Metabolic Reprogramming Triggered by Fluoride in U-87 Glioblastoma Cells: Implications for Tumor Progression?
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Intracellular Reactive Oxygen Species (ROS) Analysis
2.3. Antioxidant System Analysis
- Superoxide dismutase (SOD): Superoxide Dismutase Assay Kit (706002-96, CAYMAN, Ann Arbor, MI, USA)
- Catalase (CAT): Catalase Assay Kit (707002-96, CAYMAN)
- Glutathione (GSH and GSSG): Glutathione Assay Kit (703002-96, CAYMAN)
2.4. β-Catenin Analysis
2.5. Lysosome Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
- Tumor necrosis factor α (TNF-α): Human TNF-α ELISA Kit (E0133h, Wuhan EIAab Elisas, Wuhan, China), measured in both medium and cell lysates.
- Interleukin 1α (IL-1α): Human IL1A/Interleukin-1alpha ELISA Kit (E0071h, Wuhan EIAab Elisas), measured in both medium and cell lysates.
- Interleukin 1β (IL-1β): Human IL1B/Interleukin-1beta ELISA Kit (E0563h, Wuhan EIAab Elisas), measured in both medium and cell lysates.
- Interleukin 4 (IL-4): Human IL4/Interleukin-4 ELISA Kit (E0077h, Wuhan EIAab Elisas), measured in both medium and cell lysates.
- Interleukin 10 (IL-10): Human IL10/Interleukin-10 ELISA Kit (E0056h, Wuhan EIAab Elisas), measured in both medium and cell lysates.
- Interferon γ (IFN-γ): Human IFNG/Interferon gamma ELISA Kit (E0049h, Wuhan EIAab Elisas), measured in both medium and cell lysates.
- Interleukin 6 (IL-6): Human IL6/Interleukin-6 ELISA Kit (E0079h, Wuhan EIAab Elisas), measured in both medium and cell lysates.
- Thromboxane B2 (TXB2): Thromboxane B2 Express ELISA Kit—Monoclonal (10004023-96, CAYMAN), measured in cell lysates.
- Prostaglandin E2 (PGE2): Prostaglandin E2 ELISA Kit—Monoclonal (514010-96, CAYMAN), measured in both medium and cell lysates.
2.7. Statistical Analysis
3. Results
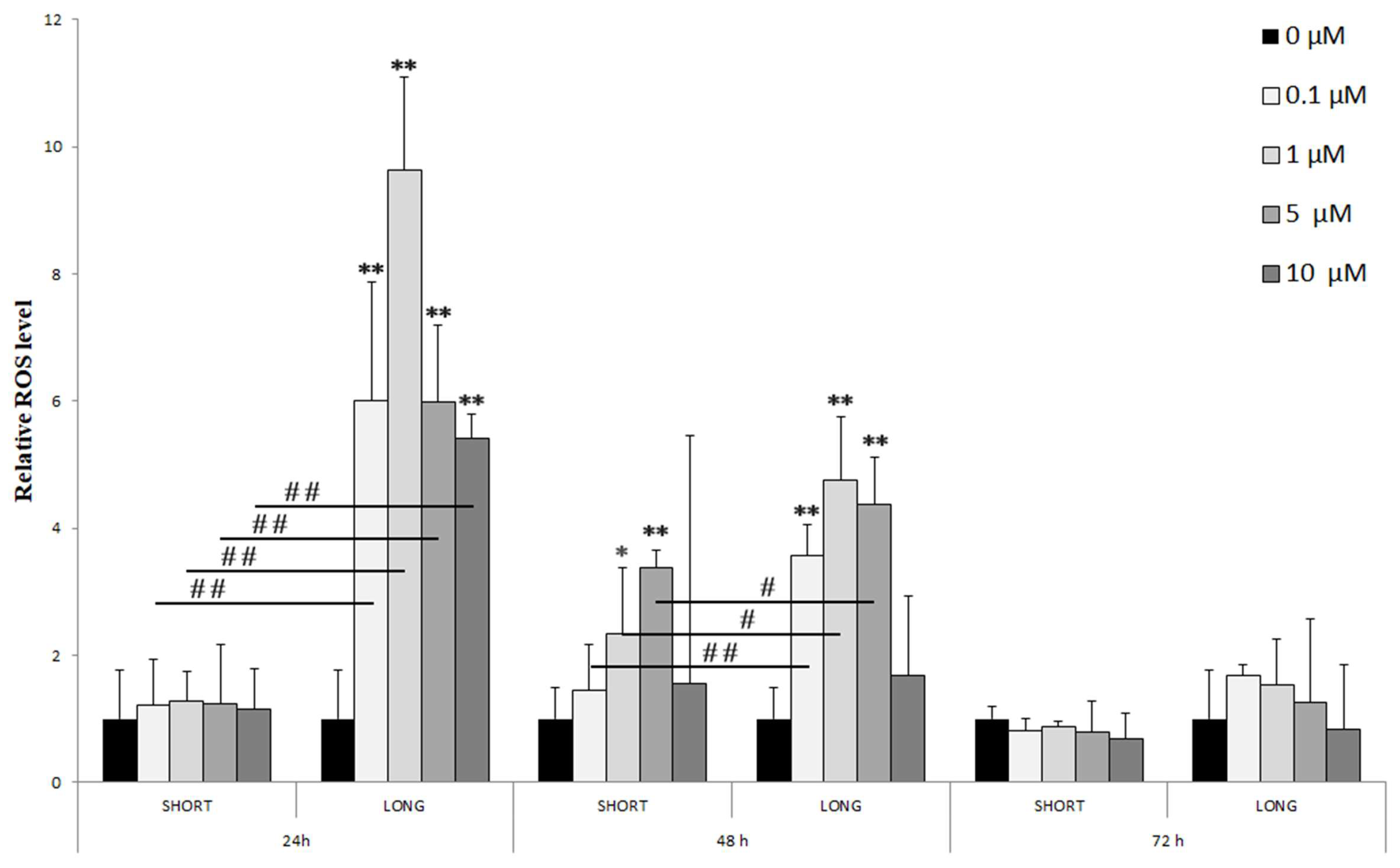
- 24 h: A significant increase in ROS levels was observed only with long-term (LONG) exposure to all tested NaF doses (0.1–10 µM), reaching a maximum at 1 µM (** p < 0.01). Significant differences between doses were marked (# p < 0.01), indicating a dose-dependency of the effect.
- 48 h: The increase in ROS levels was visible with both short (SHORT) and long (LONG) exposures, although more pronounced with LONG. The effect was again greatest for 1 µM NaF (** p < 0.01 LONG). Differences between concentration groups were also significant (# p < 0.05, ## p < 0.01).
- 72 h: After 72 h, ROS levels were significantly lower than at 24 and 48 h, with no statistically significant differences from the control, which may suggest cell adaptation to long-term exposure to NaF.
