Fh15 Reduces Colonic Inflammation and Leukocyte Infiltration in a Dextran Sulfate Sodium-Induced Ulcerative Colitis Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Recombinant F. hepatica FABP (Fh15)
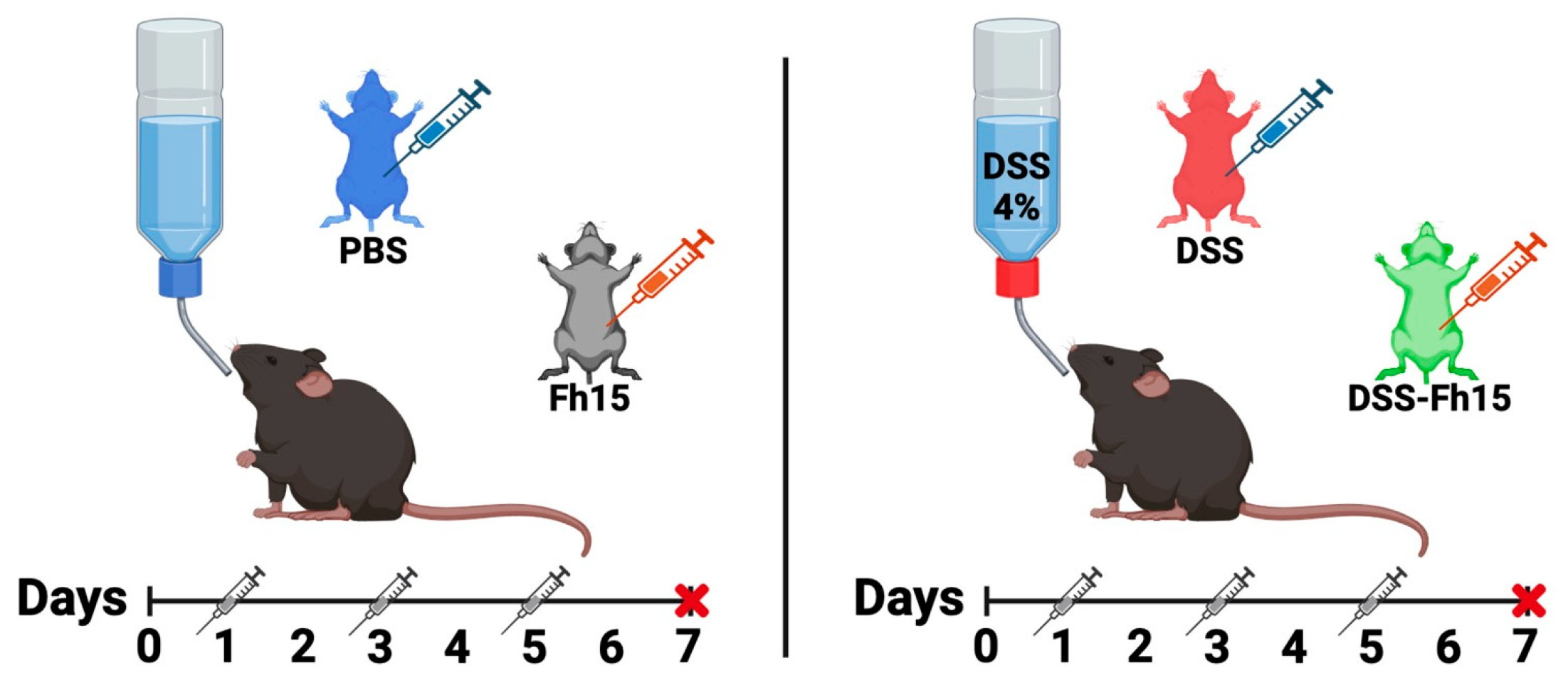
2.3. Dextran Sulfate Sodium (DSS) Colitis Induction and Fh15 Treatment
2.4. Disease Activity Index (DAI)
2.5. Macroscopic Score and Histopathological Scoring
2.6. Serum Myeloperoxidase and Chitinase-3 Like-Protein-1 Concentration
2.7. Colonic Cytokines Gene Expression
2.8. Colon Immune Cells Infiltration and Calcium Binding Protein Marker
2.9. Splenic T Cell and Leucocyte Populations Profiling
2.10. Statistical Analysis
3. Results
3.1. Fh15 Treatment Reduces Disease Activity Index in DSS-Induced UC Mice
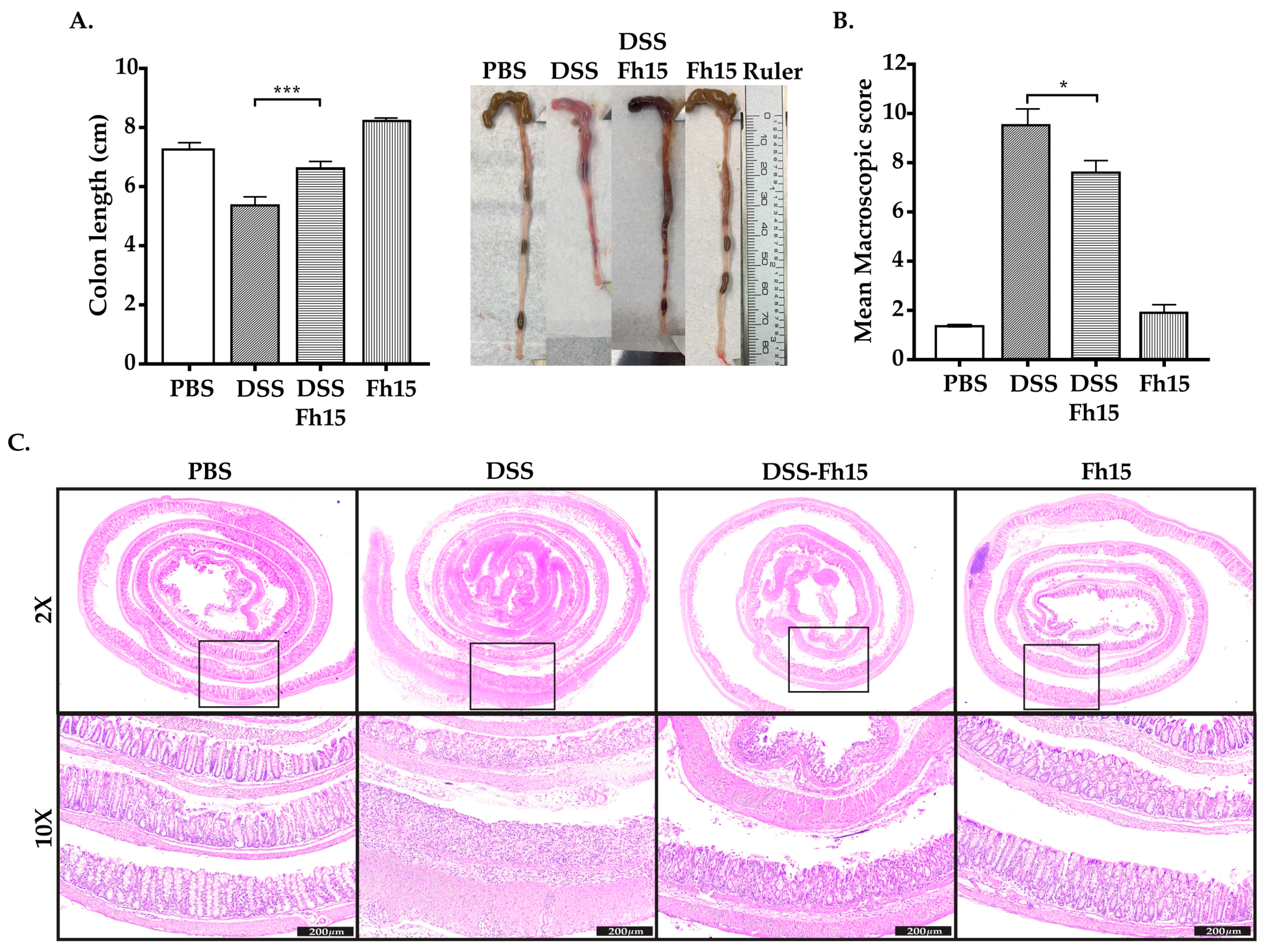
3.2. Fh15 Significantly Prevents Colon Shortening and Decreases Macroscopic Score in DSS-Induced UC Mice
3.3. Fh15 Ameliorates Histological Alterations in DSS-Induced UC Mice
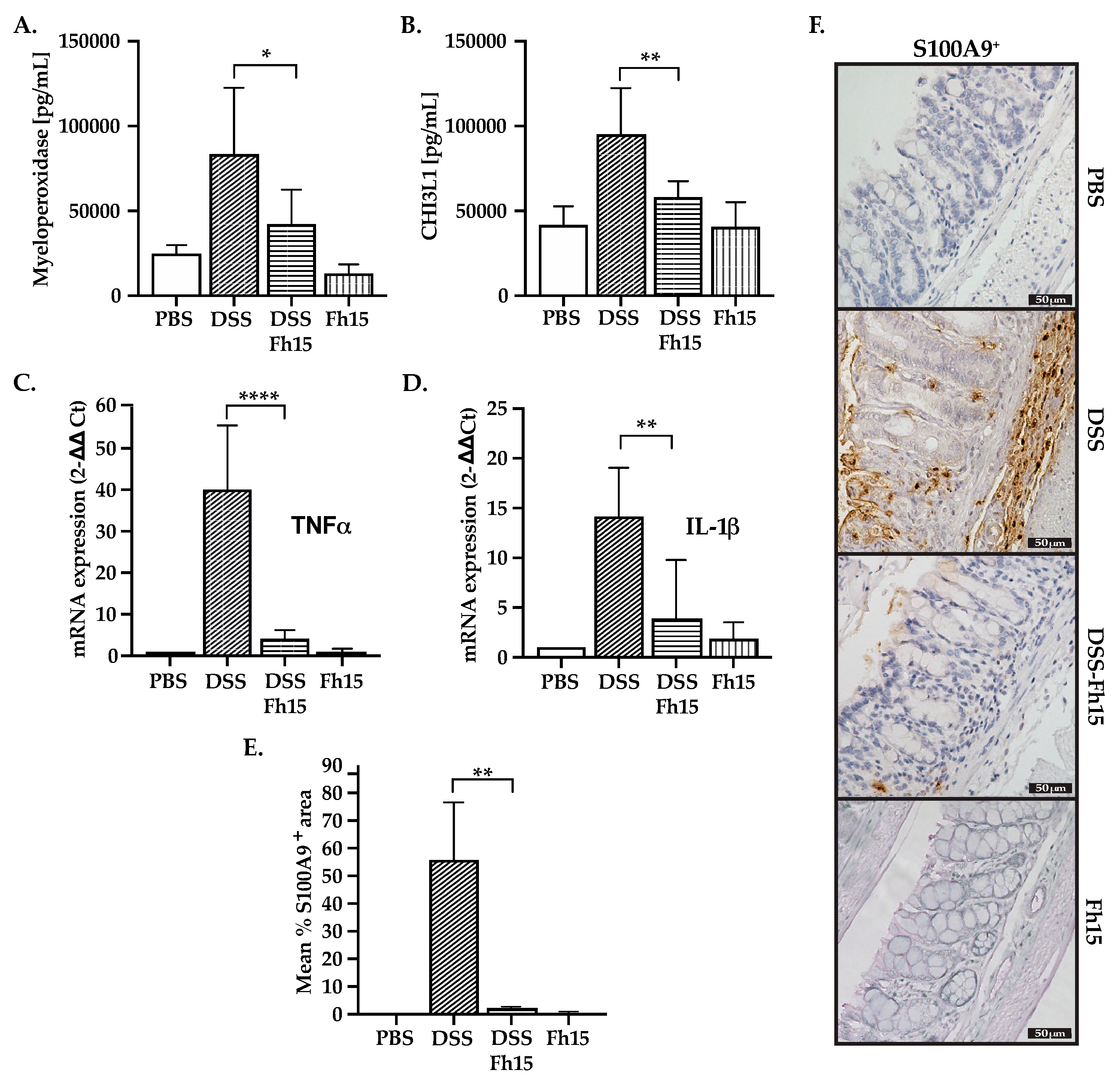
3.4. Fh15 Decreases Serum Levels of Myeloperoxidase and CHI3L1 While Suppressing S100A9 and Pro-Inflammatory Cytokines in Colonic Tissues of DSS-Induced UC Mice
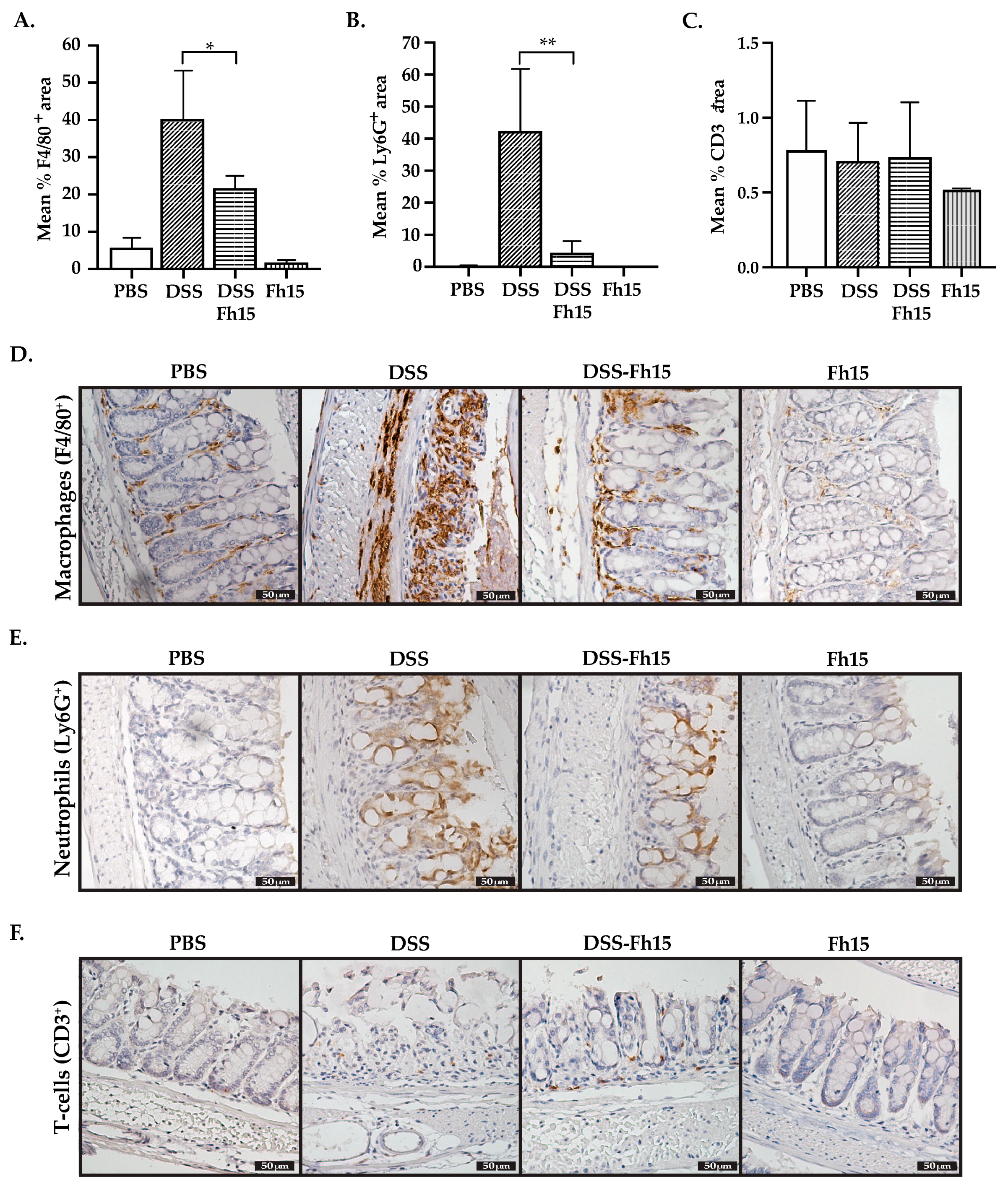
3.5. Fh15 Modulates Colonic Tissue Leukocyte Cell Infiltration of DSS-Induced UC Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.J.; Fonseca, M.T.; Bonfa, G.; Alves, V.B.; Sales-Campos, H.; Nardini, V.; Cardoso, C.R. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Braz. J. Med. Biol. Res. 2014, 47, 727–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.; Roda, G.; Peyrin-Biroulet, L.; Danese, S. Emerging therapies for the treatment of ulcerative colitis. Expert. Opin. Emerg. Drugs 2020, 25, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Reinink, A.R.; Lee, T.C.; Higgins, P.D. Endoscopic Mucosal Healing Predicts Favorable Clinical Outcomes in Inflammatory Bowel Disease: A Meta-analysis. Inflamm. Bowel Dis. 2016, 22, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Capron, M.; Beghin, L.; Leclercq, C.; Labreuche, J.; Dendooven, A.; Standaert, A.; Delbeke, M.; Porcherie, A.; Nachury, M.; Boruchowicz, A.; et al. Safety of P28GST, a Protein Derived from a Schistosome Helminth Parasite, in Patients with Crohn’s Disease: A Pilot Study (ACROHNEM). J. Clin. Med. 