Therapeutic Potential of Human Amniotic Membrane-Derived Mesenchymal Stem Cell Conditioned Medium in Combating Oxidative Stress and Age-Related Female Infertility
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Chemicals and Reagents
2.3. Culture and Characterization of AMSCs
2.4. Preparation of AMSC-CM
2.5. Experimental Animals
2.6. Intravenous Administration of AMSC-CM
2.7. Oxidative Stress and Antioxidant Marker Assays
2.8. Serum Hormone Measurement
2.9. RNA Sequencing Analysis
2.10. Functional Category Analysis
2.11. Statistical Analyses
3. Results
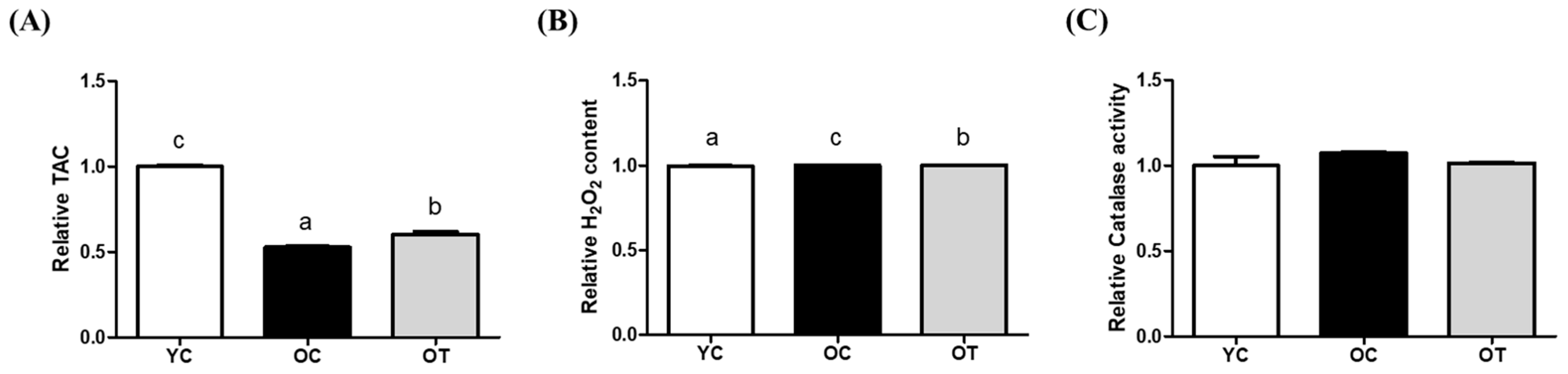
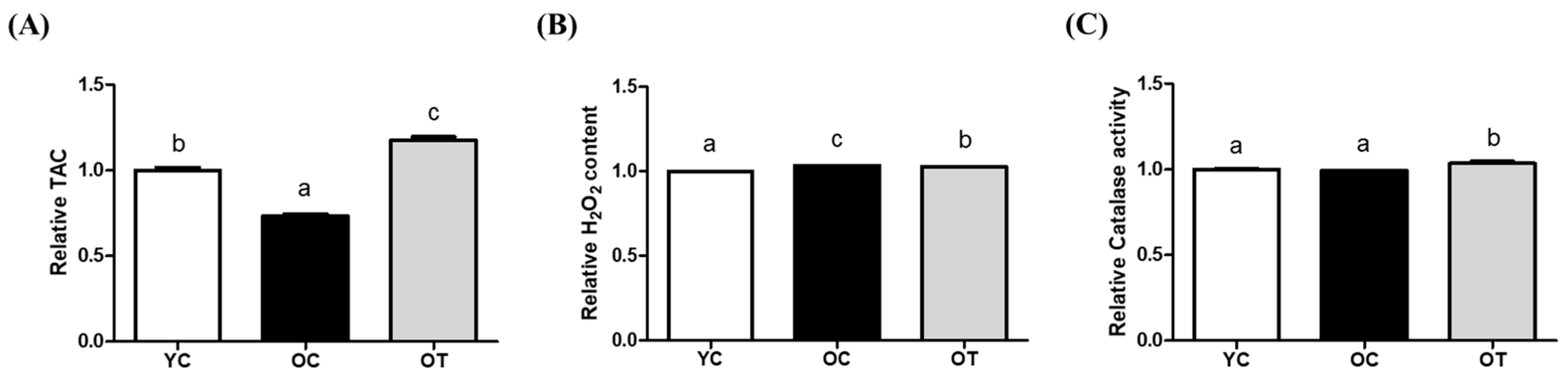
3.1. AMSC-CM Treatment Restrains Oxidative Stress and Improves Antioxidant Levels
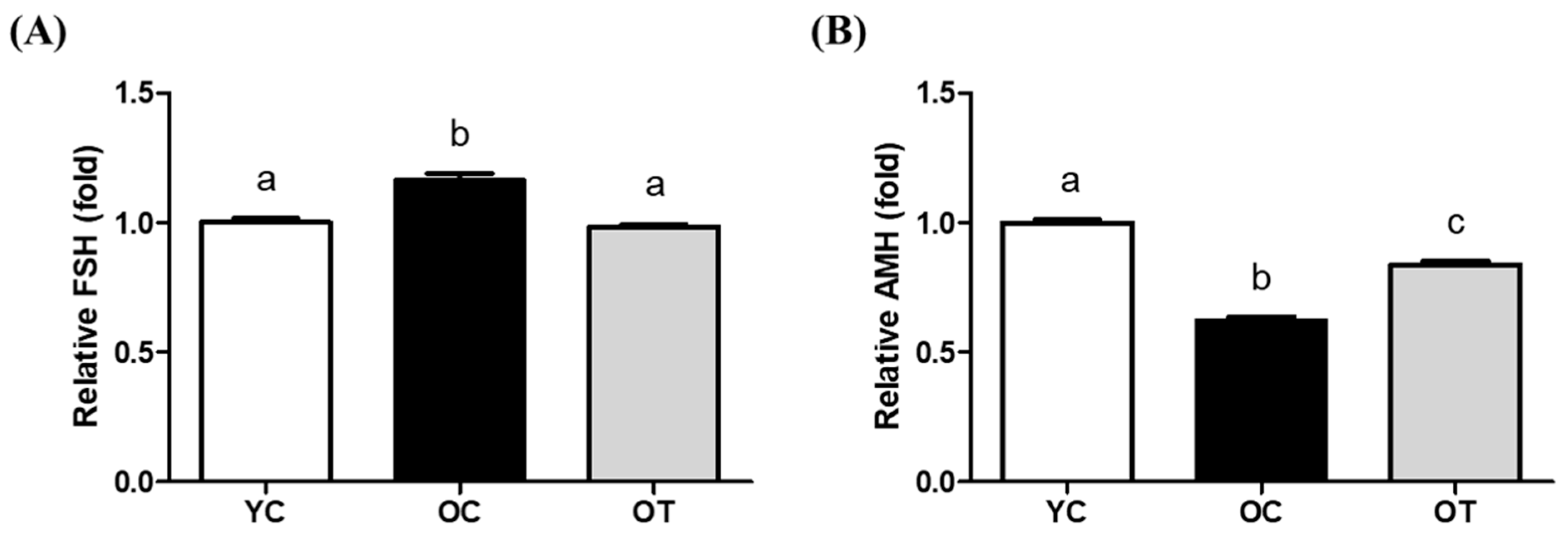
3.2. AMSC-CM Treatment Restores Serum Reproductive Hormone Levels
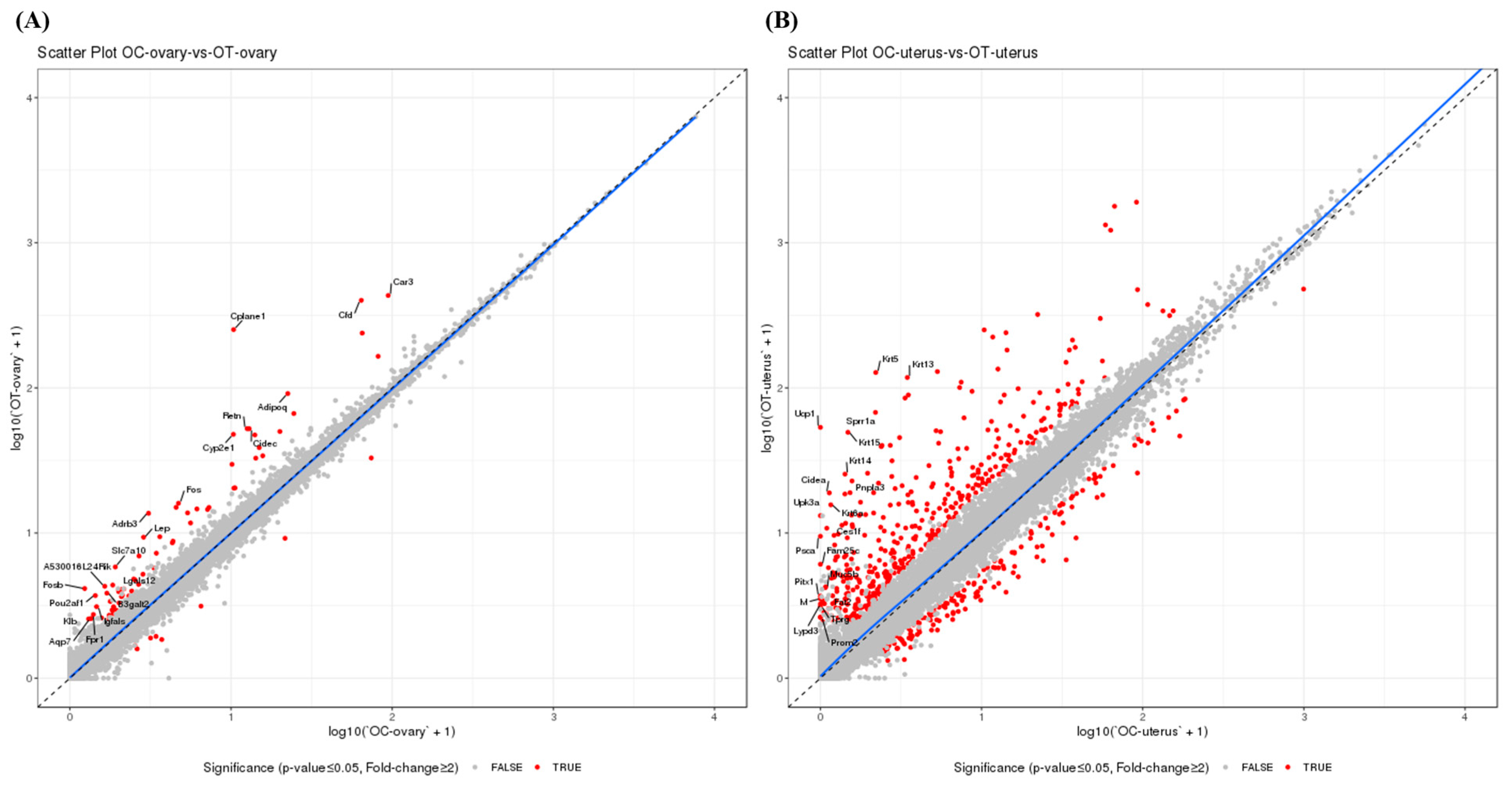
3.3. AMSC-CM Treatment Alters the Ovary and Uterus Transcriptomes
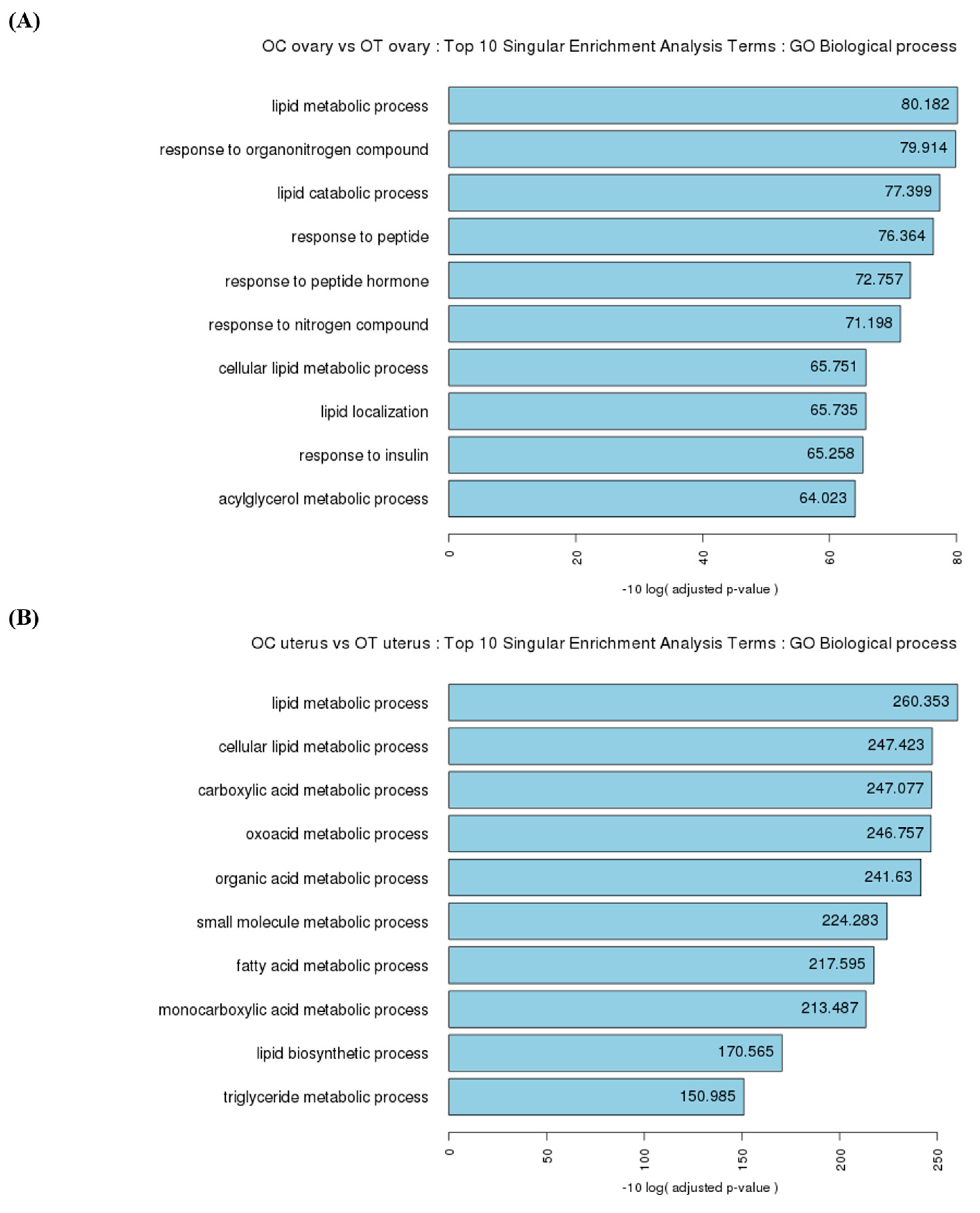
3.4. Functional Analysis of the Ovaries in Singular Enrichment Analysis Terms
3.5. Functional Analysis of the Uterus in Singular Enrichment Analysis Terms
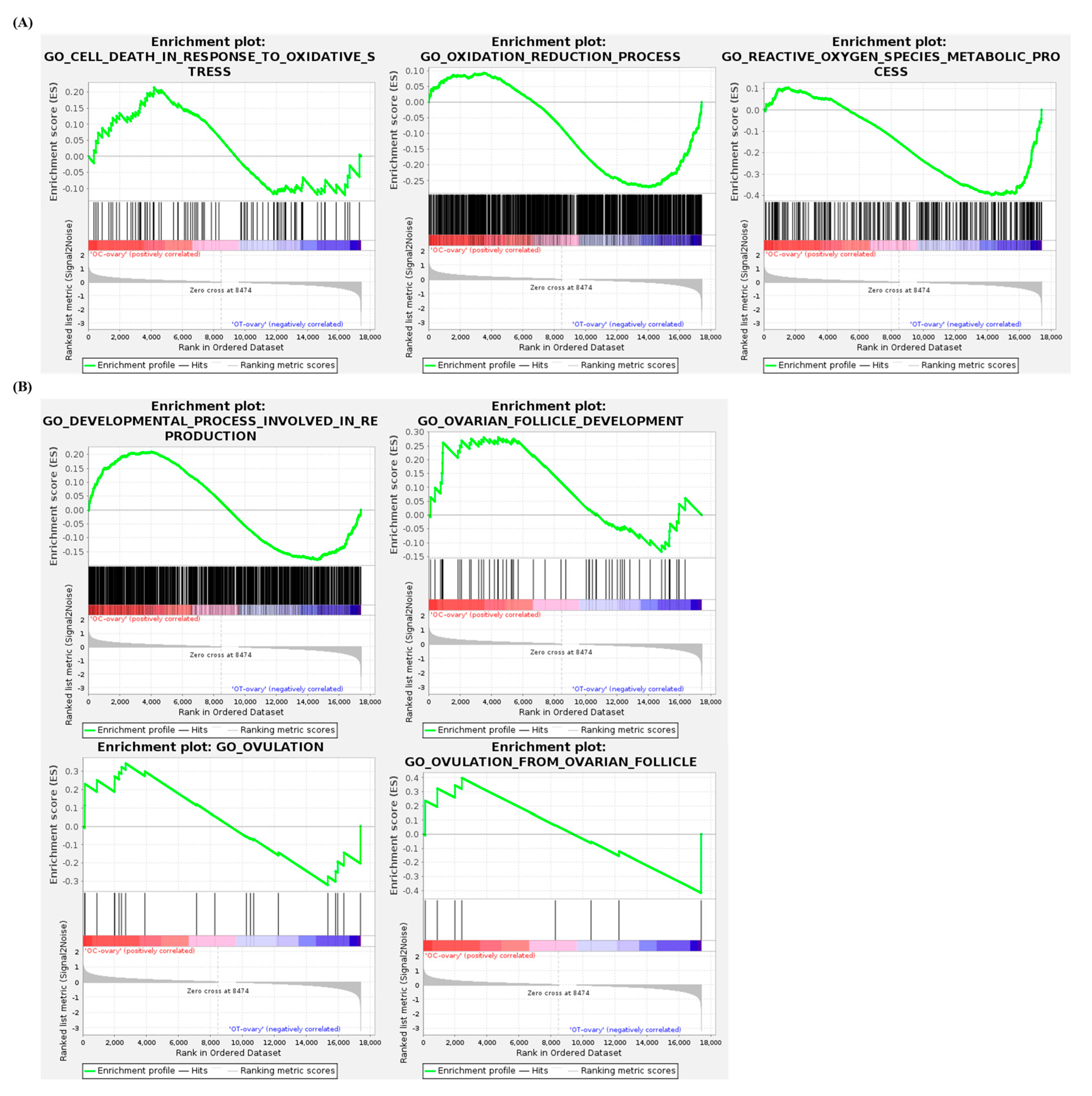
3.6. Gene Set Enrichment Analysis of the Ovaries
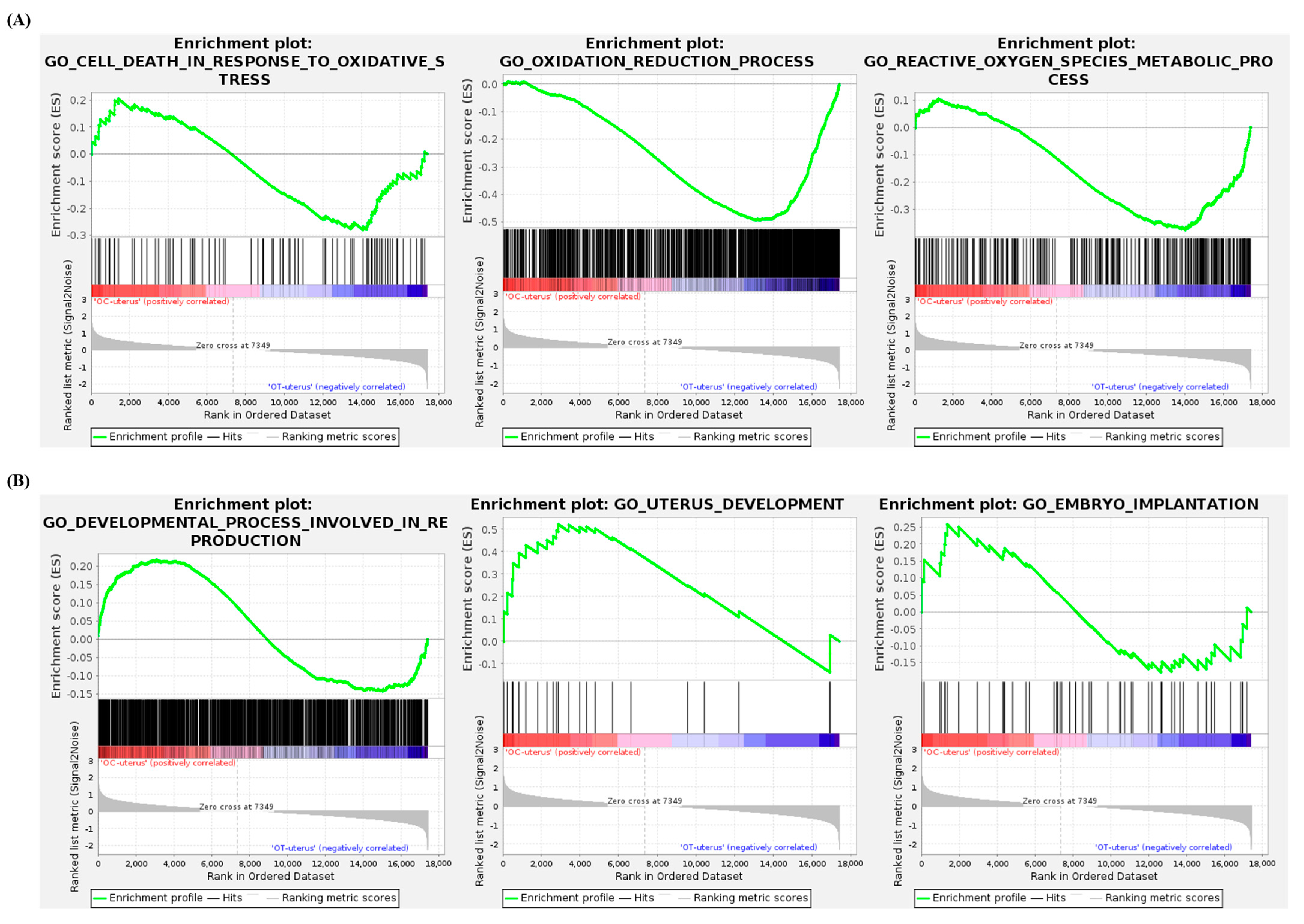
3.7. Gene Set Enrichment Analysis of the Uterus
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| AMSCs | Amniotic membrane-derived mesenchymal stem cells |
| CM | Conditioned medium |
| ROS | Reactive oxygen species |
| IV | Intravenous injection |