4. Discussion
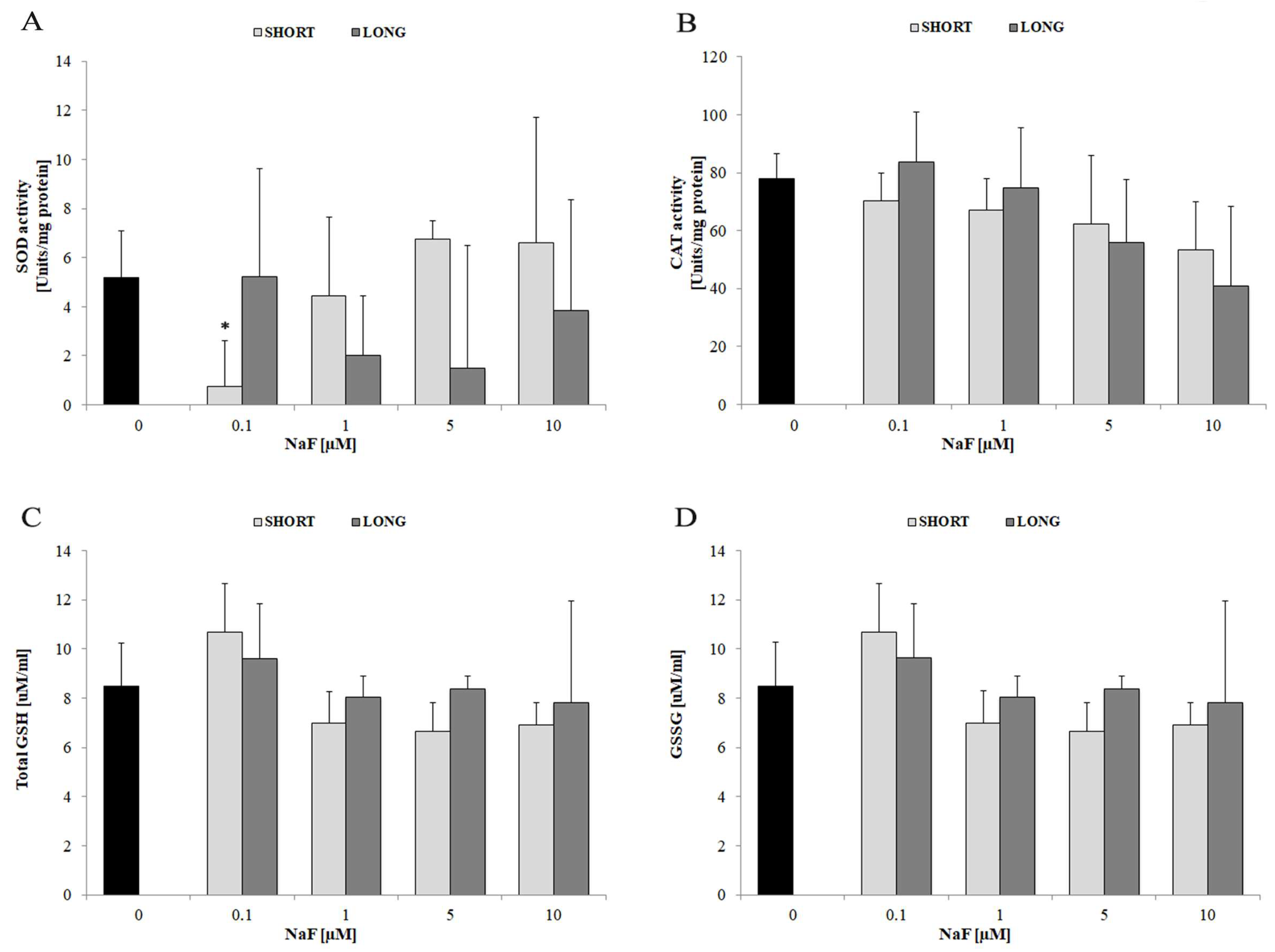
4.1. Oxidative Stress and Antioxidant Enzymes
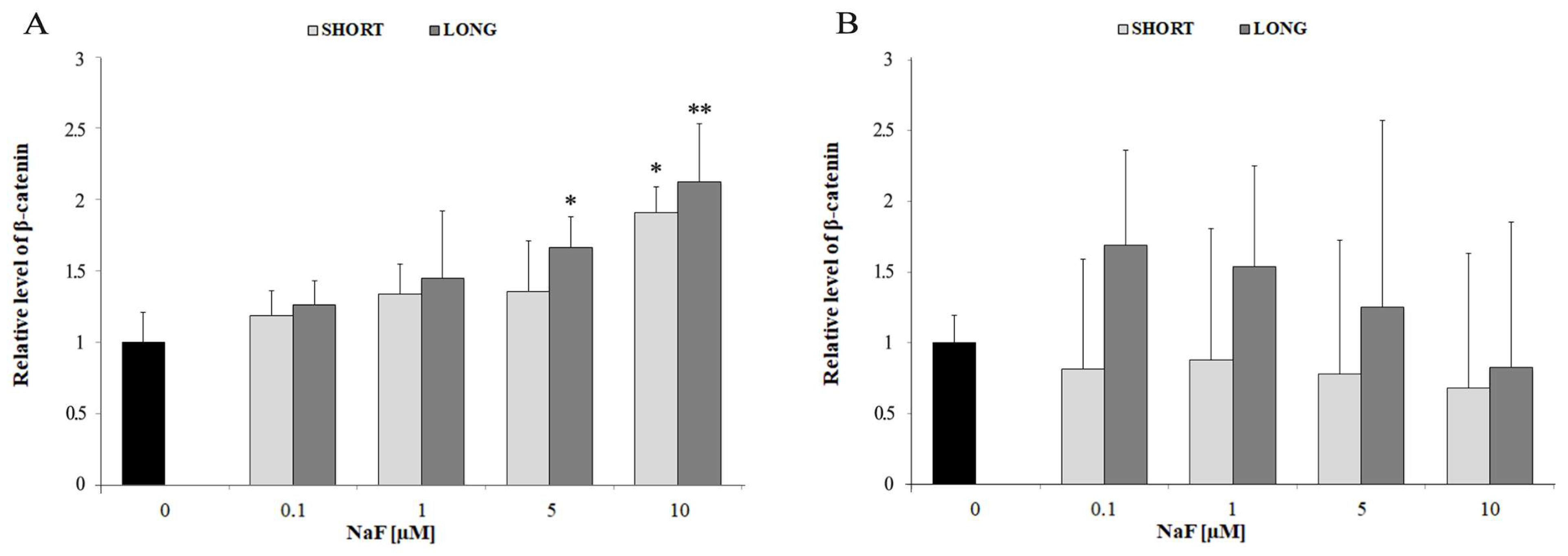
4.2. The Role of Fluorides in Regulating β-Catenin Levels
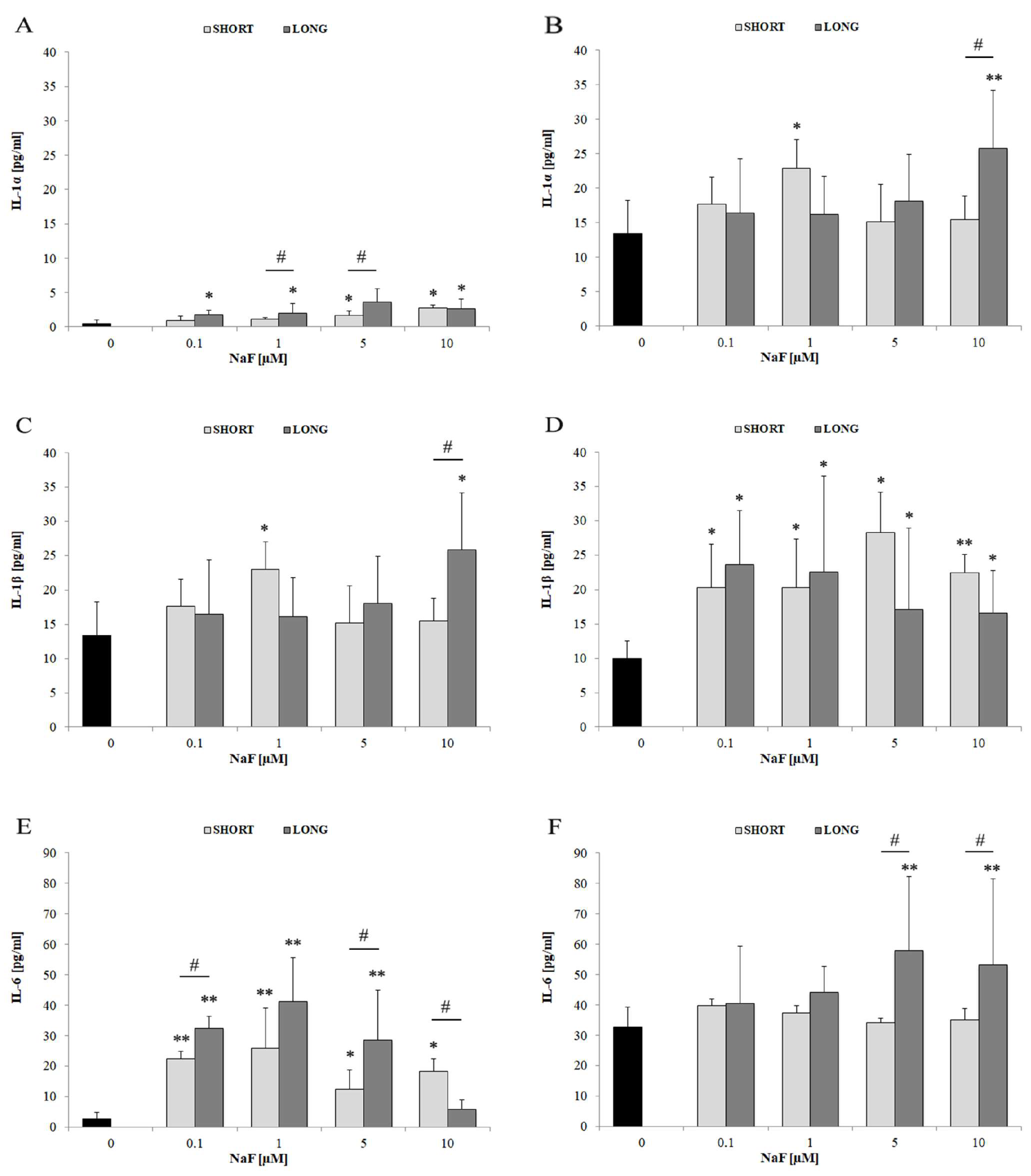
4.3. The Role of Fluorides in Regulating Inflammatory Cytokines
4.3.1. IL-6
4.3.2. IL-1α and IL-1β
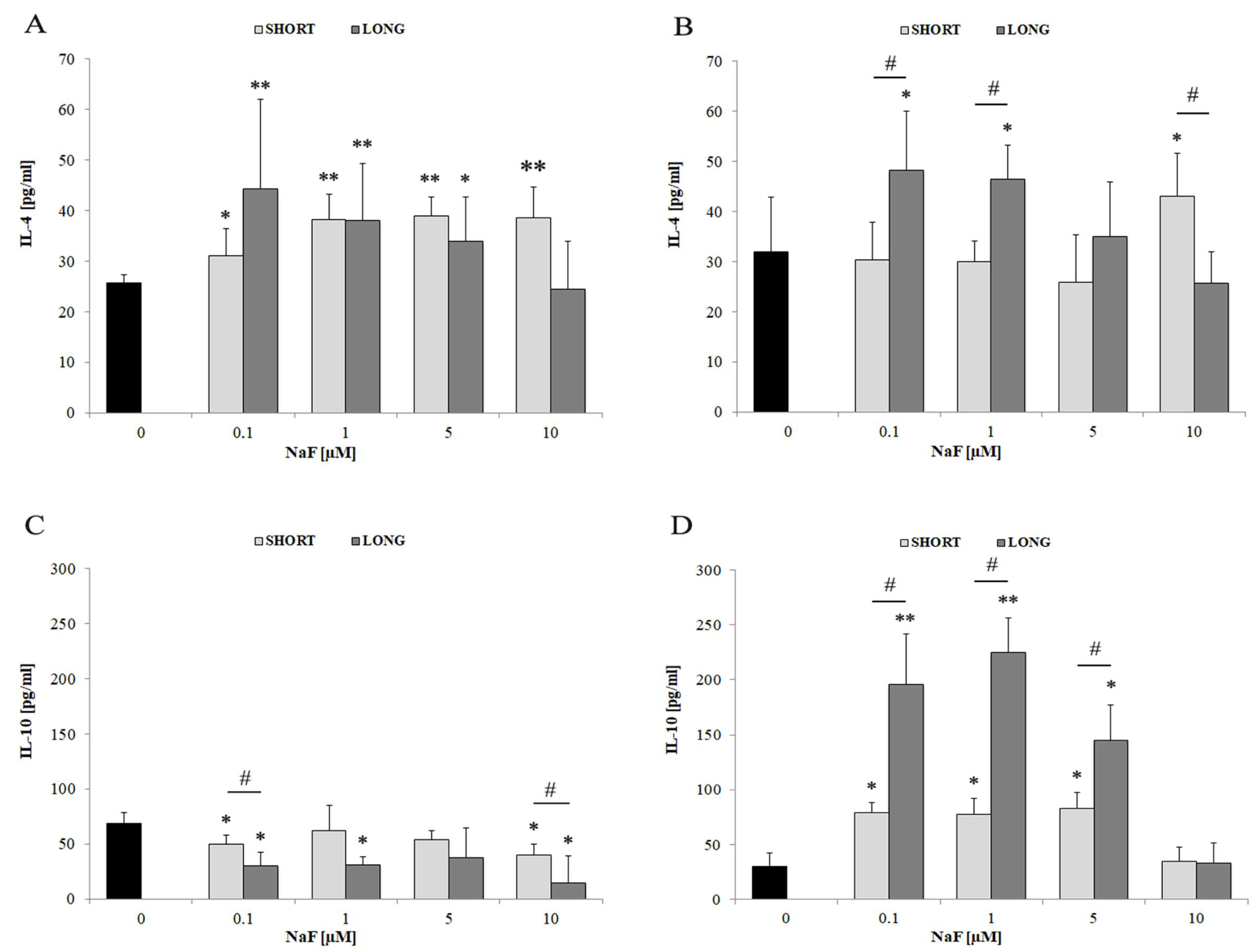
4.3.3. IL-4 and IL-10
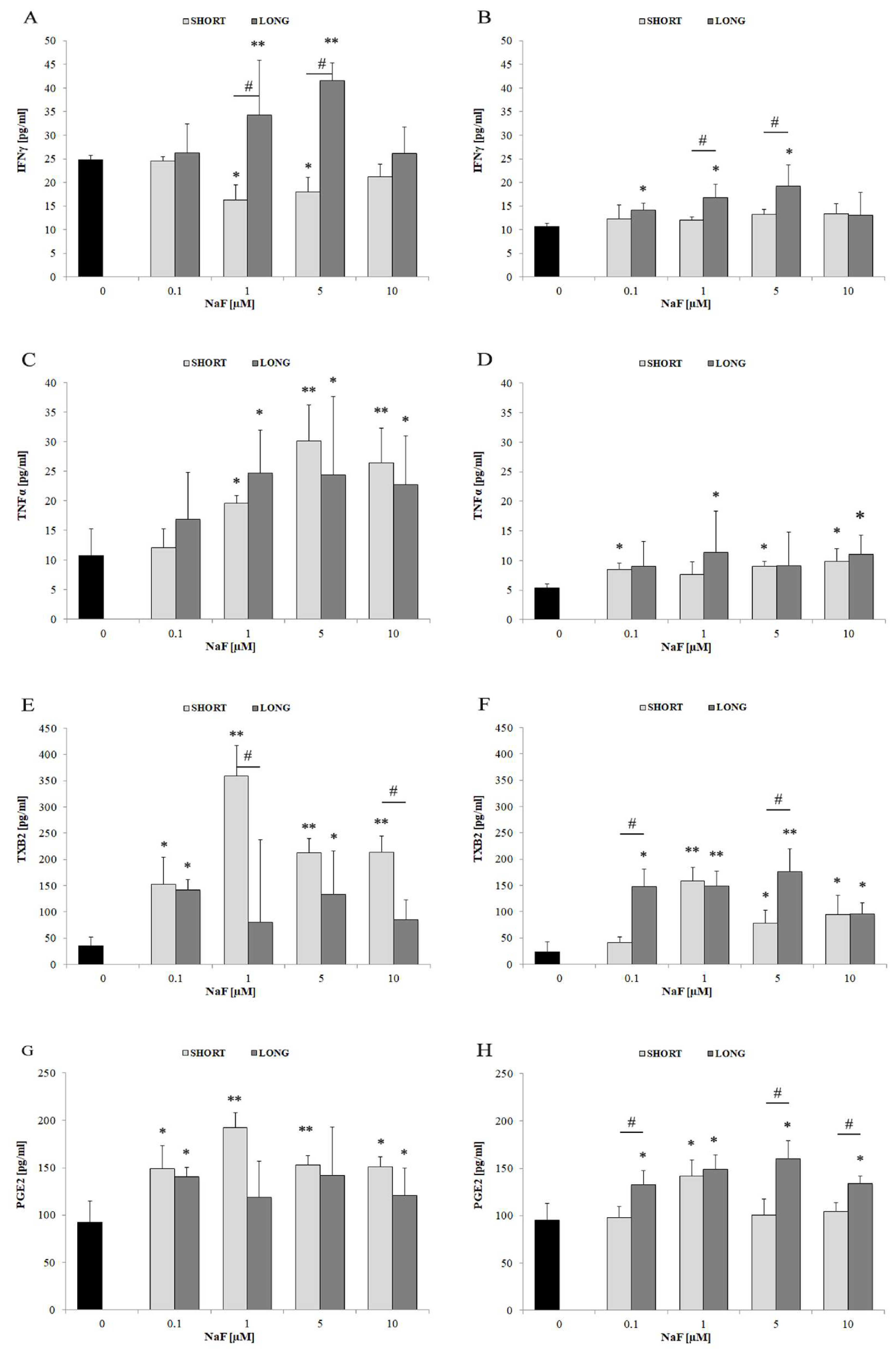
4.4. TNFα
4.5. The Role of Fluorides in Modulating Prostanoid Metabolism
4.6. The Role of Fluorides in Regulating IFN-γ Levels
5. Conclusions
6. Limitations and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miller, C.R.; Hjelmeland, A.B. Breaking the Feed Forward Inflammatory Cytokine Loop in the Tumor Microenvironment of PDGFB-Driven Glioblastomas. J. Clin. Investig. 2023, 133, e175127. [Google Scholar] [CrossRef] [PubMed]
- Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Gutowska, I. Fluoride in the Central Nervous System and Its Potential Influence on the Development and Invasiveness of Brain Tumours—A Research Hypothesis. Int. J. Mol. Sci. 2023, 24, 1558. [Google Scholar] [CrossRef] [PubMed]
- Shuhua, X.; Ziyou, L.; Ling, Y.; Fei, W.; Sun, G. A Role of Fluoride on Free Radical Generation and Oxidative Stress in BV-2 Microglia Cells. Mediat. Inflamm. 2012, 2012, 102954. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Liu, Y.; Liu, S.; Cao, S.; Wang, F.; Wang, Z.; Xi, S. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol. Neurobiol. 2016, 53, 4449–4460. [Google Scholar] [CrossRef]
- Si, J.; Guo, J.; Zhang, X.; Li, W.; Zhang, S.; Shang, S.; Zhang, Q. Hypoxia-Induced Activation of HIF-1alpha/IL-1beta Axis in Microglia Promotes Glioma Progression via NF-κB-Mediated Upregulation of Heparanase Expression. Biol. Direct 2024, 19, 45. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, L.-D.; Liu, H.; Li, H.-H.; Ren, C.; Zhang, P.; Guo, K.-T.; Zhang, H.-X.; Geng, D.-Q.; Zhang, C.-Y. Fluoride Induces Neuroinflammation and Alters Wnt Signaling Pathway in BV2 Microglial Cells. Inflammation 2017, 40, 1123–1130. [Google Scholar] [CrossRef]
- Wang, H.-W.; Zhao, W.-P.; Liu, J.; Tan, P.-P.; Zhang, C.; Zhou, B.-H. Fluoride-Induced Oxidative Stress and Apoptosis Are Involved in the Reducing of Oocytes Development Potential in Mice. Chemosphere 2017, 186, 911–918. [Google Scholar] [CrossRef]
- World Health Organization. Preventing Disease through Healthy Environments: Inadequate or Excess Fluoride: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019.