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radtke, D.; Thuma, N.; Schulein, C.; Kirchner, P.; Ekici, A.B.; Schober, K.; Voehringer, D. Th2 single-cell heterogeneity and clonal distribution at distant sites in helminth-infected mice. eLife 2022, 11, e74183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weinstock, J.V.; Summers, R.W.; Elliott, D.E.; Qadir, K.; Urban, J.F., Jr.; Thompson, R. The possible link between de-worming and the emergence of immunological disease. J. Lab. Clin. Med. 2002, 139, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.I.; Blennerhasset, P.A.; Varghese, A.K.; Chowdhury, S.K.; Omsted, P.; Deng, Y.; Collins, S.M. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun. 2002, 70, 5931–5937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, K.P.; Brady, M.T.; Finlay, C.M.; Boon, L.; Mills, K.H. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J. Immunol. 2009, 183, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.E.; O’Brien, B.A.; Hutchinson, A.T.; Robinson, M.W.; Simpson, A.M.; Dalton, J.P.; Donnelly, S. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS ONE 2014, 9, e86289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooke, A.; Tonks, P.; Jones, F.M.; O’Shea, H.; Hutchings, P.; Fulford, A.J.; Dunne, D.W. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kuijk, L.M.; Klaver, E.J.; Kooij, G.; van der Pol, S.M.; Heijnen, P.; Bruijns, S.C.; Kringel, H.; Pinelli, E.; Kraal, G.; de Vries, H.E.; et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol. Immunol. 2012, 51, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Moreels, T.G.; Nieuwendijk, R.J.; De Man, J.G.; De Winter, B.Y.; Herman, A.G.; Van Marck, E.A.; Pelckmans, P.A. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004, 53, 99–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Summer, W.R.; Elliot, D.E.; Urban, J.F.J.; Thompson, R.; Weinstock, J.V. Trichuris suis therapy in Crohn’s disease. Gut. 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.J.; Mulcahy, G.; Welsh, M.; Cassidy, J.P.; Corbett, D.; Milligan, C.; Andersen, P.; Strain, S.; McNair, J. Co-Infection of cattle with Fasciola hepatica and Mycobacterium bovis-immunological consequences. Transbound. Emerg. Dis. 2009, 56, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Roig, J.; Saiz, M.L.; Galiano, A.; Trelis, M.; Cantalapiedra, F.; Monteagudo, C.; Giner, E.; Giner, R.M.; Recio, M.C.; Bernal, D.; et al. Extracellular Vesicles From the Helminth Fasciola hepatica Prevent DSS-Induced Acute Ulcerative Colitis in a T-Lymphocyte Independent Mode. Front. Microbiol. 