| EGs | Differentially expressed genes |
| FSH | Follicle-stimulating hormone |
| AMH | Anti-mullerian hormone |
| TAC | Total antioxidant capacity |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSEA | Gene set enrichment analysis |
References
- Beshay, E.; Rehman, K.U.; Carrier, S. Impact of Aging on Reproduction and Sexual Function. In Autonomic Nervous System in Old Age; Kuchel, G.A., Hof, P.R., Eds.; Interdiscipl Top Gerontol; Karger: Basel, Switzerland, 2004; Volume 33, pp. 94–106. [Google Scholar]
- Meldrum, D.R. Female reproductive aging—Ovarian and uterine factors. Fertil. Steril. 1993, 59, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C.; Menon, R. Positive and negative effects of cellular senescence during female reproductive aging and pregnancy. J. Endocrinol. 2016, 230, R59–R76. [Google Scholar] [CrossRef]
- Shirasuna, K.; Iwata, H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.; Perez-Garcia, V.; Kieckbusch, J.; Wang, X.; DeMayo, F.; Colucci, F.; Hemberger, M. Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat. Commun. 2017, 8, 352. [Google Scholar] [CrossRef]
- Nelson, S.M.; Telfer, E.E.; Anderson, R.A. The ageing ovary and uterus: New biological insights. Hum. Reprod. Update 2013, 19, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Park, S.C.; Lee, B.C. Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant. Int. J. Mol. Sci. 2023, 24, 5053. [Google Scholar] [CrossRef]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid. Med. Cell. Longev. 