Abstract
Mitochondrial dysfunction is a pivotal driver in the pathogenesis of acute kidney injury (AKI), chronic kidney disease (CKD), and congenital anomalies of the kidney and urinary tract (CAKUT). The kidneys, second only to the heart in mitochondrial density, rely on oxidative phosphorylation to meet the high ATP demands of solute reabsorption and filtration. Disrupted mitochondrial dynamics, such as excessive fission mediated by Drp1, exacerbate tubular apoptosis and inflammation in AKI models like ischemia–reperfusion injury. In CKD, persistent mitochondrial dysfunction drives oxidative stress, fibrosis, and metabolic reprogramming, with epigenetic mechanisms (DNA methylation, histone modifications, non-coding RNAs) regulating genes critical for mitochondrial homeostasis, such as PMPCB and TFAM. Epigenetic dysregulation also impacts mitochondrial–ER crosstalk, influencing calcium signaling and autophagy in renal pathology. Mitophagy, the selective clearance of damaged mitochondria, plays a dual role in kidney disease. While PINK1/Parkin-mediated mitophagy protects against cisplatin-induced AKI by preventing mitochondrial fragmentation and apoptosis, its dysregulation contributes to fibrosis and CKD progression. For instance, macrophage-specific loss of mitophagy regulators like MFN2 amplifies ROS production and fibrotic responses. Conversely, BNIP3/NIX-dependent mitophagy attenuates contrast-induced AKI by suppressing NLRP3 inflammasome activation. In diabetic nephropathy, impaired mitophagy correlates with declining eGFR and interstitial fibrosis, highlighting its diagnostic and therapeutic potential. Emerging therapeutic strategies target mitochondrial dysfunction through antioxidants (e.g., MitoQ, SS-31), mitophagy inducers (e.g., COPT nanoparticles), and mitochondrial transplantation, which mitigates AKI by restoring bioenergetics and modulating inflammatory pathways. Nanotechnology-enhanced drug delivery systems, such as curcumin-loaded nanoparticles, improve renal targeting and reduce oxidative stress. Epigenetic interventions, including PPAR-α agonists and KLF4 modulators, show promise in reversing metabolic reprogramming and fibrosis. These advances underscore mitochondria as central hubs in renal pathophysiology. Tailored interventions—ranging from Drp1 inhibition to mitochondrial transplantation—hold transformative potential to mitigate kidney injury and improve clinical outcomes. Additionally, dietary interventions and novel regulators such as adenogens are emerging as promising strategies to modulate mitochondrial function and attenuate kidney disease progression. Future research should address the gaps in understanding the role of mitophagy in CAKUT and optimize targeted delivery systems for precision therapies.
1. Introduction
Mitochondria are double membrane-bound organelles with distinct morphological features that regulate signaling pathways, energy metabolism, cell differentiation, proliferation, and apoptosis [1,2,3,4,5,6,7]. Additionally, mitochondria are essential for energy production and serve as signaling hubs that regulate cellular responses to stress, including oxidative damage and inflammation. More than 40% of mitochondrial proteins are associated with human diseases, underscoring the crucial role of mitochondria in overall health. Mitochondrial diseases can manifest at any age and affect any organ system, either tissue-specific or multisystemic, with various inheritance patterns [8,9,10]. Due to their genetic material, mitochondria differ from nuclear components, and their dysfunction is associated with numerous diseases, including those affecting the kidneys [9].
The kidney is one of the body’s most vital organs, playing a crucial role in maintaining homeostasis within the human body. The kidneys comprise a complex three-dimensional nephron structure that responds to various extracellular, inflammatory, neurological, and hormonal signals [11,12,13]. Kidney diseases are frequently present as syndromes in clinical settings, and they have various pathological classifications. Common symptoms include unusual urination, swelling, and fatigue [14,15,16]. The percentage of deaths attributed to kidney disease has consistently risen over the last twenty years. Kidney dysfunction has now become the seventh leading risk factor for mortality [17,18,19]. One in ten people globally suffers from chronic kidney disease (CKD), which is the tenth leading cause of death worldwide. Diabetic kidney disease (DKD) and hypertensive kidney disease (HKD) account for over 75% of CKD cases. This increasing burden is further intensified by considerable disparities in treatment outcomes, with minority groups and individuals living in poverty facing limited access to renal replacement therapies and experiencing disproportionately poorer health outcomes [20,21,22,23,24].
After the heart, the kidneys have one of the highest demands on the body’s mitochondrial content and oxygen consumption. The reabsorption of solutes by the tubule cells is an energy-intensive process that requires a large amount of ATP, primarily generated through oxidative phosphorylation. This results in these cells having the highest mitochondrial content in the kidney [19,25,26]. Mitochondrial dysfunction generates excessive reactive oxygen species (ROS), disturbing redox balance and promoting oxidative stress, contributing to inflammation, fibrosis, and progressive kidney damage (Figure 1) [27]. In parallel, damaged mitochondria release mtDNA and other damage-associated molecular patterns (DAMPs) that trigger immune responses, e.g., via the cGAS-STING pathway, thereby promoting inflammation and immune cell recruitment and contributing to kidney injury and disease progression [26,28]. Furthermore, impaired mitochondrial function drives myofibroblast activation and extracellular matrix accumulation, contributing to tubulointerstitial fibrosis—a key pathological feature in the progression of chronic kidney disease (CKD) [29,30]. In addition, kidney diseases are correlated with impaired mitochondrial homeostasis, which encompasses the dysregulation of mitochondrial biogenesis, fusion dynamics, proteolytic activity, and mitophagy-mediated degradation [31,32].
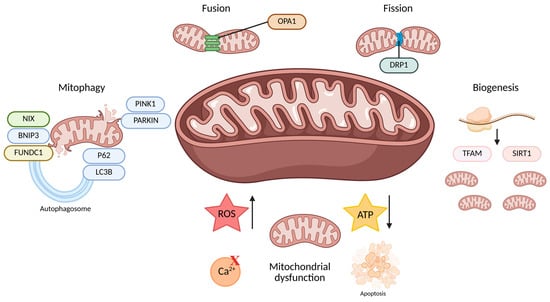
Figure 1.
This figure summarizes the mechanisms of mitochondrial quality control, including mitophagy, dynamics (fusion and fission), biogenesis, and the consequences of mitochondrial dysfunction. Fusion, mediated by OPA1, maintains mitochondrial function by merging membranes, while fission, mediated by DRP1, separates damaged mitochondria for removal. Mitophagy selectively eliminates dysfunctional mitochondria through pathways involving PINK1, PARKIN, NIX, BNIP3, FUNDC1, LC3B, and P62. Biogenesis, regulated by TFAM and SIRT1, replenishes mitochondria. Mitochondrial dysfunction leads to increased ROS, disturbed Ca2⁺ signaling, decreased ATP production, and activation of apoptosis. Created with Biorender (accessed on 1 May 2025). Abbreviations: NIX, NIP3-like protein X; BNIP3, BCL2/Adenovirus E1B 19 kDa protein-interacting protein 3; FUNDC1, FUN14 domain-containing protein 1; PINK1, PTEN-induced kinase 1; PARKIN, Parkin RBR E3 ubiquitin protein ligase; P62, Sequestosome 1 (also called SQSTM1); LC3B, Microtubule-associated protein 1A/1B-light chain 3B; OPA1, Optic atrophy 1 protein; DRP1, dynamin-related protein 1; TFAM, mitochondrial transcription factor A; SIRT1, Sirtuin 1; ROS, reactive oxygen species; ATP, adenosine triphosphate.
Given the mitochondria’s central role in kidney health and disease, targeting mitochondria for the treatment of kidney disease is crucial. A recent review by Takasu et al. provides a comprehensive overview of mitochondrial dysfunction in diabetic kidney disease, with a particular emphasis on altered mitochondrial dynamics, oxidative stress, and impaired mitophagy. They also discuss the therapeutic potential of agents such as SGLT2 inhibitors and GLP-1 receptor agonists in restoring mitochondrial function in DKD [33]. While their review focuses specifically on diabetic nephropathy, our article expands this discussion to encompass a broader range of kidney diseases, including AKI, CKD, and CAKUT anomalies, and further explores emerging mechanisms such as epigenetic regulation and mitochondrial-ER crosstalk. This broader perspective underscores the centrality of mitochondria in diverse renal pathologies and highlights additional therapeutic avenues.
2. Mitochondria in Kidney Health and Disease
The mitochondrion is a double-membrane organelle rich in enzymes, porins, and translocases in the outer mitochondrial membrane (OMM). These molecules are essential for exchanging metabolites and cations with the cytosol. In addition, apoptosis is closely linked to the OMM, as pro-apoptotic factors can be released from the intermembrane space (space between the outer and inner mitochondrial membranes) when the OMM is excessively permeabilized. In the process of apoptosis, the permeability of the OMM to a variety of pro-apoptotic proteins increases, releasing the lethal proteins into the cytoplasmic matrix and promoting apoptosis. The inner mitochondrial membrane (IMM) contains highly impermeable lipid patterns, such as the phospholipid cardiolipin. There are more proteins in the IMM (about one-fifth of all proteins contained in mitochondria) than in the OMM. Hence, the IMM is responsible for complex biochemical reactions, including transporting glutamic acid, ornithine, and nucleotides, oxidative phosphorylation, ATP synthesis, and regulating mitochondrial fission and fusion [34,35].
