Survivin Interference and SurVaxM as an Adjunct Therapy for Glioblastoma Multiforme
Abstract
1. Introduction
1.1. Overview of GBM and Current Treatment Modalities
1.2. Overview of Inhibitor of Apoptosis Protein Survivin
2. Survivin Cellular Localization Impacts Overall Survival
3. Survivin Overexpression and Immunosurveillance in Glioblastoma
4. Survivin Regulation Impacts Glioma Tumor Viability
4.1. Survivin and Radioresistance in GBM
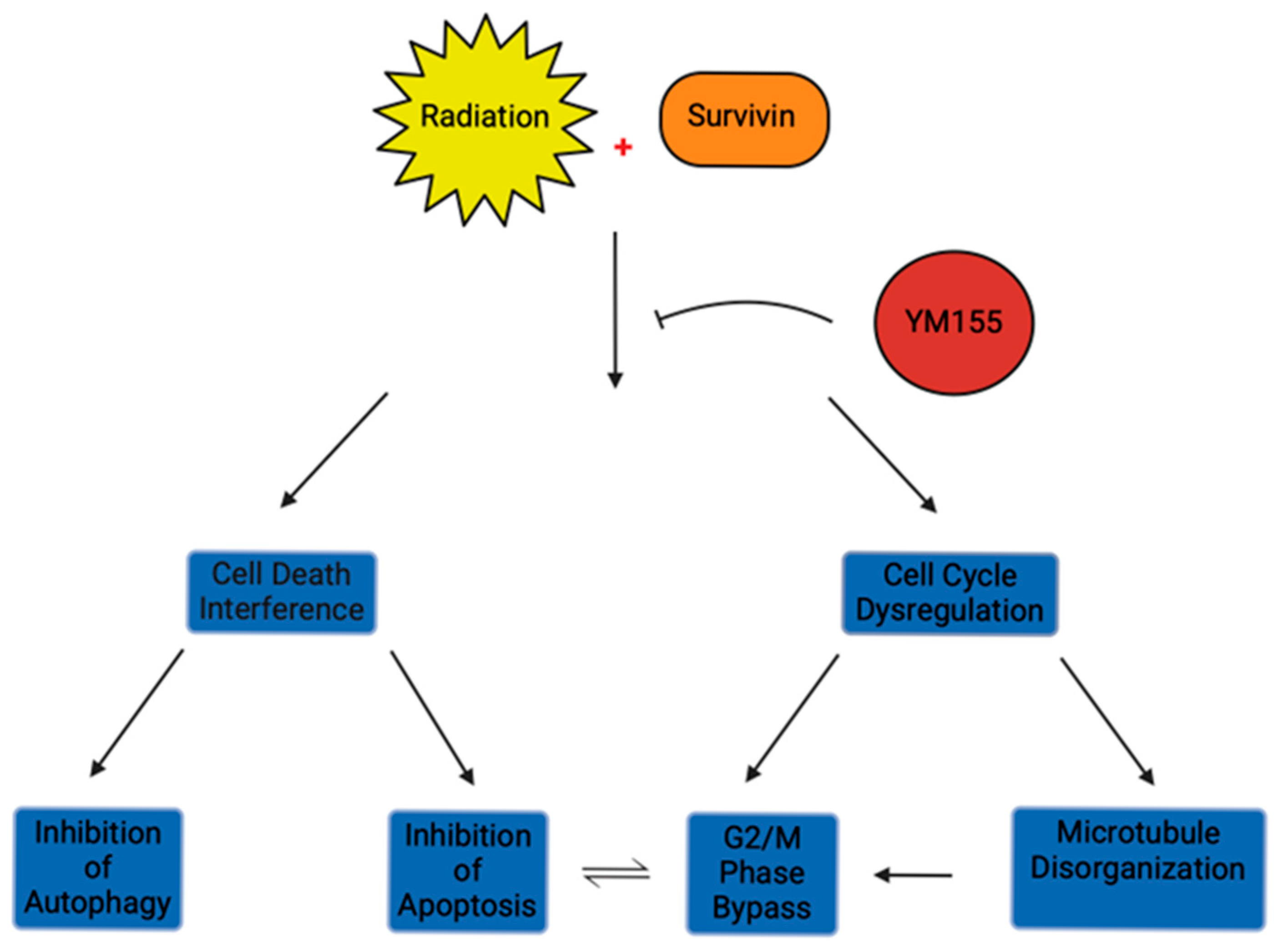
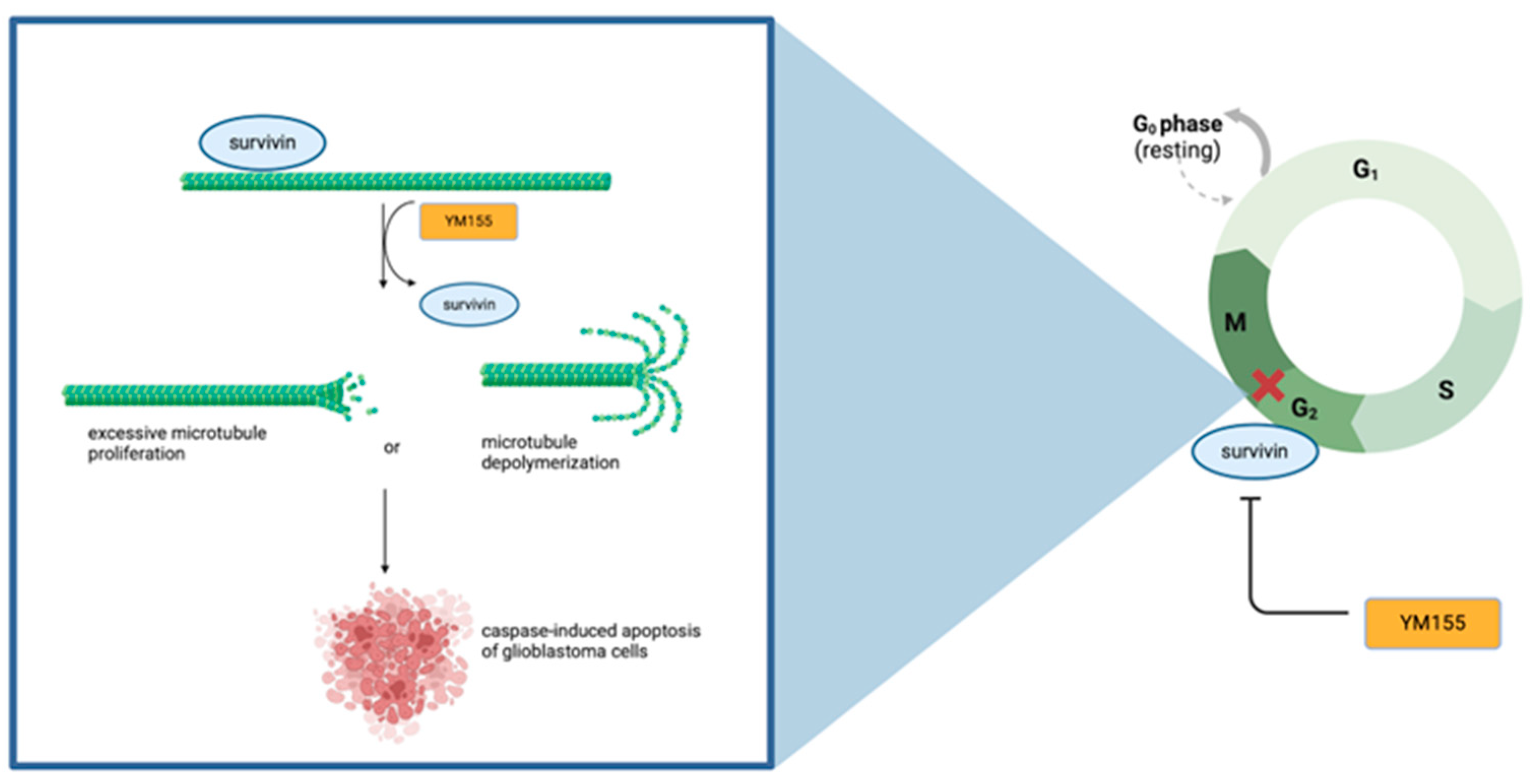
4.2. Survivin Inhibitor YM155 Enhances Radiosensitivity in Glioblastoma
4.3. Survivin Downregulation Impacts Radiosensitivity Through Inducing Apoptosis and Regulating Interphase
| Survivin Inhibitor | Molecular Structure | GBM Cell Viability IC50 | Observed Effects on Glioblastoma Tissue |
|---|---|---|---|
| YM155 | Imidazolium compound | 30–35 nM | Apoptosis induction, centrosomal duplication, radiosensitization |
| Cucurbitacin | Oxidized tetracyclic triterpenoid [91] | 2.5 µM | Mitotic spindle depletion, G2/M cell cycle arrest through increasing GAD45γ, EGF-induced FAK, AKT, and GSK3ß cell inhibition |
| AZTM | Azide-terminated survivin ligand derivative | 0.78–4.5 nM [92] | Apoptosis induction, radiosensitization |
| Parthenolide | Germacrane sequiterpenenoid | 16 µM | Mild apoptosis induction, autophagy induction, G2/M cell cycle arrest |
| Piperine | Alkaloid derivative | 120 µM | Cell migration reduction |
| Spironolactone | Antimineralicorticoid Steroid lactone | Unknown | Chemoresistance |
4.4. Survivin Regulatory Agents Show Promise for Adjunctive Therapy with Standard Treatment
5. SurVaxM Vaccine Therapy as a Treatment Modality for Refractive Glioblastoma
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wirsching, H.-G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: A retrospective study. Rep. Pract. Oncol. Radiother. J. Gt. Cancer Cent. Poznan Pol. Soc. Radiat. Oncol. 2022, 27, 1026–1036. [Google Scholar] [CrossRef]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Larjavaara, S.; Mäntylä, R.; Salminen, T.; Haapasalo, H.; Raitanen, J.; Jääskeläinen, J.; Auvinen, A. Incidence of gliomas by anatomic location. Neuro-Oncol. 2007, 9, 319–325. [Google Scholar] [CrossRef]
- Teo, M.; Martin, S.; Owusu-Agyemang, K.; Nowicki, S.; Clark, B.; Mackinnon, M.; Stewart, W.; Paul, J.; St George, J. A survival analysis of GBM patients in the West of Scotland pre- and post-introduction of the Stupp regime. Br. J. Neurosurg. 2014, 28, 351–355. [Google Scholar] [CrossRef]
- Sanson, M.; Marie, Y.; Paris, S.; Idbaih, A.; Laffaire, J.; Ducray, F.; El Hallani, S.; Boisselier, B.; Mokhtari, K.; Hoang-Xuan, K.; et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4150–4154. [Google Scholar] [CrossRef]
- Weller, M.; Felsberg, J.; Hartmann, C.; Berger, H.; Steinbach, J.P.; Schramm, J.; Westphal, M.; Schackert, G.; Simon, M.; Tonn, J.C.; et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: A prospective translational study of the German Glioma Network. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5743–5750. [Google Scholar] [CrossRef]
- Kálovits, F.; Tompa, M.; Nagy, Á.; Kálmán, B. Isocitrate dehydrogenase mutations in defining the biology of and supporting clinical decision making in glioblastoma. Ideggyogyaszati Szle. 2018, 71, 237–247. [Google Scholar] [CrossRef]
