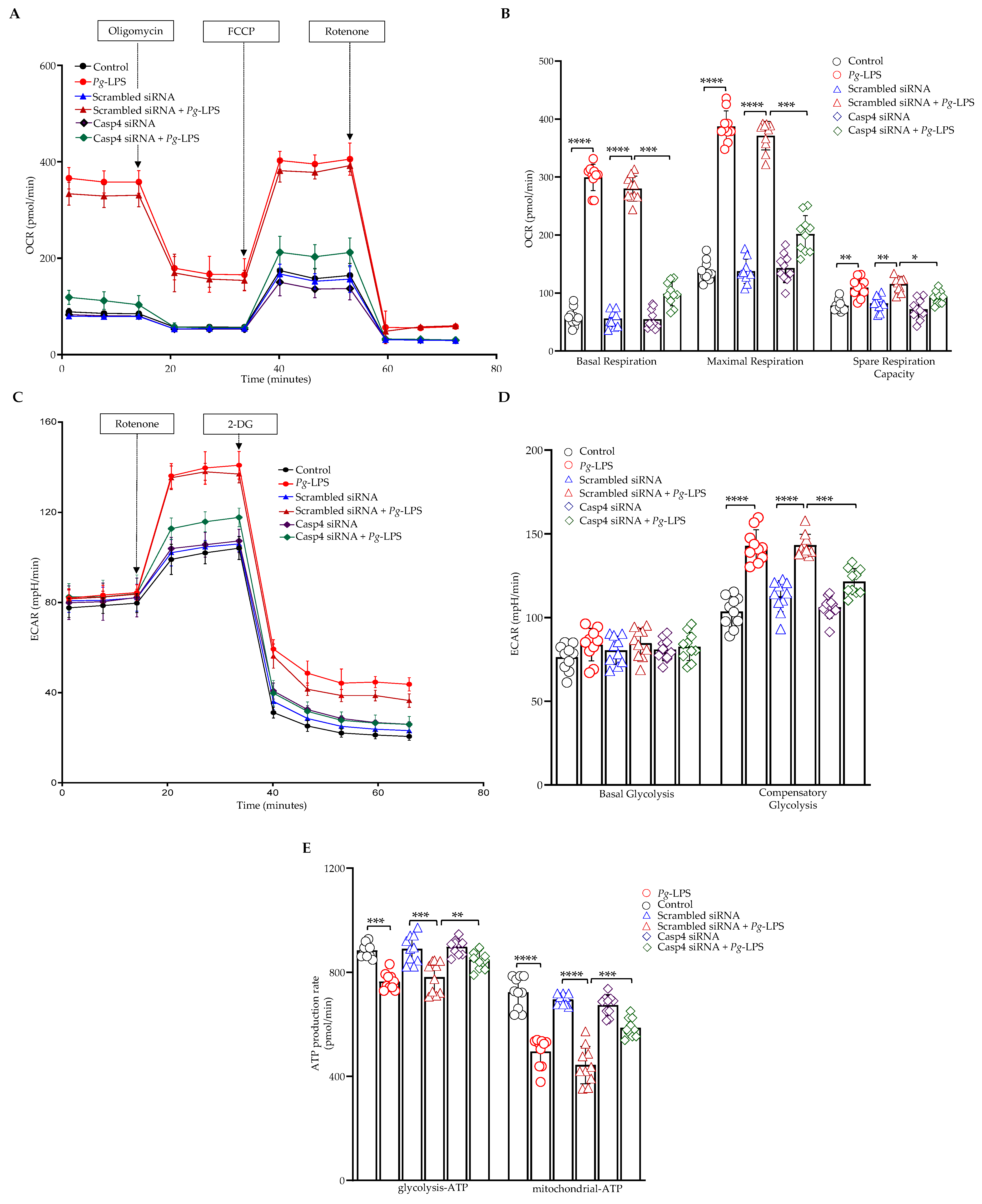
3.6. Caspase-4 Mediates the Impact of P. gingivalis-LPS on Oxidative Phosphorylation, Glycolysis, and ATP Production
3.7. P. gingivalis-LPS Specifically Regulates Mitochondrial Respiratory Complexes Through Caspase-4
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P. gingivalis | Porphyromonas gingivalis |
| LPS | Lipopolysaccharide |
| AD | Alzheimer’s disease |
| ADRD | Alzheimer’s disease and AD-related dementias |
| OMVs | Outer membrane vesicles |
| GSDMD | Gasdermin D |
| ROS | Reactive oxygen species |
| APP | Amyloid precursor protein |
| Aβ | Amyloid-β |
| PS1 | Presenilin-1 |
| iNOS | Inducible nitric oxide synthase |
| 4-HNE | 4-hydroxynonenal |
| MnSOD | Manganese superoxide dismutase |
| RT-qPCR | Real-time quantitative PCR |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TBST | Tris-buffered saline with Tween-20 |
| mtROS | Mitochondrial reactive oxygen species |
| MMP | Mitochondrial membrane potential |
| OCR | Oxygen consumption rate |
| ECAR | Extracellular acidification rate |
| ATP | Adenosine triphosphate |
| ETC | Electron transport chain |
| OXPHOS | Oxidative phosphorylation |
References
- Ponnappan, S.; Ponnappan, U. Aging and Immune Function: Molecular Mechanisms to Interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef] [PubMed]
- Bascones Martínez, A.; Figuero Ruiz, E. Periodontal Diseases as Bacterial Infection. Av. En Periodoncia 2005, 17, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas Gingivalis, Periodontal and Systemic Implications: A Systematic Review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.I. Porphyromonas gingivalis and Alzheimer Disease: Recent Findings and Potential Therapies. J. Periodontol. 2020, 91, S45–S49. [Google Scholar] [CrossRef]
- Olsen, I.; Singhrao, S.K. Is There a Link between Genetic Defects in the Complement Cascade and Porphyromonas gingivalis in Alzheimer’s Disease? J. Oral Microbiol. 2020, 12, 1676486. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Richmond, R.C.; Chen, Y.; Mai, X.-M. Mixed Evidence for the Relationship between Periodontitis and Alzheimer’s Disease: A Bidirectional Mendelian Randomization Study. PLoS ONE 2020, 15, e0228206. [Google Scholar] [CrossRef]
- Holt, S.C.; Kesavalu, L.; Walker, S.; Genco, C.A. Virulence Factors of Porphyromonas gingivalis. Periodontol. 2000 1999, 20, 168–238. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.W.; Lee, K.W.; Kim, S.-M.; Jung, I.D.; Yang, H.D.; et al. Gram-Negative Bacteria and Their Lipopolysaccharides in Alzheimer’s Disease: Pathologic Roles and Therapeutic Implications. Transl Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the Presence of Periodontopathic Virulence Factors in Short-Term Postmortem Alzheimer’s Disease Brain Tissue. J. Alzheimers Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef]