Mitochondrial dynamics, i.e., the balance between fusion and fission of mitochondria, is a tightly regulated process that plays a crucial role in maintaining cellular homeostasis. This dynamic system is essential for key physiological functions such as cell cycle progression, programmed cell death, and the maintenance of mitochondrial integrity. Under normal conditions, fusion and fission are well coordinated and ensure proper mitochondrial form and function. However, this balance often shifts towards excessive fission in pathological conditions, leading to mitochondrial fragmentation and subsequent cell damage. A key result of disrupted mitochondrial dynamics is the excessive generation of reactive oxygen species (ROS), which, when sustained, can lead to oxidative harm to proteins, lipids, and DNA, thereby playing a significant role in advancing kidney disease [36,37,38,39,40,41].
The kidneys receive around 20% of the heart’s output, processing roughly 180 liters of glomerular filtrate daily. Due to their role in waste removal, nutrient reabsorption, fluid and electrolyte balance, acid–base regulation, and blood pressure control, kidneys have a high metabolic demand and are rich in mitochondria. Mitochondria play a central role in kidney function, reflecting the organ’s high metabolic demands [42,43]. The kidney ranks just behind the heart in mitochondrial density and oxygen consumption, as large amounts of ATP are required to fuel active transport processes essential for filtration, reabsorption, and secretion. The renal cortex, densely packed with proximal and distal tubules, is particularly rich in mitochondria due to its role in reabsorbing water, ions, and nutrients. Proximal tubular cells, which perform most of this task, rely heavily on mitochondrial energy and must dynamically adjust to fluctuations in energy availability to maintain metabolic efficiency. In contrast, fewer mitochondria are present in the collecting duct cells and specific segments of the Henle loop. However, intercalated cells in the collecting duct still depend on mitochondrial activity for acid–base and electrolyte balance. In addition to ATP production, renal mitochondria are involved in apoptosis regulation, calcium and iron homeostasis, and steroid synthesis, underscoring their critical role in maintaining cellular and organ-level homeostasis [42,43,44,45,46,47].
While much of the focus on mitochondrial dysfunction in kidney disease has centered on tubular epithelial cells, emerging evidence highlights the critical role of mitochondria in glomerular podocytes, which are essential for maintaining the filtration barrier. Podocytes have high energy demands and rely heavily on mitochondrial ATP production to sustain their complex cytoskeletal architecture and slit diaphragm integrity. Disruptions in mitochondrial dynamics, including altered fusion and fission processes, as well as impaired mitophagy, contribute to podocyte injury, leading to proteinuria and glomerulosclerosis—hallmarks of chronic kidney disease (CKD) [48,49]. Moreover, mitochondrial damage can lead to the release of mitochondrial DNA (mtDNA) into the cytosol and extracellular space, acting as a damage-associated molecular pattern (DAMP). The recognition of mtDNA by innate immune receptors, such as Toll-like receptor 9 (TLR9) and the cGAS-STING pathway, triggers sterile inflammatory responses in podocytes and tubular cells, thereby exacerbating renal injury and fibrosis. This mtDNA-driven inflammation highlights a crucial connection between mitochondrial dysfunction and immune activation in the pathogenesis of kidney disease. Incorporating these insights broadens our understanding of mitochondrial contributions beyond tubular segments and highlights potential therapeutic targets for preserving mitochondrial integrity and modulating mtDNA-induced inflammation [50,51,52,53,54].
Mitochondrial dysfunction is closely associated with several kidney diseases, including acute kidney injury (AKI) and chronic kidney disease (CKD) [48,49]. AKI can occur due to ischemic, toxic, or inflammatory insults that impair mitochondrial energy production, increase oxidative stress, and lead to the apoptosis or necrosis of tubular cells. Disrupted mitochondrial dynamics, characterized by imbalances in fission and fusion and defective mitophagy, worsen AKI by allowing damaged mitochondria to accumulate, further intensifying oxidative damage and inflammation. In CKD, ongoing mitochondrial dysfunction contributes to renal fibrosis through metabolic reprogramming, persistent oxidative stress, and damage to mitochondrial DNA (mtDNA), particularly in diabetes and IgA nephropathy [19,26,55,56].
Emerging evidence also suggests that mitochondrial dysfunction plays a role in congenital anomalies of the kidney and urinary tract (CAKUT). Genetic mutations that affect mitochondrial proteins or metabolic pathways can disrupt nephrogenesis, leading to structural malformations such as hypoplastic kidneys or urinary tract obstructions. For instance, mutations in genes related to coenzyme Q10 biosynthesis or components of the mitochondrial electron transport chain have been linked to tubular dysgenesis and cystic kidney phenotypes, emphasizing the importance of mitochondrial integrity in early kidney development [57,58,59].
Mitochondrial dysfunction is increasingly recognized as a key factor in the pathogenesis and progression of acute kidney injury (AKI) and chronic kidney disease (CKD) (Figure 2).
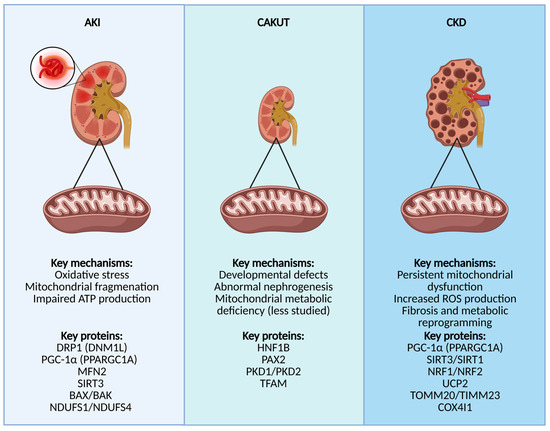
Figure 2.
This figure highlights mitochondrial dysfunction in different kidney diseases: AKI, CAKUT, and CKD. In AKI, oxidative stress, mitochondrial fragmentation, and impaired ATP production are key pathological features. In CAKUT, developmental defects and abnormal nephrogenesis are associated with mitochondrial metabolic deficiencies. In CKD, persistent mitochondrial dysfunction, increased ROS production, fibrosis, and metabolic reprogramming drive disease progression. Key regulatory proteins involved in each condition are also indicated. Created with Biorender (Accessed on 1 May 2025). Abbreviations: AKI, acute kidney injury; DRP1 (DNM1L), Dynamin-1-like protein; PGC-1α (PPARGC1A), peroxisome proliferator-activated receptor gamma coactivator 1-alpha; MFN2, Mitofusin-2; SIRT3, Sirtuin-3; BAX, Bcl-2-associated X protein; BAK, Bcl-2 homologous antagonist/killer; NDUFS1/NDUFS4, NADH:ubiquinone oxidoreductase core subunit S1/S4; CAKUT, congenital anomalies of the kidney and urinary tract; HNF1B, Hepatocyte nuclear factor 1-beta; PAX2, Paired box gene 2; PKD1/PKD2, polycystic kidney disease 1/2 proteins; TFAM, mitochondrial transcription factor A; CKD, chronic kidney disease; SIRT1, Sirtuin-1; NRF1/NRF2, Nuclear respiratory factor 1/2; UCP2, Uncoupling protein 2; TOMM20/TIMM23, Translocase of outer mitochondrial membrane 20/Translocase of inner mitochondrial membrane 23; COX4I1, Cytochrome c oxidase subunit 4 isoform 1.
2.1. AKI
Acute kidney injury (AKI), formerly known as acute renal failure, is a sudden decline in kidney function that develops within hours to days and results in the kidney’s inability to filter waste products and maintain the body’s fluid, electrolyte, and acid–base balance. AKI can range from the mild impairment of kidney function to complete kidney failure and is often triggered by another serious illness, surgery, infection, or certain medications rather than by direct physical injury to the kidneys. The leading causes of AKI are classified as pre-renal (reduced blood flow to the kidneys), intrinsic (direct damage to kidney tissue), and post-renal (obstruction of urine flow due to conditions such as kidney stones or an enlarged prostate) [60,61,62,63,64,65].
Diagnosis usually involves blood tests to measure creatinine and urea levels, urine tests, and imaging tests such as ultrasound or CT scans to determine the underlying cause and assess the extent of kidney damage. In some cases, a kidney biopsy may be performed [66,67].
2.2. CKD
In chronic kidney disease (CKD), the persistent dysregulation of mitochondrial homeostasis, including defects in mitochondrial structure, dynamics, and biogenesis, results in impaired energy metabolism, the increased production of reactive oxygen species (ROS), and heightened oxidative stress. These factors contribute to renal inflammation, tubular atrophy, and interstitial fibrosis. Reduced mitochondrial function in renal tubular cells, particularly the shift from oxidative phosphorylation to glycolysis, has been linked to tubular injury and fibrosis, the primary features of CKD progression. In addition, changes in the shape and structure of mitochondria are closely related to their loss of function, further exacerbating kidney damage [68,69].
The diagnosis of CKD typically involves blood tests to measure creatinine and calculate the estimated glomerular filtration rate (eGFR), which assesses how well the kidneys filter waste from the blood. Urine tests are also used to check for the presence of protein or blood, which can indicate kidney damage. Imaging studies such as ultrasound may be performed to evaluate the size and structure of the kidneys and rule out other causes of kidney dysfunction. Sometimes, a kidney biopsy may be necessary to determine the underlying cause and extent of kidney damage. Early detection and regular monitoring are crucial for managing CKD and slowing its progression [70,71,72].
