Inhibition of GPX4 by Toxoplasma gondii Promotes Ferroptosis and Enhances Its Proliferation in Acute and Chronic Infection
Abstract
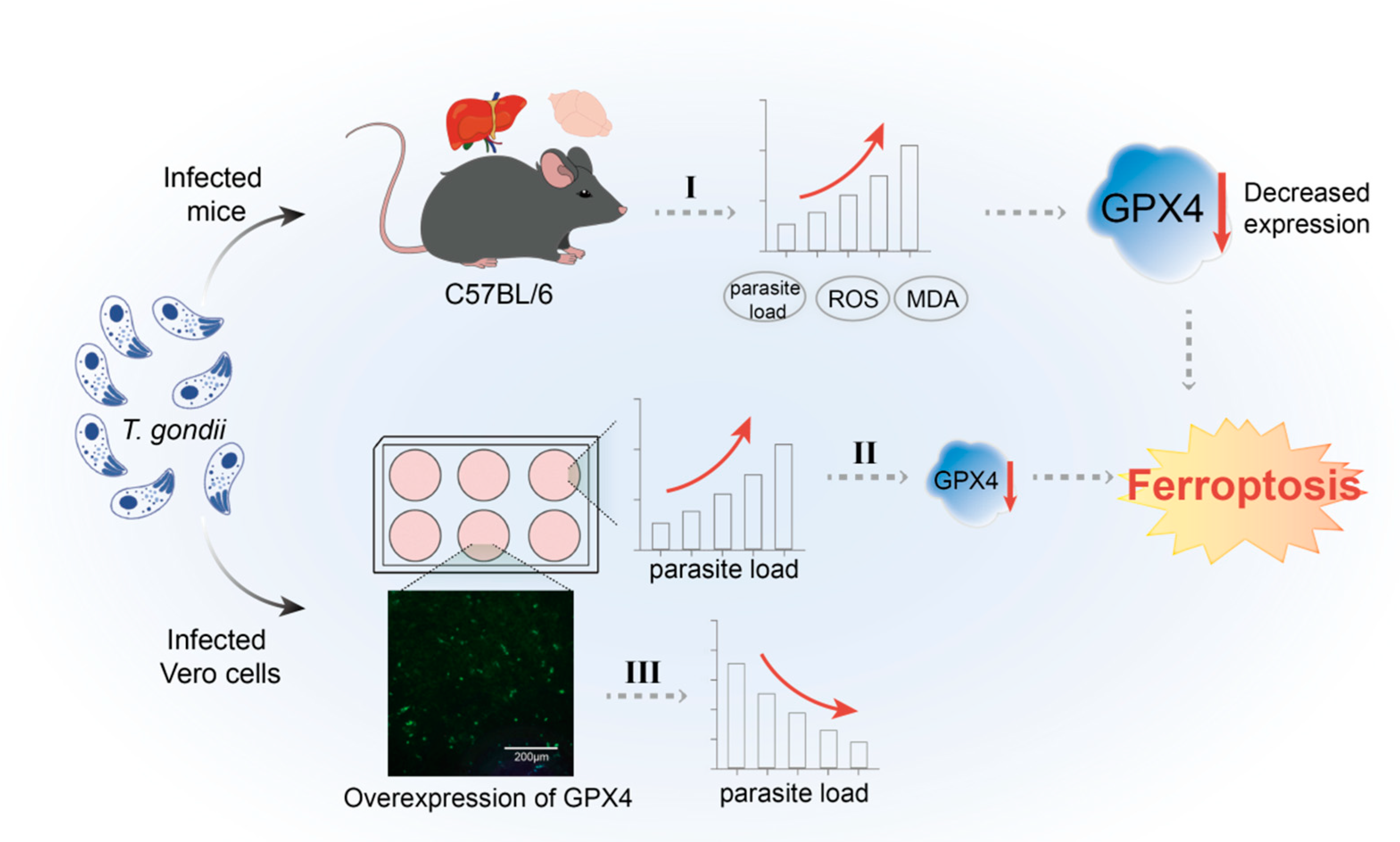
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Laboratory Animals, Parasites
2.3. Cell Culture and Treatment
2.4. Parasite Culture, Infection Model Construction, and Sample Collection
2.5. RNA Extraction and cDNA Synthesis
2.6. Plasmid Construction
2.7. Cell Transfection and Indirect Immunofluorescence (IIF)
2.8. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Western Blotting (WB)
2.11. Statistical Analysis
3. Results
3.1. T. gondii Infection Elevated the Levels of ROS and MDA in Mouse Tissues
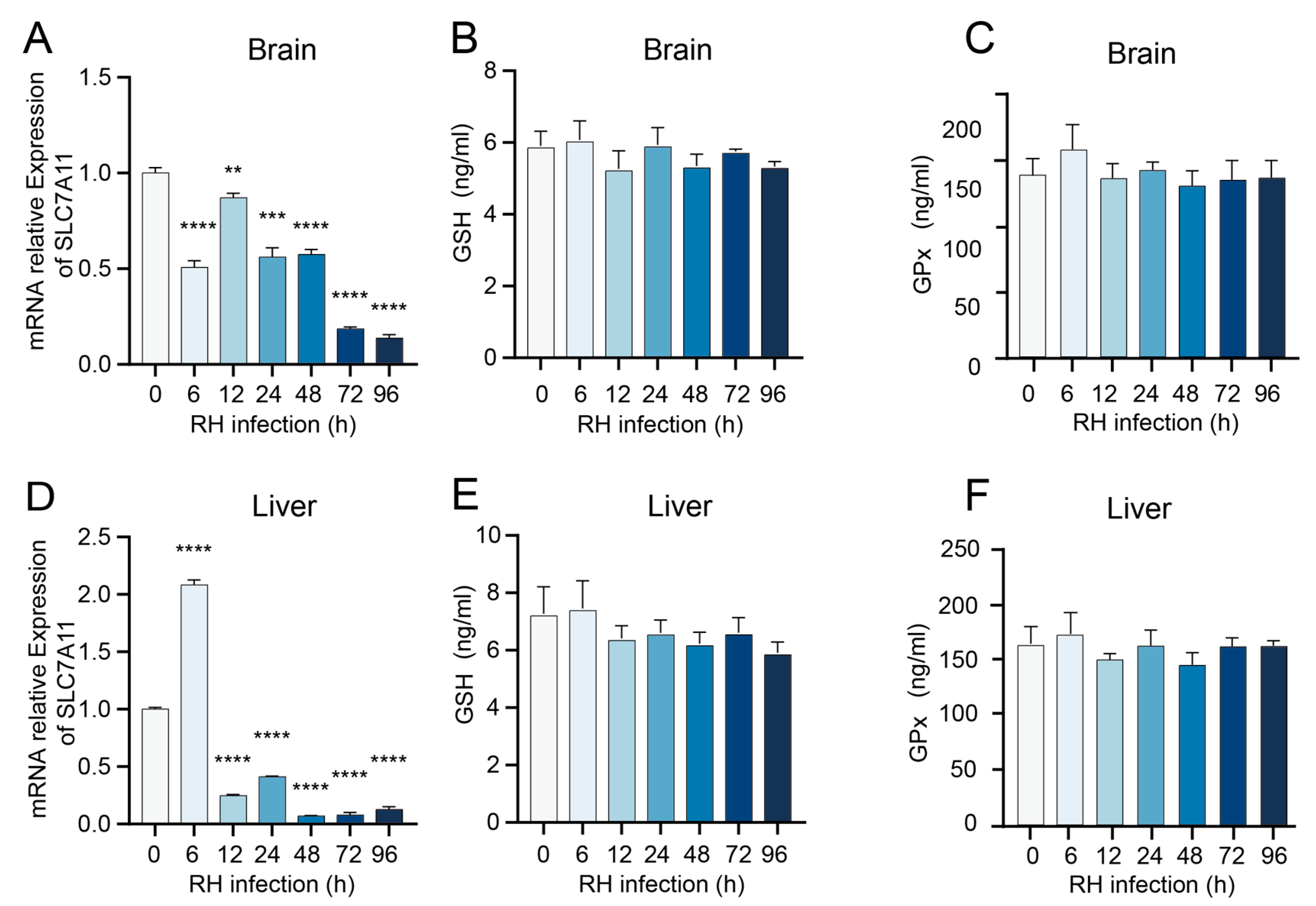
3.2. T. gondii Infection Modulates the Expression of Antioxidant System-Related Genes in Murine Tissues
3.3. T. gondii Infection Reduces the Expression of GPX4, a Key Negative Regulator of Ferroptosis
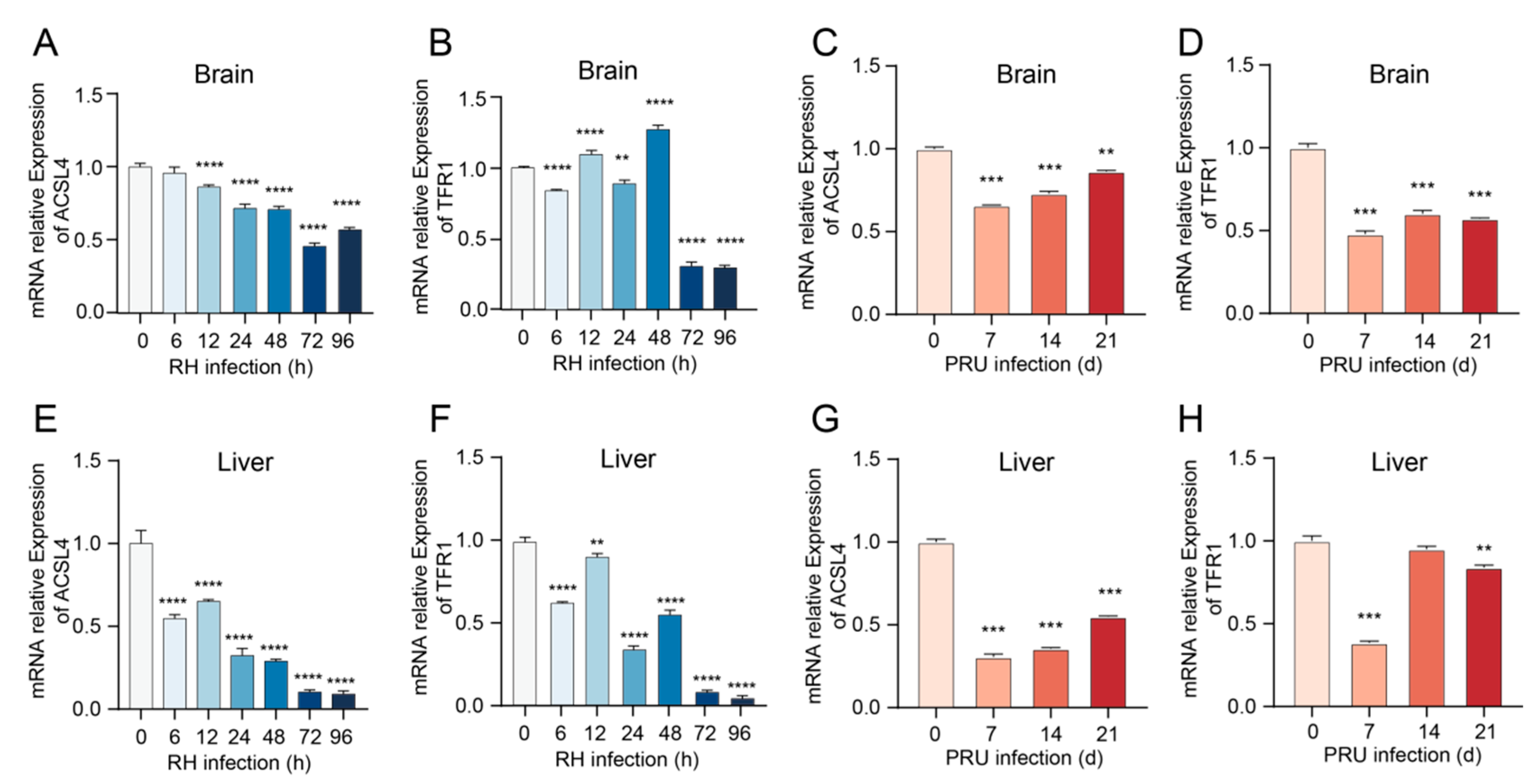
3.4. Alterations in mRNA Transcription Levels of ACSL4 and TFR1 Genes in Mice Infected with T. gondii
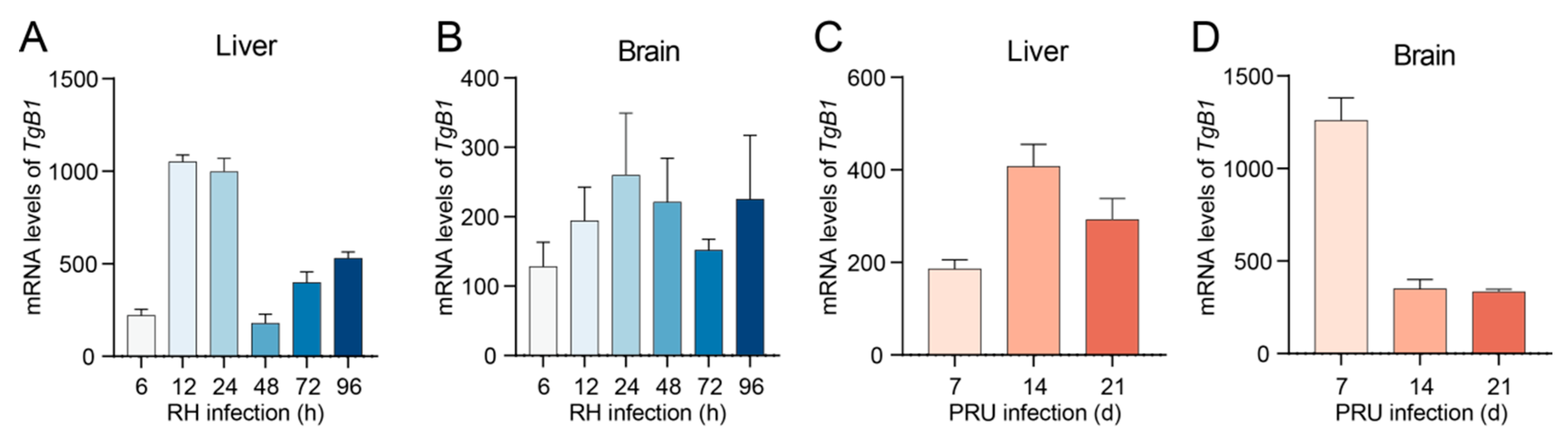
3.5. T. gondii Infection Triggers Ferroptosis in Host Cells, Thereby Facilitating Its Proliferation Within the Host Organism
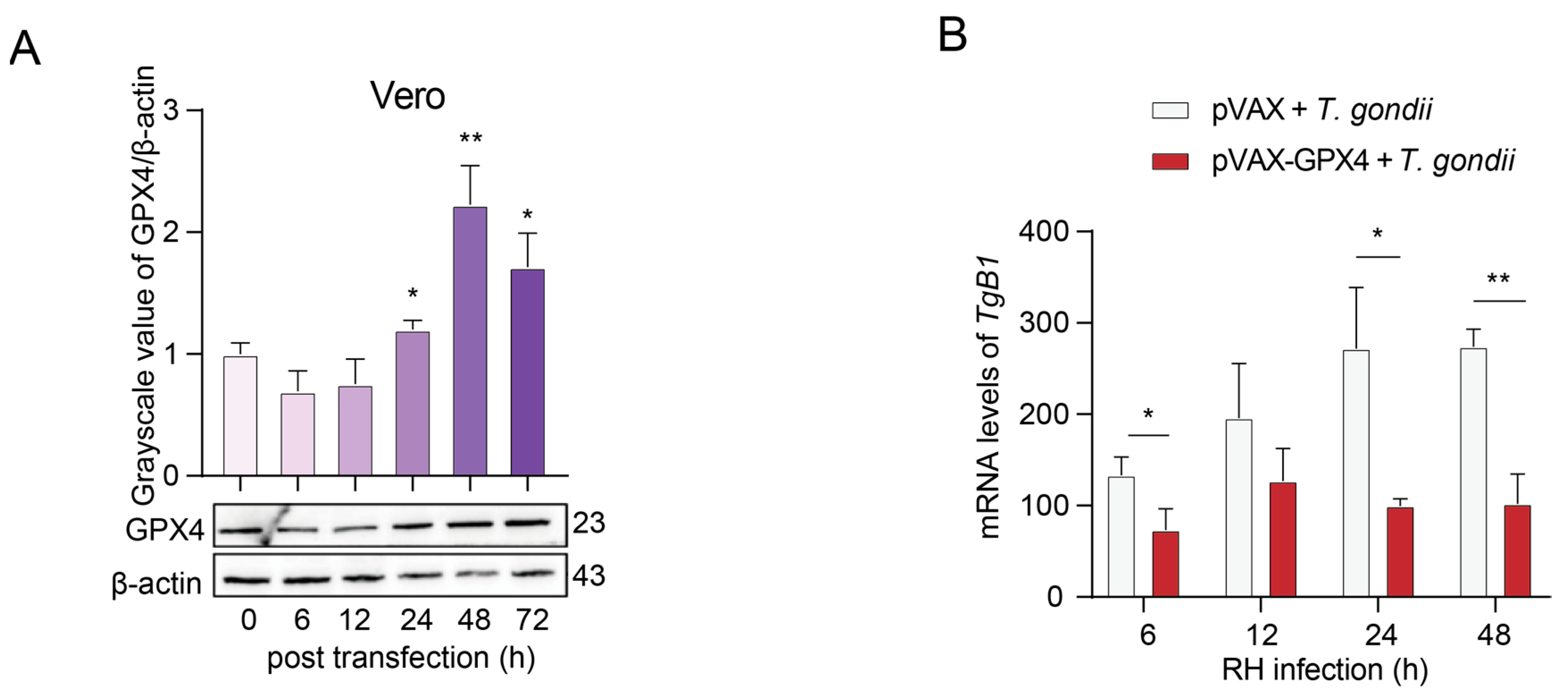