- DenBesten, P.; Li, W. Chronic Fluoride Toxicity: Dental Fluorosis. Monogr. Oral Sci. 2011, 22, 81. [Google Scholar] [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of Fluoride: Critical Evaluation of Evidence for Human Developmental Neurotoxicity in Epidemiological Studies, Animal Experiments and in Vitro Analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental Fluoride Neurotoxicity: An Updated Review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Gao, Q.; Wu, C.-X.; Guan, Z.-Z. Alterations of nAChRs and ERK1/2 in the Brains of Rats with Chronic Fluorosis and Their Connections with the Decreased Capacity of Learning and Memory. Toxicol. Lett. 2010, 192, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Reddy, K.; Kumar, K. Neurodegenerative Changes in Different Regions of Brain, Spinal Cord and Sciatic Nerve of Rats Treated with Sodium Fluoride. J. Med. Allied Sci. 2011, 1, 30–35. [Google Scholar]
- Wu, C.; Gu, X.; Ge, Y.; Jianhai, Z.; Wang, J. Effects of High Fluoride and Arsenic on Brain Biochemical Indexes and Learning-Memory in Rats. Fluoride 2006, 39, 274–279. [Google Scholar]
- Bartos, M.; Gumilar, F.; Gallegos, C.E.; Bras, C.; Dominguez, S.; Cancela, L.M.; Minetti, A. Effects of Perinatal Fluoride Exposure on Short- and Long-Term Memory, Brain Antioxidant Status, and Glutamate Metabolism of Young Rat Pups. Int. J. Toxicol. 2019, 38, 405–414. [Google Scholar] [CrossRef]
- Kupnicka, P.; Listos, J.; Tarnowski, M.; Kolasa-Wołosiuk, A.; Wąsik, A.; Łukomska, A.; Barczak, K.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats. Int. J. Mol. Sci. 2020, 21, 2361. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Xue, X.; Niu, R.; Wang, J. Maternal Fluoride Exposure during Gestation and Lactation Decreased Learning and Memory Ability, and Glutamate Receptor mRNA Expressions of Mouse Pups. Hum. Exp. Toxicol. 2018, 37, 87–93. [Google Scholar] [CrossRef]
- Lopes, G.O.; Martins Ferreira, M.K.; Davis, L.; Bittencourt, L.O.; Bragança Aragão, W.A.; Dionizio, A.; Rabelo Buzalaf, M.A.; Crespo-Lopez, M.E.; Maia, C.S.F.; Lima, R.R. Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation. Int. J. Mol. Sci. 2020, 21, 7297. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, S.; Liu, H.; Guan, Z.; Zeng, Q.; Zhang, C.; Lei, R.; Xia, T.; Wang, Z.; Yang, L.; et al. Low Glucose Utilization and Neurodegenerative Changes Caused by Sodium Fluoride Exposure in Rat’s Developmental Brain. Neuromolecular Med. 2014, 16, 94–105. [Google Scholar] [CrossRef]
- Adedara, I.A.; Olabiyi, B.F.; Ojuade, T.D.; Idris, U.F.; Onibiyo, E.M.; Farombi, E.O. Taurine Reverses Sodium Fluoride-Mediated Increase in Inflammation, Caspase-3 Activity, and Oxidative Damage along the Brain-Pituitary-Gonadal Axis in Male Rats. Can. J. Physiol. Pharmacol. 2017, 95, 1019–1029. [Google Scholar] [CrossRef]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Jakubczyk, K.; Tarnowski, M.; Lubkowska, A.; Baranowska-Bosiacka, I.; Styburski, D.; Skórka-Majewicz, M.; Maciejewska, D.; et al. Chronic Exposure to Fluoride Affects GSH Level and NOX4 Expression in Rat Model of This Element of Neurotoxicity. Biomolecules 2020, 10, 422. [Google Scholar] [CrossRef]
- Chen, L.; Kuang, P.; Liu, H.; Wei, Q.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; et al. Sodium Fluoride (NaF) Induces Inflammatory Responses Via Activating MAPKs/NF-κB Signaling Pathway and Reducing Anti-Inflammatory Cytokine Expression in the Mouse Liver. Biol. Trace Elem. Res. 2019, 189, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Guan, Z.-Z.; Gao, Q.; Pei, J.-J. Increased Level of Apoptosis in Rat Brains and SH-SY5Y Cells Exposed to Excessive Fluoride—A Mechanism Connected with Activating JNK Phosphorylation. Toxicol. Lett. 2011, 204, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xu, Z.; Xia, T.; He, P.; Gao, P.; He, W.; Zhang, M.; Guo, L.; Niu, Q.; Wang, A. Effects of the Fas/Fas-L Pathway on Fluoride-Induced Apoptosis in SH-SY5Y Cells. Environ. Toxicol. 2011, 26, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhang, Q.; Liu, Y.; Han, L.; Wang, Q.; Chen, P.; Zhang, S.; Wang, A.; Zhou, X. Fluoride Induces Apoptosis via Inhibiting SIRT1 Activity to Activate Mitochondrial P53 Pathway in Human Neuroblastoma SH-SY5Y Cells. Toxicol. Appl. Pharmacol. 2018, 347, 60–69. [Google Scholar] [CrossRef]
- Zhou, G.; Tang, S.; Yang, L.; Niu, Q.; Chen, J.; Xia, T.; Wang, S.; Wang, M.; Zhao, Q.; Liu, L.; et al. Effects of Long-Term Fluoride Exposure on Cognitive Ability and the Underlying Mechanisms: Role of Autophagy and Its Association with Apoptosis. Toxicol. Appl. Pharmacol. 2019, 378, 114608. [Google Scholar] [CrossRef]
- Bashash, M.; Thomas, D.; Hu, H.; Angeles Martinez-Mier, E.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Ettinger, A.S.; Wright, R.; Zhang, Z.; et al. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef]
- Cao, K.; Xiang, J.; Dong, Y.-T.; Xu, Y.; Li, Y.; Song, H.; Zeng, X.-X.; Ran, L.-Y.; Hong, W.; Guan, Z.-Z. Exposure to Fluoride Aggravates the Impairment in Learning and Memory and Neuropathological Lesions in Mice Carrying the APP/PS1 Double-Transgenic Mutation. Alzheimer’s Res. Ther. 2019, 11, 35. [Google Scholar] [CrossRef]
- Farmus, L.; Till, C.; Green, R.; Hornung, R.; Martinez Mier, E.A.; Ayotte, P.; Muckle, G.; Lanphear, B.P.; Flora, D.B. Critical Windows of Fluoride Neurotoxicity in Canadian Children. Environ. Res. 2021, 200, 111315. [Google Scholar] [CrossRef]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940. [Google Scholar] [CrossRef]
- Russ, T.C.; Killin, L.O.J.; Hannah, J.; Batty, G.D.; Deary, I.J.; Starr, J.M. Aluminium and Fluoride in Drinking Water in Relation to Later Dementia Risk. Br. J. Psychiatry 2020, 216, 29–34. [Google Scholar] [CrossRef]
- Malin, A.J.; Till, C. Exposure to Fluoridated Water and Attention Deficit Hyperactivity Disorder Prevalence among Children and Adolescents in the United States: An Ecological Association. Environ. Health 2015, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Agalakova, N.I.; Nadei, O.V. Inorganic Fluoride and Functions of Brain. Crit. Rev. Toxicol. 2020, 50, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, J.M.; Thomson, W.M.; Ramrakha, S.; Moffitt, T.E.; Zeng, J.; Foster Page, L.A.; Poulton, R. Community Water Fluoridation and Intelligence: Prospective Study in New Zealand. Am. J. Public Health 2015, 105, 72–76. [Google Scholar] [CrossRef]
- Saeed, M.; Malik, R.N.; Kamal, A. Fluorosis and Cognitive Development among Children (6–14 Years of Age) in the Endemic Areas of the World: A Review and Critical Analysis. Environ. Sci. Pollut. Res. 2020, 27, 2566–2579. [Google Scholar] [CrossRef]
- Żwierełło, W.; Maruszewska, A.; Skórka, M.; Wszołek, A.; Gutowska, I. Is Fluoride Blameless?—The Influence of Fluorine Compounds on the Invasiveness of the Human Glioma-like Cell Line U-87. Int. J. Mol. Sci. 2024, 25, 12773. [Google Scholar] [CrossRef]
- Gillespie, G.; Rudd, D.J.; Zhang, S.; Schaeffer, A.; Tomek, C.; Larson, P.; Stoch, S.A.; Iwamoto, M. A Phase 1 Trial to Evaluate the Relationship Between Fluoride Intake and Urinary Fluoride Excretion in Healthy Participants. J. Clin. Pharmacol. 2021, 62, 190. [Google Scholar] [CrossRef]
- ATCC Passage Number Effects in Cell Lines. Available online: https://www.atcc.org/resources/technical-documents/passage-number-effects-in-cell-lines (accessed on 2 November 2024).