2017, 2017, 4371714. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. Biomed. Online 2012, 25, 551–560. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Marcus, A.J.; Woodbury, D. Fetal stem cells from extra-embryonic tissues: Do not discard. J. Cell. Mol. Med. 2008, 12, 730–742. [Google Scholar] [CrossRef]
- Miki, T. Amnion-derived stem cells: In quest of clinical applications. Stem Cell Res. Ther. 2011, 2, 25. [Google Scholar] [CrossRef]
- Li, Z.; Han, Z.C. Introduction of Perinatal Tissue-Derived Stem Cells. In Perinatal Stem Cells: Biology, Manufacturing and Translational Medicine; Han, Z.C., Takahashi, T.A., Han, Z., Li, Z., Eds.; Springer: Singapore, 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Huang, B.; Ding, C.; Zou, Q.; Lu, J.; Wang, W.; Li, H. Human Amniotic Fluid Mesenchymal Stem Cells Improve Ovarian Function During Physiological Aging by Resisting DNA Damage. Front. Pharmacol. 2020, 11, 272. [Google Scholar] [CrossRef]
- Li, J.; Mao, Q.; He, J.; She, H.; Zhang, Z.; Yin, C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res. Ther. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zou, Q.; Wang, F.; Wu, H.; Chen, R.; Lv, J.; Ling, M.; Sun, J.; Wang, W.; Li, H.; et al. Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res. Ther. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Oh, H.J.; Kim, E.Y.; Kang, S.K.; Ra, J.C.; Kim, E.H.; Lee, B.C. Anti-Oxidative Effects of Human Adipose Stem Cell Conditioned Medium with Different Basal Medium during Mouse Embryo In Vitro