- Akkaoui, J.; Yamada, C.; Duarte, C.; Ho, A.; Vardar-Sengul, S.; Kawai, T.; Movila, A. Contribution of Porphyromonas gingivalis Lipopolysaccharide to Experimental Periodontitis in Relation to Aging. GeroScience 2021, 43, 367–376. [Google Scholar] [CrossRef]
- Yamada, C.; Akkaoui, J.; Ho, A.; Duarte, C.; Deth, R.; Kawai, T.; Nichols, F.; Lakshmana, M.K.; Movila, A. Potential Role of Phosphoglycerol Dihydroceramide Produced by Periodontal Pathogen Porphyromonas gingivalis in the Pathogenesis of Alzheimer’s Disease. Front. Immunol. 2020, 11, 591571. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of Outer Membrane Vesicle Entry into Host Cells: MicroReview–OMV Entry into Host Cells. Cell. Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate Immunity to Intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Yi, Y.-S. Caspase-11 Noncanonical Inflammasome: A Novel Key Player in Murine Models of Neuroinflammation and Multiple Sclerosis. Neuroimmunomodulation 2021, 28, 195–203. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory Caspases Are Innate Immune Receptors for Intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e20. [Google Scholar] [CrossRef]
- Rühl, S.; Broz, P. Caspase-11 Activates a Canonical NLRP3 Inflammasome by Promoting K+ Efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef]
- Ding, P.-H.; Yang, M.-X.; Wang, N.-N.; Jin, L.-J.; Dong, Y.; Cai, X.; Chen, L.-L. Porphyromonas gingivalis-Induced NLRP3 Inflammasome Activation and Its Downstream Interleukin-1β Release Depend on Caspase-4. Front. Microbiol. 2020, 11, 1881. [Google Scholar] [CrossRef]
- Rosa, C.P.; Belo, T.C.A.; Santos, N.C.D.M.; Silva, E.N.; Gasparotto, J.; Corsetti, P.P.; De Almeida, L.A. Reactive Oxygen Species Trigger Inflammasome Activation after Intracellular Microbial Interaction. Life Sci. 2023, 331, 122076. [Google Scholar] [CrossRef]
- Yi, Y. Functional Crosstalk between Non-canonical Caspase-11 and Canonical NLRP3 Inflammasomes during Infection-mediated Inflammation. Immunology 2020, 159, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome Activation and Regulation: Toward a Better Understanding of Complex Mechanisms. Cell. Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, A.; Wu, R.; Demirel, I. Porphyromonas gingivalis Triggers Microglia Activation and Neurodegenerative Processes through NOX4. Front. Cell. Infect. Microbiol. 2024, 14, 1451683. [Google Scholar] [CrossRef]
- Nie, R.; Wu, Z.; Ni, J.; Zeng, F.; Yu, W.; Zhang, Y.; Kadowaki, T.; Kashiwazaki, H.; Teeling, J.L.; Zhou, Y. Porphyromonas gingivalis Infection Induces Amyloid-β Accumulation in Monocytes/Macrophages. J. Alzheimers Dis. 2019, 72, 479–494. [Google Scholar] [CrossRef]