3.6. T. gondii Infection Suppresses the Expression of GPX4 in Vero Cells, Leading to an Elevated Parasite Load
3.7. Construction and Validation of Recombinant pVAX-GPX4 Plasmid Expression in Vero Cells
3.8. The Inhibitory Effect of GPX4 Overexpression on the Proliferation of T. gondii RH Strain in Vero Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elmore, S.A.; Jones, J.L.; Conrad, P.A.; Patton, S.; Lindsay, D.S.; Dubey, J.P. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010, 26, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Hill, D.E.; Chirukandoth, S.; Dubey, J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005, 6, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lyu, C.; Zhao, J.; Shen, B. Sixty Years (1957–2017) of Research on Toxoplasmosis in China-An Overview. Front. Microbiol. 2017, 8, 1825. [Google Scholar] [CrossRef]
- Dong, H.; Su, R.; Lu, Y.; Wang, M.; Liu, J.; Jian, F.; Yang, Y. Prevalence, Risk Factors, and Genotypes of Toxoplasma gondii in Food Animals and Humans (2000–2017) From China. Front. Microbiol. 2018, 9, 2108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, H.; Liu, D.; Huo, X.; Gao, J.; Song, X.; Xu, X.; Huang, K.; Liu, W.; Wang, Y.; et al. Genotypes and mouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS ONE 2013, 8, e53483. [Google Scholar] [CrossRef]