2.3. CAKUT
Additionally, mitochondrial dysfunction has been identified as a crucial factor in the pathogenesis of congenital anomalies of the kidney and urinary tract (CAKUT). CAKUT encompasses a broad spectrum of developmental malformations of the kidneys and urinary tract that result from impaired embryonic development. CAKUT is characterized by developmental defects and abnormal nephrogenesis related to mitochondrial metabolic deficiencies, involving proteins such as HNF1B, PAX2, PKD1/PKD2, and TFAM, although this area is less well studied. These mitochondrial abnormalities can disrupt critical developmental pathways, including branching morphogenesis and nephron formation, leading to structural abnormalities such as renal agenesis or hypoplasia/dysplasia. Understanding the role of mitochondrial defects in CAKUT provides insight into disease mechanisms. It opens potential avenues for targeted therapies to restore mitochondrial health to prevent or attenuate congenital renal malformations [73,74,75].
3. How Does Epigenetics Shape Mitochondrial Function in Renal Pathology?
Epigenetic mechanisms, including DNA methylation, post-translational modifications of histone proteins, and non-coding RNA (ncRNA) regulations, significantly influence mitochondrial function in renal pathology by regulating genes involved in mitochondrial dynamics, oxidative stress responses, and organelle interactions [76].
DNA methylation can alter the expression of key mitochondrial genes such as PMPCB, AU RNA binding protein/enoyl-CoA hydratase, and TSFM, which are involved in mitochondrial protein processing, translation, and elongation. This can impact mitochondrial morphology, energy production, and ROS generation [40]. Changes in DNA methylation patterns can either upregulate or suppress these genes, influencing mitochondrial dynamics and function in kidney cells.
Histone modifications, such as acetylation and methylation, further regulate mitochondrial biogenesis and fission–fusion processes. For example, the acetylation of mitochondrial transcription factor A (TFAM) by GCN5L1 impairs its translocation to mitochondria, reducing mtDNA replication and transcription and exacerbating kidney injury [77]. Additionally, increased histone H3K27 acetylation and phosphorylation of Drp1 at serine 616 (p-Drp1S616) promote mitochondrial fission and fibroblast activation, leading to fibrosis and oxidative stress in diabetic nephropathy [78]. Conversely, phosphorylation of Drp1 at serine 637 (p-Drp1S637) inhibits fission, favoring mitochondrial elongation and longevity. Histone methylation also influences mitochondrial gene expression; for instance, EZH2-mediated H3K27 methylation contributes to tubular damage and mitochondrial dysfunction, while mitochondrial S-adenosylmethionine (SAM) levels regulate histone methyltransferases involved in these processes [79,80].
Furthermore, ncRNAs, such as microRNAs (miRNAs), modulate mitochondrial dynamics by targeting transcripts like MTP18 and PPAR-α, affecting mitochondrial fission and fatty acid oxidation, respectively. For example, miR-668 inhibits MTP18 to prevent excessive mitochondrial fission and protect tubular cells from apoptosis during ischemic injury [81,82], whereas miR-17 downregulates PPAR-α, impairing mitochondrial energy metabolism and promoting cyst formation in polycystic kidney disease [83].
Collectively, these epigenetic modifications disrupt mitochondrial homeostasis, leading to increased oxidative stress, impaired bioenergetics, and progression of kidney disease. This highlights their potential as therapeutic targets.
4. Mitophagy in Kidney Disease
Autophagy is present in all body tissues as a means of discarding old organelles through lysosomal degradation and recycling of cellular components [84]. However, a special form of autophagy that specifically targets mitochondria is known as mitophagy. Mitophagy occurs either when mitochondrial damage exceeds the cell’s reparatory mechanisms or as a controlled process of maintaining cellular metabolic homeostasis [85]. The first mention of mitophagy dates back to 2008. In a study focusing on the ubiquitin ligase Parkin, known for its role in Parkinson’s disease, researchers discovered that Parkin is selectively recruited to dysfunctional mitochondria with low membrane potential in mammalian cells, thereby triggering the engulfment of autophagosomes [86]. A previous review article portrays a detailed summary of the known signaling pathways of mitophagy and their effector molecules [87]. Briefly, there are two signaling cascades which lead to mitophagy, namely serine/threonine PTEN-induced putative kinase 1 (PINK1)/Parkin-mediated mitophagy and PINK1/Parkin-independent mitophagy through [87]. In the former, PINK1 accumulates on depolarized mitochondria, recruits Parkin to ubiquitinate outer membrane proteins, and signals autophagosome recruitment via adaptors like OPTN/NDP52 that bind LC3. In the latter, the primary effectors are E3 ubiquitin ligases (e.g., ARIH1, MUL1) and autophagy receptors (e.g., BNIP3, FUNDC1, PHB2), which directly recruit autophagosomes, often bypassing ubiquitination [87].
Given the previously mentioned implication of mitochondria in kidney disease, the process of mitophagy in this context should be discussed. When talking about acute kidney injury (AKI), several studies on sepsis, cisplatin toxicity, and ischemia–reperfusion injury models have observed the protective effect of mitophagy in kidney disease [55]. For instance, research on cisplatin, a widely used chemotherapeutic drug with notorious toxicity in the kidneys, showed that PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. The results of one study show that PINK1 or Parkin gene knockout mice, compared to wild-type littermates, show more severe renal functional loss, tissue damage, and apoptosis during cisplatin treatment [87]. Interestingly, the level of mitophagy was reduced in the knockout mice even during basal conditions, without cisplatin treatment. Furthermore, a study published in 2024 discovered a novel protein of interest in mitophagy-related kidney disease research. It presented macrophage migration inhibitory factor (MIF) as a suppressor of mitophagy through the disruption of PINK1–Parkin protein interactions in sepsis-associated acute kidney injury, thereby promoting renal damage. This was in line with previous research that reported increased serum levels of MIF in SA-AKI [88]. In a study by Lin and coworkers, which investigated the pathogenic mechanism of contrast-induced acute kidney injury (CI-AKI), NLR family pyrin domain-containing 3 (NLRP3)-driven inflammation came into focus. It was proven on a mouse model that the inhibition of NLRP3 inflammasome attenuates apoptosis in CI-AKI through the upregulation of Hypoxia-inducible factor 1-alpha (HIF1A) and BCL2 Interacting Protein 3 (BNIP3)-mediated mitophagy [88].
On the other hand, dysregulated mitophagy correlates with disrupted mitochondrial dynamics in AKI and contributes to the development and progression of acute to chronic kidney disease (CKD). Most recent clinical studies on CKD have shown that mitochondrial function decline is associated with the degree of kidney fibrosis in CKD [89]. In line with this, in patients with diabetic nephropathy (DN), urinary mtDNA, which is an indirect indicator of impaired mitophagy, correlated inversely with the estimated glomerular filtration rate (eGFR) and positively with interstitial fibrosis [90]. Furthermore, Cui and colleagues found that the Mitofusin 2 (MFN2) downregulation in macrophages enhances ROS and inhibits mitophagy, driving renal fibrosis [91]. Interestingly, no studies have investigated the influence of mitophagy on CAKUT pathophysiology so far. An indirect implication of the autophagy process has been observed in hypoplastic kidneys (HYP), where a significantly smaller proportion of autophagy marker-positive cells, specifically immunoglobulin heavy chain binding protein (BiP)-positive cells, was found [92]. The PI3K-AKT signaling pathway has also been implicated in CAKUT development through integrative multi-omics data analysis [93]. Since this pathway also regulates mitophagy, a potential mechanistic link between mitophagy and CAKUT warrants exploration.
Mitochondrial dysfunction drives both AKI and CKD through distinct mechanisms. Both conditions feature oxidative stress from ROS overproduction, but mitophagy plays divergent roles—protective in AKI vs. maladaptive in CKD. Unresolved AKI-related mitochondrial damage promotes transition to CKD through persistent inflammation and fibrotic signaling. In summary, mitophagy is a double-edged sword in the context of kidney disease. More specifically, its precise regulation is essential for preventing mitochondrial damage-driven kidney injury, while its dysfunction accelerates disease progression.
5. Feeding the Mitochondria: Can Diet Slow Kidney Disease?
Obesity and metabolic syndrome dramatically raise the risk of diabetic nephropathy and chronic kidney disease (CKD) [94,95,96]. A diet designed explicitly for the kidney can promote health and slow its progression to failure [95]. Although mitochondrial dysfunction is a well-known pathway in obesity-related organ damage, its specific impact on the kidney is unclear.
The kidney responds early to a high-fat diet (HFD) in mice, showing increased body weight, reduced plasma adiponectin, and early renal inflammation, including elevated MCP-1 and urinary H₂O₂ levels, observed after just one week of exposure. This initial inflammation precedes albuminuria and may drive obesity-related kidney damage. HFD exerts a biphasic effect on renal mitochondrial function [96,97,98,99]. In the short term, HFD may induce an adaptive response characterized by enhanced mitochondrial antioxidant defenses, such as increased manganese superoxide dismutase (MnSOD) activity and preserved respiratory capacity despite elevated oxidative stress. Upregulated redox-regulating pathways to maintain cellular homeostasis primarily drive this temporary resilience [94,100]. However, these compensatory mechanisms wane with prolonged HFD exposure, typically observed after 16 weeks in rodent models. Mitochondrial copy number declines, biogenesis is impaired due to suppressed PGC-1α expression, and ATP production is significantly reduced. Mitochondrial dysfunction worsens over time, resulting in excessive leakage of reactive oxygen species (ROS), lipid peroxidation, and a heightened vulnerability to further injuries, such as ischemia–reperfusion injury. These maladaptive changes eventually lead to renal fibrosis, glomerular hypertrophy, and damage to tubular epithelial cells, ultimately speeding up the progression of kidney disease [94,100,101,102].