2018, 9, 1036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Yu, Z.; Wan, S.; Wu, F.; Chen, W.; Zhang, B.; Lin, D.; Liu, J.; Xie, H.; Sun, X.; et al. Exosomes Derived from Dendritic Cells Treated with Schistosoma japonicum Soluble Egg Antigen Attenuate DSS-Induced Colitis. Front. Pharmacol. 2017, 8, 651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramos-Benitez, M.J.; Ruiz-Jimenez, C.; Rosado-Franco, J.J.; Ramos-Perez, W.D.; Mendez, L.B.; Osuna, A.; Espino, A.M. Fh15 blocks the LPS-induced cytokine storm while modulating peritoneal macrophage migration and CD38 expression within spleen macrophages in a mouse model of septic shock. mSphere 2018, 3, e00548-00518. [Google Scholar] [CrossRef]
- Armina-Rodriguez, A.O.-M.C.; Méndez-Torres, L.B.; Valdés-Fernández, B.; Espino, A.M. Fasciola hepatica Fh15 promote survival in a mouse septic shock model and downregulates inflammatory cytokines. J. Immunol. 2023, 210, 82-02. [Google Scholar] [CrossRef]
- Rosado-Franco, J.J.; Armina-Rodriguez, A.; Marzan-Rivera, N.; Burgos, A.G.; Spiliopoulos, N.; Dorta-Estremera, S.M.; Mendez, L.B.; Espino, A.M. Recombinant Fasciola hepatica Fatty Acid Binding Protein as a Novel Anti-Inflammatory Biotherapeutic Drug in an Acute Gram-Negative Nonhuman Primate Sepsis Model. Microbiol. Spectr. 2021, 9, e0191021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Storr, M.A.; Keenan, C.M.; Zhang, H.; Patel, K.D.; Makriyannis, A.; Sharkey, K.A. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm. Bowel Dis. 2009, 15, 1678–1685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carson, F.L. Histotechnology: A Self-Instructional Text, 3rd ed.; American Society for Clinical Pathology Press: Hong Kong, 2009. [Google Scholar]