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis Induced by Bacterial Infection Exacerbates Features of Alzheimer’s Disease in Transgenic Mice. npj Aging Mech. Disease 2017, 3, 15. [Google Scholar] [CrossRef]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and Cognitive Impairment in Alzheimer Disease: A Complex but Coherent Relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Watanabe, K. Chronic Oral Application of a Periodontal Pathogen Results in Brain Inflammation, Neurodegeneration and Amyloid Beta Production in Wild Type Mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Chukkapalli, S.; Rivera, M.; Velsko, I.; Kesavalu, L.; Crean, S. Active Invasion of Porphyromonas gingivalis and Infection-Induced Complement Activation in ApoE-/-Mice Brains. J. Alzheimers Dis. 2014, 43, 67–80. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Zhang, X.; Chen, H.; Dong, J.; Lu, W.; Song, Z.; Zhou, W. Porphyromonas gingivalis Lipopolysaccharide Induces Cognitive Dysfunction, Mediated by Neuronal Inflammation via Activation of the TLR4 Signaling Pathway in C57BL/6 Mice. J. Neuroinflamm. 2018, 15, 37. [Google Scholar] [CrossRef]
- Verma, A.; Azhar, G.; Patyal, P.; Zhang, W.; Zhang, X.; Wei, J.Y. Proteomic Analysis of P. gingivalis-Lipopolysaccharide Induced Neuroinflammation in SH-SY5Y and HMC3 Cells. GeroScience 2024, 46, 4315–4332. [Google Scholar] [CrossRef] [PubMed]
- Annaert, W.G.; Levesque, L.; Craessaerts, K.; Dierinck, I.; Snellings, G.; Westaway, D.; George-Hyslop, P.S.; Cordell, B.; Fraser, P.; De Strooper, B. Presenilin 1 Controls γ-Secretase Processing of Amyloid Precursor Protein in Pre-Golgi Compartments of Hippocampal Neurons. J. Cell Biol. 1999, 147, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Multhaup, G.; Czech, C.; Blanchard, V.; Tremp, G.; Pradier, L.; Beyreuther, K.; Bayer, T.A. Reelin in Plaques of β-Amyloid Precursor Protein and Presenilin-1 Double-Transgenic Mice. Neurosci. Lett. 2001, 316, 145–148. [Google Scholar] [CrossRef]
- Botella-López, A.; Cuchillo-Ibáñez, I.; Cotrufo, T.; Mok, S.S.; Li, Q.-X.; Barquero, M.-S.; Dierssen, M.; Soriano, E.; Sáez-Valero, J. β-Amyloid Controls Altered Reelin Expression and Processing in Alzheimer’s Disease. Neurobiol. Dis. 2010, 37, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.X.; Zeng, L.; Wang, Q.; Tan, E.K.; Zhou, Z.D. Reelin Links Apolipoprotein E4, Tau, and Amyloid-β in Alzheimer’s Disease. Ageing Res. Rev. 2024, 98, 102339. [Google Scholar] [CrossRef]
- Cabezas-Opazo, F.A.; Vergara-Pulgar, K.; Pérez, M.J.; Jara, C.; Osorio-Fuentealba, C.; Quintanilla, R.A. Mitochondrial Dysfunction Contributes to the Pathogenesis of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1α: A Key Regulator of Energy Metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Liu, Y.; Liu, X.; Zhu, L.; Cui, Y.; Cui, A.; Qiao, A.; Kong, X.; Liu, Y.; Chen, Q.; et al. PGC-1β-Regulated Mitochondrial Biogenesis and Function in Myotubes Is Mediated by NRF-1 and ERRα. Mitochondrion 2010, 10, 516–527. [Google Scholar] [CrossRef]