Dietary interventions such as protein and salt restriction are crucial in reducing systemic stressors affecting mitochondrial function and preserving kidney health. High protein intake can elevate intraglomerular pressure, leading to glomerular damage. Simultaneously, a low-protein diet (LPD) of 0.6–0.8 g/kg/day has been shown to reduce proteinuria, slow CKD progression, and improve metabolic acidosis, supporting mitochondrial function [103,104,105,106]. Combining LPD with renin–angiotensin system (RAAS) blockade provides additional renal protection [107,108]. Salt restriction, particularly lowering sodium intake to less than 2,000 mg/day, has been linked to reduced blood pressure and decreased proteinuria, both important for slowing CKD progression. However, the long-term effects of strict salt restriction remain debated, with some studies raising concerns over potential risks such as hypotension in advanced CKD [109,110,111,112]. Key nutrients like coenzyme Q10 (CoQ10) and B vitamins (riboflavin and thiamine) support mitochondrial health and energy production. CoQ10, as an antioxidant, may diminish oxidative stress and enhance renal function, while B vitamins help alleviate energy deficits caused by mitochondrial dysfunction in CKD [113]. Antioxidant-rich foods, such as berries, red bell peppers, and leafy greens, also contribute to this protection by neutralizing reactive oxygen species (ROS), thereby further shielding kidney mitochondria from damage [114].
6. Hormonal Guardians of the Mitochondria: Estrogen and Thyroid Hormones in Kidney Disease Regulation
Hormones, particularly estrogen and thyroid hormones, are vital in mitochondrial function and kidney health. Estrogen is vital in regulating mitochondrial dynamics and oxidative stress in kidney disease through various mechanisms. It inhibits the production of ROS by blocking angiotensin II receptor type 1 (AT1R) and mineralocorticoid receptor (MR) signaling, which are typically linked to increased NADPH oxidase activity and intracellular ROS generation. This inhibition helps to reduce oxidative damage in vascular smooth muscle cells (VSMCs) and endothelial cells (ECs), thereby improving kidney function. Additionally, estrogen enhances the expression of antioxidant enzymes, such as superoxide dismutase (SOD), which further reduces oxidative stress [115,116,117,118].
Furthermore, estrogen protects against mitochondrial dysfunction by regulating calcium-induced permeability transition and promoting mitochondrial calcium sequestration through L-type calcium channels. This regulation activates protective signaling pathways, including Src/ERK/CREB via PI3K activation, which helps maintain mitochondrial integrity and prevent apoptosis [119]. By increasing the expression of Bcl-2 family proteins, estrogen inhibits the mitochondrial apoptotic pathway responsible for triggering excessive ROS and calcium concentrations. Overall, these effects preserve mitochondrial function and prevent cell death, underscoring the potential of estrogen as a therapeutic agent in treating kidney disease [120,121,122,123,124,125].
In addition to estrogen, other hormones have been shown to modulate mitochondrial function and influence the progression of kidney disease. Among these, thyroid hormones (T3 and T4) also play a critical yet dual role in mitochondrial function and kidney disease pathophysiology. T3 enhances mitochondrial ATP production and oxygen consumption through mechanisms such as mitochondrial uncoupling proteins (e.g., UCP-2), promoting energy metabolism and increasing renal oxygen demand, which may induce cortical hypoxia and exacerbate nephropathy [126,127,128]. Conversely, hypothyroidism, which is common in CKD patients, is associated with reduced mitochondrial efficiency, oxidative stress, and accelerated kidney dysfunction. Thyroid hormones modulate antioxidant defenses, such as superoxide dismutase (SOD), and interact with the renin–angiotensin–aldosterone system (RAAS), further influencing oxidative stress, and fibrosis. These findings highlight the delicate balance of thyroid hormone signaling in mitochondrial homeostasis and kidney health, underscoring their potential as therapeutic targets in CKD management [126,127,128,129,130,131,132].
7. Therapeutic Approaches for Kidney Disease
Mitochondria-targeted therapies for kidney diseases have shown promise in addressing mitochondrial dysfunction, particularly in reducing oxidative stress, improving mitochondrial dynamics, and enhancing biogenesis. However, current evidence largely stems from preclinical studies, with limited translation to clinical practice despite some advances.
Several studies assessed the effect of mitophagy as a therapy target for acute and chronic kidney injury. A study conducted on ischemic AKI in mouse models and gentamicin-induced AKI in the zebrafish model demonstrated that cobaltosilicate oxide-polyethylene glycol-triphenylphosphine (COPT) nanoparticles ameliorate the transition from acute to chronic kidney disease by inducing BNIP3-mediated mitophagy [133]. Additionally, paeoniflorin (PF), a water-soluble monoterpene glycoside extracted from Paeonia lactiflora, suppresses kidney inflammation by regulating mitophagy [134]. The mechanism through which it exerted its beneficial effect was by promoting macrophage polarization from M1 to M2 and inducing mitophagy via regulating Krüppel-like transcription factor 4 (KLF4) and upregulating mitophagy-related proteins PINK1, Parkin, Bnip3, P62, and LC3 in vivo and in vitro. In line with this, a study dealing with immune-regulatory effects on macrophages observed the ability of rapid-releasing hydrogen sulfide (H2S) donor NaSH, and a slow-releasing H2S compound S-propargyl-cysteine (SPRC) to protect the heart and kidney from tissue injury induced by LPS [135]. The study again portrayed a link between macrophage polarization from M1 to M2 and the PINK1/Parkin-mediated mitophagy pathway. Furthermore, treatment with the peroxisome proliferator-activated receptor-α (PPAR-α) agonist could reduce the pathology of polycystic kidney disease (PKD) and potentially improve the renal function of the disease by modulating mitophagy [136]. Additional information about targeting mitophagy or mitophagy-related pathways as a treatment for kidney disease can be found in Table 1.

Table 1.
Targeting mitophagy and mitophagy-related signaling in kidney disease.
Furthermore, an interesting approach to treating renal tubular injury using mitochondria transplantation (MITO) was employed [150].The study found that MITO, a process in which exogenous isolated mitochondria are taken up by cells, can mitigate AKI both in vitro and ex vivo. The molecular basis included the modulation of genes and pathways most consistent with mitochondrial biogenesis and energy metabolism, thereby reducing kidney damage. Additionally, RNAseq detected the downregulation of genes involved in neutrophil recruitment, including IL1A, CXCL8, and PIK3R1. Diabetes mellitus (DM) often leads to an increase in oxidative stress, which contributes to the development of diabetes complications, including diabetic kidney disease (DKD) [150]. One study, which investigated the effects of ethyl acetate extract of Potentilla indica on streptozotocin-induced diabetic male rats, found a protective effect [150]. The study emphasizes the importance of phenolic compounds in Potentilla indica extract for its renoprotective effect. Through their potent antioxidant activity, these compounds reduce ROS production, lipid peroxidation, and improve mitochondrial respiratory chain complex activity, as well as glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT) activities. In line with another study that dealt with obesity-linked DN, a positive effect of L-carnitine was found to be exerted through improvements in ROS production and SOD expression in the kidney, among other effects [151]. Furthermore, Hallows and coworkers found that bempedoic acid treatment in hypercholesterolemia might benefit polycystic kidney disease [152]. Specifically, in Polycystic Kidney Disease 1 (Pkd1)-null kidney cells and ATP Citrate Lyase (Acly) knockdown cells, BA inhibited mitochondrial superoxide production and promoted mitochondrial elongation, suggesting improved mitochondrial function. Although several studies have shed light on the role of Pyruvate kinase M2 (PKM2) in mitochondrial regulation, one study discovered that PKM2 binds to myosin heavy chain 9 (MYH9) to promote dynamin-related protein 1 (DRP1)-mediated mitochondrial fragmentation [153]. According to the study, in the case of staurosporine or cisplatin AKI, the regulation of PKM2 activity partially limits mitochondrial fragmentation, thereby directly decreasing the level of renal tubular injury and cell death, including apoptosis, necroptosis, and ferroptosis. Yan and colleagues reported an advanced technique for treating AKI in 2023. They studied tetrahedral framework nucleic acid (tFNA) as a vehicle and combined typhaneoside (Typ) to develop the tFNA-Typ complex (TTC) for targeting mitochondria and treating AKI, which managed to restore mitochondrial function [154]. Nanotechnology-mediated antioxidative therapy is another target of research in AKI. Curcumin-loaded nanodrug delivery system (NPS@Cur) was studied to assess its effects on apoptosis, autophagy, and ER stress in AKI [155]. The results from cisplatin-induced AKI models revealed that NPS@Cur alleviates mitochondrial injury, which subsequently leads to kidney protection through antioxidative protection, regulated autophagy, and reduced ER stress. In addition, adenosine and related purinergic molecules, which play central roles in energy metabolism, have recently been implicated in the regulation of mitochondrial function in renal cells and may represent novel therapeutic avenues [155].
8. Future Perspective
Future research on mitochondrial dysfunction in kidney disease holds promise for significant advances in diagnosis, prevention, and treatment. Mitochondrial transplantation represents a groundbreaking therapeutic approach, with preliminary studies demonstrating its ability to mitigate acute kidney injury by modulating inflammatory pathways and enhancing bioenergetics [156]. Novel nanomedicine approaches, including the COPT nanoparticles that induce BNIP3-mediated mitophagy and curcumin-loaded delivery systems, show promise in targeting mitochondrial pathology with high specificity and reduced systemic toxicity [133]. Mitochondrial biomarkers, particularly urinary mitochondrial DNA, hold promise for the early detection and monitoring of disease progression, with research suggesting a correlation with declining eGFR and interstitial fibrosis in diabetic nephropathy [157,158,159,160]. The field is moving toward personalized approaches, where treatment strategies target specific mitochondrial pathways based on individual patient profiles, including genetic background, disease etiology, and hormonal status. Epigenetic interventions, such as PPAR-α agonists and KLF4 modulators, represent another frontier in personalizing mitochondrial-targeted therapies. Translational research, which bridges preclinical discoveries to clinical applications, is essential, with emerging technologies like tFNA delivery systems progressing toward human trials. Integrating multi-omics approaches with traditional clinical parameters will likely enhance our ability to stratify patients and predict responses to mitochondrial-targeted interventions, ultimately improving outcomes in the diverse spectrum of kidney diseases where mitochondrial dysfunction plays a central role.