- Ghosh, D.; Ghosh, S. FLUORIDE AND BRAIN: A REVIEW. IJPSR 2020, 11, 2011–2017. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Priya, P.; Chattopadhyay, A. Sodium Fluoride Affects Zebrafish Behaviour and Alters mRNA Expressions of Biomarker Genes in the Brain: Role of Nrf2/Keap1. Environ. Toxicol. Pharmacol. 2015, 40, 352–359. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Lou, D.-D.; Guan, Z.-Z. Activation of the AGE/RAGE System in the Brains of Rats and in SH-SY5Y Cells Exposed to High Level of Fluoride Might Connect to Oxidative Stress. Neurotoxicol. Teratol. 2015, 48, 49–55. [Google Scholar] [CrossRef]
- Ran, L.-Y.; Xiang, J.; Zeng, X.-X.; Tang, J.-L.; Dong, Y.-T.; Zhang, F.; Yu, W.-F.; Qi, X.-L.; Xiao, Y.; Zou, J.; et al. Integrated Transcriptomic and Proteomic Analysis Indicated That Neurotoxicity of Rats with Chronic Fluorosis May Be in Mechanism Involved in the Changed Cholinergic Pathway and Oxidative Stress. J. Trace Elem. Med. Biol. 2021, 64, 126688. [Google Scholar] [CrossRef]
- Khatun, S.; Mandi, M.; Rajak, P.; Roy, S. Interplay of ROS and Behavioral Pattern in Fluoride Exposed Drosophila Melanogaster. Chemosphere 2018, 209, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Araujo, T.T.; Barbosa Silva Pereira, H.A.; Dionizio, A.; Sanchez, C.d.C.; de Souza Carvalho, T.; da Silva Fernandes, M.; Rabelo Buzalaf, M.A. Changes in Energy Metabolism Induced by Fluoride: Insights from inside the Mitochondria. Chemosphere 2019, 236, 124357. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, S.A.; Akintunde, O.W.; Ogunlade, B. Fluoride-Induced Testicular Degeneration and Sperm Quality Deteriorations: Salutary Role of Cyperus Esculentus Tubers (Tiger Nut) Extract in Animal Model. Rev. Int. Androl. 2021, 19, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Guo, Z.; Li, R.; Wang, J.; Niu, R.; Wang, J. Effects of Fluoride on SOD and CAT in Testis and Epididymis of Mice. Biol. Trace Elem. Res. 2018, 184, 148–153. [Google Scholar] [CrossRef]
- Alhusaini, A.M.; Faddah, L.M.; El Orabi, N.F.; Hasan, I.H. Role of Some Natural Antioxidants in the Modulation of Some Proteins Expressions against Sodium Fluoride-Induced Renal Injury. BioMed Res. Int. 2018, 2018, e5614803. [Google Scholar] [CrossRef]
- Miao, L.P.; Zhou, M.Y.; Zhang, X.Y.; Yuan, C.; Dong, X.Y.; Zou, X.T. Effect of Excess Dietary Fluoride on Laying Performance and Antioxidant Capacity of Laying Hens. Poult. Sci. 2017, 96, 2200–2205. [Google Scholar] [CrossRef]
- Grzegorzewska, A.K.; Ocłoń, E.; Kucharski, M.; Sechman, A. Effect of in Vitro Sodium Fluoride Treatment on CAT, SOD and Nrf mRNA Expression and Immunolocalisation in Chicken (Gallus domesticus) Embryonic Gonads. Theriogenology 2020, 157, 263–275. [Google Scholar] [CrossRef]
- Puty, B.; Bittencourt, L.O.; Nogueira, I.C.; Buzalaf, M.A.R.; Oliveira, E.H.; Lima, R.R. Human cultured IMR-32 neuronal-like and U87 glial-like cells have different patterns of toxicity under fluoride exposure. PLoS ONE 2021, 16, e0251200. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta (BBA)-General. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Campos-Pereira, F.D.; Lopes-Aguiar, L.; Renosto, F.L.; Nogueira, G.A.S.; Costa, E.F.D.; Barbieri Pulz, R.; Silva-Zacarin, E.C.M.; Oliveira, C.A.; Pigoso, A.A.; Severi-Aguiar, G.D.C. Genotoxic Effect and Rat Hepatocyte Death Occurred after Oxidative Stress Induction and Antioxidant Gene Downregulation Caused by Long Term Fluoride Exposure. Chem. Biol. Interact. 2017, 264, 25–33. [Google Scholar] [CrossRef]
- Pereira, H.A.B.d.S.; Dionizio, A.S.; Araujo, T.T.; Fernandes, M.d.S.; Iano, F.G.; Buzalaf, M.A.R. Proposed Mechanism for Understanding the Dose- and Time-Dependency of the Effects of Fluoride in the Liver. Toxicol. Appl. Pharmacol. 2018, 358, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Gao, Y.; Yang, Y.; Liu, Y.; Guo, N.; Wang, L.; Huang, W.; Wu, L.; Sun, D.; Gu, W. β-Catenin Mediates Fluoride-Induced Aberrant Osteoblasts Activity and Osteogenesis. Environ. Pollut. 2020, 265, 114734. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Cheng, X.; Yin, F.; Zhao, Y.; Zhu, Y.; Yan, Z.; Khodaei, F.; Ommati, M.M.; Manthari, R.K.; et al. Influence of Calcium Supplementation against Fluoride-Mediated Osteoblast Impairment in Vitro: Involvement of the Canonical Wnt/β-Catenin Signaling Pathway. J. Agric. Food Chem. 2019, 67, 10285–10295. [Google Scholar] [CrossRef]
- Liu, X.-L.; Li, C.-C.; Liu, K.-J.; Cui, C.-Y.; Zhang, Y.-Z.; Liu, Y. The Influence of Fluoride on the Expression of Inhibitors of Wnt/β-Catenin Signaling Pathway in Rat Skin Fibroblast Cells. Biol. Trace Elem. Res. 2012, 148, 117–121. [Google Scholar] [CrossRef]
- Zeng, Q.B.; Xu, Y.Y.; Tu, C.L.; Yu, X.; Yang, J.; Hong, F. Biological exposure limits caused by co exposure to fluoride and arsenic based on Wnt signaling pathway. Ying Yong Sheng Tai Xue Bao 2019, 30, 37–42. [Google Scholar] [CrossRef]
- Luo, K.; Qin, Y.; Ouyang, T.; Wang, X.; Zhang, A.; Luo, P.; Pan, X. Let-7c-5p Regulates CyclinD1 in Fluoride-Mediated Osteoblast Proliferation and Activation. Toxicol. Sci. 2021, 182, 275–287. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Shusterman, K.; Kuehl, M.; Gibson, C.W. Wnt-RhoA Signaling Is Involved in Dental Enamel Development. Eur. J. Oral Sci. 2011, 119 (Suppl. S1), 41–49. [Google Scholar] [CrossRef]
- Shusterman, K.; Gibson, C.W.; Li, Y.; Healey, M.; Peng, L. Wnt-RhoA Signaling Pathways in Fluoride-Treated Ameloblast-Lineage Cells. CTO 2014, 199, 159–168. [Google Scholar] [CrossRef]
- Nadei, O.V.; Khvorova, I.A.; Agalakova, N.I. Cognitive Decline of Rats with Chronic Fluorosis Is Associated with Alterations in Hippocampal Calpain Signaling. Biol. Trace Elem. Res. 2020, 197, 495–506. [Google Scholar] [CrossRef]
- Yun, E.-J.; Kim, S.; Hsieh, J.-T.; Baek, S.T. Wnt/β-Catenin Signaling Pathway Induces Autophagy-Mediated Temozolomide-Resistance in Human Glioblastoma. Cell Death Dis. 2020, 11, 771. [Google Scholar] [CrossRef]