- Sann, H.; Erichsen, J.v.; Hessmann, M.; Pahl, A.; Hoffmeyer, A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Sci. 2013, 92, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Schaid, T.R., Jr.; LaCroix, I.; Hansen, K.C.; D’Alessandro, A.; Moore, E.E.; Sauaia, A.; Dzieciatkowska, M.; DeBot, M.; Cralley, A.L.; Thielen, O.; et al. A proteomic analysis of NETosis in trauma: Emergence of serpinB1 as a key player. J. Trauma Acute Care Surg. 2022, 94, 361–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizoguchi, E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 2006, 130, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Chen, F.; Laroui, H.; Baker, M.T.; Merlin, D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: Lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res. Notes 2013, 6, 360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Wei, L.; Wang, J.; Qin, Z.; Wang, J.; Lu, Y.; Zheng, X.; Peng, Q.; Ye, Q.; Ai, F.; et al. Suppression Colitis and Colitis-Associated Colon Cancer by Anti-S100a9 Antibody in Mice. Front. Immunol. 2017, 8, 1774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, S.G.; Tittle, T.; Garcia-Prada, D.; Kordower, J.H.; Melki, R.; Killinger, B.A. Alpha-synuclein aggregates are phosphatase resistant. Acta Neuropathol. Commun. 2024, 12, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jovicic, N.; Jeftic, I.; Jovanovic, I.; Radosavljevic, G.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS ONE 2015, 10, e0134089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corral-Ruiz, G.M.; Sanchez-Torres, L.E. Fasciola hepatica-derived molecules as potential immunomodulators. Acta Trop. 2020, 210, 105548. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.; Stack, C.M.; O’Neill, S.M.; Sayed, A.A.; Williams, D.L.; Dalton, J.P. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008, 22, 4022–4032. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Driss, V.; El Nady, M.; Delbeke, M.; Rousseaux, C.; Dubuquoy, C.; Sarazin, A.; Gatault, S.; Dendooven, A.; Riveau, G.; Colombel, J.F.; et al. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal Immunol. 2016, 9, 322–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Long, S.R.; Liu, R.D.; Kumar, D.V.; Wang, Z.Q.; Su, C.W. Immune Protection of a Helminth Protein in the DSS-Induced Colitis Model in Mice. Front. Immunol. 2021, 12, 664998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coronado, S.; Barrios, L.; Zakzuk, J.; Regino, R.; Ahumada, V.; Franco, L.; Ocampo, Y.; Caraballo, L. A recombinant cystatin from Ascaris lumbricoides attenuates inflammation of DSS-induced colitis. Parasite Immunol. 