Culture. Animals 2020, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Oh, H.J.; Kim, E.Y.; Kang, S.K.; Ra, J.C.; Kim, E.H.; Park, S.C.; Lee, B.C. Comparison of Anti-Oxidative Effect of Human Adipose- and Amniotic Membrane-Derived Mesenchymal Stem Cell Conditioned Medium on Mouse Preimplantation Embryo Development. Antioxidants 2021, 10, 268. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g:Profiler—A web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Ra, K.; Oh, H.J.; Kim, G.A.; Kang, S.K.; Ra, J.C.; Lee, B.C. High Frequency of Intravenous Injection of Human Adipose Stem Cell Conditioned Medium Improved Embryo Development of Mice in Advanced Maternal Age through Antioxidant Effects. Animals 2020, 10, 978. [Google Scholar] [CrossRef]
- Azami, S.H.; Nazarian, H.; Abdollahifar, M.A.; Eini, F.; Farsani, M.A.; Novin, M.G. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reprod. Fertil. Dev. 2020, 32, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.E. The menopause and aging, a comparative perspective. J. Steroid Biochem. Mol. Biol. 2014, 142, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Gracia, C.R. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J. Clin. Endocrinol. Metab. 2012, 97, 1673–1680. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martin-Carro, B.; Cannata-Andia, J.B.; Mora-Fernandez, C.; Navarro-Gonzalez, J.F. Klotho, Oxidative Stress, and Mitochondrial Damage in Kidney Disease. Antioxidants 2023, 12, 239. [Google Scholar] [CrossRef]