- Lorenzi, H.; Khan, A.; Behnke, M.S.; Namasivayam, S.; Swapna, L.S.; Hadjithomas, M.; Karamycheva, S.; Pinney, D.; Brunk, B.P.; Ajioka, J.W.; et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat. Commun. 2016, 7, 10147. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Coutinho, L.B.; Almeida, M.P.O.; Briceño, M.P.; Araujo, E.C.B.; Silva, N.M. The Availability of Iron Is Involved in the Murine Experimental Toxoplasma gondii Infection Outcome. Microorganism 2020, 8, 560. [Google Scholar] [CrossRef]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Zhao, G. Beclin1-mediated ferroptosis activation is associated with isoflurane-induced toxicity in SH-SY5Y neuroblastoma cells. Acta Biochim. Biophys. Sin. 2019, 51, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Linghu, D.L.; Hung, M.C. Ferroptosis: A promising target for cancer immunotherapy. Am. J. Cancer Res. 2021, 11, 5856–5863. [Google Scholar] [PubMed]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Organelle-specific regulation of ferroptosis. Cell Death Differ. 2021, 28, 2843–2856. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free. Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.Y.; Pang, Y.L.; Li, W.X.; Zhao, C.X.; Zhang, Y.; Wang, X.; Ning, G.Z.; Kong, X.H.; Liu, C.; Yao, X.; et al. Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen. Res. 2021, 16, 561–566. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Xia, R.; Zhang, H.S. Ferroptosis resistance in cancer: Recent advances and future perspectives. Biochem. Pharmacol. 2024, 219, 115933. [Google Scholar] [CrossRef]
- Duan, J.Y.; Lin, X.; Xu, F.; Shan, S.K.; Guo, B.; Li, F.X.; Wang, Y.; Zheng, M.H.; Xu, Q.S.; Lei, L.M.; et al. Ferroptosis and Its Potential Role in Metabolic Diseases: A Curse or Revitalization? Front. Cell Dev. Biol. 2021, 9, 701788. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Y.; Sang, R.; Ge, B.; Wang, M.; Zhou, H.; Zhang, X. Inonotus obliquus polysaccharide protects against adverse pregnancy caused by Toxoplasma gondii infection through regulating Th17/Treg balance via TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2020, 146, 832–840. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hosseini, S.A.; Sarvi, S.; Ebrahimnejad, P.; Asgarian Omran, H.; Zare, Z.; Gholami, S.; Khalilian, A.; Tork, M.; Daryani, A.; et al. Efficacy of Clindamycin in Preventing Abortion and Vertical Transmission of Toxoplasma gondii (PRU Strain) Infection in Pregnant BALB/c Mice. Iran. J. Pharm. Res. 2024, 23, e150424. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lin, M.H.; Chen, T.C.; Kuo, T.T.; Tseng, C.C.; Tseng, C.P. Real-time PCR for quantitative detection of Toxoplasma gondii. J. Clin. Microbiol. 2000, 38, 4121–4125. [Google Scholar] [CrossRef]