2017, 39, e12425. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Schmidt, I.; Boeckx, B.; Dierckx de Casterle, I.; et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell 2018, 175, 400–415.e13. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.I.; Kuhl, A.A.; Erben, U.; May, C.; Schulzke, J.D.; Siegmund, B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm. Bowel Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webb, L.V.; Ley, S.C.; Seddon, B. TNF activation of NF-kappaB is essential for development of single-positive thymocytes. J. Exp. Med. 2016, 213, 1399–1407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, L.; Chu, C.; Teng, F.; Bessman, N.J.; Goc, J.; Santosa, E.K.; Putzel, G.G.; Kabata, H.; Kelsen, J.R.; Baldassano, R.N.; et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 2019, 568, 405–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosales, C.; Demaurex, N.; Lowell, C.A.; Uribe-Querol, E. Neutrophils: Their Role in Innate and Adaptive Immunity. J. Immunol. Res. 2016, 2016, 1469780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, X.; Manzanares, L.D.; Piccolo, E.B.; Urbanczyk, J.M.; Sullivan, D.P.; Yalom, L.K.; Bui, T.M.; Lantz, C.; Najem, H.; Dulai, P.S.; et al. Macrophage-endothelial cell crosstalk orchestrates neutrophil recruitment in inflamed mucosa. J. Clin. Investig. 2023, 133, e170733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochem. (Mosc.) 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franze, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J. Crohns Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Mizutani, T.; Mizuta, S.; Miyamoto, A.; Murata, S.; Ano, T.; Ichise, H.; Morita, D.; Yamada, H.; Hoshino, Y.; et al. Neutrophils and the S100A9 protein critically regulate granuloma formation. Blood Adv. 2016, 1, 184–192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simard, J.C.; Simon, M.M.; Tessier, P.A.; Girard, D. Damage-associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. J. Immunol. 2011, 186, 3622–3631. [Google Scholar] [CrossRef] [PubMed]