- Davis, M.; Pragani, R.; Popovici-Muller, J.; Gross, S.; Thorne, N.; Salituro, F.; Fantin, V.; Straley, K.; Su, M.; Dang, L.; et al. ML309: A potent inhibitor of R132H mutant IDH1 capable of reducing 2-hydroxyglutarate production in U87 MG glioblastoma cells. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. Available online: http://www.ncbi.nlm.nih.gov/books/NBK153220/ (accessed on 12 March 2025).
- Cohen, A.L.; Holmen, S.L.; Colman, H. IDH1 and IDH2 mutations in gliomas. Curr. Neurol. Neurosci. Rep. 2013, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.J.; Gubbiotti, M.A.; Carlin, A.M.; Nasrallah, M.P.; Van Deerlin, V.M.; Herlihy, S.E. Clinical Evaluation of IDH Mutation Status in Formalin-Fixed Paraffin-Embedded Tissue in Gliomas. Mol. Diagn. Ther. 2023, 27, 371–381. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Hainfellner, J.A.; Marosi, C.; Preusser, M. Assessing MGMT methylation status and its current impact on treatment in glioblastoma. CNS Oncol. 2015, 4, 47–52. [Google Scholar] [CrossRef]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro-Oncol. 2019, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi Mashhad, M.; Hassanian, S.M.; Anvari, K.; Avan, A. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J. Cell. Physiol. 2018, 233, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.-D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet Lond. Engl. 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Herrlinger, U.; Schäfer, N.; Steinbach, J.P.; Weyerbrock, A.; Hau, P.; Goldbrunner, R.; Friedrich, F.; Rohde, V.; Ringel, F.; Schlegel, U.; et al. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine-DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1611–1619. [Google Scholar] [CrossRef]
- Dietrich, J. Clinical presentation, diagnosis, and initial surgical management of high-grade gliomas. In UpToDate; Wolters Kluwer. Available online: https://www.uptodate.com/contents/clinical-presentation-diagnosis-and-initial-surgical-management-of-high-grade-gliomas (accessed on 12 March 2025).
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Brandner, S.; McAleenan, A.; Jones, H.E.; Kernohan, A.; Robinson, T.; Schmidt, L.; Dawson, S.; Kelly, C.; Leal, E.S.; Faulkner, C.L.; et al. Diagnostic accuracy of 1p/19q codeletion tests in oligodendroglioma: A comprehensive meta-analysis based on a Cochrane systematic review. Neuropathol. Appl. Neurobiol. 2022, 48, e12790. [Google Scholar] [CrossRef] [PubMed]
- Marker, D.F.; Agnihotri, S.; Amankulor, N.; Murdoch, G.H.; Pearce, T.M. The dominant TP53 hotspot mutation in IDH -mutant astrocytoma, R273C, has distinctive pathologic features and sex-specific prognostic implications. Neuro-Oncol. Adv. 2022, 4, vdab182. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.M.; Melamed, L.; Wetzel, E.A.; Chang, J.C.-C.; Nagashima, H.; Kitagawa, Y.; Muzyka, L.; Wakimoto, H.; Cahill, D.P.; Miller, J.J. CDKN2A/B Homozygous Deletion Sensitizes IDH-Mutant Glioma to CDK4/6 Inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Kahari, J.; Vestman, A.; Hallmans, M.; Johansson, M.; Bergenheim, A.T.; Sandström, M. Improved treatment of glioblastoma - changes in survival over two decades at a single regional Centre. Acta Oncol. Stockh. Swed. 2019, 58, 334–341. [Google Scholar] [CrossRef]
- Ståhl, P.; Henoch, I.; Smits, A.; Rydenhag, B.; Ozanne, A. Quality of life in patients with glioblastoma and their relatives. Acta Neurol. Scand. 2022, 146, 82–91. [Google Scholar] [CrossRef]
- Siragusa, G.; Tomasello, L.; Giordano, C.; Pizzolanti, G. Survivin (BIRC5): Implications in cancer therapy. Life Sci. 2024, 350, 122788. [Google Scholar] [CrossRef]