- Aw, Y.T.V.; Seidi, A.; Hayward, J.A.; Lee, J.; Makota, F.V.; Rug, M.; van Dooren, G.G. A key cytosolic iron-sulfur cluster synthesis protein localizes to the mitochondrion of Toxoplasma gondii. Mol. Microbiol. 2021, 115, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Renaud, E.A.; Pamukcu, S.; Cerutti, A.; Berry, L.; Lemaire-Vieille, C.; Yamaryo-Botté, Y.; Botté, C.Y.; Besteiro, S. Disrupting the plastidic iron-sulfur cluster biogenesis pathway in Toxoplasma gondii has pleiotropic effects irreversibly impacting parasite viability. J. Biol. Chem. 2022, 298, 102243. [Google Scholar] [CrossRef]
- Pamukcu, S.; Cerutti, A.; Bordat, Y.; Hem, S.; Rofidal, V.; Besteiro, S. Differential contribution of two organelles of endosymbiotic origin to iron-sulfur cluster synthesis and overall fitness in Toxoplasma. PLoS Pathog. 2021, 17, e1010096. [Google Scholar] [CrossRef]
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856. [Google Scholar] [CrossRef]
- Aghabi, D.; Sloan, M.; Gill, G.; Hartmann, E.; Antipova, O.; Dou, Z.; Guerra, A.J.; Carruthers, V.B.; Harding, C.R. The vacuolar iron transporter mediates iron detoxification in Toxoplasma gondii. Nat. Commun. 2023, 14, 3659. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Flegr, J.; Prandota, J.; Sovičková, M.; Israili, Z.H. Toxoplasmosis—A global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE 2014, 9, e90203. [Google Scholar] [CrossRef]
- Huang, M.; Cao, X.; Jiang, Y.; Shi, Y.; Ma, Y.; Hu, D.; Song, X. Evaluation of the Combined Effect of Artemisinin and Ferroptosis Inducer RSL3 against Toxoplasma gondii. Int. J. Mol. Sci. 2022, 24, 229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Lima, T.S.; Lodoen, M.B. Mechanisms of Human Innate Immune Evasion by Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2019, 9, 103. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal 2018, 29, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schröder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Freigang, S.; Schneider, C.; Conrad, M.; Bornkamm, G.W.; Kopf, M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015, 212, 555–568. [Google Scholar] [CrossRef]





| Primers | Sequences (5′–3′) |
|---|---|
| GPX4-F | AAAC↓TGCAGATGAACCTCGGCCGCCTTTG |
| GPX4-R | CTAGT↓CTAGACTAGAAATAGTGGGGCAGGTCCT |
| GPX4-qPCR-F | ATAAGAACGGCTGCGTGGTGAAG |
| GPX4-qPCR-R | TAGAGATAGCACGGCAGGTCCTTC |
| SLC7A11-qPCR-F | CATGGTTGTCCTCTCCCTTTAC |
| SLC7A11-qPCR-R | ACTTGGGTTTCTTGTCCCATAC |
| ACSL4-qPCR-F | TATGGGCTGACAGAATCATG |