- Hirst, J.; King, M.S.; Pryde, K.R. The Production of Reactive Oxygen Species by Complex I. Biochem. Soc. Trans. 2008, 36, 976–980. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial Complex II Can Generate Reactive Oxygen Species at High Rates in Both the Forward and Reverse Reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; De Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free. Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Carlson, E.A.; Rao, V.K.; Yan, S.S. From a Cell’s Viewpoint: Targeting Mitochondria in Alzheimer’s Disease. Drug Discov. Today: Ther. Strateg. 2013, 10, e91–e98. [Google Scholar] [CrossRef][Green Version]
- Cenini, G.; Voos, W. Mitochondria as Potential Targets in Alzheimer Disease Therapy: An Update. Front. Pharmacol. 2019, 10, 902. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, J.; Ma, L.; Wang, C.; Wang, X.; Huang, X.; Cao, Z. Mitochondrial Dysfunction in Periodontitis and Associated Systemic Diseases: Implications for Pathomechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 1024. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, T.; Zheng, Q.; Jiang, A.; Zhao, J.; Ying, Y.; Liu, N.; Pan, Y.; Zhang, D. Mitochondria: An Emerging Unavoidable Link in the Pathogenesis of Periodontitis Caused by Porphyromonas gingivalis. Int. J. Mol. Sci. 2024, 25, 737. [Google Scholar] [CrossRef]
- Gölz, L.; Memmert, S.; Rath-Deschner, B.; Jäger, A.; Appel, T.; Baumgarten, G.; Götz, W.; Frede, S. LPS from P. gingivalis and Hypoxia Increases Oxidative Stress in Periodontal Ligament Fibroblasts and Contributes to Periodontitis. Mediat. Inflamm. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Azhar, G.; Zhang, X.; Patyal, P.; Kc, G.; Sharma, S.; Che, Y.; Wei, J.Y.P. Gingivalis-LPS Induces Mitochondrial Dysfunction Mediated by Neuroinflammation through Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 950. [Google Scholar] [CrossRef]
- Charoensaensuk, V.; Chen, Y.-C.; Lin, Y.-H.; Ou, K.-L.; Yang, L.-Y.; Lu, D.-Y. Porphyromonas Gingivalis Induces Proinflammatory Cytokine Expression Leading to Apoptotic Death through the Oxidative Stress/NF-κB Pathway in Brain Endothelial Cells. Cells 2021, 10, 3033. [Google Scholar] [CrossRef]
- Platnich, J.M.; Chung, H.; Lau, A.; Sandall, C.F.; Bondzi-Simpson, A.; Chen, H.-M.; Komada, T.; Trotman-Grant, A.C.; Brandelli, J.R.; Chun, J.; et al. Shiga Toxin/Lipopolysaccharide Activates Caspase-4 and Gasdermin D to Trigger Mitochondrial Reactive Oxygen Species Upstream of the NLRP3 Inflammasome. Cell Rep. 2018, 25, 1525–1536.e7. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Katz, J.; Vogel, S.N.; Michalek, S.M. Differential Induction of Endotoxin Tolerance by Lipopolysaccharides Derived from Porphyromonas gingivalis and Escherichia coli. J. Immunol. 2001, 167, 5278–5285. [Google Scholar] [CrossRef] [PubMed]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The Role of Nitric Oxide in Inflammation and Oxidative Stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Hu, W.; Ren, L.; Jiang, P.; Margolskee, R.F.; Wang, H.; Feng, S. Lipopolysaccharide-Induced Inflammation Increases Nitric Oxide Production in Taste Buds. Brain Behav. Immun. 2022, 103, 145–153. [Google Scholar] [CrossRef]