- Shale, M.; Schiering, C.; Powrie, F. CD4(+) T-cell subsets in intestinal inflammation. Immunol. Rev. 2013, 252, 164–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, W.; Cong, Y. Exploring Colitis through Dynamic T Cell Adoptive Transfer Models. Inflamm. Bowel Dis. 2023, 29, 1673–1680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Axelsson, L.G.; Landstrom, E.; Goldschmidt, T.J.; Gronberg, A.; Bylund-Fellenius, A.C. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: Effects in CD4(+)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm. Res. 1996, 45, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Ridwan, B.U.; Tennyson, G.S.; Beagley, K.W.; Bucy, R.P.; Elson, C.O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 1994, 107, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Pena, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melgar, S.; Karlsson, A.; Michaelsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1328–G1338. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.J.; Faivre, E.; Quinlan, A.; Shanahan, F.; Nally, K.; Melgar, S. Induction and activation of adaptive immune populations during acute and chronic phases of a murine model of experimental colitis. Dig. Dis. Sci. 2011, 56, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Freise, A.C.; Zettlitz, K.A.; Salazar, F.B.; Tavare, R.; Tsai, W.K.; Chatziioannou, A.F.; Rozengurt, N.; Braun, J.; Wu, A.M. Immuno-PET in Inflammatory Bowel Disease: Imaging CD4-Positive T Cells in a Murine Model of Colitis. J. Nucl. Med. 2018, 59, 980–985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abi Abdallah, D.S.; Egan, C.E.; Butcher, B.A.; Denkers, E.Y. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 2011, 23, 317–326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.; Yang, D.; Shen, L.; Fang, J.; Khan, R.; Liu, D. Overexpression of CD86 enhances the ability of THP-1 macrophages to defend against Talaromyces marneffei. Immun. Inflamm. Dis. 2022, 10, e740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, T.S.; Goh, J.K.; Mortellaro, A.; Lim, C.T.; Hammerling, G.J.; Ricciardi-Castagnoli, P. CD80 and CD86 differentially regulate mechanical interactions of T-cells with antigen-presenting dendritic cells and B-cells. PLoS ONE 2012, 7, e45185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, I.; Caban-Hernandez, K.; Figueroa-Santiago, O.; Espino, A.M. Fasciola hepatica fatty acid binding protein inhibits TLR4 activation and suppresses the inflammatory cytokines induced by lipopolysaccharide in vitro and in vivo. J. Immunol. 