9. Conclusions
This review highlights the essential role of mitochondria in kidney health and disease. Mitochondria are key players in energy production and maintaining cellular balance, and they also interact intricately with various signaling pathways and epigenetic mechanisms. Their involvement is particularly significant in the development of acute kidney injury (AKI), chronic kidney disease (CKD), and congenital anomalies of the kidney and urinary tract (CAKUT). Mitochondrial dysfunction, characterized by impaired dynamics, increased production of reactive oxygen species (ROS), and disrupted metabolic processes, is a significant contributor to renal inflammation, fibrosis, and structural abnormalities. Therefore, targeting mitochondrial function is a promising therapeutic approach to combat kidney disease and improve patient outcomes.
Author Contributions
Conceptualization, N.P., M.K. (Marinela Križanac), M.K. (Marko Kumrić), K.V. and J.B.; methodology, N.P. and M.K. (Marinela Križanac); software, N.P.; validation, N.P., K.V. and J.B.; formal analysis, N.P.; investigation, N.P., M.K. (Marinela Križanac) and M.K. (Marko Kumrić); resources, J.B.; data curation, N.P.; writing—original draft preparation, N.P., M.K. (Marinela Križanac), M.K. (Marko Kumrić), K.V. and J.B.; writing—review and editing, N.P., M.K. (Marinela Križanac), M.K. (Marko Kumrić), K.V. and J.B.; visualization, N.P. and M.K. (Marinela Križanac); supervision, K.V. and J.B.; project administration, J.B.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Croatian Science Foundation under the project numbers IP-06–2016–2575 and IP-2022–10–8720.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Disha, B.; Mathew, R.P.; Dalal, A.B.; Mahato, A.K.; Satyamoorthy, K.; Singh, K.K.; Thangaraj, K.; Govindaraj, P. Mitochondria in biology and medicine—2023. Mitochondrion 2024, 76, 101853. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, R.M.; Seli, E. Mitochondria as therapeutic targets in assisted reproduction. Hum. Reprod. 2024, 39, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.B.; Jeffery, G. Light stimulation of mitochondria reduces blood glucose levels. J. Biophotonics 2024, 17, e202300521. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Kaynezhad, P.; Tachtsidis, I.; Jeffery, G. Optical monitoring of retinal respiration in real time: 670 nm light increases the redox state of mitochondria. Exp. Eye Res. 2016, 152, 88–93. [Google Scholar] [CrossRef]
- Begum, R.; Calaza, K.; Kam, J.H.; Salt, T.E.; Hogg, C.; Jeffery, G. Near-infrared light increases ATP, extends lifespan and improves mobility in aged Drosophila melanogaster. Biol. Lett. 2015, 11, 20150073. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, R.M.; Seli, E. The role of mitochondrial dynamics in oocyte and early embryo development. Semin. Cell Dev. Biol. 2024, 159–160, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Morgenstern, M.; Peikert, C.D.; Lübbert, P.; Suppanz, I.; Klemm, C.; Alka, O.; Steiert, C.; Naumenko, N.; Schendzielorz, A.; Melchionda, L.; et al. Quantitative high-confidence human mitochondrial proteome and its dynamics in cellular context. Cell Metab. 2021, 33, 2464–2483.e18. [Google Scholar] [CrossRef]
- Suomalainen, A.; Nunnari, J. Mitochondria at the crossroads of health and disease. Cell 2024, 187, 2601–2627. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Rizk, D.; Chapman, A.B. Cystic and inherited kidney diseases. Am. J. Kidney Dis. 2003, 42, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Narang, J.; Chhillar, A.K.; Rana, J.S.; Siddique, M.U.M.; Kenawy, E.-R.; Alkahtani, S.; Ahsan, M.N.; Nayak, A.K.; Hasnain, S. Diagnostic methods employing kidney biomarkers clinching biosensors as promising tools. Sensors Int. 2024, 5, 100253. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Mao, J.; Li, C.; Wu, F.; Wang, Y.; Zhu, J.; Wen, C. The relationship between kidney disease and mitochondria: A bibliometric study. Ren. Fail. 2024, 46, 2302963. [Google Scholar] [CrossRef]
- Chung, K.W.; Dhillon, P.; Huang, S.; Sheng, X.; Shrestha, R.; Qiu, C.; Kaufman, B.A.; Park, J.; Pei, L.; Baur, J.; et al. Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab. 2019, 30, 784–799.e5. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Renal. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef]
- Zelnick, L.R.; Weiss, N.S.; Kestenbaum, B.R.; Robinson-Cohen, C.; Heagerty, P.J.; Tuttle, K.; Hall, Y.N.; Hirsch, I.B.; de Boer, I.H. Diabetes and CKD in the United States Population, 2009–2014. Clin. J. Am. Soc. Nephrol. 2017, 12, 1984–1990. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Bleyer, A.J.; Molnar, M.Z.; Ma, J.Z.; Sim, J.J.; Cushman, W.C.; Darryl Quarles, L.; Kalantar-Zadeh, K. Blood pressure and mortality in U.S. Veterans with chronic kidney disease: A cohort study. Ann. Intern Med. 2013, 159, 4. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N. Temporal trends in the burden of chronic kidney disease in the United States. Curr. Opin. Nephrol. Hypertens. 2010, 19, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Gilbertson, D.T.; Snyder, J.J.; Chen, S.; Foley, R.N. Chronic kidney disease awareness, screening and prevention: Rationale for the design of a public education program. Nephrology 2010, 15, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Mizdrak, M.; Kumrić, M.; Kurir, T.T.; Božić, J. Emerging Biomarkers for Early Detection of Chronic Kidney Disease. J. Pers. Med. 2022, 12, 548. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- Bhatia, D.; Capili, A.; Choi, M.E. Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches. Kidney Res. Clin. Pract. 2020, 39, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11, 565023. [Google Scholar] [CrossRef]
- Ho, H.-J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef]
- Funk, J.A.; Schnellmann, R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am. J. Physiol. Physiol. 2012, 302, F853–F864. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Kanasaki, K.; Goodwin, J.E. Loss of Mitochondrial Control Impacts Renal Health. Front. Pharmacol. 2020, 11, 543973. [Google Scholar] [CrossRef]
- Tang, C.; Cai, J.; Yin, X.-M.; Weinberg, J.M.; Venkatachalam, M.A.; Dong, Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Miao, M.; Xu, X.; Bai, M.; Wu, M.; Zhang, A. From Physiology to Pathology: The Role of Mitochondria in Acute Kidney Injuries and Chronic Kidney Diseases. Kidney Dis. 2023, 9, 342–357. [Google Scholar] [CrossRef]
- Takasu, M.; Kishi, S.; Nagasu, H.; Kidokoro, K.; Brooks, C.R.; Kashihara, N. The Role of Mitochondria in Diabetic Kidney Disease and Potential Therapeutic Targets. Kidney Int. Rep. 2024, 10, 328–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, R.; Zhang, C.; Xiang, Z.; Lin, T.; Ling, J.; Hu, H. Role of mitochondria in renal ischemia–reperfusion injury. FEBS J. 2024, 291, 5365–5378. [Google Scholar] [CrossRef] [PubMed]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef]
- Jiang, Y.; Krantz, S.; Qin, X.; Li, S.; Gunasekara, H.; Kim, Y.-M.; Zimnicka, A.; Bae, M.; Ma, K.; Toth, P.T.; et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission—Fusion dynamics and mitophagy. Redox Biol. 2022, 52, 102304. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Chernikov, V.P.; Prusov, A.N.; Kireev, I.I.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mitochondrial Damage and Mitochondria-Targeted Antioxidant Protection in LPS-Induced Acute Kidney Injury. Antioxidants 2019, 8, 176. [Google Scholar] [CrossRef]
- Galloway, C.A.; Lee, H.; Nejjar, S.; Jhun, B.S.; Yu, T.; Hsu, W.; Yoon, Y. Transgenic Control of Mitochondrial Fission Induces Mitochondrial Uncoupling and Relieves Diabetic Oxidative Stress. Diabetes 2012, 61, 2093–2104. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Swan, E.J.; Maxwell, A.P.; McKnight, A.J. Distinct methylation patterns in genes that affect mitochondrial function are associated with kidney disease in blood-derived DNA from individuals with Type 1 diabetes. Diabet. Med. 2015, 32, 1110–1115. [Google Scholar] [CrossRef]
- Agil, A.; Chayah, M.; Visiedo, L.; Navarro-Alarcon, M.; Ferrer, J.M.R.; Tassi, M.; Reiter, R.J.; Fernández-Vázquez, G. Melatonin Improves Mitochondrial Dynamics and Function in the Kidney of Zücker Diabetic Fatty Rats. J. Clin. Med. 2020, 9, 2916. [Google Scholar] [CrossRef]