- Wheatley, S.P.; Altieri, D.C. Survivin at a glance. J. Cell Sci. 2019, 132, jcs223826. [Google Scholar] [CrossRef]
- Crook, N.E.; Clem, R.J.; Miller, L.K. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 1993, 67, 2168–2174. [Google Scholar] [CrossRef]
- Hrdinka, M.; Yabal, M. Inhibitor of apoptosis proteins in human health and disease. Genes Immun. 2019, 20, 641–650. [Google Scholar] [CrossRef]
- Verdecia, M.A.; Huang, H.; Dutil, E.; Kaiser, D.A.; Hunter, T.; Noel, J.P. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat. Struct. Biol. 2000, 7, 602–608. [Google Scholar] [CrossRef]
- Li, F.; Ambrosini, G.; Chu, E.Y.; Plescia, J.; Tognin, S.; Marchisio, P.C.; Altieri, D.C. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hama, S.; Izumi, H.; Yamasaki, F.; Kajiwara, Y.; Matsuura, S.; Morishima, K.; Hidaka, T.; Shrestha, P.; Sugiyama, K.; et al. Centrosome amplification induced by survivin suppression enhances both chromosome instability and radiosensitivity in glioma cells. Br. J. Cancer 2008, 98, 345–355. [Google Scholar] [CrossRef]
- de Rodrigues, F.O.; Sarmet, M.; Maldaner, V.; Yamasaki, R.; Behlau, M.; Davison Mangilli, L. Traumatic Spinal Injury: Preliminary Results of Respiratory Function, Voice and Quality of Life. J. Voice Off. J. Voice Found. 2023, 37, 469.e1–469.e10. [Google Scholar] [CrossRef]
- Beardmore, V.A.; Ahonen, L.J.; Gorbsky, G.J.; Kallio, M.J. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J. Cell Sci. 2004, 117, 4033–4042. [Google Scholar] [CrossRef] [PubMed]
- Borah, N.A.; Reddy, M.M. Aurora Kinase B Inhibition: A Potential Therapeutic Strategy for Cancer. Mol. Basel Switz. 2021, 26, 1981. [Google Scholar] [CrossRef] [PubMed]
- Chandele, A.; Prasad, V.; Jagtap, J.C.; Shukla, R.; Shastry, P.R. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia N. Y. 2004, 6, 29–40. [Google Scholar] [CrossRef]
- Dai, D.; Liang, Y.; Xie, Z.; Fu, J.; Zhang, Y.; Zhang, Z. Survivin deficiency induces apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Oncol. Rep. 2012, 27, 621–627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. J. Am. Soc. Gene Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Reddy, R.; Yan, S.C.; Hasanpour Segherlou, Z.; Hosseini-Siyanaki, M.-R.; Poe, J.; Perez-Vega, C.; Chiocca, E.A.; Lucke-Wold, B. Oncolytic viral therapy: A review and promising future directions. J. Neurosurg. 2024, 140, 319–327. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Reardon, D.A.; Abad, A.P.; Curry, W.T.; Wong, E.T.; Figel, S.A.; Mechtler, L.L.; Peereboom, D.M.; Hutson, A.D.; Withers, H.G.; et al. Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 1453–1465. [Google Scholar] [CrossRef]
- Fenstermaker, R.A.; Ciesielski, M.J.; Qiu, J.; Yang, N.; Frank, C.L.; Lee, K.P.; Mechtler, L.R.; Belal, A.; Ahluwalia, M.S.; Hutson, A.D. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol. Immunother. CII 2016, 65, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Schrama, D.; Thor Straten, P.; Becker, J.C. Cytotoxic T cells. J. Invest. Dermatol. 2006, 126, 32–41. [Google Scholar] [CrossRef]
- Saito, T.; Sugiyama, K.; Takeshima, Y.; Amatya, V.J.; Yamasaki, F.; Takayasu, T.; Nosaka, R.; Muragaki, Y.; Kawamata, T.; Kurisu, K. Prognostic implications of the subcellular localization of survivin in glioblastomas treated with radiotherapy plus concomitant and adjuvant temozolomide. J. Neurosurg. 2018, 128, 679–684. [Google Scholar] [CrossRef]