| ACSL4-qPCR-R | CAACTCTTCCAGTAGTGTAG |
| TFR1-qPCR-F | TGAACCTGGACTATGAGATG |
| TFR1-qPCR-R | TAGAAGTAGCACGGAAGTAG |
| B1-qPCR-F | ACGACATCGCATTCAAGGGA |
| B1-qPCR-R | CATGAGAGGAGGCAGCACAA |
| β-actin-qPCR-F | GGCTGTATTCCCCTCCATCG |
| β-actin-qPCR-R | CCAGTTGGTAACAATGCCATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Niu, Z.; Lu, H.-H.; Li, S.-A.; Zhou, D.-H. Inhibition of GPX4 by Toxoplasma gondii Promotes Ferroptosis and Enhances Its Proliferation in Acute and Chronic Infection. Cells 2025, 14, 756. https://doi.org/10.3390/cells14100756
Gu Y, Niu Z, Lu H-H, Li S-A, Zhou D-H. Inhibition of GPX4 by Toxoplasma gondii Promotes Ferroptosis and Enhances Its Proliferation in Acute and Chronic Infection. Cells. 2025; 14(10):756. https://doi.org/10.3390/cells14100756
Chicago/Turabian StyleGu, Yanlong, Zhipeng Niu, Hui-Hong Lu, Si-Ang Li, and Dong-Hui Zhou. 2025. "Inhibition of GPX4 by Toxoplasma gondii Promotes Ferroptosis and Enhances Its Proliferation in Acute and Chronic Infection" Cells 14, no. 10: 756. https://doi.org/10.3390/cells14100756
APA StyleGu, Y., Niu, Z., Lu, H.-H., Li, S.-A., & Zhou, D.-H. (2025). Inhibition of GPX4 by Toxoplasma gondii Promotes Ferroptosis and Enhances Its Proliferation in Acute and Chronic Infection. Cells, 14(10), 756. https://doi.org/10.3390/cells14100756