- Orozco-Ibarra, M.; Aparicio-Trejo, O.E.; Jiménez-Uribe, A.P.; Hernández-Cruz, E.Y.; Aranda-Rivera, A.K.; Amador-Martínez, I.; Fernández-Valverde, F.; Pedraza-Chaverri, J. Assessment of Kidney Mitochondrial Function by High-Resolution Respirometry, Transmission Electron Microscopy, and Histological Techniques. Methods Mol. Biol. 2023, 2664, 283–308. [Google Scholar]
- Jiang, M.; Bai, M.; Lei, J.; Xie, Y.; Xu, S.; Jia, Z.; Zhang, A. Mitochondrial dysfunction and the AKI-to-CKD transition. Am. J. Physiol. Physiol. 2020, 319, F1105–F1116. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Lee, W.-K.; Garrick, M.D. Iron and Cadmium Entry Into Renal Mitochondria: Physiological and Toxicological Implications. Front. Cell Dev. Biol. 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Gui, T.; Kullak-Ublick, G.A.; Li, Y.; Visentin, M. The Role of Mitochondria in Drug-Induced Kidney Injury. Front. Physiol. 2020, 11, 1079. [Google Scholar] [CrossRef]
- Boveris, A.; Valdez, L.B.; Zaobornyj, T.; Bustamante, J. Mitochondrial metabolic states regulate nitric oxide and hydrogen peroxide diffusion to the cytosol. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.; Ciarimboli, G. Editorial: Mitochondria in Renal Health and Disease. Front. Physiol. 2021, 12, 707175. [Google Scholar] [CrossRef]
- Ayanga, B.A.; Badal, S.S.; Wang, Y.; Galvan, D.L.; Chang, B.H.; Schumacker, P.T.; Danesh, F.R. Dynamin–Related Protein 1 Deficiency Improves Mitochondrial Fitness and Protects against Progression of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2733–2747. [Google Scholar] [CrossRef]
- Gujarati, N.A.; Vasquez, J.M.; Bogenhagen, D.F.; Mallipattu, S.K. The complicated role of mitochondria in the podocyte. Am. J. Physiol. Renal. Physiol. 2020, 319, F955–F965. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, Y.; Xue, Y.; Xing, C.; Zhang, B. Podocyte Injury in Diabetic Kidney Disease: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2022, 10, 832887. [Google Scholar] [CrossRef]
- Hejazian, S.M.; Ardalan, M.; Hosseiniyan Khatibi, S.M.; Rahbar Saadat, Y.; Barzegari, A.; Gueguen, V.; Meddahi-Pellé, A.; Anagnostou, F.; Zununi Vahed, S.; Pavon-Djavid, G. Biofactors regulating mitochondrial function and dynamics in podocytes and podocytopathies. J. Cell. Physiol. 2023, 238, 2206–2227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, J.; Zhu, W.; Niu, Y.; Wu, M.; Zhang, A. Therapeutic Potential Targeting Podocyte Mitochondrial Dysfunction in Focal Segmental Glomerulosclerosis. Kidney Dis. 2023, 9, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Dong, J.; Wang, R.; Bi, G.; Xu, D.; Zhang, Y.; Deng, Y.; Lin, W.; Yang, Z.; et al. HDAC3 aberration-incurred GPX4 suppression drives renal ferroptosis and AKI-CKD progression. Redox Biol. 2023, 68, 102939. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Tang, C.; Dong, Z. Mitophagy in Acute Kidney Injury and Kidney Repair. Cells 2020, 9, 338. [Google Scholar] [CrossRef]
- Guo, Y.; Che, R.; Wang, P.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Physiol. 2024, 326, F768–F779. [Google Scholar] [CrossRef]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef]
- Connaughton, D.M.; Hildebrandt, F. Disease mechanisms of monogenic congenital anomalies of the kidney and urinary tract. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190, 325–343. [Google Scholar] [CrossRef]
- Westland, R.; Sanna-Cherchi, S. Recessive mutations in CAKUT and VACTERL association. Kidney Int. 2014, 85, 1253–1255. [Google Scholar] [CrossRef]
- Zhan, M.; Brooks, C.; Liu, F.; Sun, L.; Dong, Z. Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013, 83, 568–581. [Google Scholar] [CrossRef]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraten, C.A.; Hoenderop, J.G.; de Baaij, J.H.F. Mitochondrial Dysfunction in Kidney Tubulopathies. Annu. Rev. Physiol. 2024, 86, 379–403. [Google Scholar] [CrossRef]
- Noble, R.A.; Lucas, B.J.; Selby, N.M. Long-Term Outcomes in Patients with Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2020, 15, 423–429. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Yao, C.; Li, Z.; Sun, K.; Zhang, Y.; Shou, S.; Jin, H. Mitochondrial dysfunction in acute kidney injury. Ren. Fail. 2024, 46, 2393262. [Google Scholar] [CrossRef]
- Clare, C.S. Identifying and managing acute kidney injury. Nurs. Stand. 2022, 37, 59–66. [Google Scholar] [CrossRef]
- Rahman, M.; Shad, F.; Smith, M.C. Acute kidney injury: A guide to diagnosis and management. Am. Fam. Physician 2012, 86, 631–639. [Google Scholar] [PubMed]
- Fontecha-Barriuso, M.; Lopez-Diaz, A.M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Tomar, B.; Sharma, D.; Rath, S.K. Mitochondrial dysfunction and oxidative stress: Role in chronic kidney disease. Life Sci. 2023, 319, 121432. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Cook, D.L.; Rivera, I.M. Chronic Kidney Disease: Detection and Evaluation. Am. Fam. Physician 2017, 96, 776–783. [Google Scholar]
- Pettitt, R.M.; Brumbaugh, A.P.; Gartman, M.F.; Jackson, A.M. Chronic kidney disease: Detection and evaluation. Am. Fam. Physician 2020, 12, 14–19. [Google Scholar]
- Chouhan, A.S.; Hingway, S.; Kaple, M.N. A Brief Review of Diagnostic Techniques and Clinical Management in Chronic Kidney Disease. Cureus 2023, 15, e49030. [Google Scholar] [CrossRef] [PubMed]
- Saisawat, P.; Kohl, S.; Hilger, A.C.; Hwang, D.-Y.; Gee, H.Y.; Dworschak, G.C.; Tasic, V.; Pennimpede, T.; Natarajan, S.; Sperry, E.; et al. Whole-exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney Int. 2014, 85, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.Y.X.; Winyard, P.; Marlais, M. Congenital anomalies of the kidney and urinary tract: Antenatal diagnosis, management and counselling of families. Pediatr. Nephrol. 2023, 39, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Soares dos Santos, A.C., Jr.; Marques de Miranda, D.; Simões e Silva, A.C. Congenital anomalies of the kidney and urinary tract: An embryogenetic review. Birth Defects Res. C Embryo Today 2014, 102, 374–381. [Google Scholar] [CrossRef]
- Lopes, A.F.C. Mitochondrial metabolism and DNA methylation: A review of the interaction between two genomes. Clin. Epigenetics 2020, 12, 182. [Google Scholar] [CrossRef]
- Lv, T.; Zhang, Y.; Ji, X.; Sun, S.; Xu, L.; Ma, W.; Liu, Y.; Wan, Q. GCN5L1-mediated TFAM acetylation at K76 participates in mitochondrial biogenesis in acute kidney injury. J. Transl. Med. 2022, 20, 571. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, M.; Xiong, L.; Fan, J.; Zhou, Y.; Li, H.; Peng, X.; Zhong, Z.; Wang, Y.; Huang, F.; et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020, 11, 29. [Google Scholar] [CrossRef]
- Yu, C.; Li, T.; Li, J.; Cui, B.; Liu, N.; Bayliss, G.; Zhuang, S. Inhibition of polycomb repressive complex 2 by targeting EED protects against cisplatin-induced acute kidney injury. J. Cell. Mol. Med. 2022, 26, 4061–4075. [Google Scholar] [CrossRef]
- de Oliveira Cruz, J.; Silva, A.O.; Ribeiro, J.M.; Luizon, M.R.; Ceron, C.S. Epigenetic Regulation of the N-Terminal Truncated Isoform of Matrix Metalloproteinase-2 (NTT-MMP-2) and Its Presence in Renal and Cardiac Diseases. Front. Genet. 2021, 12, 637148. [Google Scholar] [CrossRef]
- Ge, Q.-M.; Huang, C.-M.; Zhu, X.-Y.; Bian, F.; Pan, S.-M. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS ONE 2017, 12, e0173292. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.K.; Kota, S.B. Noncoding RNA and epigenetic gene regulation in renal diseases. Drug Discov. Today 2017, 22, 1112–1122. [Google Scholar] [CrossRef]
- Hajarnis, S.; Lakhia, R.; Yheskel, M.; Williams, D.; Sorourian, M.; Liu, X.; Aboudehen, K.; Zhang, S.; Kersjes, K.; Galasso, R.; et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 2017, 8, 14395. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.-W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Zhang, Z.; Dong, Z. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 2018, 9, 1113. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jiang, N.; Jin, H.; Shao, X.; Zhu, X.; Wu, J.; Zhang, M.; Zhang, Z.; Shen, J.; et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 2020, 17, 2975–2990. [Google Scholar] [CrossRef]