- Yim, W.W.-Y.; Mizushima, N. Lysosome Biology in Autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Widodo, S.S.; Dinevska, M.; Furst, L.M.; Stylli, S.S.; Mantamadiotis, T. IL-10 in Glioma. Br. J. Cancer 2021, 125, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; He, X.; Song, W.; Han, D.; Niu, J.; Wang, J. Role of IL-6 in the Invasiveness and Prognosis of Glioma. Int. J. Clin. Exp. Med. 2015, 8, 9114–9120. [Google Scholar] [PubMed]
- Crespo, S.; Kind, M.; Arcaro, A. The Role of the PI3K/AKT/mTOR Pathway in Brain Tumor Metastasis. J. Cancer Metast. Treat. 2016, 2, 80–89. [Google Scholar] [CrossRef]
- Melisi, D.; Niu, J.; Chang, Z.; Xia, Q.; Peng, B.; Ishiyama, S.; Evans, D.B.; Chiao, P.J. Secreted Interleukin-1alpha Induces a Metastatic Phenotype in Pancreatic Cancer by Sustaining a Constitutive Activation of Nuclear Factor-kappaB. Mol. Cancer Res. 2009, 7, 624–633. [Google Scholar] [CrossRef]
- Yoo, K.-C.; Suh, Y.; An, Y.; Lee, H.-J.; Jeong, Y.J.; Uddin, N.; Cui, Y.-H.; Roh, T.-H.; Shim, J.-K.; Chang, J.H.; et al. Proinvasive Extracellular Matrix Remodeling in Tumor Microenvironment in Response to Radiation. Oncogene 2018, 37, 3317–3328. [Google Scholar] [CrossRef]
- Lu, T.; Tian, L.; Han, Y.; Vogelbaum, M.; Stark, G.R. Dose-Dependent Cross-Talk between the Transforming Growth Factor-Beta and Interleukin-1 Signaling Pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 4365–4370. [Google Scholar] [CrossRef]
- Meini, A.; Sticozzi, C.; Massai, L.; Palmi, M. A Nitric Oxide/Ca2+/Calmodulin/ERK1/2 Mitogen-Activated Protein Kinase Pathway Is Involved in the Mitogenic Effect of IL-1beta in Human Astrocytoma Cells. Br. J. Pharmacol. 2008, 153, 1706–1717. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Bryce, N.S.; Adams, S.; Braidy, N.; Konayagi, M.; McDonald, K.L.; Teo, C.; Guillemin, G.J.; Grewal, T.; Munoz, L. P38 MAPK Inhibitors Attenuate Pro-Inflammatory Cytokine Production and the Invasiveness of Human U251 Glioblastoma Cells. J. Neurooncol. 2012, 109, 35–44. [Google Scholar] [CrossRef]
- Post, D.E.; Sandberg, E.M.; Kyle, M.M.; Devi, N.S.; Brat, D.J.; Xu, Z.; Tighiouart, M.; Van Meir, E.G. Targeted Cancer Gene Therapy Using a Hypoxia Inducible Factor–Dependent Oncolytic Adenovirus Armed with Interleukin-4. Cancer Res. 2007, 67, 6872–6881. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine To Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Gieryng, A.; Pszczolkowska, D.; Walentynowicz, K.A.; Rajan, W.D.; Kaminska, B. Immune Microenvironment of Gliomas. Lab. Investig. 2017, 97, 498–518. [Google Scholar] [CrossRef] [PubMed]
- Huettner, C.; Czub, S.; Kerkau, S.; Roggendorf, W.; Tonn, J.C. Interleukin 10 Is Expressed in Human Gliomas in Vivo and Increases Glioma Cell Proliferation and Motility in Vitro. Anticancer Res. 1997, 17, 3217–3224. [Google Scholar]
- Zhang, Z.; Huang, X.; Li, J.; Fan, H.; Yang, F.; Zhang, R.; Yang, Y.; Feng, S.; He, D.; Sun, W.; et al. Interleukin 10 Promotes Growth and Invasion of Glioma Cells by Up-Regulating KPNA 2 in Vitro. J. Cancer Res. Ther. 2019, 15, 927–932. [Google Scholar] [CrossRef]
- Cardoso, A.P.; Pinto, M.L.; Pinto, A.T.; Pinto, M.T.; Monteiro, C.; Oliveira, M.I.; Santos, S.G.; Relvas, J.B.; Seruca, R.; Mantovani, A.; et al. Matrix Metalloproteases as Maestros for the Dual Role of LPS- and IL-10-Stimulated Macrophages in Cancer Cell Behaviour. BMC Cancer 2015, 15, 456. [Google Scholar] [CrossRef]
- Teo, G.S.L.; Ankrum, J.A.; Martinelli, R.; Boetto, S.E.; Simms, K.; Sciuto, T.E.; Dvorak, A.M.; Karp, J.M.; Carman, C.V. Mesenchymal Stem Cells Transmigrate between and Directly through Tumor Necrosis Factor-α-Activated Endothelial Cells via Both Leukocyte-like and Novel Mechanisms. Stem Cells 2012, 30, 2472–2486. [Google Scholar] [CrossRef]
- Guo, G.; Gong, K.; Ali, S.; Ali, N.; Shallwani, S.; Hatanpaa, K.J.; Pan, E.; Mickey, B.; Burma, S.; Wang, D.H.; et al. A TNF-JNK-Axl-ERK Signaling Axis Mediates Primary Resistance to EGFR Inhibition in Glioblastoma. Nat. Neurosci. 2017, 20, 1074–1084. [Google Scholar] [CrossRef]
- Nabors, L.B.; Suswam, E.; Huang, Y.; Yang, X.; Johnson, M.J.; King, P.H. Tumor Necrosis Factor α Induces Angiogenic Factor Up-Regulation in Malignant Glioma Cells: A Role for RNA Stabilization and HuR. Cancer Res. 2003, 63, 4181–4187. [Google Scholar]
- Waugh, D.T. The Contribution of Fluoride to the Pathogenesis of Eye Diseases: Molecular Mechanisms and Implications for Public Health. Int. J. Environ. Res. Public Health 2019, 16, 856. [Google Scholar] [CrossRef]
- Wu, P.; Sun, Z.; Lv, X.; Pei, X.; Manthari, R.K.; Wang, J. Fluoride Induces Autoimmune Orchitis Involved with Enhanced IL-17A Secretion in Mice Testis. J. Agric. Food Chem. 2019, 67, 13333–13343. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B.-H.; Tan, P.-P.; Chen, Y.; Miao, C.-Y.; Wang, H.-W. Key Role of Pro-Inflammatory Cytokines in the Toxic Effect of Fluoride on Hepa1-6 Cells. Biol. Trace Elem. Res. 2020, 197, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Becher, R.; Lâg, M.; Skuland, T.; Schwarze, P.E. Fluoride-Induced Interleukin-6 and Interleukin-8 Synthesis in Human Epithelial Lung Cells. Hum. Exp. Toxicol. 1999, 18, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium Fluoride Induces Renal Inflammatory Responses by Activating NF-κB Signaling Pathway and Reducing Anti-Inflammatory Cytokine Expression in Mice. Oncotarget 2017, 8, 80192–80207. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, S.; Wang, C.; Wang, F.; Song, Y.; Yan, N.; Xi, S.; Liu, Z.; Sun, G. JNK and NADPH Oxidase Involved in Fluoride-Induced Oxidative Stress in BV-2 Microglia Cells. Mediat. Inflamm. 2013, 2013, e895975. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Goschorska, M.; Kolasa, A.; Łukomska, A.; Jakubczyk, K.; Dec, K.; Chlubek, D. Fluoride as a Factor Initiating and Potentiating Inflammation in THP1 Differentiated Monocytes/Macrophages. Toxicol. In Vitro 2015, 29, 1661–1668. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Siennicka, A. Fluoride and generation of pro-inflammatory factors in human macrophages. Fluoride 2011, 44, 125. [Google Scholar]