- Olejnik, A.; Banaszkiewicz, M.; Krzywonos-Zawadzka, A.; Bil-Lula, I. The Klotho protein supports redox balance and metabolic functions of cardiomyocytes during ischemia/reperfusion injury. Cardiol. J. 2022, 29, 836–849. [Google Scholar] [CrossRef]
- Meroni, M.; Dongiovanni, P.; Tiano, F.; Piciotti, R.; Alisi, A.; Panera, N. beta-Klotho as novel therapeutic target in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A narrative review. Biomed. Pharmacother. 2024, 180, 117608. [Google Scholar] [CrossRef]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Idrees, M.; Kumar, V.; Khan, A.M.; Joo, M.D.; Lee, K.W.; Sohn, S.H.; Kong, I.K. Cycloastragenol activation of telomerase improves beta-Klotho protein level and attenuates age-related malfunctioning in ovarian tissues. Mech. Ageing Dev. 2023, 209, 111756. [Google Scholar] [CrossRef]
- Xu, C.; Messina, A.; Somm, E.; Miraoui, H.; Kinnunen, T.; Acierno, J., Jr.; Niederlander, N.J.; Bouilly, J.; Dwyer, A.A.; Sidis, Y.; et al. KLB, encoding beta-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol. Med. 2017, 9, 1379–1397. [Google Scholar] [CrossRef]
- Konishi, M.; Haraguchi, G.; Ohigashi, H.; Ishihara, T.; Saito, K.; Nakano, Y.; Isobe, M. Adiponectin protects against doxorubicin-induced cardiomyopathy by anti-apoptotic effects through AMPK up-regulation. Cardiovasc. Res. 2011, 89, 309–319. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Chen, Y.; Dong, Y. Unraveling the AMPK-SIRT1-FOXO Pathway: The In-Depth Analysis and Breakthrough Prospects of Oxidative Stress-Induced Diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Park, S.; Kim, M.J.; Yang, W.K.; Im, D.U.; Yang, K.R.; Hong, J.; Choe, W.; Kang, I.; Kim, S.S.; et al. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014, 281, 4421–4438. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Azzu, V.; Valencak, T.G. Energy Metabolism and Ageing in the Mouse: A Mini-Review. Gerontology 2017, 63, 327–336. [Google Scholar] [CrossRef]
- Reverchon, M.; Rame, C.; Bertoldo, M.; Dupont, J. Adipokines and the female reproductive tract. Int. J. Endocrinol. 2014, 2014, 232454. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Zeng, W.; Yuan, X.; Li, B.; Li, T.; Zu, Y.; Jiang, M.; Li, Y. Field experiment on the effects of sepiolite and biochar on the remediation of Cd- and Pb-polluted farmlands around a Pb-Zn mine in Yunnan Province, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 7743–7751. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Ge, S.Q.; Chen, L.; Cai, L.S.; Hwang, M.F.; Wang, C.L. Relationships between female infertility and female genital infections and pelvic inflammatory disease: A population-based nested controlled study. Clinics 2018, 73, e364. [Google Scholar] [CrossRef]
- Xing, X.; Su, L.; Asare, P.F.; Wang, L.; Li, L.; Liu, E.; Yu, B.; Zhu, Y.; Gao, X.; Fan, G. Danzhi Qing’e (DZQE) activates AMPK pathway and regulates lipid metabolism in a rat model of perimenopausal hyperlipidaemia. Exp. Physiol. 