- Yang, K.; Li, T.; Geng, Y.; Zou, X.; Peng, F.; Gao, W. The role of mitophagy in the development of chronic kidney disease. PeerJ 2024, 12, e17260. [Google Scholar] [CrossRef]
- Bhatia, D.; Choi, M.E. Autophagy and mitophagy: Physiological implications in kidney inflammation and diseases. Am. J. Physiol. Physiol. 2023, 325, F1–F21. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, Z.; Tu, H.; Wu, J.; Zhou, J.; Yi, Q.; Liu, O.; Dai, X. Mitophagy in fibrotic diseases: Molecular mechanisms and therapeutic applications. Front. Physiol. 2024, 15, 1430230. [Google Scholar] [CrossRef]
- Maglica, M.; Kelam, N.; Perutina, I.; Racetin, A.; Rizikalo, A.; Filipović, N.; Prusac, I.K.; Mišković, J.; Vukojević, K. Immunoexpression Pattern of Autophagy-Related Proteins in Human Congenital Anomalies of the Kidney and Urinary Tract. Int. J. Mol. Sci. 2024, 25, 6829. [Google Scholar] [CrossRef] [PubMed]
- Bayjanov, J.R.; Doornbos, C.; Ozisik, O.; Shin, W.; Queralt-Rosinach, N.; Wijnbergen, D.; Saulnier-Blache, J.-S.; Schanstra, J.P.; Buffin-Meyer, B.; Klein, J.; et al. Integrative analysis of multi-omics data reveals importance of collagen and the PI3K AKT signalling pathway in CAKUT. Sci. Rep. 2024, 14, 20731. [Google Scholar] [CrossRef]
- Ruggiero, C.; Ehrenshaft, M.; Cleland, E.; Stadler, K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am. J. Physiol. Metab. 2011, 300, E1047–E1058. [Google Scholar] [CrossRef]
- Chen, J.; Muntner, P.; Hamm, L.L.; Jones, D.W.; Batuman, V.; Fonseca, V.; Whelton, P.K.; He, J. The Metabolic Syndrome and Chronic Kidney Disease in U.S. Adults. Ann. Intern. Med. 2004, 140, 167–174. [Google Scholar] [CrossRef]
- Abrass, C.K. Overview: Obesity: What does it have to do with kidney disease? J. Am. Soc. Nephrol. 2004, 15, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Effect of Diet on the Survival of Patients with Chronic Kidney Disease. Nutrients 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.-E.; Mathew, A.V.; Cunard, R.; Sharma, K. AMPK Mediates the Initiation of Kidney Disease Induced by a High-Fat Diet. J. Am. Soc. Nephrol. 2011, 22, 1846–1855. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-Aminoimidazole-4-Carboxamide Ribonucleoside: A Specific Method for Activating AMP-Activated Protein Kinase in Intact Cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Prem, P.N.; Kurian, G.A. High-Fat Diet Increased Oxidative Stress and Mitochondrial Dysfunction Induced by Renal Ischemia-Reperfusion Injury in Rat. Front. Physiol. 2021, 12, 715693. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, J.; Li, S.; Guo, F.; Li, A.; Wu, H.; Chen, J.; Pan, Q.; Liao, S.; Liu, H.-F.; et al. High-Fat Diet-Induced Renal Proximal Tubular Inflammatory Injury: Emerging Risk Factor of Chronic Kidney Disease. Front. Physiol. 2021, 12, 786599. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Back to the future: Restricted protein intake for conservative management of CKD, triple goals of renoprotection, uremia mitigation, and nutritional health. Int. Urol. Nephrol. 2016, 48, 725–729. [Google Scholar] [CrossRef]
- Bellizzi, V. Low-protein diet or nutritional therapy in chronic kidney disease? Blood Purif. 2013, 36, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, Y.-S.; Kim, Y.H.; Chung, W.; Park, S.K.; Choi, K.H.; Ahn, C.; Oh, K.-H. Dietary Protein Intake, Protein Energy Wasting, and the Progression of Chronic Kidney Disease: Analysis from the KNOW-CKD Study. Nutrients 2019, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Riccio, E.; Di Nuzzi, A.; Pisani, A. Nutritional treatment in chronic kidney disease: The concept of nephroprotection. Clin. Exp. Nephrol. 2014, 19, 161–167. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Moore, L.W.; Tortorici, A.R.; Chou, J.A.; St-Jules, D.E.; Aoun, A.; Rojas-Bautista, V.; Tschida, A.K.; Rhee, C.M.; Shah, A.A.; et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016, 17, 90. [Google Scholar] [CrossRef]
- Iseki, K. Nutrition and quality of life in chronic kidney disease patients: A practical approach for salt restriction. Kidney Res. Clin. Pract. 2022, 41, 657–669. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef]
- Crawford-Faucher, A.; Finer, L.B.; Zolna, M.R. Declines in unintended pregnancy in the United States. N. Engl. J. Med. 2017, 374, 843–852. Available online: www.aafp.org/afp (accessed on 1 May 2025).
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W.; Kelly, J.T. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2021, 6, CD010070. [Google Scholar] [CrossRef]
- Abe, Y.; Nishiwaki, H.; Suzuki, T.; Noma, H.; Watanabe, Y.; Ota, E.; Hasegawa, T. Renoprotective effects of coenzyme Q10 supplementation in patients with chronic kidney disease: A protocol for a systematic review. BMJ Open 2024, 14, e084088. [Google Scholar] [CrossRef] [PubMed]
- Tkaczenko, H.; Kurhaluk, N. Antioxidant-Rich Functional Foods and Exercise: Unlocking Metabolic Health Through Nrf2 and Related Pathways. Int. J. Mol. Sci. 2025, 26, 1098. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Chen, S.; Du, Y. Estrogen and estrogen receptors in kidney diseases. Ren. Fail. 2021, 43, 619–642. [Google Scholar] [CrossRef] [PubMed]
- Kafami, M.; Hosseini, M.; Niazmand, S.; Farrokhi, E.; Hajzadeh, M.A.-R.; Nazemi, S. The effects of estradiol and testosterone on renal tissues oxidative after central injection of angiotensin II in female doca—Salt treated rats. Horm. Mol. Biol. Clin. Investig. 2019, 37, 3. [Google Scholar] [CrossRef]
- Rzewuska-Lech, E.; Jayachandran, M.; Fitzpatrick, L.A.; Miller, V.M. Differential effects of 17β-estradiol and raloxifene on VSMC phenotype and expression of osteoblast-associated proteins. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E105–E112. [Google Scholar] [CrossRef]
- Chang, Y.; Han, Z.; Zhang, Y.; Zhou, Y.; Feng, Z.; Chen, L.; Li, X.; Li, L.; Si, J.-Q. G protein-coupled estrogen receptor activation improves contractile and diastolic functions in rat renal interlobular artery to protect against renal ischemia reperfusion injury. Biomed. Pharmacother. 2019, 112, 108666. [Google Scholar] [CrossRef]
- Guajardo-Correa, E.; Silva-Agüero, J.F.; Calle, X.; Chiong, M.; Henríquez, M.; García-Rivas, G.; Latorre, M.; Parra, V. Estrogen signaling as a bridge between the nucleus and mitochondria in cardiovascular diseases. Front. Cell Dev. Biol. 2022, 10, 968373. [Google Scholar] [CrossRef]
- Tian, H.; Gao, Z.; Wang, G.; Li, H.; Zheng, J. Estrogen potentiates reactive oxygen species (ROS) tolerance to initiate carcinogenesis and promote cancer malignant transformation. Tumor Biol. 2015, 37, 141–150. [Google Scholar] [CrossRef]
- Razmara, A.; Duckles, S.P.; Krause, D.N.; Procaccio, V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007, 1176, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Estrogen and mitochondria function in cardiorenal metabolic syndrome. Prog. Mol. Biol. Transl. Sci. 2014, 127, 229–249. [Google Scholar] [PubMed]
- Wang, X.X.; Myakala, K.; Libby, A.E.; Krawczyk, E.; Panov, J.; Jones, B.A.; Bhasin, K.; Shults, N.; Qi, Y.; Krausz, K.W.; et al. Estrogen-Related Receptor Agonism Reverses Mitochondrial Dysfunction and Inflammation in the Aging Kidney. Am. J. Pathol. 2023, 193, 1969–1987. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Liu, H.; Kim, J.K. Estrogen Protects the Female Heart from Ischemia/Reperfusion Injury through Manganese Superoxide Dismutase Phosphorylation by Mitochondrial p38β at Threonine 79 and Serine 106. PLoS ONE 2016, 11, e0167761. [Google Scholar] [CrossRef]
- Lee, W.-L.; Cheng, M.-H.; Tarng, D.-C.; Yang, W.-C.; Lee, F.-K.; Wang, P.-H. The benefits of estrogen or selective estrogen receptor modulator on kidney and its related disease—Chronic kidney disease—Mineral and bone disorder: Osteoporosis. J. Chin. Med. Assoc. 2013, 76, 365–371. [Google Scholar] [CrossRef]
- Rhee, C.M. The interaction between thyroid and kidney disease: An overview of the evidence. Curr. Opin. Endocrinol. Diabetes 2016, 23, 407–415. [Google Scholar] [CrossRef]
- Basu, G.; Mohapatra, A. Interactions between thyroid disorders and kidney disease. Indian J. Endocrinol. Metab. 2012, 16, 204–213. [Google Scholar] [CrossRef]