- Praticò, D.; Uryu, K.; Leight, S.; Trojanoswki, J.Q.; Lee, V.M.-Y. Increased Lipid Peroxidation Precedes Amyloid Plaque Formation in an Animal Model of Alzheimer Amyloidosis. J. Neurosci. 2001, 21, 4183–4187. [Google Scholar] [CrossRef]
- Galbusera, C.; Facheris, M.; Magni, F.; Galimberti, G.; Sala, G.; Tremolada, L.; Isella, V.; Guerini, F.; Appollonio, I.; Galli-Kienle, M.; et al. Increased Susceptibility to Plasma Lipid Peroxidation in Alzheimer Disease Patients. CAR 2004, 1, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St. Clair, D.K. Manganese Superoxide Dismutase: Guardian of the Powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, X.; Azhar, G.; Patyal, P.; Verma, A.; Kc, G.; Wei, J.Y. Valine Improves Mitochondrial Function and Protects against Oxidative Stress. Biosci. Biotechnol. Biochem. 2024, 88, 168–176. [Google Scholar] [CrossRef]
- Patyal, P.; Nguyen, B.; Zhang, X.; Azhar, G.; Ameer, F.S.; Verma, A.; Crane, J.; Kc, G.; Che, Y.; Wei, J.Y. Rho/SRF Inhibitor Modulates Mitochondrial Functions. Int. J. Mol. Sci. 2022, 23, 11536. [Google Scholar] [CrossRef]
- Patyal, P.; Zhang, X.; Verma, A.; Azhar, G.; Wei, J.Y. Inhibitors of Rho/MRTF/SRF Transcription Pathway Regulate Mitochondrial Function. Cells 2024, 13, 392. [Google Scholar] [CrossRef]
- Patyal, P.; Ameer, F.S.; Verma, A.; Zhang, X.; Azhar, G.; Shrivastava, J.; Sharma, S.; Zhang, R.; Wei, J.Y. The Role of Sirtuin-1 Isoforms in Regulating Mitochondrial Function. Curr. Issues Mol. Biol. 2024, 46, 8835–8851. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Azhar, G.; Patyal, P.; Zhang, X.; Wei, J.Y. P. gingivalis-LPS promotes AD/ADRD via caspase-4-mediated noncanonical inflammasome pathway. Innov. Aging 2024, 8, 956. [Google Scholar] [CrossRef]
- Kajiwara, Y.; McKenzie, A.; Dorr, N.; Gama Sosa, M.A.; Elder, G.; Schmeidler, J.; Dickstein, D.L.; Bozdagi, O.; Zhang, B.; Buxbaum, J.D. The Human-Specific CASP4 Gene Product Contributes to Alzheimer-Related Synaptic and Behavioural Deficits. Hum. Mol. Genet. 2016, 25, 4315–4327. [Google Scholar] [CrossRef]
- Moonen, S.; Koper, M.J.; Van Schoor, E.; Schaeverbeke, J.M.; Vandenberghe, R.; Von Arnim, C.A.F.; Tousseyn, T.; De Strooper, B.; Thal, D.R. Pyroptosis in Alzheimer’s Disease: Cell Type-Specific Activation in Microglia, Astrocytes and Neurons. Acta Neuropathol. 2023, 145, 175–195. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Kim, S.H.; Hwang, D.; Lee, K.E.; Kim, M.J.; Yang, E.G.; Kim, S.Y.; Chung, H.S. Caspase-4 Disaggregates Lipopolysaccharide Micelles via LPS-CARD Interaction. Sci. Rep. 2019, 9, 826. [Google Scholar] [CrossRef]