- Faccion, R.S.; Bernardo, P.S.; de Lopes, G.P.F.; Bastos, L.S.; Teixeira, C.L.; de Oliveira, J.A.; Fernandes, P.V.; Dubois, L.G.; Chimelli, L.; Maia, R.C. p53 expression and subcellular survivin localization improve the diagnosis and prognosis of patients with diffuse astrocytic tumors. Cell. Oncol. Dordr. Neth. 2018, 41, 141–157. [Google Scholar] [CrossRef]
- Saito, T.; Arifin, M.T.; Hama, S.; Kajiwara, Y.; Sugiyama, K.; Yamasaki, F.; Hidaka, T.; Arita, K.; Kurisu, K. Survivin subcellular localization in high-grade astrocytomas: Simultaneous expression in both nucleus and cytoplasm is negative prognostic marker. J. Neurooncol. 2007, 82, 193–198. [Google Scholar] [CrossRef]
- Sasaki, T.; Lopes, M.B.S.; Hankins, G.R.; Helm, G.A. Expression of survivin, an inhibitor of apoptosis protein, in tumors of the nervous system. Acta Neuropathol. 2002, 104, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-E.; Kim, T.-K.; Lee, J.-S.; Oh, S.-Y.; Kwak, S.; Jin, X.; Sohn, J.-Y.; Song, M.-K.; Sohn, Y.-W.; Lee, S.-Y.; et al. Survivin inhibits anti-growth effect of p53 activated by aurora B. Biochem. Biophys. Res. Commun. 2005, 336, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Amini, J.; Zafarjafarzadeh, N.; Ghahramanlu, S.; Mohammadalizadeh, O.; Mozaffari, E.; Bibak, B.; Sanadgol, N. Role of Circular RNA MMP9 in Glioblastoma Progression: From Interaction With hnRNPC and hnRNPA1 to Affecting the Expression of BIRC5 by Sequestering miR-149. J. Mol. Recognit. JMR 2025, 38, e3109. [Google Scholar] [CrossRef]
- Lebelt, A.; Rutkowski, R.; Och, W.; Jaczun, K.; Dziemiańczyk-Pakieła, D.; Milewski, R.; Mariak, Z.; Reszeć, J. Survivin, caspase-3 and MIB-1 expression in astrocytic tumors of various grades. Adv. Med. Sci. 2016, 61, 237–243. [Google Scholar] [CrossRef]
- Das, A.; Tan, W.-L.; Teo, J.; Smith, D.R. Expression of survivin in primary glioblastomas. J. Cancer Res. Clin. Oncol. 2002, 128, 302–306. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Yamasaki, F.; Hama, S.; Yahara, K.; Yoshioka, H.; Sugiyama, K.; Arita, K.; Kurisu, K. Expression of survivin in astrocytic tumors: Correlation with malignant grade and prognosis. Cancer 2003, 97, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Poiret, T.; Persson, O.; Meng, Q.; Rane, L.; Bartek, J.; Karbach, J.; Altmannsberger, H.-M.; Illies, C.; Luo, X.; et al. NY-ESO-1- and survivin-specific T-cell responses in the peripheral blood from patients with glioma. Cancer Immunol. Immunother. CII 2018, 67, 237–246. [Google Scholar] [CrossRef]
- Kleinschmidt-DeMasters, B.K.; Heinz, D.; McCarthy, P.J.; Bobak, J.B.; Lillehei, K.O.; Shroyer, A.L.W.; Shroyer, K.R. Survivin in glioblastomas. Protein and messenger RNA expression and comparison with telomerase levels. Arch. Pathol. Lab. Med. 2003, 127, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kuroiwa, T.; Nakagawa, T.; Kajimoto, Y.; Dohi, T.; Azuma, H.; Tsuji, M.; Kami, K.; Miyatake, S.-I. Transcriptional expression of survivin and its splice variants in brain tumors in humans. J. Neurosurg. 2003, 99, 738–745. [Google Scholar] [CrossRef]
- Tong, X.; Yang, P.; Wang, K.; Liu, Y.; Liu, X.; Shan, X.; Huang, R.; Zhang, K.; Wang, J. Survivin is a prognostic indicator in glioblastoma and may be a target of microRNA-218. Oncol. Lett. 2019, 18, 359–367. [Google Scholar] [CrossRef]