2016, 101, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Almada, M.; Domingues, M.R.; Doria, M.L.; Fonseca, B.M.; Teixeira, N.A.; Correia-da-Silva, G. Lipidomic approach towards deciphering anandamide effects in rat decidual cell. J. Cell. Physiol. 2015, 230, 1549–1557. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Chen, C.; Qi, H.; Baker, P.N.; Liu, X.; Zhang, H.; Han, T.L. Metabolic Changes of Maternal Uterine Fluid, Uterus, and Plasma during the Peri-implantation Period of Early Pregnancy in Mice. Reprod. Sci. 2020, 27, 488–502. [Google Scholar] [CrossRef]
| OC vs. OT | |||||
|---|---|---|---|---|---|
| Official Gene Symbol | Log2 (Fold Change) | Official Gene Symbol | Log2 (Fold Change) | ||
| Ovary | Uterus | Ovary | Uterus | ||
| Slc7a10 | 2.406 | 5.256 | Ffar2 | 1.260 | 3.868 |
| A530016L24Rik | 2.35 | 5.092 | Lpl | 1.362 | 3.758 |
| Cfd | 2.66 | 4.759 | Rbp4 | 1.689 | 3.423 |
| Adrb3 | 2.61 | 4.793 | Fosb | 3.741 | 1.284 |
| Klb | 2.35 | 5.009 | Retnla | 1.219 | 3.601 |
| Pnpla3 | 1.819 | 5.391 | Abcd2 | 1.184 | 3.334 |
| Pou2af1 | 2.623 | 4.517 | Trarg1 | 1.421 | 3.089 |
| Aqp7 | 2.163 | 4.924 | Cdo1 | 1.829 | 2.349 |
| Cyp2e1 | 2.327 | 4.736 | Cd36 | 1.48 | 2.575 |
| Car3 | 2.211 | 4.396 | Irs3 | 1.481 | 2.518 |
| Cidec | 2.102 | 4.377 | Jchain | 1.653 | 2.341 |
| Plin1 | 1.438 | 4.895 | Fcor | 1.21 | 2.779 |
| Retn | 2.147 | 4.182 | Ces1d | 1.022 | 2.706 |
| Fabp4 | 1.889 | 4.293 | Mzb1 | 1.359 | 2.265 |
| B3galt2 | 2.034 | 4.098 | Cd79b | 1.544 | 2.017 |
| Lgals12 | 2.000 | 4.037 | Acp5 | 1.14 | 2.398 |
| Adipoq | 2.071 | 3.909 | Ifi27l2a | 1.022 | 2.368 |