- Daily, K.P.; Badr, A.; Eltobgy, M.; Estfanous, S.; Whitham, O.; Tan, M.H.; Carafice, C.; Krause, K.; McNamara, A.; Hamilton, K.; et al. DNA Hypomethylation Promotes the Expression of CASPASE-4 Which Exacerbates Inflammation and Amyloid-β Deposition in Alzheimer’s Disease. Alz. Res. Therapy 2024, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hao, M.; Shi, N.; Wang, X.; Yuan, L.; Yuan, H.; Wang, X. Porphyromonas gingivalis: A Potential Trigger of Neurodegenerative Disease. Front. Immunol. 2025, 16, 1482033. [Google Scholar] [CrossRef]
- Liao, Y.-F.; Wang, B.-J.; Cheng, H.-T.; Kuo, L.-H.; Wolfe, M.S. Tumor Necrosis Factor-α, Interleukin-1β, and Interferon-γ Stimulate γ-Secretase-Mediated Cleavage of Amyloid Precursor Protein through a JNK-Dependent MAPK Pathway. J. Biol. Chem. 2004, 279, 49523–49532. [Google Scholar] [CrossRef] [PubMed]
- Goldgaber, D.; Harris, H.W.; Hla, T.; Maciag, T.; Donnelly, R.J.; Jacobsen, J.S.; Vitek, M.P.; Gajdusek, D.C. Interleukin 1 Regulates Synthesis of Amyloid Beta-Protein Precursor mRNA in Human Endothelial Cells. Proc. Natl. Acad. Sci. USA 1989, 86, 7606–7610. [Google Scholar] [CrossRef]
- Fan, L.; Liu, H.; Zhu, G.; Singh, S.; Yu, Z.; Wang, S.; Luo, H.; Liu, S.; Xu, Y.; Ge, J.; et al. Caspase-4/11 Is Critical for Angiogenesis by Repressing Notch1 Signaling via Inhibiting Γ-secretase Activity. British J. Pharmacol. 2022, 179, 4809–4828. [Google Scholar] [CrossRef]
- Chin, J.; Massaro, C.M.; Palop, J.J.; Thwin, M.T.; Yu, G.-Q.; Bien-Ly, N.; Bender, A.; Mucke, L. Reelin Depletion in the Entorhinal Cortex of Human Amyloid Precursor Protein Transgenic Mice and Humans with Alzheimer’s Disease. J. Neurosci. 2007, 27, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Knuesel, I.; Nyffeler, M.; Mormède, C.; Muhia, M.; Meyer, U.; Pietropaolo, S.; Yee, B.K.; Pryce, C.R.; LaFerla, F.M.; Marighetto, A.; et al. Age-Related Accumulation of Reelin in Amyloid-like Deposits. Neurobiol. Aging 2009, 30, 697–716. [Google Scholar] [CrossRef]
- Doehner, J.; Madhusudan, A.; Konietzko, U.; Fritschy, J.-M.; Knuesel, I. Co-Localization of Reelin and Proteolytic AβPP Fragments in Hippocampal Plaques in Aged Wild-Type Mice. J. Alzheimers Dis. 2010, 19, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Casson, C.N.; Yu, J.; Reyes, V.M.; Taschuk, F.O.; Yadav, A.; Copenhaver, A.M.; Nguyen, H.T.; Collman, R.G.; Shin, S. Human Caspase-4 Mediates Noncanonical Inflammasome Activation against Gram-Negative Bacterial Pathogens. Proc. Natl. Acad. Sci. USA 2015, 112, 6688–6693. [Google Scholar] [CrossRef]
- Oh, C.; Verma, A.; Hafeez, M.; Hogland, B.; Aachoui, Y. Shigella OspC3 Suppresses Murine Cytosolic LPS Sensing. iScience 2021, 24, 102910. [Google Scholar] [CrossRef] [PubMed]