- Chakravarti, A.; Noll, E.; Black, P.M.; Finkelstein, D.F.; Finkelstein, D.M.; Dyson, N.J.; Loeffler, J.S. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Brun, S.N.; Markant, S.L.; Esparza, L.A.; Garcia, G.; Terry, D.; Huang, J.-M.; Pavlyukov, M.S.; Li, X.-N.; Grant, G.A.; Crawford, J.R.; et al. Survivin as a therapeutic target in Sonic hedgehog-driven medulloblastoma. Oncogene 2015, 34, 3770–3779. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Zhou, D.; Zhou, Y.; Ye, M.; Wang, H.; Du, Z. Downregulation of Survivin Gene Expression Affects Ionizing Radiation Resistance of Human T98 Glioma Cells. Cell. Mol. Neurobiol. 2018, 38, 861–868. [Google Scholar] [CrossRef]
- McLaughlin, N.; Annabi, B.; Bouzeghrane, M.; Temme, A.; Bahary, J.-P.; Moumdjian, R.; Béliveau, R. The Survivin-mediated radioresistant phenotype of glioblastomas is regulated by RhoA and inhibited by the green tea polyphenol (-)-epigallocatechin-3-gallate. Brain Res. 2006, 1071, 1–9. [Google Scholar] [CrossRef]
- Reichert, S.; Rödel, C.; Mirsch, J.; Harter, P.N.; Tomicic, M.T.; Mittelbronn, M.; Kaina, B.; Rödel, F. Survivin inhibition and DNA double-strand break repair: A molecular mechanism to overcome radioresistance in glioblastoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2011, 101, 51–58. [Google Scholar] [CrossRef]
- Anandharaj, A.; Cinghu, S.; Park, W.-Y. Rapamycin-mediated mTOR inhibition attenuates survivin and sensitizes glioblastoma cells to radiation therapy. Acta Biochim. Biophys. Sin. 2011, 43, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kita, A.; Yamanaka, K.; Mori, M.; Amino, N.; Takeuchi, M.; Tominaga, F.; Hatakeyama, S.; Kinoyama, I.; Matsuhisa, A.; et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007, 67, 8014–8021. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.-T.; Chang, L.-S. ONC212 enhances YM155 cytotoxicity by triggering SLC35F2 expression and NOXA-dependent MCL1 degradation in acute myeloid leukemia cells. Biochem. Pharmacol. 2024, 224, 116242. [Google Scholar] [CrossRef]
- Li, X.; Yang, F.; He, N.; Zhang, M.; Lv, Y.; Yu, Y.; Dong, Q.; Hou, X.; Hao, Y.; An, Z.; et al. YM155 inhibits neuroblastoma growth through degradation of MYCN: A new role as a USP7 inhibitor. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 181, 106343. [Google Scholar] [CrossRef]
- Hou, L.-J.; Huang, X.-X.; Xu, L.-N.; Zhang, Y.-Y.; Zhao, N.; Ou, R.-Y.; Li, W.-F.; Zhang, W.-J.; Jiang, Q.-W.; Yang, Y.; et al. YM155 enhances docetaxel efficacy in ovarian cancer. Am. J. Transl. Res. 2018, 10, 696–708. [Google Scholar]
- Rauch, A.; Hennig, D.; Schäfer, C.; Wirth, M.; Marx, C.; Heinzel, T.; Schneider, G.; Krämer, O.H. Survivin and YM155: How faithful is the liaison? Biochim. Biophys. Acta 2014, 1845, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.C.; Chen, S.H.; Yang, S.H.; Cheng, C.C.; Chiu, T.H.; Huang, Y.T. Novel survivin inhibitor YM155 elicits cytotoxicity in glioblastoma cell lines with normal or deficiency DNA-dependent protein kinase activity. Pediatr. Neonatol. 2012, 53, 199–204. [Google Scholar] [CrossRef]
- Dahan, P.; Martinez Gala, J.; Delmas, C.; Monferran, S.; Malric, L.; Zentkowski, D.; Lubrano, V.; Toulas, C.; Cohen-Jonathan Moyal, E.; Lemarie, A. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: Possible involvement in radioresistance. Cell Death Dis. 2014, 5, e1543. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Xu, R.; Ji, J.; Xu, Y.; Han, M.; Wei, Y.; Huang, B.; Chen, A.; Zhang, Q.; et al. YM155 decreases radiation-induced invasion and reverses epithelial-mesenchymal transition by targeting STAT3 in glioblastoma. J. Transl. Med. 2018, 16, 79. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Song, T.; Xin, T.; Zheng, Z.; Zhong, P.; Zhang, X. Silencing of survivin using YM155 inhibits invasion and suppresses proliferation in glioma cells. Cell Biochem. Biophys. 2015, 71, 587–593. [Google Scholar] [CrossRef]