| Adig | 1.407 | 4.516 | Hcar1 | 1.023 | 2.289 |
| Lep | 2.163 | 3.73 | Fos | 2.016 | 1.125 |
| Acvr1c | 1.483 | 4.181 | Cd209g | 1.029 | 1.871 |
| Pck1 | 1.545 | 4.068 | Pxmp2 | 1.279 | 1.493 |
| Apoc1 | 1.154 | 4.404 | Tmem45b | 1.023 | 1.676 |
| Igfals | 2.183 | 3.335 | Ccl2 | 1.284 | 1.384 |
| Orm1 | 1.36 | 4.096 | Dgat2 | 1.166 | 1.411 |
| Serpina3c | 1.284 | 4.144 | Thrsp | 1.265 | 1.218 |
| Myl1 | 1.039 | 4.181 | Pla2g2d | 1.066 | 1.218 |
| Sncg | 1.979 | 3.201 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ra, K. Therapeutic Potential of Human Amniotic Membrane-Derived Mesenchymal Stem Cell Conditioned Medium in Combating Oxidative Stress and Age-Related Female Infertility. Cells 2025, 14, 801. https://doi.org/10.3390/cells14110801
Ra K. Therapeutic Potential of Human Amniotic Membrane-Derived Mesenchymal Stem Cell Conditioned Medium in Combating Oxidative Stress and Age-Related Female Infertility. Cells. 2025; 14(11):801. https://doi.org/10.3390/cells14110801
Chicago/Turabian StyleRa, Kihae. 2025. "Therapeutic Potential of Human Amniotic Membrane-Derived Mesenchymal Stem Cell Conditioned Medium in Combating Oxidative Stress and Age-Related Female Infertility" Cells 14, no. 11: 801. https://doi.org/10.3390/cells14110801
APA StyleRa, K. (2025). Therapeutic Potential of Human Amniotic Membrane-Derived Mesenchymal Stem Cell Conditioned Medium in Combating Oxidative Stress and Age-Related Female Infertility. Cells, 14(11), 801. https://doi.org/10.3390/cells14110801