- Sivertsson, E.; Friederich-Persson, M.; Persson, P.; Nangaku, M.; Hansell, P.; Palm, F. Thyroid hormone increases oxygen metabolism causing intrarenal tissue hypoxia; A pathway to kidney disease. PLoS ONE 2022, 17, e0264524. [Google Scholar] [CrossRef]
- Amorim Amato, A.; Martins Santos, G.; de Assis Rocha Neves, F. Thyroid hormone action in chronic kidney disease. Curr. Opin. Endocrinol. Diabetes 2008, 15, 459–465. [Google Scholar] [CrossRef]
- Harper, M.-E.; Seifert, E.L. Thyroid Hormone Effects on Mitochondrial Energetics. Thyroid 2008, 18, 145–156. [Google Scholar] [CrossRef]
- Rajeev, G.; Rayappa, W.D.S.C.; Vijayalakshmi, R.; Swathi, M.; Kumar, S. Evaluation of thyroid hormone levels in chronic kidney disease patients. Saudi J. Kidney Dis. Transplant. 2015, 26, 90–93. [Google Scholar] [CrossRef]
- Alsagheer, M.M.M.; Rahman, A.A.A.; Abdelmeguid, M.M.; Abdelhafez, A.A. Evaluation of Thyroid Hormones Level in Chronic Kidney Disease Patients. Al-Azhar Int. Med. J. 2023, 4, 9. [Google Scholar] [CrossRef]
- Qin, S.; Liu, C.; Chen, Y.; Yao, M.; Liao, S.; Xin, W.; Gong, S.; Guan, X.; Li, Y.; Xiong, J.; et al. Cobaltosic oxide-polyethylene glycol-triphenylphosphine nanoparticles ameliorate the acute-to-chronic kidney disease transition by inducing BNIP3-mediated mitophagy. Kidney Int. 2023, 103, 903–916. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, J.; Guan, X.; Yin, S.; Chen, J.; Yuan, S.; Liu, H.; Lin, S.; Zhou, Y.; Qiu, J.; et al. Paeoniflorin suppresses kidney inflammation by regulating macrophage polarization via KLF4-mediated mitophagy. Phytomedicine 2023, 116, 154901. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, W.; Li, B.; Qiao, X.; Wang, X.; Yang, G.; Li, S. The potential role of hydrogen sulfide in regulating macrophage phenotypic changes via PINK1/parkin-mediated mitophagy in sepsis-related cardiorenal syndrome. Immunopharmacol. Immunotoxicol. 2024, 46, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Suga, N.; Ikeda, Y.; Yoshikawa, S.; Matsuda, S. Inspiring Tactics with the Improvement of Mitophagy and Redox Balance for the Development of Innovative Treatment against Polycystic Kidney Disease. Biomolecules 2024, 14, 207. [Google Scholar] [CrossRef]
- Fan, X.; Wu, L.; Wang, F.; Liu, D.; Cen, X.; Xia, H. Mitophagy Regulates Kidney Diseases. Kidney Dis. 2024, 10, 73–587. [Google Scholar] [CrossRef]
- Tang, C.-Y.; Lai, C.-C.; Yang, A.-H.; Chiang, S.-C.; Huang, P.-C.; Tseng, K.-W.; Huang, C.-H. Magnolol reduces myocardial injury induced by renal ischemia and reperfusion. J. Chin. Med. Assoc. 2022, 85, 584–596. [Google Scholar] [CrossRef]
- Zhu, Z.; Luan, G.; Peng, S.; Fang, Y.; Fang, Q.; Shen, S.; Wu, K.; Qian, S.; Jia, W.; Ye, J.; et al. Huangkui capsule attenuates diabetic kidney disease through the induction of mitophagy mediated by STING1/PINK1 signaling in tubular cells. Phytomedicine 2023, 119, 154975. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Feng, X.; Xu, Y.; Zhou, L.; Wang, C.; Wang, M. Astragaloside IV attenuates fatty acid-induced renal tubular injury in diabetic kidney disease by inhibiting fatty acid transport protein-2. Phytomedicine 2024, 134, 155991. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M. Metformin rescues Parkin protein expression and mitophagy in high glucose-challenged human renal epithelial cells by inhibiting NF-κB via PP2A activation. Life Sci. 2020, 246, 117382. [Google Scholar] [CrossRef]
- Hurtado, K.A.; Schnellmann, R.G. Mitophagy regulates mitochondrial number following pharmacological induction of mitochondrial biogenesis in renal proximal tubule cells. Front. Pharmacol. 2024, 15, 1344075. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, M.; Bai, X.; Li, J.; Nie, P.; Li, B.; Luo, P. SS-31, a Mitochondria-Targeting Peptide, Ameliorates Kidney Disease. Oxid. Med. Cell. Longev. 2022, 2022, 1295509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Song, Y.; Chen, L.; Chen, P.; Yuan, M.; Meng, Y.; Wang, Q.; Zheng, G.; Qiu, Z. Urolithin A Attenuates Hyperuricemic Nephropathy in Fructose-Fed Mice by Impairing STING-NLRP3 Axis-Mediated Inflammatory Response via Restoration of Parkin-Dependent Mitophagy. Front. Pharmacol. 2022, 13, 907209. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, R.; Zhou, J.; Guo, P.; Li, P.; Ye, M.; Liu, Y.; Shi, S. Pharmacokinetics and safety of pirfenidone in individuals with chronic kidney disease stage G2 and G3a: A single-dose, Phase I, bridging study. J. Pharm. Sci. 2025, 114, 1087–1094. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perkovic, V.; Tuttle, K.R.; Pergola, P.E.; Mahaffey, K.W.; Patel, U.D.; Ishida, J.H.; Kuo, A.; Chen, F.; Kustra, F.; et al. Selonsertib in Patients with Diabetic Kidney Disease: A Phase 2b Randomized Active Run-In Clinical Trial. J. Am. Soc. Nephrol. 2024, 35, 1726–1736. [Google Scholar] [CrossRef]
- Dagar, N.; Habshi, T.; Shelke, V.; Jadhav, H.R.; Gaikwad, A.B. Renoprotective effect of esculetin against ischemic acute kidney injury-diabetic comorbidity. Free. Radic. Res. 2024, 58, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Gale, D.P.; Gross, O.; Wang, F.; de la Rosa, R.J.E.; Hall, M.; Sayer, J.A.; Appel, G.; Hariri, A.; Liu, S.; Maski, M.; et al. A Randomized Controlled Clinical Trial Testing Effects of Lademirsen on Kidney Function Decline in Adults with Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2024, 19, 995–1004. [Google Scholar] [CrossRef]
- Landa-Moreno, C.I.; Trejo-Hurtado, C.M.; la Cruz, J.L.-D.; Peña-Montes, D.J.; Murillo-Villicaña, M.; Huerta-Cervantes, M.; Montoya-Pérez, R.; Salgado-Garciglia, R.; Manzo-Avalos, S.; Cortés-Rojo, C.; et al. Antioxidant Effect of the Ethyl Acetate Extract of Potentilla indica on Kidney Mitochondria of Streptozotocin-Induced Diabetic Rats. Plants 2023, 12, 3196. [Google Scholar] [CrossRef]
- Ito, S.; Nakashima, M.; Ishikiriyama, T.; Nakashima, H.; Yamagata, A.; Imakiire, T.; Kinoshita, M.; Seki, S.; Kumagai, H.; Oshima, N. Effects of L-Carnitine Treatment on Kidney Mitochondria and Macrophages in Mice with Diabetic Nephropathy. Kidney Blood Press. Res. 2022, 47, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Hallows, K.R.; Li, H.; Saitta, B.; Sepehr, S.; Huang, P.; Pham, J.; Wang, J.; Mancino, V.; Chung, E.J.; Pinkosky, S.L.; et al. Beneficial effects of bempedoic acid treatment in polycystic kidney disease cells and mice. Front. Mol. Biosci. 2022, 9, 1001941. [Google Scholar] [CrossRef]
- Xie, W.; He, Q.; Zhang, Y.; Xu, X.; Wen, P.; Cao, H.; Zhou, Y.; Luo, J.; Yang, J.; Jiang, L. Pyruvate kinase M2 regulates mitochondrial homeostasis in cisplatin-induced acute kidney injury. Cell Death Dis. 2023, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Cui, W.; Ma, W.; Li, J.; Liu, Z.; Lin, Y. Typhaneoside-Tetrahedral Framework Nucleic Acids System: Mitochondrial Recovery and Antioxidation for Acute Kidney Injury treatment. ACS Nano 2023, 17, 8767–8781. [Google Scholar] [CrossRef]
- Lan, T.; Guo, H.; Lu, X.; Geng, K.; Wu, L.; Luo, Y.; Zhu, J.; Shen, X.; Guo, Q.; Wu, S. Dual-Responsive Curcumin-Loaded Nanoparticles for the Treatment of Cisplatin-Induced Acute Kidney Injury. Biomacromolecules 2022, 23, 5253–5266. [Google Scholar] [CrossRef]
- Burnstock, G.; Evans, L.C.; Bailey, M.A. Purinergic signalling in the kidney in health and disease. Purinergic Signal. 2013, 10, 71–101. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.I.; Tam, F.W.; Unwin, R.J.; Bailey, M.A. Purinergic signaling in kidney disease. Kidney Int. 2017, 91, 315–323. [Google Scholar] [CrossRef]
- Jabbari, H.; Roushandeh, A.M.; Rostami, M.K.; Razavi-Toosi, M.T.; Shokrgozar, M.A.; Jahanian-Najafabadi, A.; Kuwahara, Y.; Roudkenar, M.H. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165809. [Google Scholar] [CrossRef]
- Cho, J.; Doo, S.W.; Song, N.; Lee, M.; Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Kwon, S.H. Dapagliflozin Reduces Urinary Kidney Injury Biomarkers in Chronic Kidney Disease Irrespective of Albuminuria Level. Clin. Pharmacol. Ther. 2024, 115, 1441–1449. [Google Scholar] [CrossRef]
- Yadav, B.; Prasad, N.; Kushwaha, R.S.; Patel, M.R.; Bhadauria, D.S.; Kaul, A. Urinary Mitochondrial Deoxyribonucleic Acid: A Novel Biomarker of Coronavirus Disease 2019-associated Acute Kidney Injury in Renal Transplant Recipients. Indian J. Transplant. 2023, 17, 287–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).