2015, 194, 3924–3936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramos-Benitez, M.J.; Ruiz-Jimenez, C.; Aguayo, V.; Espino, A.M. Recombinant Fasciola hepatica fatty acid binding protein suppresses toll-like receptor stimulation in response to multiple bacterial ligands. Sci. Rep. 2017, 7, 5455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dejban, P.; Nikravangolsefid, N.; Chamanara, M.; Dehpour, A.; Rashidian, A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF-kappaB pathway. Phytother. Res. 2021, 35, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zou, K.F.; Qian, W.; Chen, S.; Hou, X.H. Expression and implication of toll-like receptors TLR2, TLR4 and TLR9 in colonic mucosa of patients with ulcerative colitis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, R.; Chen, L.; Peng, Q.; Li, S.; Zhang, Y.; Zhou, L.; Duan, L. S100A9 Regulates MDSCs-Mediated Immune Suppression via the RAGE and TLR4 Signaling Pathways in Colorectal Carcinoma. Front. Immunol. 2019, 10, 2243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamba, A.; Lee, I.A.; Mizoguchi, E. Potential association between TLR4 and chitinase 3-like 1 (CHI3L1/YKL-40) signaling on colonic epithelial cells in inflammatory bowel disease and colitis-associated cancer. Curr. Mol. Med. 2013, 13, 1110–1121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, Y.J.; Gong, H.F.; Zhao, Q.Q.; Liu, X.S.; Liu, C.; Wang, H. Critical role of toll-like receptor 4 (TLR4) in dextran sulfate sodium (DSS)-Induced intestinal injury and repair. Toxicol. Lett. 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Vlk, A.M.; Prantner, D.; Shirey, K.A.; Perkins, D.J.; Buzza, M.S.; Thumbigere-Math, V.; Keegan, A.D.; Vogel, S.N. M2a macrophages facilitate resolution of chemically-induced colitis in TLR4-SNP mice. mBio 2023, 14, e0120823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mottet, C.; Uhlig, H.H.; Powrie, F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003, 170, 3939–3943. [Google Scholar] [CrossRef] [PubMed]


| Macroscopic Damage | Score |
|---|---|
| Colon length | ≥6 cm = 0 pt, <6 cm = 1 pt, or <5 cm = 2 pt |
| Inflamed length | Measurement in centimeters |
| Bowel thickness | Measurement in millimeters |