- Fournier, T.; Riches, D.W.; Winston, B.W.; Rose, D.M.; Young, S.K.; Noble, P.W.; Lake, F.R.; Henson, P.M. Divergence in Macrophage Insulin-like Growth Factor-I (IGF-I) Synthesis Induced by TNF-Alpha and Prostaglandin E2. J. Immunol. 1995, 155, 2123–2133. [Google Scholar] [CrossRef]
- Zou, J.; Lin, X.; Bian, J.; Zhu, Q.; Zou, X. The Effect of Selenium on the Blood Radioimmunological Indexes Induced by High Dose of Fluorine. Nat. Environ. Pollut. Technol. 2013, 12, 4. [Google Scholar]
- Dec, K.; Łukomska, A.; Baranowska-Bosiacka, I.; Pilutin, A.; Maciejewska, D.; Skonieczna-Żydecka, K.; Derkacz, R.; Goschorska, M.; Wąsik, A.; Rębacz-Maron, E.; et al. Pre-and Postnatal Exposition to Fluorides Induce Changes in Rats Liver Morphology by Impairment of Antioxidant Defense Mechanisms and COX Induction. Chemosphere 2018, 211, 112–119. [Google Scholar] [CrossRef]
- Ridley, W.; Matsuoka, M. Fluoride-Induced Cyclooxygenase-2 Expression and Prostaglandin E2 Production in A549 Human Pulmonary Epithelial Cells. Toxicol. Lett. 2009, 188, 180–185. [Google Scholar] [CrossRef]
- Lee, H.-J.; Choi, C.-H. Anti-Inflammatory Effects of Bamboo Salt and Sodium Fluoride in Human Gingival Fibroblasts–An in Vitro Study. Kaohsiung J. Med. Sci. 2015, 31, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Garetto, L.P.; Dunipace, A.J.; Zhang, W.; Wilson, M.E.; Grynpas, M.D.; Chachra, D.; McClintock, R.; Peacock, M.; Stookey, G.K. Fluoride Treatment Increased Serum IGF-1, Bone Turnover, and Bone Mass, but Not Bone Strength, in Rabbits. Calcif. Tissue Int. 1997, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.T.; Gomes, R.N.; Panagopoulos, A.T.; de Almeida, F.G.; Veiga, J.C.E.; Colquhoun, A. Opposing Roles of PGD2 in GBM. Prostaglandins Other Lipid Mediat. 2018, 134, 66–76. [Google Scholar] [CrossRef]
- Jiang, J.; Qiu, J.; Li, Q.; Shi, Z. Prostaglandin E2 Signaling: Alternative Target for Glioblastoma? Trends Cancer 2017, 3, 75–78. [Google Scholar] [CrossRef]
- Giese, A.; Hagel, C.; Kim, E.L.; Zapf, S.; Djawaheri, J.; Berens, M.E.; Westphal, M. Thromboxane Synthase Regulates the Migratory Phenotype of Human Glioma Cells. Neuro Oncol. 1999, 1, 3–13. [Google Scholar] [CrossRef]
- MacKenzie, K.F.; Clark, K.; Naqvi, S.; McGuire, V.A.; Nöehren, G.; Kristariyanto, Y.; van den Bosch, M.; Mudaliar, M.; McCarthy, P.C.; Pattison, M.J.; et al. PGE2 Induces Macrophage IL-10 Production and a Regulatory-like Phenotype via a Protein Kinase A–SIK–CRTC3 Pathway. J. Immunol. 2013, 190, 565–577. [Google Scholar] [CrossRef]
- Wolff, J.E.A.; Wagner, S.; Reinert, C.; Gnekow, A.; Kortmann, R.-D.; Kühl, J.; Van Gool, S.W. Maintenance Treatment with Interferon-Gamma and Low-Dose Cyclophosphamide for Pediatric High-Grade Glioma. J. Neurooncol. 2006, 79, 315–321. [Google Scholar] [CrossRef]
- Loftenius, A.; Andersson, B.; Butler, J.; Ekstrand, J. Fluoride Augments the Mitogenic and Antigenic Response of Human Blood Lymphocytes in Vitro. Caries Res. 1999, 33, 148–155. [Google Scholar] [CrossRef]
| Parameter | Marker | Effect | NaF (µM) | Exposure Time | Comments |
|---|---|---|---|---|---|
| Oxidative Stress | Reactive oxygen species (ROS) | ↑↑ | 0.1–10 | mainly LONG | ROS adaptation observed after 72 h |
| Superoxide dismutase (SOD) activity | ↓ | 0.1 | SHORT | No significant effect at higher doses | |
| Catalase (CAT) activity | ↓ | 5–10 | SHORT and LONG | Observed trend without statistical significance | |
| Total glutathione (GSH/GSSG levels) | ↔ | all doses | SHORT and LONG | Stable glutathione system | |
| Wnt Signaling Pathway | Intracellular β-catenin | ↑ | 5–10 | SHORT and LONG | Intracellular activation of the Wnt pathway |
| Extracellular β-catenin (medium) | ↔ | all doses | SHORT and LONG | No significant extracellular release | |
| Pro-inflammatory Markers | IFN-γ | ↑ | 1–5 | mainly LONG | Moderate extracellular secretion |
| TNF-α | ↑↑ | 1–10 | SHORT and LONG | Higher levels intracellularly than extracellularly | |
| IL-1α | ↑ | mainly 10 | mainly LONG | Extracellular secretion observed | |
| IL-1β | ↑ | 1–10 | SHORT and LONG | More evident extracellular secretion | |
| IL-6 | ↑↑ | 0.1–10 | SHORT and LONG | Strongest response among IL cytokines | |
| TXB2 | ↑↑↑ | mainly 1 | SHORT and LONG | Prominent role of COX/eicosanoid pathway | |
| PGE2 | ↑↑↑ | 0.1–10 | mainly LONG | Active secretion into the medium | |
| Anti-inflammatory Markers | IL-4 | ↑ | 0.1–1 | SHORT and LONG | Higher intracellular vs. medium levels |
| IL-10 | ↑↑ | 0.1–5 | LONG | Strong extracellular secretion despite decreased intracellular levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Wszołek, A.; Gutowska, I. Metabolic Reprogramming Triggered by Fluoride in U-87 Glioblastoma Cells: Implications for Tumor Progression? Cells 2025, 14, 800. https://doi.org/10.3390/cells14110800
Żwierełło W, Maruszewska A, Skórka-Majewicz M, Wszołek A, Gutowska I. Metabolic Reprogramming Triggered by Fluoride in U-87 Glioblastoma Cells: Implications for Tumor Progression? Cells. 2025; 14(11):800. https://doi.org/10.3390/cells14110800
Chicago/Turabian StyleŻwierełło, Wojciech, Agnieszka Maruszewska, Marta Skórka-Majewicz, Agata Wszołek, and Izabela Gutowska. 2025. "Metabolic Reprogramming Triggered by Fluoride in U-87 Glioblastoma Cells: Implications for Tumor Progression?" Cells 14, no. 11: 800. https://doi.org/10.3390/cells14110800
APA StyleŻwierełło, W., Maruszewska, A., Skórka-Majewicz, M., Wszołek, A., & Gutowska, I. (2025). Metabolic Reprogramming Triggered by Fluoride in U-87 Glioblastoma Cells: Implications for Tumor Progression? Cells, 14(11), 800. https://doi.org/10.3390/cells14110800