- Temme, A.; Herzig, E.; Weigle, B.; Morgenroth, A.; Schmitz, M.; Kiessling, A.; Rieger, M.A.; Schackert, H.K.; Rieber, E.P. Inhibition of malignant glioma cell growth by a survivin mutant retrovirus. Hum. Gene Ther. 2005, 16, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gamero, A.M.; Borges, K.S.; Moreno, D.A.; Suazo, V.K.; Fujinami, M.M.; de Paula Gomes Queiroz, R.; de Oliveira, H.F.; Carlotti, C.G.; Scrideli, C.A.; Tone, L.G. Tetra-O-methyl nordihydroguaiaretic acid, an inhibitor of Sp1-mediated survivin transcription, induces apoptosis and acts synergistically with chemo-radiotherapy in glioblastoma cells. Invest. New Drugs 2013, 31, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Hendruschk, S.; Wiedemuth, R.; Aigner, A.; Töpfer, K.; Cartellieri, M.; Martin, D.; Kirsch, M.; Ikonomidou, C.; Schackert, G.; Temme, A. RNA interference targeting survivin exerts antitumoral effects in vitro and in established glioma xenografts in vivo. Neuro-Oncol. 2011, 13, 1074–1089. [Google Scholar] [CrossRef]
- George, J.; Banik, N.L.; Ray, S.K. Survivin knockdown and concurrent 4-HPR treatment controlled human glioblastoma in vitro and in vivo. Neuro-Oncol. 2010, 12, 1088–1101. [Google Scholar] [CrossRef]
- Gaca, S.; Reichert, S.; Multhoff, G.; Wacker, M.; Hehlgans, S.; Botzler, C.; Gehrmann, M.; Rödel, C.; Kreuter, J.; Rödel, F. Targeting by cmHsp70.1-antibody coated and survivin miRNA plasmid loaded nanoparticles to radiosensitize glioblastoma cells. J. Control. Release Off. J. Control. Release Soc. 2013, 172, 201–206. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Lin, X.; Li, L.; Chen, W.; Zhang, T.; Liu, Y.; Niu, L.; Zhang, Y.; Hu, P. Cucurbitacin E inhibits the proliferation of glioblastoma cells via FAK/AKT/GSK3β pathway. Oncol. Rep. 2023, 50, 221. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Chen, M.-J.; Huang, T.-Y. Inducement of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene from climbing stem of Cucumis melo L., through GADD45γ in human brain malignant glioma (GBM) 8401 cells. Cell Death Dis. 2014, 5, e1087. [Google Scholar] [CrossRef]
- Zhu, N.; Shao, Y.; Xu, L.; Yu, L.; Sun, L. Gadd45-alpha and Gadd45-gamma utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol. Biol. Rep. 2009, 36, 2075–2085. [Google Scholar] [CrossRef]
- Premkumar, D.R.; Jane, E.P.; Pollack, I.F. Cucurbitacin-I inhibits Aurora kinase A, Aurora kinase B and survivin, induces defects in cell cycle progression and promotes ABT-737-induced cell death in a caspase-independent manner in malignant human glioma cells. Cancer Biol. Ther. 2015, 16, 233–243. [Google Scholar] [CrossRef]
- Vader, G.; Kauw, J.J.W.; Medema, R.H.; Lens, S.M.A. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 2006, 7, 85–92. [Google Scholar] [CrossRef]
- Carmena, M.; Wheelock, M.; Funabiki, H.; Earnshaw, W.C. The chromosomal passenger complex (CPC): From easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 2012, 13, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Labarrade, F.; Botto, J.-M.; Domloge, N. CRM1 and chromosomal passenger complex component survivin are essential to normal mitosis progress and to preserve keratinocytes from mitotic abnormalities. Int. J. Cosmet. Sci. 2016, 38, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Knauer, S.K.; Bier, C.; Habtemichael, N.; Stauber, R.H. The Survivin-Crm1 interaction is essential for chromosomal passenger complex localization and function. EMBO Rep. 2006, 7, 1259–1265. [Google Scholar] [CrossRef]