| Adhesion | 0 = No Adhesion, 1 = Mild, 2 = Moderate, or 3 = Severe |
| Hemorrhage | Present = 1 or Absent = 0 |
| Fecal blood | Present = 1 or Absent = 0 |
| Diarrhea | Present = 1 or Absent = 0 |
| Gene | Primers | Sequence 5′-3′ |
|---|---|---|
| GAPDH | Forward | CATGGCCTTCCGTGTTCCTA |
| Reverse | CCTGCTTCACCACCTTCTTGAT | |
| TNF-α | Forward | AAGCCTGTAGCCCACGTCGTA |
| Reverse | AGGTACAACCCATCGGCTGG | |
| IL-1β | Forward | GAAATGCCACCTTTTGACAGTG |
| Reverse | TGGATGCTCTCATCAGGACAG |
| Marker | Target | Host Species | IHC Dilution | Cat. No. | Vendor |
|---|---|---|---|---|---|
| CD3 F4/80 | T-cells Macrophages | Rabbit Rabbit | 1:100 1:100 | ab16669 70076S | Abcam CST |
| LY6G | Neutrophils | Rabbit | 1:75 | 87048S | CST |
| S100A9 | Calcium binding protein A9 | Rabbit | 1:800 | 73425S | CST |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Gispert, M.D.M.; Ramos-Lugo, C.M.; Ocasio-Malavé, C.; Scott, R.P.; Ahrendsen, J.T.; Gomez-Samblas, M.; Osuna, A.; Dorta-Estremera, S.M.; Espino, A.M. Fh15 Reduces Colonic Inflammation and Leukocyte Infiltration in a Dextran Sulfate Sodium-Induced Ulcerative Colitis Mouse Model. Cells 2025, 14, 799. https://doi.org/10.3390/cells14110799
Figueroa-Gispert MDM, Ramos-Lugo CM, Ocasio-Malavé C, Scott RP, Ahrendsen JT, Gomez-Samblas M, Osuna A, Dorta-Estremera SM, Espino AM. Fh15 Reduces Colonic Inflammation and Leukocyte Infiltration in a Dextran Sulfate Sodium-Induced Ulcerative Colitis Mouse Model. Cells. 2025; 14(11):799. https://doi.org/10.3390/cells14110799
Chicago/Turabian StyleFigueroa-Gispert, María Del Mar, Claudia M. Ramos-Lugo, Carlimar Ocasio-Malavé, Rizaldy P. Scott, Jared T. Ahrendsen, Mercedes Gomez-Samblas, Antonio Osuna, Stephanie M. Dorta-Estremera, and Ana M. Espino. 2025. "Fh15 Reduces Colonic Inflammation and Leukocyte Infiltration in a Dextran Sulfate Sodium-Induced Ulcerative Colitis Mouse Model" Cells 14, no. 11: 799. https://doi.org/10.3390/cells14110799
APA StyleFigueroa-Gispert, M. D. M., Ramos-Lugo, C. M., Ocasio-Malavé, C., Scott, R. P., Ahrendsen, J. T., Gomez-Samblas, M., Osuna, A., Dorta-Estremera, S. M., & Espino, A. M. (2025). Fh15 Reduces Colonic Inflammation and Leukocyte Infiltration in a Dextran Sulfate Sodium-Induced Ulcerative Colitis Mouse Model. Cells, 14(11), 799. https://doi.org/10.3390/cells14110799