- Viganò, E.; Diamond, C.E.; Spreafico, R.; Balachander, A.; Sobota, R.M.; Mortellaro, A. Human Caspase-4 and Caspase-5 Regulate the One-Step Non-Canonical Inflammasome Activation in Monocytes. Nat. Commun. 2015, 6, 8761. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Zhang, J.; Zhang, X.; Xia, X.; Qiu, C.; Liao, Y.; Chen, H.; Song, Z.; Zhou, W. Periodontitis Induced by P. gingivalis-LPS Is Associated With Neuroinflammation and Learning and Memory Impairment in Sprague-Dawley Rats. Front. Neurosci. 2020, 14, 658. [Google Scholar] [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Tredici, K.D.; et al. Correlation of Alzheimer Disease Neuropathologic Changes With Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Tang, Z.; Liang, D.; Cheng, M.; Su, X.; Liu, R.; Zhang, Y.; Wu, H. Effects of Porphyromonas gingivalis and Its Underlying Mechanisms on Alzheimer-Like Tau Hyperphosphorylation in Sprague-Dawley Rats. J. Mol. Neurosci. 2021, 71, 89–100. [Google Scholar] [CrossRef]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial Dysfunction in Chronic Neuroinflammatory Diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef]
- Shao, G.; Wang, L.; Wang, X.; Fu, C. Apaf-1/Caspase-4 Pyroptosome: A Mediator of Mitochondrial Permeability Transition-Triggered Pyroptosis. Sig. Transduct. Target. Ther. 2021, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Dong, Q.; Luo, Y.; Liu, Y.; Gao, L.; Pan, Y.; Zhang, D. Porphyromonas gingivalis Infection Promotes Mitochondrial Dysfunction through Drp1-Dependent Mitochondrial Fission in Endothelial Cells. Int. J. Oral Sci. 2021, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Patyal, P.; Azhar, G.; Zhang, X.; Verma, A.; Wei, J.Y. Cardiac-Specific Overexpression of Serum Response Factor Regulates Age-Associated Decline in Mitochondrial Function. GeroScience 2025. [Google Scholar] [CrossRef]
- Napa, K.; Baeder, A.C.; Witt, J.E.; Rayburn, S.T.; Miller, M.G.; Dallon, B.W.; Gibbs, J.L.; Wilcox, S.H.; Winden, D.R.; Smith, J.H.; et al. LPS from P. gingivalis Negatively Alters Gingival Cell Mitochondrial Bioenergetics. Int. J. Dent. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.; Azhar, G.; Patyal, P.; Zhang, X.; Wei, J.Y. Porphyromonas gingivalis-Lipopolysaccharide Induced Caspase-4 Dependent Noncanonical Inflammasome Activation Drives Alzheimer’s Disease Pathologies. Cells 2025, 14, 804. https://doi.org/10.3390/cells14110804
Verma A, Azhar G, Patyal P, Zhang X, Wei JY. Porphyromonas gingivalis-Lipopolysaccharide Induced Caspase-4 Dependent Noncanonical Inflammasome Activation Drives Alzheimer’s Disease Pathologies. Cells. 2025; 14(11):804. https://doi.org/10.3390/cells14110804
Chicago/Turabian StyleVerma, Ambika, Gohar Azhar, Pankaj Patyal, Xiaomin Zhang, and Jeanne Y. Wei. 2025. "Porphyromonas gingivalis-Lipopolysaccharide Induced Caspase-4 Dependent Noncanonical Inflammasome Activation Drives Alzheimer’s Disease Pathologies" Cells 14, no. 11: 804. https://doi.org/10.3390/cells14110804
APA StyleVerma, A., Azhar, G., Patyal, P., Zhang, X., & Wei, J. Y. (2025). Porphyromonas gingivalis-Lipopolysaccharide Induced Caspase-4 Dependent Noncanonical Inflammasome Activation Drives Alzheimer’s Disease Pathologies. Cells, 14(11), 804. https://doi.org/10.3390/cells14110804