- Jenkins, R.; Bandera, Y.P.; Daniele, M.A.; Ledford, L.L.; Tietje, A.; Kelso, A.A.; Sehorn, M.G.; Wei, Y.; Chakrabarti, M.; Ray, S.K.; et al. Sequestering survivin to functionalized nanoparticles: A strategy to enhance apoptosis in cancer cells. Biomater. Sci. 2016, 4, 614–626. [Google Scholar] [CrossRef]
- Samad, R.; Rahman, M.R.; Yunus, E.B.; Hussain, M.A.; Arif, S.M.; Islam, M.N.; Hafiz, S.a.M.M.A.; Hossain, M.M.; Faiz, M.A. An open randomized controlled trial to compare the efficacy of two fixed dose combinations of artemesinin based combinations for uncomplicated falciparum malaria in Bangladesh. Bangladesh Med. Res. Counc. Bull. 2013, 39, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Reichert, S.; Reinboldt, V.; Hehlgans, S.; Efferth, T.; Rödel, C.; Rödel, F. A radiosensitizing effect of artesunate in glioblastoma cells is associated with a diminished expression of the inhibitor of apoptosis protein survivin. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 103, 394–401. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef]
- Tang, T.-K.; Chiu, S.-C.; Lin, C.-W.; Su, M.-J.; Liao, M.-H. Induction of survivin inhibition, G2/M cell cycle arrest and autophagic on cell death in human malignant glioblastoma cells. Chin. J. Physiol. 2015, 58, 95–103. [Google Scholar] [CrossRef]
- Dai, S.; Wang, C.; Zhao, X.; Ma, C.; Fu, K.; Liu, Y.; Peng, C.; Li, Y. Cucurbitacin B: A review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol. Res. 2023, 187, 106587. [Google Scholar] [CrossRef]
- Cui, Q.; Huang, C.; Liu, J.-Y.; Zhang, J.-T. Small Molecule Inhibitors Targeting the “Undruggable” Survivin: The Past, Present, and Future from a Medicinal Chemist’s Perspective. J. Med. Chem. 2023, 66, 16515–16545. [Google Scholar] [CrossRef]
- Cruz, R.Q.; Morais, C.M.; Cardoso, A.M.; Silva, S.G.; Vale, M.L.; Marques, E.F.; Pedroso de Lima, M.C.; Jurado, A.S. Enhancing glioblastoma cell sensitivity to chemotherapeutics: A strategy involving survivin gene silencing mediated by gemini surfactant-based complexes. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2016, 104, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-Y.; Yeh, M.; Wang, Y.-Y.; Oh, C.; Zhao, Z.-M.; Kaur, B.; Lee, T.-J. MicroRNA-138 Increases Chemo-Sensitivity of Glioblastoma through Downregulation of Survivin. Biomedicines 2021, 9, 780. [Google Scholar] [CrossRef]
- Haq, I.-U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. PTR 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Warrier, N.M.; Krishnan, R.K.; Prabhu, V.; Hariharapura, R.C.; Agarwal, P.; Kumar, P. Survivin Inhibition by Piperine Sensitizes Glioblastoma Cancer Stem Cells and Leads to Better Drug Response. Int. J. Mol. Sci. 2022, 23, 7604. [Google Scholar] [CrossRef] [PubMed]
- Sanomachi, T.; Suzuki, S.; Togashi, K.; Sugai, A.; Seino, S.; Okada, M.; Yoshioka, T.; Kitanaka, C.; Yamamoto, M. Spironolactone, a Classic Potassium-Sparing Diuretic, Reduces Survivin Expression and Chemosensitizes Cancer Cells to Non-DNA-Damaging Anticancer Drugs. Cancers 2019, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Winograd, E.K.; Ciesielski, M.J.; Fenstermaker, R.A. Novel vaccines for glioblastoma: Clinical update and perspective. Immunotherapy 2016, 8, 1293–1308. [Google Scholar] [CrossRef]
- Galbo, P.M.; Ciesielski, M.J.; Figel, S.; Maguire, O.; Qiu, J.; Wiltsie, L.; Minderman, H.; Fenstermaker, R.A. Circulating CD9+/GFAP+/survivin+ exosomes in malignant glioma patients following survivin vaccination. Oncotarget 2017, 8, 114722–114735. [Google Scholar] [CrossRef]
- Pollack, I.F.; Jakacki, R.I.; Butterfield, L.H.; Hamilton, R.L.; Panigrahy, A.; Normolle, D.P.; Connelly, A.K.; Dibridge, S.; Mason, G.; Whiteside, T.L.; et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro-Oncol. 2016, 18, 1157–1168. [Google Scholar] [CrossRef]
- Ahmed, A.U.; Thaci, B.; Tobias, A.L.; Auffinger, B.; Zhang, L.; Cheng, Y.; Kim, C.K.; Yunis, C.; Han, Y.; Alexiades, N.G.; et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J. Natl. Cancer Inst. 2013, 105, 968–977. [Google Scholar] [CrossRef]
- Tobias, A.L.; Thaci, B.; Auffinger, B.; Rincón, E.; Balyasnikova, I.V.; Kim, C.K.; Han, Y.; Zhang, L.; Aboody, K.S.; Ahmed, A.U.; et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl. Med. 2013, 2, 655–666. [Google Scholar] [CrossRef]
- Ahluwalia, M.; Peereboom, D.; Rauf, Y.; Schilero, C.; Ciolfi, M.; Ciesielski, M.; Fenstermaker, R. CTIM-10. Phase II study of pembrolizumab plus SurVaxM for glioblastoma at first recurrence. Neuro-Oncol. 2020, 22, ii34–ii35. [Google Scholar] [CrossRef]
- Fenstermaker, R.; Ahluwalia, M.; Ciesielski, M.; SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma (SURVIVE). ClinicalTrials/gov. Available online: https://aging.networkofcare.org/riverside/CommunityResources/ClinicalTrials/Detail/NCT05163080?keyword=%22Newly%20Diagnosed%22 (accessed on 17 April 2025).
| Trial Name/NCT Number | Clinical Phase | Status | Notable Discoveries |
|---|---|---|---|
| Phase I Study of Safety, Tolerability, and Immunologic Effects of a Survivin Peptide Mimic Vaccine (SurVaxM) in Patients with Recurrent Malignant Glioma | Phase I | Completed | Adverse effects elicited from patients include erythema (reddening) of the injection site, fatigue, myalgia, lymphopenia, leukopenia. |
| Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma | Phase II | Completed | No serious adverse effects elicited. Ninety-five percent of patients remained progression-free after diagnosis. Median progression-free survival (PFS) was 11.4 months, with an overall survival of 25.9 months. Survivin elicited a cytotoxic cellular and humoral response in vaccinated participants. |
| SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma [104] | Phase II | In Progress | N/A |
| Phase II Study of Pembrolizumab Plus SurVaxM for Glioblastoma at First Recurrence | Phase II | In Progress | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, W.J.; Gurramkonda, N.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. Survivin Interference and SurVaxM as an Adjunct Therapy for Glioblastoma Multiforme. Cells 2025, 14, 755. https://doi.org/10.3390/cells14100755
Elliott WJ, Gurramkonda N, Guda MR, Tsung AJ, Velpula KK. Survivin Interference and SurVaxM as an Adjunct Therapy for Glioblastoma Multiforme. Cells. 2025; 14(10):755. https://doi.org/10.3390/cells14100755
Chicago/Turabian StyleElliott, Willie James, Nandini Gurramkonda, Maheedhara R. Guda, Andrew J. Tsung, and Kiran K. Velpula. 2025. "Survivin Interference and SurVaxM as an Adjunct Therapy for Glioblastoma Multiforme" Cells 14, no. 10: 755. https://doi.org/10.3390/cells14100755
APA StyleElliott, W. J., Gurramkonda, N., Guda, M. R., Tsung, A. J., & Velpula, K. K. (2025). Survivin Interference and SurVaxM as an Adjunct Therapy for Glioblastoma Multiforme. Cells, 14(10), 755. https://doi.org/10.3390/cells14100755







